Abstract
Measles remains a leading cause of child mortality in developing countries. Residual maternal measles antibodies and immunologic immaturity dampen immunogenicity of the current vaccine in young infants. Because cotton rat respiratory tract is susceptible to measles virus (MV) replication after intranasal (i.n.) challenge, this model can be used to assess the efficacy of MV vaccines. Pursuing a new measles vaccine strategy that might be effective in young infants, we used attenuated Salmonella enterica serovar Typhi CVD 908-htrA and Shigella flexneri 2a CVD 1208 vaccines to deliver mucosally to cotton rats eukaryotic expression plasmid pGA3-mH and Sindbis virus-based DNA replicon pMSIN-H encoding MV hemagglutinin (H). The initial i.n. dose-response with bacterial vectors alone identified a well-tolerated dosage (1 × 109 to 7 × 109 CFU) and a volume (20 μl) that elicited strong antivector immune responses. Animals immunized i.n. on days 0, 28, and 76 with bacterial vectors carrying DNA plasmids encoding MV H or immunized parenterally with these naked DNA vaccine plasmids developed MV plaque reduction neutralizing antibodies and proliferative responses against MV antigens. In a subsequent experiment of identical design, cotton rats were challenged with wild-type MV 1 month after the third dose of vaccine or placebo. MV titers were significantly reduced in lung tissue of animals immunized with MV DNA vaccines delivered either via bacterial live vectors or parenterally. Since attenuated serovar Typhi and S. flexneri can deliver measles DNA vaccines mucosally in cotton rats, inducing measles immune responses (including neutralizing antibodies) and protection, boosting strategies can now be evaluated in animals primed with MV DNA vaccines.
The current parenteral attenuated measles vaccine is well tolerated and highly effective in infants 9 months of age or older (25). Nevertheless, measles remains the highest cause of infant and young child mortality for a vaccine-preventable disease in the world, causing ca. 1 million deaths annually (1, 21, 28). In developing countries where measles transmission is rampant, approximately one-third of measles cases occur during the first year of life (21). One reason for this is the “window of vulnerability” in young infants that lasts from ca. 4 to 9 months of age. During this period, declining maternally derived anti-measles antibodies fail to protect against wild-type measles virus (MV), and yet the infant is below the minimum age (9 months) that the World Health Organization recommends for measles vaccination (25).
In infants younger than 9 months of age, residual maternally derived antibodies and immaturity of the immune system limit the ability of the vaccine to elicit seroconversion (12). Attempts to overcome these immunologic barriers in young infants by using a higher-than-usual titer of parenteral attenuated vaccine were abandoned because of questions of long-term safety (2, 14, 17). For these reasons, research is under way in several laboratories to develop a new generation of measles vaccine that would be safe and effective in young infants during their window of susceptibility.
Among rodents, the cotton rat (Sigmodon hispidus) is extraordinary in its susceptibility to pulmonary infection with MV after intranasal (i.n.) challenge, thereby allowing studies of pathogenesis, infection, and immunity elicited by vaccines (31, 33, 51-53). After i.n. inoculation of cotton rats with wild-type MV, virus replication ensues, allowing recovery of virus from lung tissue, bronchial cells, and draining lymph nodes, as well as from the spleen (31, 51, 52). Vaccine candidates that elicit sufficiently high titers of neutralizing antibodies and MV-specific T-cell responses can confer protection against subsequent challenge with wild-type virus (41, 42, 50, 53).
We investigated whether the cotton rat model could be adapted to test the ability of attenuated Shigella flexneri 2a and Salmonella enterica serovar Typhi to serve as live vectors to deliver measles DNA vaccines to prime MV-specific immune responses (5, 10, 35), in particular, plaque reduction virus neutralizing antibody, an immunologic correlate of protection in cotton rats and humans (6, 32).
MATERIALS AND METHODS
Plasmid construction and purification.
Plasmid pGA3-mH encoding MV hemagglutinin (H) from the Edmonston wild-type strain was constructed in the laboratory of Harriet Robinson as a derivative of an earlier DNA plasmid construct (Fig. 1). To produce pGA3-mH, the MV H sequence was PCR amplified from pJW4303/H (24, 54) by using the 5′ primer AAGCTTATGTCCCCCCAGCGCGACCGCATCAACGCCTTCTACAAGGACAACCCCC and the 3′ primer GGATCCCTATCTGCGATTGGTTCCATCTTCCCG. The amplified sequence was digested with HindIII and BamHI and cloned into HindIII- and BamHI-digested pGA3 vector in keeping with U.S. Food and Drug Administration Points to Consider on Plasmid DNA Vaccines (1996) (40). The 5′ primer optimized the codons for the first 14 amino acids of the MV H gene for codons most frequently used in highly expressed human genes (15, 48). Sequence analyses confirmed the introduction of the 5′ codon-optimized sequence. However, Western blot analyses comparing expression of the 5′ codon-optimized H gene with the non-codon-optimized version revealed that the 5′codon optimization did not increase expression of the MV H gene.
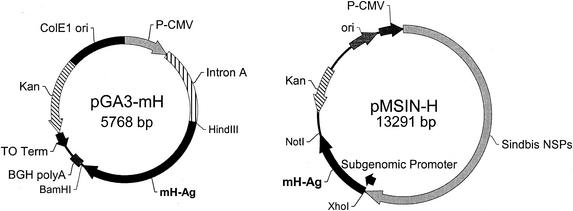
FIG. 1.
Schematic representation of eukaryotic expression vectors pGA3-mH and pMSIN-H encoding MV H. (A) The pGA3-mH vector contains a CMV immediate-early promoter plus intron A for initiating transcription of eukaryotic inserts and bovine growth hormone polyadelylation signal (BGH polyA) for termination of transcription. The vector also contains the ColE1 origin of replication for prokaryotic replication, as well as the kanamycin resistance gene (Kanr) for selection in antibiotic media. The T0 terminator has been placed 3′ to Kanr to increase the stability of eukaryotic inserts. The MV H insert was cloned into pGA3 vector by using HindIII and BamHI restriction endonuclease sites. (B) Plasmid pMSIN-H is a Sindbis virus-based vector with the MV H gene cloned as an XhoI-NotI fragment. The replicon contains the ColE1 origin of replication and Kanr marker. Replication of alphavirus genome is directed by the CMV promoter, whereas the expression of MV H is under the control of the subgenomic promoter.
To construct plasmid pMSIN-H, investigators at the Center for Vaccine Development removed the cDNA gene encoding MV H antigen from peH1SK1 (kindly provided by Alexandra Valsamakis, Johns Hopkins University) as a 1.85-kb XhoI-NotI fragment and ligated it into pSINCP (Chiron Corp., Emeryville, Calif.) digested with XhoI and NotI (39). The DNA vaccine plasmid pSINCP consists of a conventional polymerase II cytomegalovirus (CMV) promoter and cDNA copy of a self-replicating alphavirus (Sindbis virus) genome in which the structural protein genes are removed and replaced with a heterologous gene (or genes) of interest (7, 19, 39). Plasmid pSINCP is a modified plasmid replicon that incorporates nonstructural protein gene sequences from a human dendritic cell tropic strain of Sindbis virus (13). Transcription from the CMV promoter within a mammalian cell gives rise to a Sindbis virus RNA replicon vector, which programs its own cytoplasmic RNA amplification and high-level expression of the introduced heterologous gene via the alphavirus subgenomic promoter.
