Abstract
The matrix (M) protein of vesicular stomatitis virus (VSV) expressed in the absence of other viral components causes many of the cytopathic effects of VSV, including an inhibition of host gene expression and the induction of cell rounding. It was recently shown that M protein also induces apoptosis in the absence of other viral components. This raises the possibility that the activation of apoptotic pathways causes the inhibition of host gene expression and cell rounding by M protein. To test this hypothesis, host gene expression and cell rounding were analyzed after the transfection of M mRNA into HeLa cells stably overexpressing Bcl-2 (HeLa-Bcl-2 cells). We have shown previously that Bcl-2 inhibits M-protein-induced apoptosis. Here, we show that activation of the apoptotic pathways downstream of Bcl-2 is not required for the inhibition of host gene expression by M protein. In contrast, overexpression of Bcl-2 inhibited cell rounding induced by M protein, indicating that apoptotic pathways downstream of Bcl-2 are required for the cell-rounding activities of M protein.
The matrix (M) protein of vesicular stomatitis virus (VSV) is remarkable for the number of different roles it plays in virus-infected cells. M protein's multiple activities can be broadly classified into viral assembly functions and activities that lead to cytopathogenesis. The viral assembly functions of M protein include the ability to condense the viral nucleocapsid into a tightly coiled helix and the ability to interact with host plasma membranes to generate the viral envelope (3, 12, 20). M protein's activities that contribute to cytopathogenesis include an ability to induce cell rounding, the ability to inhibit host gene expression, and the ability to induce apoptosis (5-7, 9, 14, 15, 21). The viral assembly functions of M protein are genetically separable from its cytopathic functions (6, 15). Thus, the activities of M protein that are involved in virus assembly do not cause the cytopathic effects of M protein. However, the cell-rounding activity and the induction of apoptosis by M protein are genetically correlated with its ability to inhibit host gene expression, raising the possibility that these activities are related to each other (6, 14, 15). The many effects of M protein raise the question of how one protein can have so many activities. One possibility is that what appear to be multiple separate activities are actually related by cause and effect. The goal of the experiments here was to determine the role of the induction of apoptosis by M protein in two other cytopathic effects caused by M protein: the inhibition of host gene expression and the induction of cell rounding.
The first cytopathic activity described for M protein was its ability to induce cell rounding (7). It was subsequently shown that M protein interacts with tubulin in vitro and that tubulin coimmunoprecipitates with M protein from VSV-infected cells (17). These results suggested that the cell-rounding activity by M protein is due, in part, to its interaction with tubulin. However, cell rounding requires disruption of multiple cytoskeletal elements as well as disruption of cell-substrate adhesion. Thus, an interaction with tubulin is not sufficient to induce cell rounding and multiple cellular targets must be affected in order for cell rounding to occur.
The second cytopathic activity described for M protein was its ability to inhibit host gene expression (5). M protein inhibits transcription by all three host RNA polymerases in the absence of other viral components (1). In the case of host RNA polymerase II, the target of the inhibition was identified as the general transcription factor, TFIID (27, 28). However, the inactivation of TFIID appears to be an indirect effect of M protein, mediated by host factors that have yet to be identified. The inhibition of host gene expression also involves an inhibition of the nucleocytoplasmic transport of RNAs and proteins (10). M-protein-induced inhibition of nucleocytoplasmic transport appears to be caused by its interaction with one or more nuclear pore components, including the nucleoporin Nup98 (22, 25).
It was recently reported that M protein contributes to cytopathogenesis through its ability to induce apoptosis in the absence of other viral components (14). We hypothesize that the multiple pathways involved in the process of apoptosis may be responsible for many of M protein's effects. For example, cell rounding is a prominent feature of apoptosis and, therefore, the induction of apoptosis may account for the cell-rounding activity of M protein. Likewise, the activation of the apoptotic pathways may cause the inhibition of host gene expression by inactivating host transcription factors such as TFIID. Alternatively, the inhibition of host gene expression may cause the induction of apoptosis. Indeed, our recent data showed that the induction of apoptosis by M protein resembles that induced by the pharmacologic inhibitors of host transcription, such as actinomycin D. For example, both M protein and actinomycin D activate the caspase-9 pathway, and apoptosis can be inhibited by the overexpression of Bcl-2, a host protein that prevents the activation of apoptotic pathways by inhibiting the release of proapoptotic factors from mitochondria (13).
