Abstract
Inhibition of the accumulation of protease-resistant prion protein (PrP-res) is a prime strategy in the development of potential transmissible spongiform encephalopathy (TSE) therapeutics. Here we show that curcumin (diferoylmethane), a major component of the spice turmeric, potently inhibits PrP-res accumulation in scrapie agent-infected neuroblastoma cells (50% inhibitory concentration, ∼10 nM) and partially inhibits the cell-free conversion of PrP to PrP-res. In vivo studies showed that dietary administration of curcumin had no significant effect on the onset of scrapie in hamsters. Nonetheless, other studies have shown that curcumin is nontoxic and can penetrate the brain, properties that give curcumin advantages over inhibitors previously identified as potential prophylactic and/or therapeutic anti-TSE compounds.
Transmissible spongiform encephalopathies (TSE) or prion diseases are untreatable, fatal neurodegenerative diseases that include bovine spongiform encephalopathy, chronic wasting disease, scrapie, and Creutzfeldt-Jakob disease. A central event in TSE diseases is the conversion of the normal, protease-sensitive isoform of prion protein (PrP-sen or PrPC) to an abnormal, protease-resistant form, PrP-res or PrPSc. Numerous compounds have been identified as inhibitors of PrP-res formation in scrapie agent-infected murine neuroblastoma (ScNB) cells (1-3, 5). The most potent of these inhibitors can also protect rodents against scrapie if they are administered near the time of infection (7, 8, 10, 11, 14). Unfortunately, none of these compounds are known to be both safe and effective for use in humans and animals (8, 10, 11). One therapeutic weakness of most of these compounds is likely an inability to penetrate the brain where most of the PrP-res accumulates and TSE pathogenesis occurs.
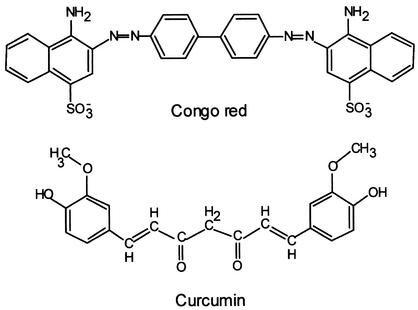
Curcumin, the major component of the spice turmeric and the yellow pigment in curry powder, has several properties that make it of interest as a possible anti-TSE drug. First, its structure resembles Congo red, the most potent of the small-molecule PrP-res inhibitors that have been assayed in ScNB cells (Fig. 1) in that both are potentially planar compounds that have two aromatic rings or ring systems with conjugated linkers. Structure-activity studies have provided evidence that the potential for coplanarity of the rings and linker is important for the inhibitory potency of Congo red (6). Second, unlike Congo red, curcumin is uncharged and is thought to have at least limited bioavailability to the brain after consumption. Indeed, recent studies with a rat model of Alzheimer's disease reported that dietary curcumin reduces β-peptide deposition in the brain as well as associated neuropathology and cognitive deficits (9, 12). Third, curcumin has antioxidant activity, a factor that may be important given that oxidative damage is a feature in TSE neuropathogenesis (13). Fourth, humans consume curcumin in large amounts with no apparent toxicity. Toxicology studies have indicated that rodents can tolerate for a long period up to 5% of their diet being turmeric oleoresin (∼80% curcumin) without their life spans being shortened (http://ntp-server.niehs.nih.gov/htdocs/LT-studies/tr427.html). These considerations prompted us to test whether curcumin could inhibit the formation and accumulation of PrP-res.
FIG. 1.
Structures of curcumin and Congo red.