Upon entry into a mammalian cell, transcription of the Sindbis virus DNA replicon is initiated by the conventional polymerase II CMV promoter (Fig. 1). This results in mRNA that encodes the alphavirus nonstructural replicase proteins which, in turn, drive cytoplasmic amplification of the alphavirus genome sequences. High-level expression of the introduced heterologous gene is achieved via the highly active subgenomic promoter. After transformation into Escherichia coli DH5α, transformants were selected on Luria-Bertani (LB; Fisher Biotech, Fair Lawn, N.J.) agar plates containing 40 μg of kanamycin (Sigma Chemical Co., St. Louis, Mo.)/ml. The resultant plasmid, pMSIN-H, was confirmed by restriction digest analysis and then electroporated into serovar Typhi CVD 908-htrA and S. flexneri 2a CVD 1208. pSINCP (negative control), pMSIN-H, pGA3 (negative control), and pGA3-mH used for intramuscular (i.m.) immunization were purified by using Qiagen columns as indicated by the manufacturer (Qiagen, Inc., Valencia, Calif.) and resuspended in sterile phosphate-buffered saline (PBS) to a final concentration of 1 μg/μl.
Bacterial strains and culture conditions.
Serovar Typhi strain CVD 908-htrA, a ΔaroC ΔaroD ΔhtrA mutant (47), and the derivatives CVD 908-htrA(pTETlpp) (expressing tetanus toxin [TT] fragment C under control of a powerful constitutive prokaryotic promoter) (11, 36, 37, 46), CVD 908-htrA(pGA3), CVD 908-htrA(pGA3-mH), CVD 908-htrA(pSINCP), and CVD 908-htrA(pMSIN-H) were streaked from a glycerol stock onto 2× LB agar plates containing 20 g of Bacto tryptone/liter, 10 g of Bacto yeast extract (Difco, Detroit, Mich.)/liter, and 50 mM NaCl (Sigma) supplemented with 0.0001% (wt/vol) 2,3-dihydroxybenzoic acid (DHB; Sigma) and kanamycin (40 μg/ml), where necessary. Colonies were suspended in LB broth supplemented with DHB and kanamycin and grown overnight.
Shigella strain CVD 1208 (23), which harbors deletions in chromosomal genes guaBA (34) and set (encoding Shigella enterotoxin 1) (8, 9), and invasiveness plasmid gene sen (encoding Shigella enterotoxin 2) (29), as well as derivatives CVD 1208(pTETlpp), CVD 1208(pGA3), CVD 1208(pGA3-mH), CVD 1208(pSINCP), and CVD 1208(pMSIN-H) were streaked from frozen stock onto Trypticase soy agar (Becton Dickinson, Cockeysville, Md.)-Congo red plates supplemented with guanine (0.001%) plus kanamycin (40 μg/ml), as necessary. Single colonies were seeded in LB broth supplemented with guanine (0.001%) plus kanamycin (40 μg/ml), where necessary. Shigella strains were screened to ensure that their ∼140 mDa enteroinvasiveness plasmids were not lost during the genetic manipulations (16). Congo red-positive colonies (denoting the expression of plasmid-encoded Shigella virulence determinants) were used to inoculate broth cultures, and PCR was performed to amplify plasmid virulence gene virG in master seeds.
Vaccine inocula were prepared from fresh broth cultures that were allowed to reach an optical density at 600 nm (OD600) of 1.3 (late log phase). Cultures were centrifuged and resuspended in 1 to 2 ml of sterile PBS to the desired CFU/ml. The number of viable organisms was calculated by plating serial dilutions of the inoculum onto l-agar plates.
Animals and immunization.
Groups of four to five male and female cotton rats (Virion Systems, Gaithersburg, Md.), 6 to 12 weeks old (∼60 to 250 g), were immunized i.n. with CVD 908-htrA or CVD 1208 alone or with these bacterial vectors harboring MV H-DNA vaccine plasmids. In initial experiments, the bacterial strains were administered in 20-, 50-, or 100-μl volumes that were gradually introduced into the cotton rat nare with a micropipette. Control groups received PBS i.n. Other groups were immunized with naked DNA vaccine plasmids i.m. in doses of 100 μg in 100 μl (50 μl per leg). Animals were anesthetized with isofluorane for immunization and the collection of blood samples. Blood samples were collected from the retro-orbital vein prior to immunization and every 2 weeks thereafter for 16 weeks. Sera were stored at −70°C until tested. Animals were sacrificed 120 days after primary immunization, and spleens from each group were pooled.
To compare the immunogenicity of the two measles DNA vaccine plasmids in another species, female BALB/c mice (Charles River, Wilmington, Mass.), 6 to 8 weeks old, were inoculated i.m. on days 0 and 28 with the MV H DNA vaccine plasmids pGA3-mH or pMSIN-H or with the empty plasmids pGA3 and pSINCP, as described above. Blood was collected prior to immunization and every 2 weeks thereafter for up to 8 weeks. For immunization and collection of blood samples, animals were anesthetized as described above. The cotton rat and mouse study protocols were approved by the University of Maryland and Virion Systems Institutional and Animal Care and Use Committees.
Measurement of MV-specific plaque reduction neutralizing (PRN) antibodies.
Cotton rat serum dilutions from different groups were incubated with 100 PFU of wild-type MV (Edmonston strain) for 1 h at 37°C in 5% CO2 and then plated in duplicate onto confluent (∼90% density monolayers) Vero cells (American Type Culture Collection, Manassas, Va.) in 12-well plates in an adaptation of the method of Albrecht et al. (3). After 1 h of incubation, cells were overlaid with 2 ml of agar/well and incubated for 5 days. Wells were stained with neutral red (Gibco Invitrogen Corp., Grand Island, N.Y.) and incubated overnight, and plaques were then counted.
Measurement of antibodies to LPS, TT fragment C, and MV in cotton rat sera.
The total immunoglobulin G (IgG) antibodies in serum against serovar Typhi and S. flexneri 2a lipopolysaccharide (LPS), TT fragment C, and MV H antigens were measured by enzyme-linked immunosorbent assay (ELISA) as previously described (5, 35, 42). Briefly, 96-well plates were coated with 100 μl of serovar Typhi LPS (Difco, Detroit, Mich.) or S. flexneri LPS (purified at the CVD) at 10 μg/ml in carbonate buffer (pH 9), TT fragment C (Boehringer Mannheim, Indianapolis, Ind.) at 5 μg/ml in PBS, and MV Edmonston strain lysate (Biodesign, Saco, Maine) at 5 μg/ml in carbonate buffer (pH 9) for 3 h at 37°C and then blocked overnight with 10% milk (Nestle USA, Inc., Glendale, Calif) in PBS. After each incubation, plates were washed six times with PBS containing 0.05% Tween 20 (PBST). To determine endpoint titers, eight twofold dilutions of sera in 10% milk PBST were tested. To reduce nonspecific binding of cotton rat serum proteins, plates were incubated for 1 h at 4°C. After a washing step, plates were incubated with 100 μl of rabbit serum specific for cotton rat IgG (Virion Systems) diluted 1/2,000 in 10% dried milk in PBST (PBST-M) for 1 h at room temperature. Peroxidase-conjugated goat serum specific for rabbit IgG (Zymed, San Francisco, Calif.) was diluted 1/1,000 in the same diluent and then incubated for 1 h at room temperature. The substrate solution used was TMB Microwell Peroxidase (KPL Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.). After a 15-min incubation, the reaction was stopped by the addition of 100 μl of 1 M H2PO4, and the OD450 nm was determined in an ELISA micro plate reader (Multiskan Ascent; Thermo Labsystems, Helsinki, Finland). Sera were run in duplicate. Negative and positive control sera were included in each assay. Linear regression curves were plotted for each serum sample, and titers were calculated (through equation parameters) as the inverse of the serum dilution that produces an OD of 0.2 above the blank and expressed as ELISA units (EU)/ml.
