Abstract
The use of a human chromosome or its fragment as a vector for animal transgenesis may facilitate functional studies of large human genomic regions. We describe here the generation and analysis of double trans-chromosomic (Tc) mice harboring two individual human chromosome fragments (hCFs). Two transmittable hCFs, one containing the Ig heavy chain locus (IgH, ≈1.5 Mb) and the other the κ light chain locus (Igκ, ≈2 Mb), were introduced into a mouse strain whose endogenous IgH and Igκ loci were inactivated. In the resultant double-Tc/double-knockout mice, substantial proportion of the somatic cells retained both hCFs, and the rescue in the defect of Ig production was shown by high level expression of human Ig heavy and κ chains in the absence of mouse heavy and κ chains. In addition, serum expression profiles of four human Ig γ subclasses resembled those seen in humans. They mounted an antigen-specific human antibody response upon immunization with human serum albumin, and human serum albumin-specific human monoclonal antibodies with various isotypes were obtained from them. These results represent a generation of mice with “humanized” loci by using the transmittable hCFs, which suggest that the Tc technology may allow for the humanization of over megabase-sized, complex loci in mice or other animals. Such animals may be useful not only for studying in vivo functions of the human genome but also for obtaining various therapeutic products.
Technical advances that enable larger stretches of human DNA to be introduced into mice allow not only for introduction of large genes or gene clusters but also correct expression of transgenes by inclusion of essential remote regulatory elements (1). This also facilitates the generation of mice with “humanized” loci whose endogenous loci are functionally substituted for intact human equivalents in combination with targeted inactivation of endogenous loci, thereby providing valuable experimental animals for gaining insight into in vivo functions of human genes and for studying human genetic disorders (2, 3). Particularly, much effort has been made by a number of groups to create mice with humanized Ig (Ig) loci for obtaining therapeutic human mAbs (hu-mAbs) monoclonal antibodies (4, 5). Their studies established that transgenic mice carrying a portion of human IgH (14q32.33, ≈1.5 Mb) and Igκ (2q12, ≈2 Mb) loci in the endogenous Ig-knockout (KO) background were successfully used for the production of antigen-specific fully human antibodies. They also showed that the use of larger transgenes containing a larger number of V-gene segments resulted in mice exhibiting more efficient humoral response to a wide range of antigens. Although the introduction of entire human Ig loci into mice to reconstitute full diverse human antibody repertoires has been a next major challenge, this has never been achieved because the cloning of over megabase-sized DNA fragments encompassing whole human Ig loci remains difficult even with the use of yeast artificial chromosomes (1, 5). In addition, the constant region of the human IgH locus is known to contain sequences difficult to be cloned (6).
To circumvent such a DNA cloning step, we have developed a procedure utilizing a human chromosome fragment (hCF) as a vector for transgenesis. In our previous study (7), various hCFs were introduced into mouse embryonic stem (ES) cells via microcell-mediated chromosome transfer, and viable chimeric mice were produced from them. Transferred hCFs were stably retained, and human genes, including the Igκ, heavy, and -λ genes, were expressed in a proper tissue-specific manner in adult chimeric tissues. In the case of a human chromosome 2 (hChr.2)-derived hCF [hCF(2-W23), ≈5–20 Mb] (8) containing the Igκ locus, it was found to be transmitted to the offspring through the germ line, demonstrating the establishment of a trans-chromosomic (Tc) mouse [Tc(W23)] expressing the human Ig κ light chain (hκ) (7). Another group also employed the microcell-mediated chromosome transfer to produce chimeric mice containing a hChr.21 or its fragment in a recent report (9).
This procedure was anticipated to be used to generate mice with humanized Ig loci; however, several issues remained to be explored to attain this goal. For example, the somatic mosaicism and the transmission efficiency of hCF(2-W23) were not evaluated, and the hChr.14-derived hCF (>50 Mb) containing the IgH locus was not transmittable (7). In the present study, we therefore examined: (i) germ-line transmission of another hCF with the IgH locus, (ii) stability of hCFs in the somatic cells, (iii) transmission efficiency and functional stability of hCFs during several passages through the germ line, and (iv) functioning of the two individual hCFs in mice. Indeed, such studies are prerequisite to the generation of mice containing four distinct genetic modifications (IgH-Tc, Igκ-Tc, IgH-KO, Igκ-KO) and expressing fully human Ig molecules comprising human Ig heavy and κ light chains. They are also crucial to demonstrate that the Tc technology can be generally used for humanizing the mouse genome.
Here, double-Tc/double-KO mice expressing fully human antibodies have been successfully generated by the establishment of a Tc strain that retains a hChr.14-derived hCF with the IgH locus, and the breeding of this strain with the Tc(W23) strain on a IgH- and Igκ-KO background. ELISAs showed the high level expression of human Igμ, all four Igγ subclasses, and Igκ in the sera of resultant double-Tc/KO mice. Furthermore, hybridomas secreting antigen-specific hu-mAbs with various isotypes were obtained from them, indicating the reconstitution of a functional repertoire of fully human Igs. A description regarding the in vivo stability of hCFs is also provided.
Materials and Methods
Genomic DNA Analysis by PCR.