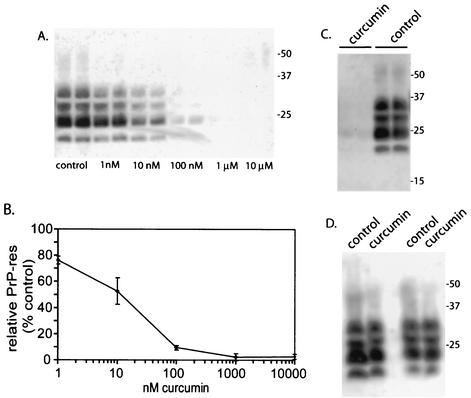
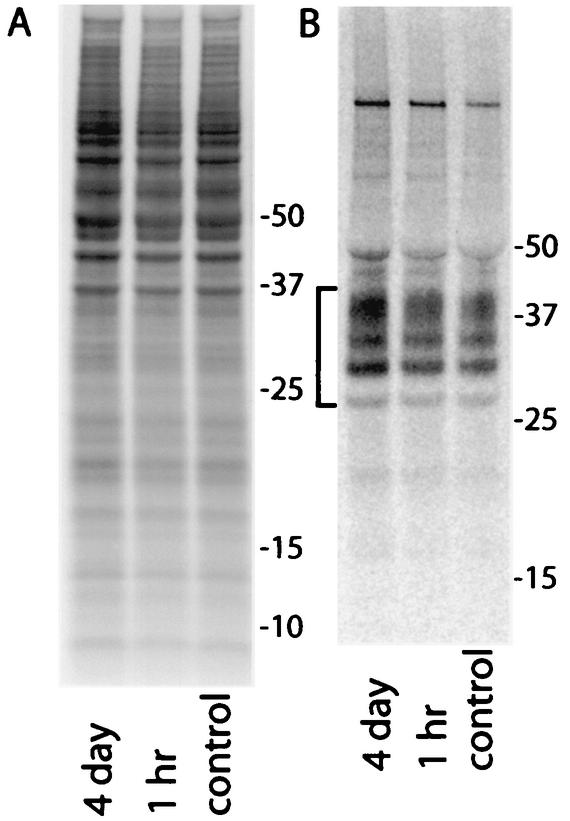
Curcumin was added to the culture medium of ScNB cells seeded at a density of 1/10 confluence and grown to near confluence for 3 to 4 days. Approximately 90% of the ScNB cells used to seed these cultures were infected, as was indicated by the fact 9 out of 10 single-cell subclones from concurrent passes of this ScNB cell line were highly positive for PrP-res (data not shown). The accumulation of PrP-res during the growth of the ScNB cultures was assayed by detergent extraction, proteinase K (PK) treatment, and immunoblotting by previously described methods (5). A curcumin concentration-dependent reduction of PrP-res accumulation was observed with a concentration giving half maximal inhibition of ∼10 nM (Fig. 2A and B). This 50% inhibitory concentration rivals that of Congo red (∼10 nM) (2, 3) and is 2,500-fold lower than the concentration of curcumin (25 μM) that began to show evidence of cytotoxicity in the ScNB cells (not shown). The curcumin-induced reduction in PrP-res was long-lasting because the PrP-res levels in cultures treated with 1 μM curcumin for 4 days (one pass) remained low after four subsequent passes in the absence of curcumin (Fig. 2C). The observed effects of curcumin were not due to artifactual interference with the detection of PrP-res or an enhancement of the protease sensitivity of PrP-res after lysis of the cells, because addition of 1 μM curcumin to the otherwise untreated control cell lysates prior to PK digestion did not affect the amount of PrP-res detected (Fig. 2D). Furthermore, incubation of purified hamster 263K strain PrP-res (0.13 μM) with curcumin concentrations of up to 5 mM in 50 mM Tris-HCl-150 mM NaCl, pH 8, for 16 h at room temperature did not enhance its sensitivity to PK or reduce its detection by immunoblotting (data not shown). The inhibition of PrP-res accumulation in the ScNB cells by curcumin was not due to a reduction of protein synthesis generally or PrP-sen biosynthesis specifically because there was no reduction in the metabolic labeling of cellular proteins (Fig. 3A) or PrP-sen (Fig. 3B) after either 1-h or 4-day treatments with 1 μM curcumin, a concentration that fully inhibited PrP-res accumulation in the intact cells. Taken together, these results provide evidence that curcumin inhibits PrP-res accumulation potently, irreversibly, and selectively in live ScNB cells.
FIG. 2.
Inhibition of PrP-res formation in ScNB cells by curcumin. ScNB cells were cultured for 4 days in the presence of curcumin at the designated concentrations in duplicate and analyzed for the accumulation of PrP-res by immunoblotting (A) by previously described methods (5). Briefly, detergent lysates of the cells were treated with PK, and PrP-res-containing pellets were solubilized and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. (B) Quantitation of the concentration dependence of the effects of curcumin from data shown in panel A and from other experiments. PrP-res band intensities are presented as means (± SD; n = 4) relative to the band intensities of untreated control ScNB cells. (C) Long-term effects of curcumin. ScNB cultures were grown for 4 days in the presence or absence (control) of 1 μM curcumin and then passed four times in the absence of curcumin prior to analysis for the accumulation of PrP-res as described for panel A. The results of duplicate experiments are shown. (D) Effect of treatment of ScNB cell lysates with 1 μM curcumin for 1 h prior to PK treatment and extraction for the detection of PrP-res by immunoblotting by previously described methods (5). The results of duplicate experiments are shown. With all of the immunoblots, the primary antibody R30 (4) was used to identify PrP-res in the PK-digested cell extracts. Results similar to those in panel A were obtained with a polyclonal antiserum directed against the C terminus of the PrP molecule (R20 [4]) (data not shown).