Measurement of antibodies to MV H antigen in mouse sera.
IgG against MV antigens was measured by ELISA as described above, with the following modifications: serum dilutions were incubated for 1 h at 37°C in plates previously coated with MV lysate and blocked with 10% dried milk in PBS. After a washing step, 100 μl of goat anti-mouse IgG (Boehringer Mannheim) diluted 1/1,000 in PBST-M was added to the wells, followed by incubation for 1 h at 37°C. Incubation with the substrate solution, measurement of the OD, and calculation of the antibody titers were performed as described above.
Proliferative responses.
Spleens from different groups were pooled, and single-cell suspensions were prepared. Cells were resuspended in RPMI 1640 supplemented with 2 mM l-glutamine, 10 mM HEPES, 50 μg of gentamicin/ml, and 10% heat-inactivated fetal calf serum (HyClone, Logan, Utah). Antigen-specific proliferative responses were measured by culturing 2 × 105 cells/well (triplicate wells) in 96-well round bottom plates with MV H antigen (BioWhittaker, Walkersville, Md.) diluted 1/20 to 1/80,000 in complete RPMI in a final volume of 200 μl. Cells were cultured for 6 days at 37°C in 5% CO2. Cultures were pulsed with 1 μCi of tritiated thymidine/well and then harvested 18 to 20 h later. Cell proliferation was assessed by determining the incorporation of [3H]thymidine in a Wallac Microbeta counter (Wallac, Turku, Finland). Results are expressed as the stimulation index, which was calculated as the ratio of counts per minute measured in cells stimulated with MV H antigen to the counts per minute of the cells incubated with medium alone.
i.n. challenge and MV quantification.
In a second experiment, cotton rats were immunized as described above with MV H-DNA vaccines delivered i.n. by bacterial vectors or administered i.m. Controls received PBS i.n. One month after the third dose, the cotton rats were challenged i.n. with 100 μl containing 107 PFU of wild-type MV (Edmonston strain). Lungs were harvested from euthanized animals 4 days after challenge, weighed, and homogenized in 2 ml of Eagle minimal essential medium supplemented with 5% fetal calf serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml (all from Gibco). Lung homogenates were centrifuged for 20 min at 4°C, and supernatants were collected. Virus load was measured by incubating 100 μl of 10-fold dilutions of individual lung supernatants in 48-well plates containing confluent monolayers of Vero cells (triplicate wells). The wells were observed for cytopathic effect 7 days later. Infectivity titers were calculated by the Karber method (20) and are expressed as the 50% tissue culture infective doses (TCID50) per g of lung tissue.
Statistical analysis.
Individual antibody titers were log transformed and group titers expressed as geometric mean (GM) ± the standard error (SE). Antibody titers measured prior to immunization (day 0) and after each dose were compared by using the Student t test and the Mann-Whitney rank sum test (when the normality test failed). Multiple comparisons of PRN antibody and ELISA titers in experimental groups versus control groups were performed by using Kruskal-Wallis nonparametric one-way analysis of variance (Dunnett's method). The virus titers measured after challenge in experimental versus control groups were compared by using Kruskal-Wallis one-way analysis of variance by ranks (Dunn's method). In all of the tests, a P value of <0.05 was considered to be statistically significant. Statistical analyses were performed by using SigmaStat 2.0 software (SPSS, Inc., Chicago, Ill.).
RESULTS
Salmonella and Shigella live vector strains in cotton rats: role of vaccine inoculum volume and dosage level.
In a series of preliminary studies, animals were immunized i.n. with different doses of serovar Typhi CVD 908-htrA or S. flexneri CVD 1208 alone that ranged from 1.0 × 106 to 7.4 × 109 CFU delivered in a volume of either 100, 50, or 20 μl. Animals inoculated with vaccine dosage levels of 4.0 × 108 to 7.4 × 109 CFU contained in a volume of 50 or 100 μl died within 48 h of inoculation. In contrast, when similar vaccine dosage levels (3.0 × 108 to 7.4 × 109 CFU) were delivered in a volume of 20 μl, the vaccines were well tolerated (no animals died or exhibited untoward reactions) and strong antivector immune responses (P < 0.05 compared to preimmunization levels and control group) were observed (Fig. 2). Consequently, all subsequent experiments utilized vaccine delivered in a total volume of 20 μl.
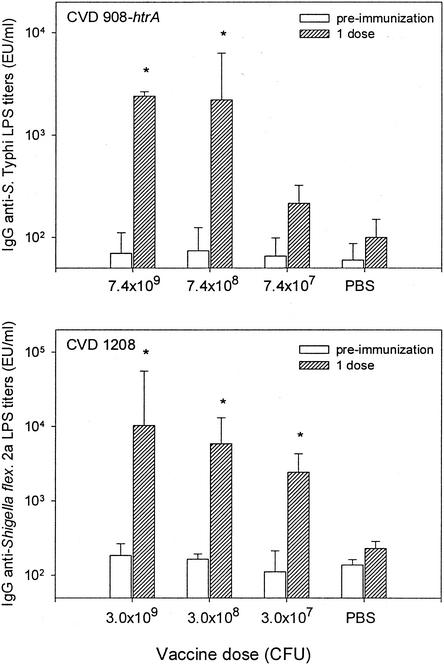
FIG. 2.
IgG anti-LPS responses elicited in serum in cotton rats immunized i.n. with various doses of vaccine strain serovar Typhi (upper panel) or S. flexneri 2a (lower panel). Cotton rats were inoculated i.n. with 7.4 × 107 to 7.4 × 109 CFU of CVD 908-htrA or 3.0 × 107 to 3.0 × 109 CFU of CVD 1208 suspended in a 20-μl volume. A control group received 20 μl of PBS i.n. IgG anti-LPS titers were measured by ELISA in serum samples collected 21 days after immunization. Bars indicate the GM titers ± the SE from four animals. ✽, Significant increases (P < 0.05) in antibody titers compared to preimmunization levels and control group. EU, ELISA unit(s).
Immunogenicity of single i.n. doses of serovar Typhi and Shigella live vectors carrying a prokaryotic expression system plasmid encoding TT fragment C.
We next investigated the dosage level of live vector bacteria that would induce the strongest immune response against both vector antigens and a foreign antigen after i.n. immunization with a single dose. Immune responses in groups of cotton rats were measured 21 days after i.n. immunization with various dosage levels of serovar Typhi CVD 908-htrA or S. flexneri CVD 1208 alone (Fig. 2) or expressing TT fragment C from prokaryotic expression plasmid pTETlpp (Fig. 3). As shown in Fig. 2, cotton rats immunized i.n. with a single dose of either CVD 908-htrA (7.4 × 108 to 7.4 × 109 CFU) or CVD 1208 (3.0 × 107 to 3.0 × 109 CFU), but not PBS control groups, mounted significant rises in anti-vector LPS antibodies responses. Postimmunization titers in animals that received ca. 108 to 109 CFU of CVD 908-htrA vaccine were significantly higher than those that received ca. 106 to 107 CFU (P < 0.05).