Reaction conditions for PCR amplification and the primer sequence of markers except for AKT1 were described in our previous report (7) and The Genome Database (http://www.gdb.org). Seven markers for the primary analysis of A9/SC20 were NP, MYH6, D14S75, D14S66, D14S43, D14S78, and IGHMC (IgM). D14S826, D14S1419, and D14S1420 were mapped within 200 kb of the telomere of chromosome 14q, which is at the 5′ end of IgH locus (10). D14S543 and AKT1 are mapped 14q32.1–32.2 and 14q32.32, respectively (http://www.gdb.org). The AKT1 primer pair was 5′-ACGGGCACATTAAGATCACA-3′, 5′-TGCCGCAAAAGGTCTTCATG-3′.
Generation of Tc Mice.
The microcell-mediated chromosome transfer, ES cell manipulation and chimera production were carried out as described (7).
Fluorescence in Situ Hybridization (FISH) Analysis.
Preparation of chromosome samples and FISH analysis were carried out essentially as described (7). Probes are as follows: digoxigenin (Boehringer Mannheim)-labeled human COT-1 DNA (BRL), digoxigenin-labeled 14/22cen (hChr.14/22 α-satellite, Oncor), biotin-labeled 14qter (ID Labs Biotech, Biotechnology, ON, Canada), digoxigenin-labeled mouse major satellite (11), and digoxigenin-labeled mouse minor satellite (11). For two-color FISH analysis, digoxigenin-labeled 14/22cen (Oncor) and biotin-labeled 2cen (hChr.2 α-satellite, Oncor) probes were used. Digoxigenin- and biotin-labeled probes were detected with anti-digoxigenin-rhodamine (Boehringer Mannheim) and FITC-avidin (Vector Laboratories), respectively. Fibroblasts prepared from tail of 4- to 6-week-old mice were cultured in DMEM containing 10% FCS for 2 weeks and then were used for preparation of metaphase chromosomes and interphase nuclei. Fifty metaphase spreads were scored to determine somatic mosaicism of each hCF. Mitotic stability of hCFs in ES cells was determined as follows. The clone #21 and microcell-hybrid ES [MH(ES)] 2-21 (7) were cultured for a week in the presence of 300 μg/ml G418 (GIBCO/BRL) and 0.75 μg/ml puromycin (Sigma), respectively. Then the plate was split 1/8 into a 35-mm plate and was grown to 80% confluency (2 days) in the media lacking the drugs. This process was repeated for 45 days. The retention of the hCF in the cells sampled at day 0, day 30, and day 45 was analyzed by FISH using the COT-1 probe. At each time point, 50 metaphase spreads were scored. The loss rate of each hCF was calculated in three independent experiments and was averaged.
Generation of Ig KO Strains.
Detailed protocol for generating IgH- and Igκ-KO strains is described elsewhere (K.T. and I.I., unpublished work). The essential genetic modification of each strain is as follows. In the IgH-KO (ΔH−/−) strain, a BamHI-XhoI genomic segment (3.7 kb) including a portion of Cμ2, Cμ3-Cμ4, and Mμ1-Mμ2 exons was replaced by a neor cassette. Absence of Igμ and -γ chain expression in the sera and B220+ cells in peripheral blood mononuclear cells in the homozygotes (ΔH−/−) was confirmed (data not shown; see Fig. 2). In the Igκ-KO strain (Δκ−/−), a SacII-BglII segment (2 kb) including the Cκ exon was replaced by a neor cassette. Absence of the Igκ light chain expression in the mice that lack Cκ exon was reported previously (12). The ΔH−/− and Δκ−/− strains were intercrossed to obtain double-KO (ΔH−/−, Δκ−/−) strain. The double-KO strain has been maintained on a mixed background of C57BL/6, CBA, and MCH(ICR). To isolate λ1low mutants, CD-1 stocks obtained from Charles River Breeding Laboratories (Tokyo) were examined by Southern blots (13). Approximately half of the tested CD-1 animals were found to be λ1low homozygotes, and they were bred with double-Tc/KO animals to generate double-Tc/KO(λ1low/low) strain.
Figure 2.
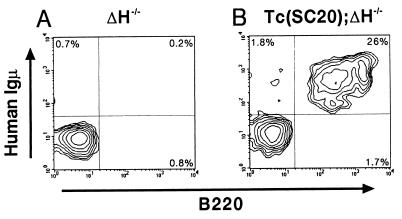
Rescue of B-cell development in ΔH−/− mice by introduction of hCF(SC20). Peripheral blood mononuclear cells from ΔH−/− mouse (A) and Tc(SC20);ΔH−/− mouse (B) were assayed for surface expression of hμ and B220. Representative FACS results using 3-month-old animals are shown. The net percentage of positively stained cells is given in each quadrant.
FACS Analysis.
Peripheral blood mononuclear cells were prepared from 12-week-old Tc(SC20);ΔH−/− and ΔH−/− mice, were treated with Fc Block (PharMingen), were stained with antibodies, and were analyzed on a FACS vantage (Becton Dickinson, cell quest software). Antibodies used were FITC anti-human IgM (PharMingen); phycoerythin (PE) anti-B220 (PharMingen).
ELISAs, Immunization, and Hybridoma Production.