The activation of apoptotic pathways downstream of Bcl-2 is not required for the inhibition of host gene expression by M protein.
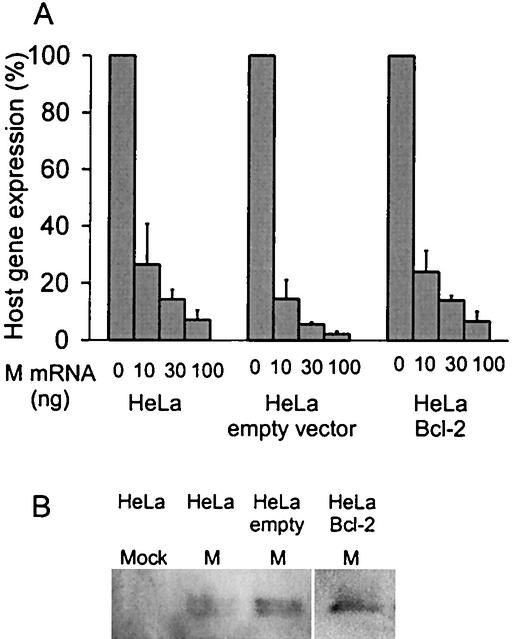
We have shown previously that the induction of apoptosis by M protein is dramatically reduced in HeLa cell lines (HeLa-Bcl-2 cells) that stably overexpress Bcl-2 (13). To determine whether the activation of apoptotic pathways is required for the inhibition of host gene expression by M protein, HeLa-Bcl-2 cells were transfected with M mRNA and the level of host gene expression was measured (Fig. 1A). M protein was expressed from transfected mRNA rather than plasmid DNA, since M protein inhibits its own expression from DNA vectors that depend on host transcription (4, 5). The methods for the in vitro transcription of M mRNA containing a 5′ cap and a 3′ poly(A) tail were as described previously (4, 14).
FIG. 1.
Overexpression of Bcl-2 does not affect the inhibition of host gene expression by M protein. (A) Stable HeLa-Bcl-2 cells and HeLa-empty-vector cells were generated and maintained as previously described (13). HeLa cells, HeLa-empty-vector cells, and HeLa-Bcl-2 cells grown in six-well plates were transfected as described previously (4) with yeast RNA as a negative control or with increasing amounts of M mRNA as indicated. All samples were transfected with 500 ng of a plasmid containing a luciferase reporter gene under the control of an SV40 promoter, a typical polymerase II promoter (2). The total amount of RNA transfected was held constant at 1 μg with yeast RNA. Host gene expression was measured by analyzing luciferase activity with the luciferase assay system (Promega) as previously described (2). The data for each cell line are expressed as a percentage of the yeast RNA control. The data represent the averages ± standard deviations from three experiments. (B) HeLa cells, HeLa-empty-vector cells, and HeLa-Bcl-2 cells were transfected with M mRNA for 24 h. Cells were harvested, and equal amounts of lysates were analyzed for Western blot analysis with an antibody for M protein. Lysates from mock-treated HeLa cells were used as a control.