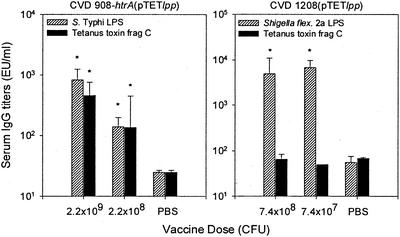
FIG. 3.
IgG responses in serum to serovar Typhi LPS and TT fragment C in cotton rats immunized i.n. with CVD 908-htrA(pTETlpp) and CVD 1208(pTETlpp). Cotton rats received one dose of 2.2 × 108 to 2.2 × 109 CFU of CVD 908-htrA(pTETlpp) or 7.4 × 107 to 7.4 × 108 of CVD 1208(pTETlpp). Titers of antibody to serovar Typhi, S. flexneri 2a LPS, and TT fragment C were measured by ELISA in serum samples collected 21 days after immunization. GM titers ± the SE from four animals are shown. ✽, Significant increases (P < 0.05) in antibody titers after immunization compared to preimmunization levels and control group. EU, ELISA unit(s).
Cotton rats immunized with CVD 908-htrA(pTETlpp) expressing TT fragment C under control of a powerful constitutive promoter induced strong antibody responses to this foreign antigen (P = 0.029). In contrast, a single i.n. dose of S. flexneri strain CVD 1208(pTETlpp) failed to elicit serologic responses to fragment C, even though strong anti-vector LPS responses were observed (P < 0.05) (Fig. 3).
Immunogenicity of two spaced i.n. doses of serovar Typhi live vector carrying a prokaryotic expression system encoding TT fragment C.
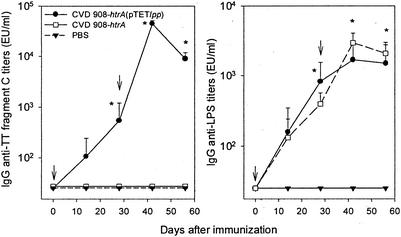
We next studied the kinetics of serologic responses to fragment C after i.n. immunization of cotton rats with two doses of CVD 908-htrA(pTETlpp) on days 0 and 28. High titers of anti-fragment C antibody were observed on day 28 after the first dose compared with preimmunization levels (P < 0.03); titers significantly increased after the booster (P < 0.001), reaching a peak on day 42 (Fig. 4, left panel). No antibody responses to fragment C were detected in cotton rats that received CVD 908-htrA alone or PBS. Strong responses to serovar Typhi LPS (P < 0.03 on days 28, 42, and 56 compared to preimmunization levels) were also induced (Fig. 4, right panel).
FIG. 4.
IgG responses in serum to serovar Typhi LPS and TT fragment C in cotton rats immunized i.n. with CVD 908-htrA(pTETlpp). Cotton rats were immunized i.n. on days 0 and 28 (indicated by arrows) with 2 to 5 × 109 CFU of CVD 908-htrA or CVD 908-htrA(pTETlpp). Curves indicate GM titers ± the SE from four animals. Titers were measured by ELISA in serum samples collected on days 0, 14, 28, 42, and 56 after primary immunization. ✽, Significant increases (P < 0.03) in antibody titers after immunization compared to preimmunization levels. EU, ELISA unit(s).
Immunogenicity of DNA vaccine plasmids encoding MV H administered parenterally to cotton rats.
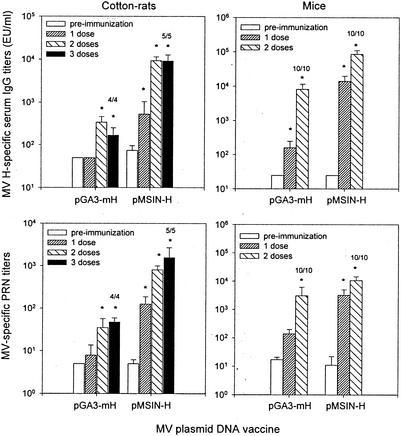
Experiments were undertaken in cotton rats to compare the immunogenicity of pMSIN-H (the DNA-based Sindbis virus replicon system encoding MV H) and pGA3-mH (the “traditional” DNA plasmid encoding MV H) when these DNA vaccines were administered parenterally. Cotton rats received three doses of DNA vaccine i.m., 28 days apart; control animals received empty plasmid pGA3 or pSINCP, or PBS. A significant increase in MV H antibody titers (including neutralizing antibodies) was observed after immunization with either of the two DNA vaccine plasmids encoding MV H (Fig. 5). No responses were observed in control cotton rats. Significantly higher antibody titers were observed in cotton rats immunized with pMSIN-H compared to pGA3-mH (P < 0.004 measured by ELISA and P < 0.003 measured by PRN assay, after the second dose) (Fig. 5).
FIG. 5.
IgG responses in serum to MV H antigen in cotton rats and mice immunized with MV H DNA vaccine plasmids. Cotton rats were immunized i.m. on days 0, 28, and 56 with 100 μg of pGA3-mH or pMSIN-H in a volume of 100 μl. IgG responses to MV H antigen in serum were measured by ELISA and PRN assay on days 0, 28, 56, and 84. Mice were immunized in a similar way but received only two doses (on days 0 and 28). IgG responses to MV H antigen in serum were measured by ELISA and PRN assay on days 0, 28, and 56. Negative control groups included animals immunized with 100 μg of empty plasmids (pGA3 and pSINCP) or PBS. GM titers ± the SE from four to five animals are indicated. The numbers of animals that seroconverted within each group (i.e., that manifested a fourfold increase in antibody titers compared to preimmunization levels) are indicated. ✽, Significant increases (P < 0.05) in antibody titers after immunization. EU, ELISA unit(s).
Immunogenicity in mice of DNA vaccine plasmids encoding MV H administered parenterally.
The significant difference in immunogenicity noted between the two measles H DNA vaccine constructs in parenterally immunized cotton rats prompted us to undertake a similar comparison of immunogenicity in mice. Mice were immunized i.m. with two doses of either pGA3-mH or pMSIN-H, given 28 days apart; control animals received empty plasmid pSINCP, pGA3, or PBS. As shown in Fig. 5 (right panel), both DNA vaccines stimulated rises in serum MV H antibody titers but, as in cotton rats, the titers were significantly higher in mice that received pMSIN-H (P < 0.002 for both PRN antibody and ELISA titers after each dose). No responses were observed in animals immunized with empty plasmids or PBS.
Antibody responses in cotton rats against MV H elicited by Salmonella and Shigella live vectors delivering MV H DNA vaccine plasmids.