Hμ, hγ, and hκ were assayed as described (7). Mλ, human Igα (hα), and Igɛ (hɛ) were assayed by using anti-mλ (Caltag, South San Francisco, CA), anti-hα (α1 + α2, Kirkegaard & Perry Laboratories), and anti-hɛ (PharMingen) immobilized on the plate and detected with peroxidase-conjugated anti-mλ (Caltag), peroxidase-conjugated anti-hα (Kirkegaard & Perry Laboratories), and alkaline phosphatase-conjugated anti-hɛ (PharMingen), respectively. Similarly, mμ and mγ were assayed by using anti-mμ (Kirkegaard & Perry Laboratories), anti-mγ (Sigma) for capture and anti-mμ (Kirkegaard & Perry Laboratories), anti-mγ (Caltag) for detection, respectively. Mouse IgG3/λ (Sigma), human IgA (Athens Research & Technology, Athens, GA), human IgE (Chemicon), mouse IgM (Sigma), and mouse IgG (PharMingen) were used as standards. The samples, standards, and antibody conjugates were diluted with mouse serum (Sigma)-supplemented PBS. Human γ chain subclasses were assayed by using a Human IgG Subclass Profile ELISA Kit (Zymed).
Mice were immunized twice with human serum albumin (HSA) (Sigma, 50 μg/injection) in Titer Max Gold (CytRx, Norcross, GA) subcutaneously (day 0, day 21). A final intraperitoneal injection of 50 μg of HSA in PBS was given at day 34, three days before fusion. Serum samples, collected at approximately weekly intervals, were diluted 1:1,800, and antigen-specific ELISAs were performed on HSA-coated plates. The presence of HSA-specific human antibody was detected with horseradish peroxidase-conjugated antibodies specific for hγ (Sigma), hμ (Southern Biotechnology Associates), hκ (Southern Biotechnology Associates), and mλ (Caltag). To produce hybridomas, splenocytes from immunized mice were fused with SP2/0-Ag14 myeloma cells by using PEG4000 (Merck). After 14 days the supernatants from hybrids growing in G418 (1 mg/ml) selection medium were first screened for the presence of HSA-specific hμ and hγ. The resulting HSA-specific hγ+ hybridomas were then assayed by using peroxidase-conjugated antibody specific for hκ, mλ, hγ1 (Zymed), hγ2 (Zymed), hγ3 (Zymed), and hγ4 (Zymed). TMB (Sumitomo bakelite) or BCIP (Kirkegaard & Perry Laboratories) was used for substrates, and the absorbance at 450 nm or 630 nm was measured by using a spectrophotometer (Bio-tek Instruments, Luton, U.K.).
Breeding Analysis.
Chimeras and Tc mice were mated with MCH(ICR), mice and the resultant offspring were examined by PCR and ELISAs [hμ for Tc(SC20), hκ for Tc(W23)]. PCR markers used were D14S543 and IGHMC for Tc(SC20); D2S1331 and IGKC for Tc(W23). MCH(ICR) and C57BL/6N mice were purchased from Japan Crea (Tokyo).
Results and Discussion
Generation of Tc Mice Containing the Human IgH Locus.
A microcell-hybrid mouse A9 cell line, A9/SC20, was a subclone isolated from A9/14-C11 (7), which retained a G418r-tagged hCF whose size was estimated to be approximately one-fifth of an intact hChr.14 [hCF(SC20), see Fig. 1A] and slightly larger than that of the hCF(2-W23). Of seven PCR markers of hChr.14 that were examined, only IgM was detected in this hybrid. Further analysis revealed that it also contained three markers (D14S826, S1419, S1420) residing within the most distal portion of the IgH locus (10) and two proximal markers (D14S543, AKT1), suggesting that the hCF(SC20) included the whole human IgH locus. The hCF(SC20) was introduced into a female mouse ES cell line, TT2F(39,XO), by microcell-mediated chromosome transfer (7) to generate G418r MH(ES) clones retaining the transferred hCF as an independent chromosome (Fig. 1A). Stability tests under the nonselective condition using one of the MH(ES) clones (#21) revealed that the hCF(SC20) was highly stable in mouse ES cells (<0.1% loss/doubling) in contrast to the hCF(2-W23) (3.2% loss/doubling) and the hChr.Y-derived minichromosome reported recently (14). Structural analyses of the hCF(SC20) by FISH showed the absence of murine centromeric sequences (major and minor satellites) (data not shown) and the presence of 14cen (Fig. 1B) and 14qter (Fig. 1C) sequences, indicating that it was generated as a consequence of an interstitial deletion between 14cen and 14q32-qter regions. Therefore, the human centromeric sequence of the hCF(SC20) is likely to be sufficient for stable maintenance in mouse ES cells.
Figure 1.