FIG. 3.
Phosphor autoradiographic images of 35S metabolic labeling of total proteins (A) and PrP-sen (B) in ScNB cells after pretreatments with 1 μM curcumin. Duplicate cultures were seeded and grown to confluence as was done in experiments such as those presented in Fig. 2. Curcumin was added to the culture medium either 4 days or 1 h prior to labeling of the cells at confluence with [35S]methionine. Curcumin was also maintained at the same concentration in the labeling medium. (A) Five-microliter aliquots of 1-ml cell lysates were run directly on the SDS-PAGE gel, and the remainder of each lysate was used for the immunoprecipitation of the 35S-PrP-sen samples shown in panel B. The bracket on the left in panel B marks PrP bands. Except for the test compound, the experimental protocols were the same as those described previously (5).
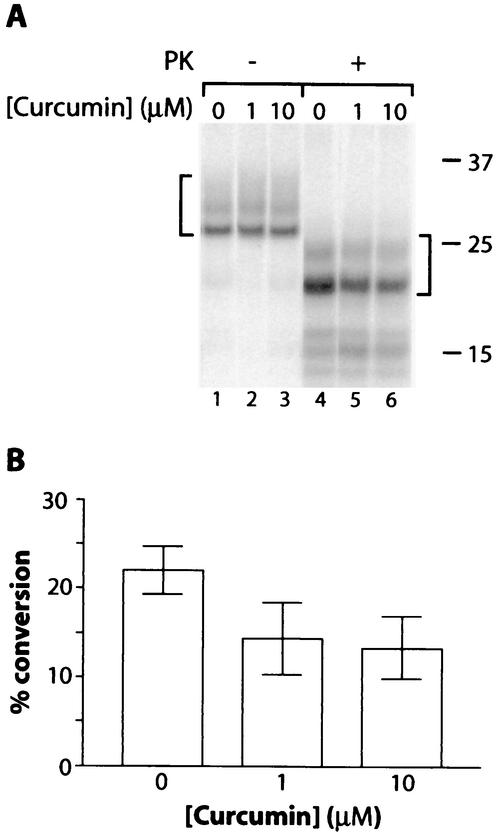
To test the direct effects of curcumin on the conversion of PrP-sen to PrP-res, we used cell-free conversion reaction mixtures in which purified hamster PrP-res (263K strain) induces the conversion of hamster 35S-PrP-sen to 35S-PrP-res. Partial mean inhibition of up to 41% (at 10 μM curcumin) was observed under previously described conditions with guanidine hydrochloride and detergents (6) (Fig. 4). A similar inhibition of PrP conversion reactions was observed under other conditions, including those without guanidine hydrochloride (data not shown). These results indicated that curcumin partially inhibits the direct interactions between PrP-sen and PrP-res that lead to conversion. Whether this direct inhibition accounts mechanistically for the more complete inhibition observed in ScNB cells is not clear.
FIG. 4.
Curcumin inhibition of the cell-free conversion of 35S-PrP-sen to the protease-resistant state. (A) Cell-free PrP conversion reactions between Syrian hamster 35S-PrP-sen and PrP-res were as described previously (6) except for the presence of the indicated concentration of curcumin. Briefly, hamster 35S-PrP-sen lacking a glycophosphatidylinositol anchor (20,000 cpm) was immunoprecipitated from fibroblast cells and incubated with PrP-res that had been isolated from the brains of 263K scrapie-infected hamsters and pretreated with 2 M guanidine HCl (6). The final conditions of the conversion incubation were 300 nM PrP-res, ∼5 nM PrP-sen, 0.5 M guanidine hydrochloride, 50 mM sodium citrate [pH 6.5], 1 mM cetyl pyridinium chloride, 0.6% N-lauroyl sarkosine, and 37°C. The formation of 35S-PrP-res (marked by the right bracket) was detected by treating 90% of the reaction equivalents with PK (10 μg/ml) for 1 h at 37°C, followed by the inactivation of the PK with Pefabloc (Boehringer Mannheim), methanol precipitation, and SDS-PAGE analysis as described previously (6). The remaining 10% of the reaction mixture was analyzed without PK digestion to show the input 35S-PrP (left bracket). (B) Quantitation of the proportion of input 35S-PrP-sen converted to the PK-resistant form (% conversion). Values are means ± SD (n = 3).