HeLa-Bcl-2 cells, control HeLa cells stably transfected with the empty vector (HeLa-empty-vector cells), and the original HeLa cells were transfected with increasing amounts of M mRNA and with a constant amount of a plasmid containing a luciferase reporter gene under the control of a simian virus 40 (SV40) promoter (pGL3) that serves as an example of a typical RNA polymerase II-dependent promoter. Data from other studies indicate that cotransfection of mRNA and plasmid DNA results in expression from both in transfected cells (1, 4, 15, 16). It was also previously determined by flow cytometry that the transfection of enhanced green fluorescent protein (EGFP) mRNA results in approximately 30% EGFP-positive cells for all three cell lines, indicating that all three cell types are transfected with similar transfection efficiencies (13). At 24 h posttransfection, the cells were lysed and gene expression from the SV40 promoter was measured by assaying luciferase activity (Luciferase Assay System; Promega). Figure 1A shows the levels of luciferase expression as a percentage of a negative control transfected with yeast RNA. There was a titratable decrease in gene expression in all three cell lines after transfection with increasing amounts of M mRNA. This level of inhibition of host gene expression by M protein is similar to that discussed in previously published reports (5, 6, 16). There was no difference in the extent of inhibition in HeLa-Bcl-2 cells compared to that in the original HeLa cells and HeLa-empty-vector cells. These results indicate that the inhibition of host gene expression by M protein cannot be inhibited by the overexpression of Bcl-2. Thus, the activation of apoptotic pathways downstream of Bcl-2 is not necessary for the inhibition of host gene expression by M protein.
To determine whether the levels of M protein were similar in the three HeLa cell lines, Western blot analysis was performed. The three cell lines were transfected with M mRNA; equal amounts of cell lysates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and a Western blot was performed with the anti-M-protein monoclonal antibody (23H12) as described previously (8). Figure 1B shows a Western blot of mock-treated HeLa cells and of HeLa cells, HeLa-empty-vector cells, and HeLa-Bcl-2 cells transfected with M mRNA. These data show that the levels of M protein expression in the three cell types were similar.
The cell-rounding activity of M protein is dependent on the induction of apoptosis.
Cell rounding is a prominent morphological change that occurs during apoptosis. Thus, it is possible that M protein may have two mechanisms for causing cell rounding, one involving an interaction with cytoskeletal proteins and the other due to the induction of apoptosis. To determine if M protein has cell-rounding activity independent of the induction of apoptosis, HeLa-Bcl-2 cells and HeLa-empty-vector cells were cotransfected with M mRNA and EGFP mRNA so that transfected cells could be identified by EGFP fluorescence, and the percentage of fluorescent cells that were round was determined by fluorescence microscopy. Negative controls were transfected with EGFP mRNA alone. Transfected cells were incubated for 18 or 24 h and then analyzed for cell rounding and the morphological changes associated with apoptosis by using a 20× objective on a Nikon microscope (13). Between 70 and 200 fluorescent cells in six representative images for each sample were scored as either flat or round for each experiment. The criteria for the evaluation of cell rounding were described previously (15).
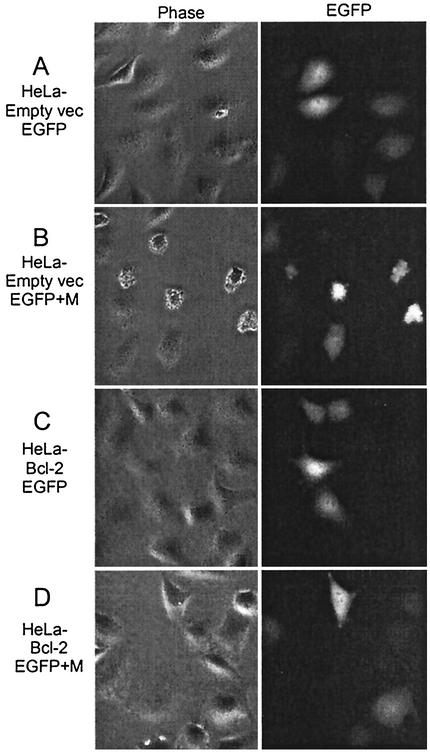
Representative phase-contrast images and fluorescence images taken of the same fields at 18 h posttransfection are shown in Fig. 2. Nearly all of the HeLa-empty-vector cells and HeLa-Bcl-2 cells transfected with EGFP mRNA alone were flat (Fig. 2A and C). HeLa-empty-vector cells transfected with EGFP and M mRNA for 18 h displayed an increase in the number of round cells (Fig. 2B). The cells also were undergoing other morphological changes associated with apoptosis, such as cell shrinking and membrane blebbing. It had previously been determined that the expression of M protein in HeLa-empty-vector cells results in morphological and biochemical changes associated with apoptosis, such as the activation of caspases 3, 8, and 9. We also showed previously that overexpression of Bcl-2 dramatically reduces caspase activation (13). In contrast, HeLa-Bcl-2 cells transfected with EGFP and M mRNA displayed few, if any, round cells (Fig. 2D). These data indicate that Bcl-2 overexpression decreases the cell-rounding activity of M protein.