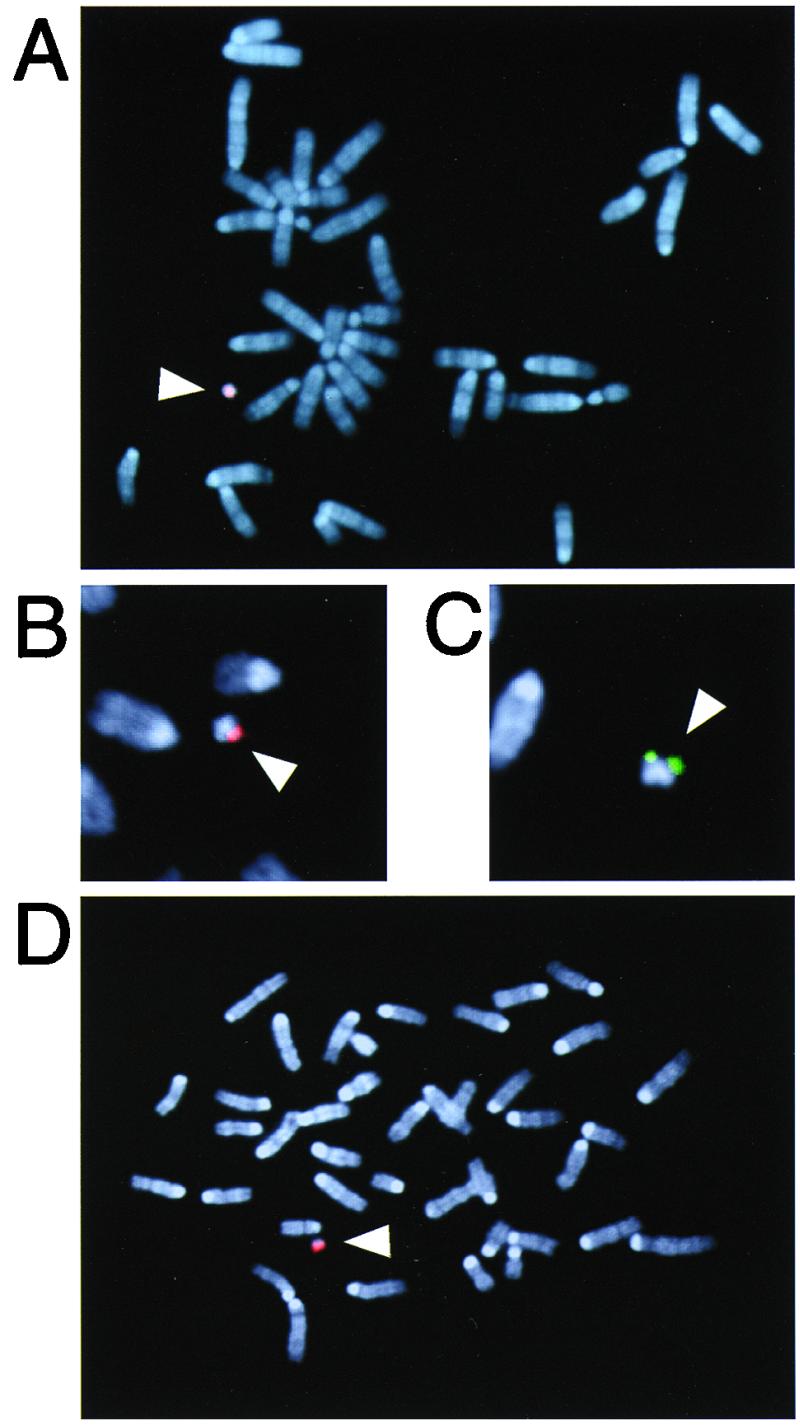
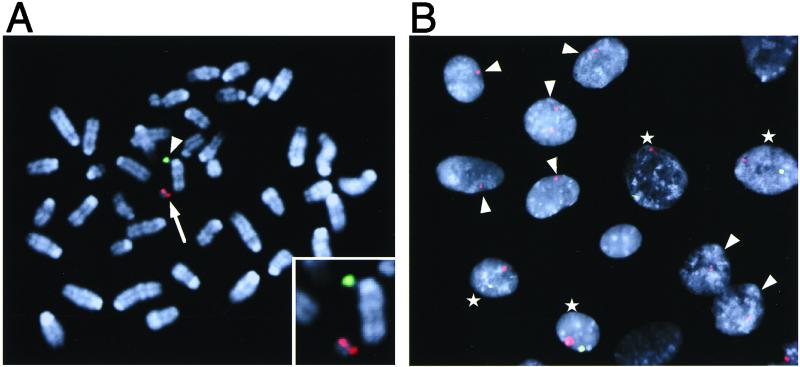
FISH analysis of MH(ES) clone (#21) and tail fibroblasts prepared from a Tc(SC20) mouse. Shown are photomicrographs of representative metaphase spreads from clone #21 hybridized to human COT-1 DNA (A), 14/22cen (B, partial spread), or 14qter (C, partial spread) probes. Hybridization signals (red, arrowhead) were detected on the transferred hCF(SC20) in mouse chromosomes stained with DAPI (blue). (D) Metaphase spreads from tail fibroblasts of a Tc(SC20) animal hybridized to human COT-1 DNA. An extra hCF with similar size to that in the clone #21 (see A) was detected (red, arrowhead).
Twenty phenotypically normal chimeras (15–100% agouti coats) expressing human Igμ (hμ, 2.1–26 mg/liter) and γ (hγ, 0.2–8.3 mg/liter) heavy chains in the sera were obtained from the clone #21, indicating that the hCF(SC20) included a functional human IgH locus. The mating of two female chimeras (100% chimerism) with albino MCH(ICR) males resulted in 30 agouti F1 offspring with normal external appearance. Retention of the hCF(SC20) was confirmed in 10 of 30 (33%) offspring by PCR (IgM and D14S543), ELISA (hμ, 2.4–12.8 mg/liter), and FISH (Fig. 1D), demonstrating the establishment of a second Tc strain, Tc(SC20). These results suggest that no apparent structural and functional change of the hCF(SC20) occurred during germ-line transmission. Further crossing revealed that the hCF(SC20) could be transmitted through the male germ line (see Table 1).
Table 1.