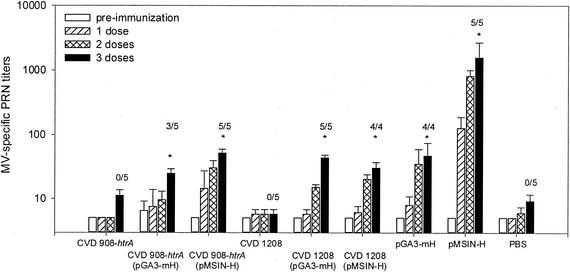
Based on results of experiments that documented the ability of the parenterally administered DNA vaccines to elicit MV PRN antibody, we proceeded to evaluate Salmonella and Shigella as mucosal live vectors to deliver these DNA vaccines encoding MV H antigen. Cotton rats were immunized i.n. with CVD 908-htrA or CVD 1208 harboring plasmids pGA3-mH or pMSIN-H; negative control groups received either CVD 908-htrA or CVD 1208 without DNA plasmids, or they received PBS. Positive control animals were immunized i.m. with pGA3-mH or pMSIN-H. The induction of MV PRN antibodies was measured as the critical readout, since there is strong correlation between the levels of neutralizing antibodies and protection from measles (6, 30). As shown in Fig. 6, a significant increase in MV PRN antibody titers was observed after the third dose in cotton rats immunized with Salmonella and Shigella live vectors delivering the MV H DNA vaccine plasmids (P < 0.004 and P < 0.03, respectively). All animals in groups that received CVD 908-htrA(pMSIN-H) and CVD 1208(pGA3-mH) or CVD 1208(pMSIN-H) seroconverted after the third dose (Fig. 6). The highest MV PRN antibody responses were observed in rats immunized i.m. with pMSIN-H (Fig. 6). PRN antibody responses elicited by live vectors carrying DNA plasmids were similar in magnitude to those induced by i.m. administration of pGA3-mH (Fig. 6). Strong immune responses to the LPS of the bacterial vectors were also observed (data not shown).
FIG. 6.
Antibody responses to MV as measured by PRN assay. Groups of cotton rats were immunized i.n. on days 0, 28, and 76 with 4 × 108 to 5 × 109 CFU/20 μl of CVD 908-htrA(pGA3-mH), CVD 908-htrA(pMSIN-H), CVD 1208(pGA3-mH), and CVD 1208(pMSIN-H). Cotton rat groups serving as negative controls received CVD 908-htrA or CVD 1208 without plasmids and PBS i.n. Positive control groups included animals immunized i.m. with 100 μl (1 μg/μl) of plasmids pGA3-mH or pMSIN-H. PRN antibody titers were measured on days 0 (□), 28 ( ), 65 (
), 65 ( ), and 104 (▪). Responses are expressed as GM titers ± the SE from four to five animals in each group. The numbers of animals that seroconverted, i.e., manifested a fourfold increase in antibody titers, after the third dose within each group are indicated. ✽, Significant increases (P < 0.05) in antibody titers after immunization compared to preimmunization levels and control groups.
), and 104 (▪). Responses are expressed as GM titers ± the SE from four to five animals in each group. The numbers of animals that seroconverted, i.e., manifested a fourfold increase in antibody titers, after the third dose within each group are indicated. ✽, Significant increases (P < 0.05) in antibody titers after immunization compared to preimmunization levels and control groups.
Proliferative responses to MV H induced by live vectors delivering measles DNA vaccines.
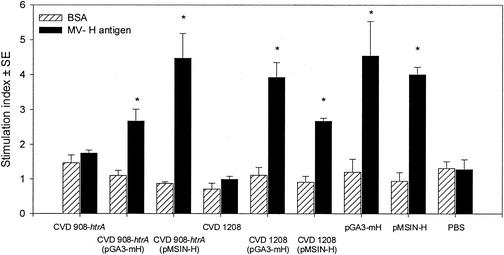
Significant T-cell proliferation was observed in cells from cotton rats immunized with CVD 908-htrA and CVD 1208 carrying plasmids pGA3-mH and pMSIN-H. In agreement with the serologic responses, specific T-cell responses were also observed in cotton rats immunized i.m. with plasmids pGA3-mH and pMSIN-H (Fig. 7).
FIG. 7.
Proliferative responses against MV H antigen. Splenocytes from cotton rats immunized i.n. with three doses of CVD 908-htrA and CVD 1208 alone or carrying MV DNA vaccine plasmids, and i.m. with plasmids pGA3-mH or pMSIN-H, as described in Fig. 6, were incubated in vitro in the presence of MV H antigen or bovine serum albumin (BSA) as control. Responses were studied 4 months after primary immunization. Bars indicate peak responses within the range of concentrations tested (0.01 to 10 μg/ml for BSA and 1/20 to 1/1/80,000 for MV H antigen) and are expressed as the stimulation index ± the SE of triplicate wells. ✽, Significant responses (P < 0.05) compared to control groups. The results shown are representative of two separate experiments.
Protection from challenge.
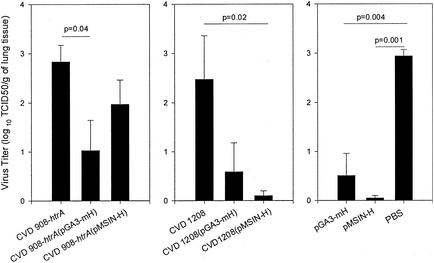
These early experiments with the MV H DNA vaccines delivered mucosally by bacterial vectors were originally merely intended to document a priming of the cotton rat immune system that could then be boosted by MV H delivered by an alternative presentation, such as by attenuated MV or adenovirus encoding MV H administered by the respiratory route, following a prime-boost strategy. However, the demonstration that cotton rats develop modest to moderate MV PRN antibody titers in response to MV H DNA vaccines delivered mucosally via bacterial vectors prompted us to perform a preliminary challenge study. In the present study, groups of cotton rats immunized identically as in the groups described in the first immunogenicity study were challenged i.n. with wild-type MV. As summarized in Fig. 8, a significant decrease in virus recovery was observed in the positive control cotton rats that had been immunized with MV H DNA vaccines i.m., particularly those that received pMSIN-H (Fig. 8). Similarly, all cotton rat groups immunized with either Salmonella or Shigella live vectors carrying the MV H DNA vaccines also showed decreased virus titers compared to control animals immunized with bacteria alone (Fig. 8). Despite the small numbers of animals involved, the diminution in viral load (TCID50) observed in cotton rats immunized with CVD 908-htrA(pGA3-mH) and CVD 1208(pMSIN-H) was statistically significant (P = 0.04 and P = 0.02, respectively). Results of this experiment (Fig. 8) document the ability of mucosal immunization with Shigella and Salmonella live vectors carrying MV H DNA vaccine plasmids to decrease virus replication in cotton rat lung after i.n. challenge with wild-type virus.
FIG. 8.
i.n. challenge of immunized cotton rats with wild-type MV. Groups of four to five cotton rats were immunized i.n. with 2 to 7 × 109 CFU of CVD 908-htrA and CVD 1208 alone or carrying plasmids pGA3-mH and pMSIN-H; i.m. with 100 μl (1 μg/μl) of plasmids pGA3-mH and pMSIN-H and i.n. with PBS (control) as described in Fig. 6. One month after the third dose, animals were challenged i.n. with wild-type MV (107 PFU in 100 μl). Pulmonary virus was measured in lung homogenates 4 days after challenge. The results are reported as the mean log10 TCID50/g of lung tissue ± the SE. The data represent the average for four to five animals. Significant differences between groups, when present, are indicated.
DISCUSSION
These experiments mark an early milestone in an ambitious project to develop a new vaccination strategy that could allow infants in developing countries who are too young to receive the current measles vaccines to be immunized against measles and to accomplish this without the use of needles and syringes. Recognizing that residual maternal antibodies in young infants constitute a formidable barrier that has frustrated attempts to immunize with existing parenteral attenuated MV vaccines, we explored the use of DNA vaccines (26). DNA vaccines are particularly suitable for priming the immune system of young animals, as long as titers of maternally derived circulating antibodies are not high (26, 44, 45). Once immunological priming has occurred, boosting can be achieved by using other vaccine moieties in prime-boost strategies (18, 27, 43, 49). Since protection against measles is mainly mediated by neutralizing antibodies directed against the H antigen, in these early studies we compared two different DNA vaccines encoding MV H for their ability to stimulate MV neutralizing antibodies. We also compared two different attenuated bacterial vectors, S. flexneri 2a strain CVD 1208 and serovar Typhi strain CVD 908-htrA, for their efficiency in delivering the DNA vaccine plasmids to antigen-presenting cells (APC) after mucosal (i.n.) administration. Finally, these immunogenicity studies were carried out in cotton rats, since this small animal model allows assessment of the efficacy of measles vaccines by means of a respiratory i.n. challenge.