FIG. 2.
Images of HeLa-empty-vector cells and HeLa-Bcl-2 cells expressing M protein. HeLa-empty-vector cells and HeLa-Bcl-2 cells were transfected with 270 ng of EGFP mRNA or with 30 ng of M mRNA and 240 ng of EGFP as indicated. At 18 h posttransfection, cells were analyzed by phase-contrast (left) or fluorescence (right) microscopy with a 20× objective. Representative digital phase-contrast and fluorescence images of the same field are shown in each row. Cells were analyzed by using phase-contrast and fluorescence microscopy with a 20× objective on a Nikon microscope.
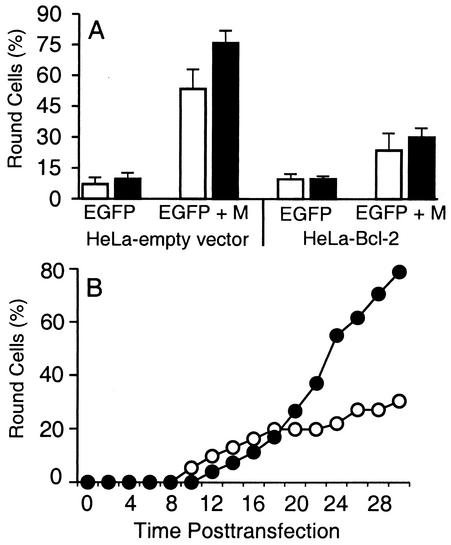
The extent of cell rounding was quantified by evaluating transfected cells for cell rounding in multiple static images (Fig. 3A), and in addition, the time course of cell rounding and other morphological changes associated with apoptosis was determined by time lapse microscopy (Fig. 3B). In Fig. 3A, the numbers of round and flat cells were counted in multiple images taken from four experiments and the percentages of fluorescent cells that were round are shown at 18 and 24 h posttransfection. Most of the fluorescent HeLa-empty-vector cells and HeLa-Bcl-2 cells transfected with EGFP mRNA alone were flat at 18 and 24 h, but there were a few round cells in these samples that were primarily cells undergoing cell division (Fig. 3A). HeLa-empty-vector cells cotransfected with EGFP and M mRNA displayed an increase in the percent round cells to 53% at 18 h and 76% by 24 h (Fig. 3A). In contrast, most of the HeLa-Bcl-2 cells transfected with EGFP and M mRNA were flat. At 18 h posttransfection, approximately 23% of HeLa-Bcl-2 cells were round, and by 24 h, approximately 30% of HeLa-Bcl-2 cells were round (Fig. 3A). These results indicate that Bcl-2 overexpression dramatically reduces the amount of round cells, indicating that the induction of apoptosis by M protein is important for most of its cell-rounding activity. However, overexpression of Bcl-2 did not completely inhibit the cell rounding induced by M protein, since there were more round cells when HeLa-Bcl-2 cells were transfected with M mRNA than in the negative control cells.
FIG. 3.