Breeding analysis of Tc mice
| Sex | No. of tested Tc mice | No. of pups* | No. of hCF+ | Transmission efficiency, %† | |
|---|---|---|---|---|---|
| Tc(SC20) | |||||
| Chimera-F1‡ | f | 2 | 30 | 10 | 33 |
| F1-F2 | m | 3 | 142 | 46 | 32 |
| f | 4 | 60 | 20 | 33 | |
| F2-F3 | m | 2 | 74 | 25 | 34 |
| f§ | 5 | 42 | 16 | 38 | |
| Tc(W23) | |||||
| Chimera-F1 | m | 1 | 25 | 3 | 12 |
| f | 4 | 67 | 22 | 33 | |
| F1-F2 | m | 8 | 324 | 25 | 8 |
| f | 6 | 148 | 32 | 22 | |
| F2-F3 | m | 8 | 346 | 30 | 9 |
| f | 8 | 202 | 48 | 24 |
m, male; f, female.
*In the case of chimeras, only ES-derived agouti offspring were examined.
† The expected transmission efficiency in chimeras and Tc mice hemizygous for the hCF is 50% when mitotic stability of the hCF is perfect and it can properly segregate in meiosis. Because 62 and 25 cell divisions are required for generation of mature sperm and oocyte, respectively (26), in mice, expected efficiencies in Tc(W23), deduced from the mitotic loss rate in (MH)ES cells (3.2% loss/doubling), are 7% in male and 22% in female. These figures are very consistent with those observed in the Tc(W23). On the other hand, the difference of transmission efficiency between male and female in the Tc(SC20) is unclear.
‡ Because male MH(ES) cells retaining the hCF(SC20) were not generated, only female chimeras that contained it were used in this study.
§The Tc(SC20) F2 female mice were mated with C57BL/6N male.
Stability of hCFs in Vivo.
Metaphase spreads of tail fibroblasts prepared from the Tc(SC20) and Tc(W23) mice were examined by FISH to evaluate the stability of hCFs in somatic cells. The percentage of the spreads containing the hCF averaged 78 ± 13% (n = 6) and 30 ± 11% (n = 3) in Tc(SC20) and Tc(W23), respectively. Next, we carried out breeding analyses to determine transmission efficiencies of these hCFs through the male and female germ line, which represent their stability in germ cells (Table 1). These results showed that each hCF was retained in a significant proportion of somatic and germ cells in both Tc mice. The overall stability of the hCF(2-W23) was lower than that of the hCF(SC20) in both somatic and germ cells, which may reflect the mitotic instability of the hCF(2-W23) that was observed in the cultured MH(ES) cells. On the other hand, the moderate loss of the hCF(SC20) was unexpected because of its perfect stability in the ES cells. Although many factors might affect mosaicism of the transferred hCFs in the somatic and germ cells, one possible explanation is that the expression of some human genes included in the hCF(SC20) might confer a selective disadvantage to the cells retaining the hCF in mice.
Genetic Rescue of IgH-KO Mice by Introducing the hCF(SC20).
The Tc(SC20) was bred with the IgH-KO (ΔH−/−) strain in which functional B-lymphocytes and Ig production are absent (Fig. 2A) to examine whether the introduction of hCF(SC20) with the human IgH locus could rescue its phenotypes. As a result, reconstitution of mature B cells (B220+, hμ+) was observed in peripheral blood lymphocytes prepared from the Tc(SC20);ΔH−/− mouse (Fig. 2B). ELISA analyses also showed the restoration of the serum Igs with hμ or hγ (data not shown; see Fig. 4A). Thus, the introduction of hCF(SC20) rescued defects in the IgH-KO strain, indicating that the stability of the hCF(SC20) is likely to be sufficient for its persistence in the B cells of adult mice. This demonstrates that the extrachromosomally maintained transgene rescues genetic defects in mice, which may have an implication on the development of mammalian artificial chromosome vectors for gene therapy (15).
Figure 4.
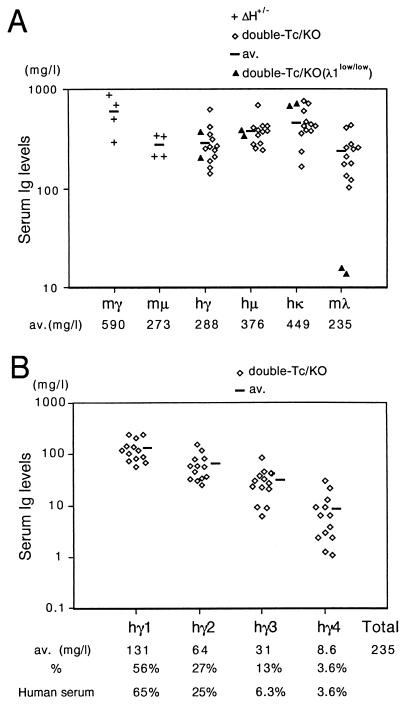
Human Ig expression in the sera of nonimmunized double-Tc/KO mice. (A) Hγ, hμ, hκ, and mλ in serum samples prepared from 8- to 13-week-old double-Tc/KO (n = 12, λ1low/+ or λ1+/+) and double-Tc/KO(λ1low/low) (n = 2) individuals. mμ and mγ from 8- to 13-week-old ΔH+/− individuals (n = 4). The average level of each Ig (av.) in double-Tc/KO or ΔH+/− individuals is also given below the graph. mγ and mμ levels in double-Tc/KO mice are <15 mg/liter and <1 mg/liter, respectively. (B) Four hγ subclasses from the double-Tc/KO individuals (n = 13). The average level of each hγ subclass (av.) is also given below the graph. The percentage of the average concentration for each hγ subclass to the total of them in double-Tc/KO mice and control human serum (Zymed) are also shown.
Generation of Double-Tc Mice.