Since there were no prior reports of the use of either Shigella or serovar Typhi live vaccine strains to deliver MV antigens in cotton rats, the first order of business was to ascertain whether the highly human host restricted Shigella and serovar Typhi vaccine strains could be administered safely to these animals. We pursued the i.n. immunization route, borrowing from the success of i.n. administration of serovar Typhi in mice and of Shigella in guinea pigs (4, 5, 11, 22, 34-36). By careful titration, a dosage level (109 CFU) and an inoculum volume (20 μl) were identified that were well tolerated and elicited immune responses to the live vector strain. Based on experiences in other animal models with i.n. administration of bacterial live vectors, we presume that the deaths observed in some anesthetized animals given higher doses of bacteria in larger inoculum volumes resulted from aspiration into the lungs resulting in adverse consequences (37). The fact that the identical dosages (CFU) of bacteria administered in a much smaller volume were completely well tolerated supports this assumption. In a next step with bacteria carrying a prokaryotic expression system encoding TT fragment C, it was shown that a single i.n. dose of live vector could elicit serum tetanus antitoxin in cotton rats.
Finally, after the above preliminary experiments, the two DNA vaccine plasmids were administered to cotton rats, either mucosally by means of the bacterial live vectors or parenterally, on three occasions 1 month apart. As shown in Fig. 5 and 6, parenteral inoculation with the Sindbis virus DNA replicon pMSIN-H elicited the highest titers of measles neutralizing antibody; these titers were significantly greater than the serologic response to the pGA3-mH DNA vaccine. Extremely encouraging results were obtained with both the attenuated S. flexneri 2a and the serovar Typhi live vectors as mucosal delivery vehicles for the two DNA vaccine plasmids; both succeeded in eliciting seroconversions of measles neutralizing antibody (Fig. 6). Notably, the titers achieved by using mucosal live vectors to deliver the pGA3-mH DNA vaccine were similar to those observed after parenteral inoculation. In contrast, mucosal delivery of the Sindbis virus-based measles DNA vaccine was inferior to parenteral administration. These results corroborate our earlier reports of the successful stimulation of serum antibodies to antigens encoded by DNA vaccines administered mucosally in mice and guinea pigs by means of attenuated Shigella and serovar Typhi live vectors (5, 35).
The Sindbis virus replicon system makes use of the propensity of alphaviruses to generate multiple copies of mRNA encoding structural proteins (38, 39). A foreign gene is inserted in place of the Sindbis virus structural protein genes in the DNA replicon that is based on the Sindbis virus genome. In addition to mRNA amplification, the potency of pSIN may be aided by the potential immunostimulatory effects of the double-stranded RNA intermediate and extra helper T-cell epitopes in the alphavirus nonstructural proteins encoded by the vaccine. The advantages of the alphavirus amplification were evident in the comparison of parenteral administration of pMSIN-H versus conventional DNA vaccine pGA3-mH in both cotton rats and mice. In these early studies with the bacterial vectors, this difference between the two measles DNA vaccine constructs was not noted. The explanation may, in part, reside in the distinct ways that the DNA vaccine plasmids are delivered by these two approaches. After i.m. inoculation, a small fraction of DNA vaccine plasmid is taken up by APCs, and the first steps toward induction of an immune response are initiated. Delivered in this way, it may be that the ability of the Sindbis virus vector to more effectively stimulate the innate immune system is critical in determining the ultimate adaptive immune response. When attenuated Shigella and serovar Typhi contact a mucosal surface (such as the intestine in humans or the nasal mucosa in rodents inoculated i.n.), the bacteria are taken up by M cells that overlay the mucosa-associated lymphoid tissue. The bacteria are then passed to the underlying lymphoid tissue, where they are internalized by professional APCs such as macrophages and dendritic cells. Whatever proportion of live vector bacteria is taken up by APCs (that ultimately allows release of the DNA vaccine plasmids and activation of their eukaryotic expression systems), activation occurs in a local environment in which the innate immune system has been highly stimulated by the LPS, flagella, and other pathogen-associated molecular patterns of the serovar Typhi and Shigella live vectors. The potent stimulation of the innate immune system by the bacterial live vectors may make subtler differences in the inherent immunostimulatory capacity of the plasmids themselves irrelevant. If this proves to be true, ways will have to be sought to increase the ability of the bacterial live vectors carrying DNA vaccines to be taken up by APCs. The final experiment involved i.n. challenge of immunized and control cotton rats with wild-type MV. The fundamental strategy that we are pursuing is to design a prime-boost approach in which mucosally administered MV DNA vaccines are intended to prime the infant immune system to respond to a boost with MV H delivered by another means. Nevertheless, the detection of MV PRN antibody titers in sera of cotton rats that received the MV H DNA vaccines delivered mucosally via bacterial vectors motivated us to perform a preliminary experiment to assess whether the serologic responses elicited by the priming immunization could mediate protection. Although the number of animals in each group was small, a protective effect was nevertheless observed. Cotton rats that received either the pGA3-mH or the Sindbis virus H DNA vaccine delivered by CVD 1208 S. flexneri 2a vector had significantly decreased titers of MV in their lungs (Fig. 8).
We have demonstrated that the cotton rat can be used as a practical animal model for evaluation of measles DNA vaccines delivered mucosally by either Shigella or serovar Typhi live vectors, resulting in the elicitation of serum neutralizing antibodies and protection against i.n. challenge with wild-type MV (as evidenced by diminished viral load in lung). These results pave the way for future studies that will evaluate accelerated immunization schedules and will compare the effectiveness of various types of booster vaccination to pursue a “prime-boost” vaccination strategy.
Acknowledgments
We thank Gregory Prince, Lorraine Ward, and Kevin Yim from Virion Systems for assistance with cotton rat experiments.
This work was supported by a grant from the Bill and Melinda Gates Foundation (to M.M.L.).