Overexpression of Bcl-2 reduces the amount of cell rounding after expression of M protein. (A) HeLa-empty-vector cells and HeLa-Bcl-2 cells were transfected with EGFP mRNA or with EGFP and M mRNA as indicated for 18 h (white bars) or 24 h (black bars) as in Fig. 2. Between 70 and 200 fluorescent cells in six representative images for each sample were scored as either flat or round for each experiment. The criteria for the evaluation of cell rounding were described previously (15). The data are expressed as the percentage of fluorescent cells that were round and represent the averages ± standard deviations from four experiments. (B) HeLa-empty-vector cells (closed circles) and HeLa-Bcl-2 cells (open circles) were transfected with EGFP and M mRNA and analyzed by fluorescence and phase-contrast time lapse microscopy. Fluorescence time lapse microscopy was performed as previously described (14). The time that each of 30 to 50 fluorescent cells became round was determined from the time-date record on the videotape. Data shown are the cumulative percentages of cells undergoing cell rounding as a function of time postinfection. The data represent the averages from two experiments.
Time lapse microscopy was used to determine the time course of cell rounding of HeLa-Bcl-2 cells and HeLa-empty-vector cells expressing M protein, as well as to determine the subsequent fate of cells that underwent cell rounding. HeLa-empty-vector cells and HeLa-Bcl-2 cells were transfected with EGFP and M mRNA, and fluorescence images were captured to indicate transfected cells. The progression of the transfected cells was followed with phase-contrast time lapse microscopy. The time that each of 30 to 50 transfected cells in the field underwent cell rounding and other morphological changes associated with apoptosis was determined from the time-date record on the videotape. The timing of the onset of cell rounding of HeLa-empty-vector cells and HeLa-Bcl-2 cells after transfection with EGFP and M mRNA is shown in Fig. 3B. The data are expressed as the cumulative percentage of round cells as a function of time posttransfection. Between 12 and 30 h posttransfection, approximately 80% of HeLa-empty-vector cells transfected with EGFP and M mRNA underwent cell rounding. In contrast, only approximately 30% of HeLa-Bcl-2 cells underwent cell rounding during this time period (Fig. 3B), similar to the results shown in Fig. 3A.
Despite the pronounced inhibition of cell rounding by Bcl-2 overexpression, Bcl-2 did not completely reduce the amount of round cells to the levels of the negative controls. One possible explanation for the incomplete reduction in cell rounding is that Bcl-2 may not completely inhibit apoptosis by M protein in all cells. In fact, previous results indicate that the caspase activation and cell death induced by M protein are dramatically reduced by the overexpression of Bcl-2 but not completely abolished. Another possible explanation for the incomplete reduction in cell rounding is that M protein has cell-rounding activities independent of apoptosis in a small percentage of cells.
To distinguish between these two possibilities, the transfected cells that underwent cell rounding were also examined for other morphological changes associated with apoptosis, such as membrane blebbing, a key morphological change associated with the induction of apoptosis. Cells undergoing apoptosis proceeded through a characteristic sequence of morphological changes, beginning with cell rounding, followed by membrane blebbing and cell shrinkage, and ultimately concluding in cell membrane rupture. By using time lapse microscopy, it could be distinguished whether the HeLa-Bcl-2 cells that became round after the expression of M protein also underwent the other morphological changes associated with apoptosis or were rounded without undergoing apoptosis. If M protein has cell-rounding activities independent of apoptotic pathways downstream of Bcl-2, we would expect that HeLa-Bcl-2 cells transfected with M mRNA would become round but not undergo the other morphological changes associated with apoptosis.