Tests of the prophylactic anti-TSE effects of curcumin in vivo are in progress with hamsters inoculated with the 263K strain of scrapie. Hamsters were fed ad libitum diets containing 0.2% (wt/wt) curcumin beginning 14 days before infection, on the day of infection, or 40 days postinfection (dpi) by the intracerebral route with 50 μl of 1% (vol/vol) brain homogenate (∼107 50% lethal doses) from clinically ill hamsters infected with the 263K strain of scrapie. Incubation periods to end-stage clinical disease (recumbent animals) of the hamsters fed a control diet (no curcumin) or curcumin at days −14 and 0 and 45 dpi were 82.6 ± 3.9, 85.3 ± 4.0, 84.1 ± 4.3, and 83.5 ± 4.1 days (means ± standard deviations [SD]), respectively, with 10 to 12 animals in each group. A 10-fold-higher dose of curcumin (2% [wt/wt]) beginning at 45 dpi gave an average incubation period of 85.4 ± 4.7 days and, thus, did not substantially improve the efficacy of the late curcumin treatment. Similar relative effects were observed in the incubation periods to the first detectable clinical signs. Thus, curcumin had little or no protective effect against a large intracerebral dose of scrapie.
Further experiments are under way to determine whether curcumin is more effective against lower oral, intraperitoneal, and intracerebral doses of scrapie agents of different strains and in different host species. Furthermore, it is possible that other routes of administration of curcumin may be more effective than mixing it with feed. Although curcumin has been demonstrated to exert some beneficial effects in the brains of rats with Alzheimer's disease (9, 12), it remains to be determined how well it accumulates in the brain after oral administration to other species (e.g., hamsters and mice). Disease state might also impact curcumin's blood-brain-barrier permeability in a species-dependent manner.
In conclusion, curcumin is among the smallest and most potent inhibitors of PrP-res formation to be identified in vitro. Our initial tests have not indicated that dietary curcumin is effective against high intracerebral doses of scrapie in vivo. However, continued study of curcumin as a potential lead compound for TSE prophylaxis and therapeutics seems warranted because of the major advantages that it has over other known PrP-res inhibitors, namely, lack of toxicity and carcinogenicity, apparent oral bioavailability to the brain (9, 12), and antioxidant activity.
REFERENCES
- 1.Caughey, B., D. Ernst, and R. E. Race. 1993. Congo red inhibition of scrapie agent replication. J. Virol. 67:6270-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caughey, B., and R. E. Race. 1992. Potent inhibition of scrapie-associated PrP accumulation by Congo red. J. Neurochem. 59:768-771. [DOI] [PubMed] [Google Scholar]
- 3.Caughey, B., and G. J. Raymond. 1993. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J. Virol. 67:643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caughey, B., G. J. Raymond, D. Ernst, and R. E. Race. 1991. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J. Virol. 65:6597-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caughey, W. S., L. D. Raymond, M. Horiuchi, and B. Caughey. 1998. Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Proc. Natl. Acad. Sci. USA 95:12117-12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demaimay, R., J. Harper, H. Gordon, D. Weaver, B. Chesebro, and B. Caughey. 1998. Structural aspects of Congo red as an inhibitor of protease-resistant prion protein formation. J. Neurochem. 71:2534-2541. [DOI] [PubMed] [Google Scholar]
- 7.Ehlers, B., and H. Diringer. 1984. Dextran sulphate 500 delays and prevents mouse scrapie by impairment of agent replication in spleen. J. Gen. Virol. 65:1325-1330. [DOI] [PubMed] [Google Scholar]
- 8.Farquhar, C. F., and A. G. Dickinson. 1986. Prolongation of scrapie incubation period by an injection of dextran sulphate 500 within the month before or after infection. J. Gen. Virol. 67:463-473. [DOI] [PubMed] [Google Scholar]
- 9.Frautschy, S. A., W. Hu, P. Kim, S. A. Miller, T. Chu, M. E. Harris-White, and G. M. Cole. 2001. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol. Aging 22:993-1005. [DOI] [PubMed] [Google Scholar]
- 10.Ingrosso, L., A. Ladogana, and M. Pocchiari. 1995. Congo red prolongs the incubation period in scrapie-infected hamsters. J. Virol. 69:506-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimberlin, R. H., and C. A. Walker. 1986. Suppression of scrapie infection in mice by heteropolyanion 23, dextran sulfate, and some other polyanions. Antimicrob. Agents Chemother. 30:409-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim, G. P., T. Chu, F. Yang, W. Beech, S. A. Frautschy, and G. M. Cole. 2001. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 21:8370-8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milhavet, O., and S. Lehmann. 2002. Oxidative stress and the prion protein in transmissible spongiform encephalopathies. Brain Res. Rev. 38:328-339. [DOI] [PubMed] [Google Scholar]
- 14.Priola, S. A., A. Raines, and W. S. Caughey. 2000. Porphyrin and phthalocyanine anti-scrapie compounds. Science 287:1503-1506. [DOI] [PubMed] [Google Scholar]