REFERENCES
- 1.Aaby, P., K. Knudsen, T. G. Jensen, J. Tharup, A. Poulsen, M. Sodemann, M. C. da Silva, and H. Whittle. 1990. Measles incidence, vaccine efficacy, and mortality in two urban African areas with high vaccination coverage. J. Infect. Dis. 162:1043-1048. [DOI] [PubMed] [Google Scholar]
- 2.Aaby, P., K. Knudsen, H. Whittle, I. M. Lisse, J. Thaarup, A. Poulsen, M. Sodemann, M. Jakobsen, L. Brink, U. Gansted, A. Permin, T. G. Jensen, H. Andersen, and M. C. da Silva. 1993. Long-term survival after Edmonston-Zagreb measles vaccination in Guinea-Bissau: increased female mortality rate. J. Pediatr. 122:904-908. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht, P., K. Herrmann, and G. R. Burns. 1981. Role of virus strain in conventional and enhanced measles plaque neutralization test. J. Virol. Methods 3:251-260. [DOI] [PubMed] [Google Scholar]
- 4.Altboum, Z., E. M. Barry, G. Losonsky, J. E. Galen, and M. M. Levine. 2001. Attenuated Shigella flexneri 2a ΔguaBA strain CVD 1204 expressing enterotoxigenic Escherichia coli (ETEC) CS2 and CS3 fimbriae as a live mucosal vaccine against Shigella and ETEC infection. Infect. Immun. 69:3150-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson, R., M. F. Pasetti, M. B. Sztein, M. M. Levine, and F. N. Noriega. 2000. Δgua attenuated Shigella flexneri 2a strain CVD 1204 as a Shigella vaccine and as a live mucosal delivery system for fragment C of tetanus toxin. Vaccine 18:2193-2202. [DOI] [PubMed] [Google Scholar]
- 6.Chen, R. T., L. E. Markowitz, P. Albrecht, J. A. Stewart, L. M. Mofenson, S. R. Preblud, and W. A. Orenstein. 1990. Measles antibody: reevaluation of protective titers. J. Infect. Dis. 162:1036-1042. [DOI] [PubMed] [Google Scholar]
- 7.Dubensky, T. W., Jr., D. A. Driver, J. M. Polo, B. A. Belli, E. M. Latham, C. E. Ibanez, S. Chada, D. Brumm, T. A. Banks, S. J. Mento, D. J. Jolly, and S. M. Chang. 1996. Sindbis virus DNA-based expression vectors: utility for in vitro and in vivo gene transfer. J. Virol. 70:508-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fasano, A., F. R. Noriega, F. M. Liao, W. Wang, and M. M. Levine. 1997. Effect of shigella enterotoxin 1 (ShET1) on rabbit intestine in vitro and in vivo. Gut 40:505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fasano, A., F. R. Noriega, D. R. Maneval, Jr., S. Chanasongcram, R. Russell, S. Guandalini, and M. M. Levine. 1995. Shigella enterotoxin 1: an enterotoxin of Shigella flexneri 2a active in rabbit small intestine in vivo and in vitro. J. Clin. Investig. 95:2853-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fennelly, G. J., S. A. Khan, M. A. Abadi, T. F. Wild, and B. R. Bloom. 1999. Mucosal DNA vaccine immunization against measles with a highly attenuated Shigella flexneri vector. J. Immunol. 162:1603-1610. [PubMed] [Google Scholar]
- 11.Galen, J. E., O. G. Gomez-Duarte, G. A. Losonsky, J. L. Halpern, C. S. Lauderbaugh, S. Kaintuck, M. K. Reymann, and M. M. Levine. 1997. A murine model of intranasal immunization to assess the immunogenicity of attenuated Salmonella typhi live vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine 15:700-708. [DOI] [PubMed] [Google Scholar]
- 12.Gans, H. A., A. M. Arvin, J. Galinus, L. Logan, R. DeHovitz, and Y. Maldonado. 1998. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA 280:527-532. [DOI] [PubMed] [Google Scholar]
- 13.Gardner, J. P., I. Frolov, S. Perri, Y. Ji, M. L. MacKichan, M. J. zur, M. Chen, B. A. Belli, D. A. Driver, S. Sherrill, C. E. Greer, G. R. Otten, S. W. Barnett, M. A. Liu, T. W. Dubensky, and J. M. Polo. 2000. Infection of human dendritic cells by a Sindbis virus replicon vector is determined by a single amino acid substitution in the E2 glycoprotein. J. Virol. 74:11849-11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garenne, M., O. Leroy, J. P. Beau, and I. Sene. 1991. Child mortality after high-titre measles vaccines: prospective study in Senegal. Lancet 338:903-907. [DOI] [PubMed] [Google Scholar]
- 15.Haas, J., E. C. Park, and B. Seed. 1996. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr. Biol. 6:315-324. [DOI] [PubMed] [Google Scholar]
- 16.Hale, T. L., E. V. Oaks, and S. B. Formal. 1985. Identification and antigenic characterization of virulence-associated, plasmid-coded proteins of Shigella spp. and enteroinvasive Escherichia coli. Infect. Immun. 50:620-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halsey, N. A. 1993. Increased mortality after high titer measles vaccines: too much of a good thing. Pediatr. Infect. Dis. J. 12:462-465. [DOI] [PubMed] [Google Scholar]
- 18.Hammond, J. M., E. S. Jansen, C. J. Morrissy, W. V. Goff, G. C. Meehan, M. M. Williamson, C. Lenghaus, K. W. Sproat, M. E. Andrew, B. E. Coupar, and M. A. Johnson. 2001. A prime-boost vaccination strategy using naked DNA followed by recombinant porcine adenovirus protects pigs from classical swine fever. Vet. Microbiol. 80:101-119. [DOI] [PubMed] [Google Scholar]
- 19.Hariharan, M. J., D. A. Driver, K. Townsend, D. Brumm, J. M. Polo, B. A. Belli, D. J. Catton, D. Hsu, D. Mittelstaedt, J. E. McCormack, L. Karavodin, T. W. Dubensky, Jr., S. M. Chang, and T. A. Banks. 1998. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J. Virol. 72:950-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawkes, R. A. 2002. General principles underlying laboratory diagnosis of viral infections, p. 3-48. In E. H. Lennette and N. J. Schmidt (ed.), APHA, Washington, D.C.
- 21.Katz, S. L., and B. G. Gellin. 1994. Measles vaccine: do we need new vaccines or new programs? Science 265:1391-1392. [DOI] [PubMed] [Google Scholar]
- 22.Koprowski, H. 2., M. M. Levine, R. J. Anderson, G. Losonsky, M. Pizza, and E. M. Barry. 2000. Attenuated Shigella flexneri 2a vaccine strain CVD 1204 expressing colonization factor antigen I and mutant heat-labile enterotoxin of enterotoxigenic Escherichia coli. Infect. Immun. 68:4884-4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine, M. M. 2000. Immunization against bacterial diseases of the intestine. J. Pediatr. Gastroenterol. Nutr. 31:336-355. [DOI] [PubMed] [Google Scholar]
- 24.Lu, S., J. Arthos, D. C. Montefiori, Y. Yasutomi, K. Manson, F. Mustafa, E. Johnson, J. C. Santoro, J. Wissink, J. I. Mullins, J. R. Haynes, N. L. Letvin, M. Wyand, and H. L. Robinson. 1996. Simian immunodeficiency virus DNA vaccine trial in macaques. J. Virol. 70:3978-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markowitz, L. E., and P. Nieburg. 1991. The burden of acute respiratory infection due to measles in developing countries and the potential impact of measles vaccine. Rev. Infect. Dis. 13(Suppl. 6):S555-S561. [DOI] [PubMed] [Google Scholar]
- 26.Martinez, X., C. Brandt, F. Saddallah, C. Tougne, C. Barrios, F. Wild, G. Dougan, P. H. Lambert, and C. A. Siegrist. 1997. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte responses in neonates and during early life. Proc. Natl. Acad. Sci. USA 94:8726-8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez, X., X. Li, J. Kovarik, M. Klein, P. H. Lambert, and C. A. Siegrist. 1999. Combining DNA and protein vaccines for early life immunization against respiratory syncytial virus in mice. Eur. J. Immunol. 29:3390-3400. [DOI] [PubMed] [Google Scholar]