Of the 80% of transfected HeLa-empty-vector cells that became round (Fig. 3B), 90% of these round cells expressing M protein also underwent membrane blebbing by 30 h. These cells also underwent other morphological changes associated with apoptosis, such as cell shrinkage, and many showed signs of membrane blistering and rupture. Examination of the round HeLa-Bcl-2 cells expressing M protein revealed that, of the 30% of transfected cells that became round (Fig. 3B), approximately 88% of these cells also underwent membrane blebbing and the other morphological changes associated with apoptosis. These results indicate that the round HeLa-Bcl-2 cells expressing M protein were undergoing apoptosis, supporting the idea that overexpressing Bcl-2 does not completely inhibit apoptosis by M protein in all cells. Thus, the residual 30% cell rounding induced by M protein in HeLa-Bcl-2 cells was of cells undergoing apoptosis. Together, the data in Fig. 2 and 3 indicate that the cell-rounding activity by M protein is caused by the induction of apoptosis and that M protein does not have cell-rounding activity independent of the induction of apoptosis. This is the first report in which the inhibition of host gene expression has been separated experimentally from the cell-rounding activity of M protein.
Cause and effect relationships among the cytopathic effects of M protein.
The goal of the experiments here was to determine the role of the induction of apoptosis by M protein in two other cytopathic effects caused by M protein: the inhibition of host gene expression and the induction of cell rounding. Our hypothesis was that what appear to be multiple separate cytopathic effects are actually related by cause and effect. We determined whether the inhibition of M-protein-induced apoptosis prevented the inhibition of host gene expression and cell rounding. Our conclusion is that apoptotic pathways downstream of Bcl-2 do not cause the inhibition of host gene expression by M protein but that the induction of apoptosis does cause the cell-rounding activity of M protein.
It was recently determined that M protein induces apoptosis when expressed in the absence of other viral components and that M-protein-induced apoptosis is genetically correlated with its ability to inhibit host gene expression and not with its viral assembly functions (14). In addition, earlier data had shown that the cell-rounding activity of M protein is also genetically correlated with its ability to inhibit host gene expression (15). Collectively, these genetic correlations suggested that these cytopathic activities are related by cause and effect. Analysis of caspase activation supports the hypothesis that the induction of apoptosis by M protein is caused by its ability to inhibit host gene expression, since the induction of apoptosis by M protein has several similarities to that induced by the pharmacologic inhibitors of host gene expression. M protein and pharmacologic inhibitors of host gene expression, such as actinomycin D and 5,6-dichlorobenzimidazole riboside, primarily induce the activation of the caspase-9 pathway (13). In addition, the induction of apoptosis by both M protein and pharmacologic inhibitors of host gene expression can be inhibited by the overexpression of Bcl-2 (13). However, it remained possible that the inhibition of host gene expression was a consequence rather than a cause of the induction of apoptosis by M protein. Even though apoptosis induced by M protein was inhibited by the overexpression of Bcl-2, the inhibition of host gene expression was not prevented by Bcl-2 overexpression (Fig. 1). This result rules out the hypothesis that the induction of apoptotic pathways downstream of Bcl-2 is responsible for the inhibition of host gene expression by M protein.
The relationship of apoptosis induction to another prominent cytopathic effect of M protein, the induction of cell rounding, was also determined. It has previously been reported that M protein interacts with tubulin, and this interaction was believed to be responsible, at least in part, for the cell-rounding activity observed by M protein (17). However, in order for cells to become round, there must be both a disruption of the cytoskeletal elements, such as actin and tubulin, and also a disruption of cell adhesion molecules, which assist in maintaining cell shape by adherence to the substrate. Therefore, it is not likely that the disruption of the microtubule network alone would be sufficient to cause cell rounding. This is supported by evidence that the severe disruption of microtubules by different chemical agents, such as colchicine, 4-hydroxy-2(E)-nonenal, and tyrphostin AG-1714, does not correlate with cell rounding (19, 24, 26). However, cell rounding is a prominent cytopathic effect that occurs during the induction of apoptosis. Our data indicate that the activation of the apoptotic pathways downstream of Bcl-2 is required for cell rounding by M protein, indicating that M protein does not have additional cell-rounding activities independent of the induction of apoptosis (Fig. 2 and 3).