To obtain mice producing fully human Igs in the absence of endogenous mouse Ig heavy and κ light chains, the Tc(W23) and Tc(SC20) were bred together with the double-KO homozygous strain whose endogenous IgH and Igκ loci were deleted in both alleles. Screening of 720 offspring obtained from the crossing of Tc(SC20);(ΔH+/−, Δκ+/−) and Tc(W23);(ΔH+/−, Δκ+/−) animals, by PCR [IgM and D14S543 for hCF(SC20), IGKC and D2S1331 for hCF(2-W23)] and ELISA (hμ and hκ) analysis, showed that 58 individuals were double-Tc that contained all of the PCR markers and produced both hμ and hκ in the sera (data not shown). Two-color FISH analysis of tail fibroblasts prepared from the double-Tc animals by using hChr.2- and hChr.14-specific probes (Fig. 3) showed the retention of each hCF as an independent chromosome. Average mosaicism of the hCF(SC20) and hCF(2-W23) in metaphase spreads were 75 ± 10% and 32 ± 13% (n = 4), respectively, similar to those in each Tc strain with a single hCF as described in the text. The percentage of the spreads containing both hCFs averaged 21 ± 7%, which is in good agreement with that deduced from the mosaicism of each hCF (75% × 32% = 24%). Thus, two individual, transferred hCFs could be retained and function in the mouse. Preliminary breeding analysis showed that both hCFs could be transmitted through the germ line of male and female double-Tc animals and that the transmission efficiency of each hCF was similar to that in single-Tc animals (data not shown; see Table 1).
Figure 3.
Two-color FISH analysis of tail fibroblasts prepared from a 4-week-old double-Tc mouse. (A) Metaphase spreads were hybridized to 14/22cen (red, arrow) and 2cen (green, arrowhead) probes to detect hCF(SC20) and hCF(2-W23), respectively. A photomicrograph of a representative spread is shown. A magnified (×2) image of the hCFs is provided in the inset. (B) Interphase nuclei were hybridized to 14/22cen (red) and 2cen (green) probes. Nuclei containing both signals and a red signal only are indicated with stars and arrowheads, respectively.
Expression of Human Igs in Double-Tc/double-KO Mice.
Double-Tc mice with the double-KO background (double-Tc/KO) were identified by Southern blots (data not shown) and were examined by ELISAs to determine serum concentrations of human Igs. Compared with the ΔH+/− mice kept under similar conditions, the average levels of hμ and hγ were equivalent to the mouse μ chain (mμ) level and a half of the mouse γ chain (mγ) level, respectively (Fig. 4A). All four hγ subclasses were produced and, interestingly, the average proportion of each subclass relative to total hγ concentration was similar to that observed in human serum (Fig. 4B). This suggests that the regulatory mechanism determining the expression level of each hγ subclass is similar between human and mouse. Human Igα (α1 + α2, 79–502 μg/liter) and -ɛ (13–97 μg/liter) chains were also detectable in 8 and 6 of 10 tested double-Tc/KO animals, respectively. Considering that the normal levels of Igα chain are similar to those of Igμ chain in the sera of mice and humans, observed levels of Igα chain in the double-Tc/KO mice are very low. Although the reason for this phenomenon is elusive at present, it should be noted that such a selective defect in IgA production (IgA deficiency) is the most common form of primary immunodeficiency in human, in which an impaired class-switching to IgA is supposed to be involved in the pathogenesis (16). The average level of hκ was higher than that of the mouse λ light chain (mλ), which indicates that the Igκ locus of the hCF(2-W23) can compete well with the intact mouse Igλ locus despite the incomplete stability of the hCF in somatic cells (Fig. 4A). Furthermore, the hκ to mλ ratio was greatly improved by introducing a λ1low allele (17) into the double-Tc/KO strain [Fig. 4A, double-Tc/KO(λ1low/low)].
Production of HSA-specific hu-mAbs.
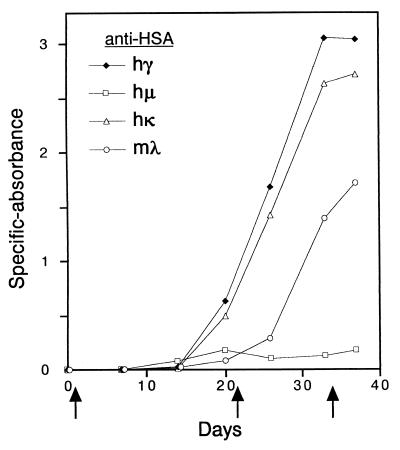
The double-Tc/KO mice were challenged with HSA to examine whether the reconstituted repertoire of human Igs is sufficient for obtaining antigen-specific hu-mAbs. After immunization, HSA-specific hγ, hμ, and hκ were readily detected in the sera (Fig. 5). Furthermore, a second immunization resulted in greater hγ and hκ responses. Splenic hybridomas were prepared from one of the HSA-immunized double-Tc/KO animal, and the resulting hybridoma supernatants were screened for the production of HSA-specific human Igs. Analysis of 3,300 wells with hybridomas revealed 11 hμ+ wells and 39 hγ+ wells. Of 39 hγ+ wells, 14 were hκ+, and the others were mλ+. There was no well positive for both Ig light chains, indicating the production of HSA-specific fully human IgG/κ antibodies in these 14 hκ+ wells. Further ELISAs of them showed that 7, 2, and 5 wells were hγ1+, hγ2+, and hγ4+, respectively. Representative human IgG/κ hybridomas were subcloned by limiting dilution, and their supernatants were subjected to the affinity measurement. The affinity constant (Ka), measured by using surface plasmon resonance in BIAcore, ranged from 1.1 × 1010 to 6.6 × 1010 M−1.