- 28.Murray, C. J., and A. D. Lopez. 1997. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 349:1436-1442. [DOI] [PubMed] [Google Scholar]
- 29.Nataro, J. P., J. Seriwatana, A. Fasano, D. R. Maneval, L. D. Guers, F. Noriega, F. Dubovsky, M. M. Levine, and J. G. Morris, Jr. 1995. Identification and cloning of a novel plasmid-encoded enterotoxin of enteroinvasive Escherichia coli and Shigella strains. Infect. Immun. 63:4721-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neumann, P. W., J. M. Weber, A. G. Jessamine, and M. V. O'Shaughnessy. 1985. Comparison of measles antihemolysin test, enzyme-linked immunosorbent assay, and hemagglutination inhibition test with neutralization test for determination of immune status. J. Clin. Microbiol. 22:296-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niewiesk, S. 1999. Cotton rats (Sigmodon hispidus): an animal model to study the pathogenesis of measles virus infection. Immunol. Lett. 65:47-50. [DOI] [PubMed] [Google Scholar]
- 32.Niewiesk, S., and P. G. Germann. 2000. Development of neutralizing antibodies correlates with resolution of interstitial pneumonia after measles virus infection in cotton rats. J. Exp. Anim. Sci. 40:201-210. [Google Scholar]
- 33.Niewiesk, S., H. Ohnimus, J. J. Schnorr, M. Gotzelmann, S. Schneider-Schaulies, C. Jassoy, and V. ter Meulen. 1999. Measles virus-induced immunosuppression in cotton rats is associated with cell cycle retardation in uninfected lymphocytes. J. Gen. Virol. 80:2023-2029. [DOI] [PubMed] [Google Scholar]
- 34.Noriega, F. R., G. Losonsky, C. Lauderbaugh, F. M. Liao, M. S. Wang, and M. M. Levine. 1996. Engineered ΔguaB-A, ΔvirG Shigella flexneri 2a strain CVD 1205: construction, safety, immunogenicity and potential efficacy as a mucosal vaccine. Infect. Immun. 64:3055-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasetti, M. F., R. J. Anderson, F. R. Noriega, M. M. Levine, and M. B. Sztein. 1999. Attenuated ΔguaBA Salmonella typhi vaccine strain CVD 915 as a live vector utilizing prokaryotic or eukaryotic expression systems to deliver foreign antigens and elicit immune responses. Clin. Immunol. 92:76-89. [DOI] [PubMed] [Google Scholar]
- 36.Pasetti, M. F., T. E. Pickett, M. M. Levine, and M. B. Sztein. 2000. A comparison of immunogenicity and in vivo distribution of Salmonella enterica serovar Typhi and Typhimurium live vector vaccines delivered by mucosal routes in the murine model. Vaccine 18:3208-3213. [DOI] [PubMed] [Google Scholar]
- 37.Pickett, T. E., M. F. Pasetti, J. E. Galen, M. B. Sztein, and M. M. Levine. 2000. In vivo characterization of the murine intranasal model for assessing the immunogenicity of attenuated Salmonella enterica serovar Typhi strains as live mucosal vaccines and as live vectors. Infect. Immun. 68:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polo, J. M., and T. W. Dubensky. 2002. Virus-based vectors for human vaccine applications. Drug Discov. Today 7:719-727. [DOI] [PubMed] [Google Scholar]
- 39.Polo, J. M., J. P. Gardner, Y. Ji, B. A. Belli, D. A. Driver, S. Sherrill, S. Perri, M. A. Liu, and T. W. Dubensky, Jr. 2000. Alphavirus DNA and particle replicons for vaccines and gene therapy. Dev. Biol. 104:181-185. [PubMed] [Google Scholar]
- 40.Ross, T. M., Y. Xu, R. A. Bright, and H. L. Robinson. 2000. C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge. Nat. Immunol. 1:127-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlereth, B., P. G. Germann, V. ter Meulen, and S. Niewiesk. 2000. DNA vaccination with both the haemagglutinin and fusion proteins but not the nucleocapsid protein protects against experimental measles virus infection. J. Gen. Virol. 81(Pt. 5):1321-1325. [DOI] [PubMed] [Google Scholar]
- 42.Schlereth, B., J. K. Rose, L. Buonocore, V. ter Meulen, and S. Niewiesk. 2000. Successful vaccine-induced seroconversion by single-dose immunization in the presence of measles virus-specific maternal antibodies. J. Virol. 74:4652-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T-cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 44.Siegrist, C. A. 1997. Potential advantages and risks of nucleic acid vaccines for infant immunization. Vaccine 15:798-800. [DOI] [PubMed] [Google Scholar]
- 45.Siegrist, C. A., C. Barrios, X. Martinez, C. Brandt, M. Berney, M. Cordova, J. Kovarik, and P. H. Lambert. 1998. Influence of maternal antibodies on vaccine responses: inhibition of antibody but not T-cell responses allows successful early prime-boost strategies in mice. Eur. J. Immunol. 28:4138-4148. [DOI] [PubMed] [Google Scholar]
- 46.Tacket, C. O., J. Galen, M. B. Sztein, G. Losonsky, T. L. Wyant, J. Nataro, S. S. Wasserman, R. Edelman, S. Chatfield, G. Dougan, and M. M. Levine. 2000. Safety and immune responses to attenuated Salmonella enterica serovar Typhi oral live vector vaccines expressing tetanus toxin fragment C. Clin. Immunol. 97:146-153. [DOI] [PubMed] [Google Scholar]
- 47.Tacket, C. O., M. B. Sztein, G. A. Losonsky, S. S. Wasserman, J. P. Nataro, R. Edelman, D. Pickard, G. Dougan, S. N. Chatfield, and M. M. Levine. 1997. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect. Immun. 65:452-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vervoort, E. B., A. van Ravestein, N. N. van Peij, J. C. Heikoop, P. J. van Haastert, G. F. Verheijden, and M. H. Linskens. 2000. Optimizing heterologous expression in dictyostelium: importance of 5′ codon adaptation. Nucleic Acids Res. 28:2069-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webster, D. E., M. L. Cooney, Z. Huang, D. R. Drew, I. A. Ramshaw, I. B. Dry, R. A. Strugnell, J. L. Martin, and S. L. Wesselingh. 2002. Successful boosting of a DNA measles immunization with an oral plant-derived measles virus vaccine. J. Virol. 76:7910-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weidinger, G., M. Ohlmann, B. Schlereth, G. Sutter, and S. Niewiesk. 2001. Vaccination with recombinant modified vaccinia virus Ankara protects against measles virus infection in the mouse and cotton rat model. Vaccine 19:2764-2768. [DOI] [PubMed] [Google Scholar]
- 51.Wyde, P. R., M. W. Ambrose, T. G. Voss, H. L. Meyer, and B. E. Gilbert. 1992. Measles virus replication in lungs of hispid cotton rats after intranasal inoculation. Proc. Soc. Exp. Biol. Med. 201:80-87. [DOI] [PubMed] [Google Scholar]
- 52.Wyde, P. R., D. K. Moore-Poveda, N. J. Daley, and H. Oshitani. 1999. Replication of clinical measles virus strains in hispid cotton rats. Proc. Soc. Exp. Biol. Med. 221:53-62. [DOI] [PubMed] [Google Scholar]
- 53.Wyde, P. R., K. J. Stittelaar, A. D. Osterhaus, E. Guzman, and B. E. Gilbert. 2000. Use of cotton rats for preclinical evaluation of measles vaccines. Vaccine 19:42-53. [DOI] [PubMed] [Google Scholar]
- 54.Yang, K., F. Mustafa, A. Valsamakis, J. C. Santoro, D. E. Griffin, and H. L. Robinson. 1997. Early studies on DNA-based immunizations for measles virus. Vaccine 15:888-891. [DOI] [PubMed] [Google Scholar]