While the cell-rounding activity by M protein is caused by apoptosis, the possibility remains that the interaction of M protein with tubulin may have an important role in the pathogenesis of VSV in intact organisms. In intact organisms, VSV is neurotropic, infecting neurons and other cells of the central nervous system (11). Disruption of microtubules does not cause neurons to become round, but it dramatically reduces the number of cells that extend neurite processes such as axons and dendrites (19). One possibility is that disruption of the neurites may interfere with the signaling required for the host antiviral response by disrupting synaptic activity. Receptors for antiviral ligands, such as the gamma interferon receptor, have been shown to be localized to the synapses of interneuronal cells (23). Interferons are important antiviral agents in limiting VSV replication and pathogenesis (18). Thus, the disruption of microtubules by M protein may inhibit interferon signaling, and possibly other antiviral signaling, in the central nervous system. Therefore, the interaction of M protein with tubulin may be important for VSV to evade the host antiviral response in the central nervous system.
In summary, we analyzed the cause and effect relationships of three cellular effects of M protein: inhibition of host gene expression, cell rounding, and apoptosis. We determined that the induction of apoptosis accounts for the cell-rounding activity but not the inhibition of host gene expression. The experiments presented here, combined with our previous experiment and those of others, suggest that the inhibition of host gene expression causes apoptosis, which in turn causes the induction of cell rounding.
Acknowledgments
We acknowledge the Microscopy Core Laboratory at Wake Forest University. We thank Griffith Parks and Mark Willingham for helpful advice and comments on the manuscript.
This work was supported by Public Health Service grants AI 32983 and AI 15892 from the National Institute of Allergy and Infectious Diseases. S.A.K. was supported by National Institutes of Health Training Grant T32 A107401. The Microscopy Core Laboratory was supported in part by the core grant for the Comprehensive Cancer Center of Wake Forest University CA12197 for the National Cancer Institute.
REFERENCES
- 1.Ahmed, M., and D. S. Lyles. 1998. Effect of vesicular stomatitis virus matrix protein on transcription directed by host RNA polymerases I, II, and III. J. Virol. 72:8413-8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, M., M. O. McKenzie, S. Puckett, M. Hojnacki, L. Poliquin, and D. S. Lyles. Ability of matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 3.Barge, A., Y. Gaudin, P. Coulon, and R. H. Ruigrok. 1993. Vesicular stomatitis virus M protein may be inside the ribonucleocapsid coil. J. Virol. 67:7246-7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black, B. L., G. Brewer, and D. S. Lyles. 1994. Effect of vesicular stomatitis virus matrix protein on host-directed translation in vivo. J. Virol. 68:555-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black, B. L., and D. S. Lyles. 1992. Vesicular stomatitis virus matrix protein inhibits host cell-directed transcription of target genes in vivo. J. Virol. 66:4058-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black, B. L., R. B. Rhodes, M. McKenzie, and D. S. Lyles. 1993. The role of vesicular stomatitis virus matrix protein in inhibition of host-directed gene expression is genetically separable from its function in virus assembly. J. Virol. 67:4814-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blondel, D., G. G. Harmison, and M. Schubert. 1990. Role of matrix protein in cytopathogenesis of vesicular stomatitis virus. J. Virol. 64:1716-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor, J. H., and D. S. Lyles. 2002. Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1. J. Virol. 76:10177-10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferran, M. C., and J. M. Lucas-Lenard. 1997. The vesicular stomatitis virus matrix protein inhibits transcription from the human beta interferon promoter. J. Virol. 71:371-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Her, L. S., E. Lund, and J. E. Dahlberg. 1997. Inhibition of Ran guanosine triphosphatase-dependent nuclear transport by the matrix protein of vesicular stomatitis virus. Science 276:1845-1848. [DOI] [PubMed] [Google Scholar]