Figure 5.
Time course of antigen-specific human antibody responses in double-Tc/KO mouse. Shown are HSA-reactive hγ, hμ, hκ, and mλ in the serum of a representative double-Tc/KO animal (10 weeks old, male) immunized on days 0, 21, and 34 (indicated with arrows).
These results strongly indicate that the double-Tc/KO mice can be used to obtain antigen-specific hu-mAbs with various isotypes exhibiting desired effector functions. Successful expression of all four hγ subclasses represents an advantage of using hCF vectors to bypass cloning steps because some sequences within the constant region of human IgH locus was found to be unclonable by conventional cloning systems (6). V gene complexity is supposed to be essential for restoration of normal humoral immune response (5), which is important for the production of high affinity hu-mAbs against variety of antigens. Therefore, high affinities of the resultant hu-mAbs suggest that the authentic repertoire of fully human Igs was reconstituted in the double-Tc/KO mice. Although more detailed structural analysis of hCFs may be required to determine whether human Ig loci contained in the double-Tc/KO mice are completely intact, the data presented here and in our previous report (7) suggest that they include almost all, if not all, of the sequence for the human Ig loci. In addition, hybridomas producing human IgG/κ antibodies against human proteins other than HSA, human tumor necrosis factor α (TNFα), and granulocyte colony stimulating factor (G-CSF) have been obtained.
Instability of the hCF(2-W23) could be a impediment to optimal hκ expression and production of hκ+ hybridomas. Although we successfully obtained anti-HSA hκ+ hybridomas, the following observations indicate that the loss of the hCF(2-W23) was actually compensated by the mλ expression. (i) The proportion of mλ to total Ig light chain concentration in the sera of double-Tc/KO mice [average: 34% (Fig. 4)] was higher than those in wild-type and YAC-transgenic mice (<10%) (5). (ii) Delayed but significant response of mλ against HSA-immunization was observed (Fig. 5). (iii) Two-thirds of anti-HSA IgG hybridomas obtained were mλ+. (iv) A majority (83%) of IgG/mλ hybridomas was found to have lost the hCF(2-W23). Therefore, the use of more stable hCF with the Igκ locus should be desirable for improving the hκ expression and the production of antigen-specific fully human monoclonals. Site-directed translocation of hCF containing the Igκ locus to a mouse chromosome in ES cells (18, 19) may be one of the solutions for this issue.
There has been no report describing transmittable foreign chromosomes in mice since we demonstrated the transmission of hCF(2-W23) through the germ line of male and female chimeras (7). Now, the hCF(SC20) has been shown to be a second transmittable hCF. The result that the hCF(SC20) could be transmitted through the germ line, whereas the larger hCFs derived from hChr.14 failed (7), implies that the use of small hCFs (≈20 Mb) may increase the probability of successful germ-line transmission. The monochromosomal hybrid library we generated previously (7) can be screened to obtain such small hCFs containing the desired loci. Hence, the procedure presented here should give us an application to humanize other large loci or gene clusters (e.g., T-cell receptors, major histocompatibility antigens, P450 gene clusters) and, ultimately, specific chromosomal regions sintenic between human and mouse (20) in combination with procedures to generate large chromosomal deletions in the mouse (21, 22). Furthermore, an increasing amount of sequence information provided by the Human Genome Project should facilitate not only engineering the hCFs with desired chromosomal regions (15, 23) but also elucidating the structural basis required for stable maintenance and germ-line transmission of the hCFs in mice.
Although the ES cells with germ-line differentiating potency are currently available only in the mouse, the chromosome transgenesis in large farm animals may be feasible using the cloning technology (24, 25). For example, cows or sheep producing human Igs may be generated by nuclear transfer from the microcell-hybrid fibroblast cells retaining the hCFs with human Ig loci, from which pathogen-specific human γ-globulins would be obtained for the treatment of infectious diseases. Because the use of hCFs allows for the expression of all four human IgG subclasses, our procedure may be most suitable for this purpose.
Our study has demonstrated the utility of the Tc technology as a complementary approach to conventional transgenic techniques using the cloned DNA fragments. Such a “top-down” approach (15) should be a promising way for large-scale genome manipulations of mice or other animals to generate genetically modified animals useful for laboratory and industrial uses.
Acknowledgments
We thank K. Hanaoka, M. Hayasaka, and Y. Kuroiwa for technical advice and valuable discussions; H. Masumoto for providing PCR primers to amplify mouse major and minor satellite sequences; and R. Akasu and M. Hirayama for technical assistance.