- 11.Huneycutt, B. S., Z. Bi, C. J. Aoki, and C. S. Reiss. 1993. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. J. Virol. 67:6698-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaptur, P. E., R. B. Rhodes, and D. S. Lyles. 1991. Sequences of the vesicular stomatitis virus matrix protein involved in binding to nucleocapsids. J. Virol. 65:1057-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopecky, S. A., and D. S. Lyles. Contrasting effects of matrix protein on apoptosis in HeLa and BHK cells infected with vesicular stomatitis virus are due to inhibition of host gene expression. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 14.Kopecky, S. A., M. C. Willingham, and D. S. Lyles. 2001. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J. Virol. 75:12169-12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyles, D. S., and M. O. McKenzie. 1997. Activity of vesicular stomatitis virus M protein mutants in cell rounding is correlated with the ability to inhibit host gene expression and is not correlated with virus assembly function. Virology 299:77-89. [DOI] [PubMed] [Google Scholar]
- 16.Lyles, D. S., M. O. McKenzie, M. Ahmed, and S. C. Woolwine. 1996. Potency of wild-type and temperature-sensitive vesicular stomatitis virus matrix protein in the inhibition of host-directed gene expression. Virology 225:172-180. [DOI] [PubMed] [Google Scholar]
- 17.Melki, R., Y. Gaudin, and D. Blondel. 1994. Interaction between tubulin and the viral matrix protein of vesicular stomatitis virus: possible implications in the viral cytopathic effect. Virology 202:339-347. [DOI] [PubMed] [Google Scholar]
- 18.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 19.Neely, M. D., K. R. Sidell, D. G. Graham, and T. J. Montine. 1999. The lipid peroxidation product 4-hydroxynonenal inhibits neurite outgrowth, disrupts neuronal microtubules, and modifies cellular tubulin. J. Neurochem. 72:2323-2333. [DOI] [PubMed] [Google Scholar]
- 20.Newcomb, W. W., and J. C. Brown. 1981. Role of the vesicular stomatitis virus matrix protein in maintaining the viral nucleocapsid in the condensed form found in native virions. J. Virol. 39:295-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paik, S. Y., A. C. Banerjea, G. G. Harmison, C. J. Chen, and M. Schubert. 1995. Inducible and conditional inhibition of human immunodeficiency virus proviral expression by vesicular stomatitis virus matrix protein. J. Virol. 69:3529-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen, J. M., L. S. Her, V. Varvel, E. Lund, and J. E. Dahlberg. 2000. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol. Cell. Biol. 20:8590-8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vikman, K., B. Robertson, G. Grant, A. Liljeborg, and K. Kristensson. 1998. Interferon-gamma receptors are expressed at synapses in the rat superficial dorsal horn and lateral spinal nucleus. J. Neurocytol. 27:749-759. [DOI] [PubMed] [Google Scholar]
- 24.Volberg, T., A. D. Bershadsky, M. Elbaum, A. Gazit, A. Levitzki, and B. Geiger. 2000. Disruption of microtubules in living cells by tyrphostin AG-1714. Cell Motil. Cytoskeleton 45:223-234. [DOI] [PubMed] [Google Scholar]
- 25.von Kobbe, C., J. M. van Deursen, J. P. Rodrigues, D. Sitterlin, A. Bachi, X. Wu, M. Wilm, M. Carmo-Fonseca, and E. Izaurralde. 2000. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin nup98. Mol. Cell 6:1243-1252. [DOI] [PubMed] [Google Scholar]
- 26.Wang, E., and R. D. Goldman. 1978. Functions of cytoplasmic fibers in intracellular movements in BHK-21 cells. J. Cell Biol. 79:708-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan, H., S. Puckett, and D. S. Lyles. 2001. Inhibition of host transcription by vesicular stomatitis virus involves a novel mechanism that is independent of phosphorylation of TATA-binding protein (TBP) or association of TBP with TBP-associated factor subunits. J. Virol. 75:4453-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan, H., B. K. Yoza, and D. S. Lyles. 1998. Inhibition of host RNA polymerase II-dependent transcription by vesicular stomatitis virus results from inactivation of TFIID. Virology 251:383-392. [DOI] [PubMed] [Google Scholar]