Abbreviations
- Tc mouse
trans-chromosomic mouse
- hCF
human chromosome fragment
- KO mouse
knockout mouse
- HSA
human serum albumin
- hu-mAbs
human monoclonal antibodies
- ES cells
mouse embryonic stem cells
- hChr.2
human chromosome 2
- MH(ES) cells
microcell-hybrid ES cells
- FISH
fluorescence in situ hybridization
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Peterson K R, Clegg C H, Stamatoyannopoulous G. Trends Genet. 1997;13:61–66. doi: 10.1016/s0168-9525(97)01003-2. [DOI] [PubMed] [Google Scholar]
- 2.Jakobovits A. Curr Biol. 1994;4:761–763. doi: 10.1016/s0960-9822(00)00172-x. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson J G, Smith D J, McCutcheon K, Koide H B, Nishiyama K, Dinulos M B, Stevens M E, Bissada N, Nasir J, Kanazawa I, et al. Hum Mol Genet. 1996;5:1875–1885. doi: 10.1093/hmg/5.12.1875. [DOI] [PubMed] [Google Scholar]
- 4.Fishwild D M, O'Donnell S L, Bengoechea T, Hudson D V, Harding F, Bernhard S L, Jones D, Kay R M, Higgins K M, Schramm S R, Lonberg N. Nat Biotechnol. 1996;14:845–851. doi: 10.1038/nbt0796-845. [DOI] [PubMed] [Google Scholar]
- 5.Mendez M J, Green L L, Corvalan J R, Jia X C, Maynard-Currie C E, Yang X D, Gallo M L, Louie D M, Lee D V, Erickson K L, et al. Nat Genet. 1997;15:146–156. doi: 10.1038/ng0297-146. [DOI] [PubMed] [Google Scholar]
- 6.Kang H K, Cox D W. Genomics. 1996;35:189–195. doi: 10.1006/geno.1996.0338. [DOI] [PubMed] [Google Scholar]
- 7.Tomizuka K, Yoshida H, Uejima H, Kugoh H, Sato K, Ohguma A, Hayasaka M, Hanaoka K, Oshimura M, Ishida I. Nat Genet. 1997;16:133–143. doi: 10.1038/ng0697-133. [DOI] [PubMed] [Google Scholar]
- 8.Rastan S. Nat Genet. 1997;16:113–114. doi: 10.1038/ng0697-113. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez D, Mee P J, Martin J E, Tybulewicz V L J, Fisher E M C. Hum Mol Genet. 1999;8:923–933. doi: 10.1093/hmg/8.5.923. [DOI] [PubMed] [Google Scholar]
- 10.Wintle R F, Nygaard T G, Herbrick J S, Kvaloy K, Cox D W. Genomics. 1997;40:409–414. doi: 10.1006/geno.1996.4572. [DOI] [PubMed] [Google Scholar]
- 11.Ikeno M, Masumoto H, Okazaki T. Hum Mol Genet. 1994;3:1245–1257. doi: 10.1093/hmg/3.8.1245. [DOI] [PubMed] [Google Scholar]
- 12.Zou Y-R, Takeda S, Rajewsky K. EMBO J. 1993;12:811–820. doi: 10.1002/j.1460-2075.1993.tb05721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ju S T, Selsing E, Huang M C, Kelly K, Dorf M E. J Immunol. 1986;136:2684–2688. [PubMed] [Google Scholar]
- 14.Shen M H, Yang J, Loupart M L, Smith A, Brown W. Hum Mol Genet. 1997;6:1375–1382. doi: 10.1093/hmg/6.8.1375. [DOI] [PubMed] [Google Scholar]
- 15.Grimes B, Cooke H. Hum Mol Genet. 1998;7:1635–1640. doi: 10.1093/hmg/7.10.1635. [DOI] [PubMed] [Google Scholar]
- 16.Islam K B, Baskin B, Nilsson L, Hammarstrom L, Sideras P, Smith C I. J Immunol. 1994;152:1442–1452. [PubMed] [Google Scholar]
- 17.Kim J Y, Kurtz B, Huszar D, Storb U. EMBO J. 1994;13:827–834. doi: 10.1002/j.1460-2075.1994.tb06325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith A J H, De Sousa M A, Kwabi-Addo B, Heppell-Parton A, Impey H, Rabbitts P. Nat Genet. 1995;9:376–385. doi: 10.1038/ng0495-376. [DOI] [PubMed] [Google Scholar]
- 19.Deursen J V, Fornerod M, Rees B V, Grosveld G. Proc Natl Acad Sci USA. 1995;92:7376–7380. doi: 10.1073/pnas.92.16.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeBry R W, Seldin M F. Genomics. 1996;33:337–351. doi: 10.1006/geno.1996.0209. [DOI] [PubMed] [Google Scholar]
- 21.Ramirez-Solis R, Liu P, Bradley A. Nature (London) 1995;378:720–724. doi: 10.1038/378720a0. [DOI] [PubMed] [Google Scholar]
- 22.You Y, Bergstrom R, Klemm M, Lederman B, Nelson H, Ticknor C, Jaenisch R, Schimenti J. Nat Genet. 1997;15:285–288. doi: 10.1038/ng0397-285. [DOI] [PubMed] [Google Scholar]
- 23.Kuroiwa Y, Shinohara T, Notsu T, Tomizuka K, Yoshida H, Takeda S, Oshimura M, Ishida I. Nucleic Acids Res. 1998;26:3447–3448. doi: 10.1093/nar/26.14.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnieke A E, Kind A J, Ritchie W A, Mycock K, Scott A R, Ritchie M, Wilmut I, Colman A, Campbell K H. Science. 1997;278:2130–2133. doi: 10.1126/science.278.5346.2130. [DOI] [PubMed] [Google Scholar]
- 25.Cibelli J B, Stice S L, Golueke P J, Kane J J, Jerry J, Blackwell C, Ponce de Leon F A, Robl J M. Science. 1998;280:1256–1258. doi: 10.1126/science.280.5367.1256. [DOI] [PubMed] [Google Scholar]
- 26.Drost J B, Lee W R. Environ Mol Mutagen. 1995;25:48–64. doi: 10.1002/em.2850250609. [DOI] [PubMed] [Google Scholar]