Abstract
Herpes simplex virus (HSV) infection of many cultured cells, e.g., Vero cells, can be initiated by receptor binding and pH-neutral fusion with the cell surface. Here we report that a major pathway for HSV entry into the HeLa and CHO-K1 cell lines is dependent on endocytosis and exposure to a low pH. Enveloped virions were readily detected in HeLa or receptor-expressing CHO cell vesicles by electron microscopy at <30 min postinfection. As expected, images of virus fusion with the Vero cell surface were prevalent. Treatment with energy depletion or hypertonic medium, which inhibits endocytosis, prevented uptake of HSV from the HeLa and CHO cell surface relative to uptake from the Vero cell surface. Incubation of HeLa and CHO cells with the weak base ammonium chloride or the ionophore monensin, which elevate the low pH of organelles, blocked HSV entry in a dose-dependent manner. Noncytotoxic concentrations of these agents acted at an early step during infection by HSV type 1 and 2 strains. Entry mediated by the HSV receptor HveA, nectin-1, or nectin-2 was also blocked. As analyzed by fluorescence microscopy, lysosomotropic agents such as the vacuolar H+-ATPase inhibitor bafilomycin A1 blocked the delivery of virus capsids to the nuclei of the HeLa and CHO cell lines but had no effect on capsid transport in Vero cells. The results suggest that HSV can utilize two distinct entry pathways, depending on the type of cell encountered.
Many viruses utilize the cellular endocytic machinery to transport their genetic material to the cell interior. In addition to depending on this machinery, enveloped viruses such as influenza virus and nonenveloped viruses such as rhinovirus 2 require an acid pH for escape from the endocytic pathway to the host cytosol (35). For some enveloped viruses, the low-pH environment of the endosome triggers fusion of the virion envelope with cellular membranes. For other viruses, endocytic entry is not followed by a pH-triggered step. For example, Epstein-Barr virus and duck hepatitis B virus enter certain types of cells by endocytosis yet they do not require exposure to a low pH to penetrate the cytosol (27, 38). By contrast, viruses such as human immunodeficiency virus (HIV) and Sendai virus usually fuse with the plasma membrane in a pH-independent manner without a requirement for endocytosis (19).
Entry of herpes simplex virus type 1 (HSV-1) and HSV-2 is initiated by interaction of virions with cell surface glycosaminoglycans (48). This is followed by binding to one of several entry receptors utilized by HSV, including HveA (herpesvirus entry mediator A, also known as HVEM), a member of the tumor necrosis factor receptor family (39), and the immunoglobulin-like intercellular adhesion molecules nectin-1 (10, 18, 33, 55) and nectin-2 (32, 58), also known as HveC/HIgR/PRR1 and HveB/PRR2, respectively. Chinese hamster ovary (CHO) cells are naturally resistant to HSV entry and require expression of a receptor to remove the block to entry (39, 47). Thus, they have been used as a model system in which to study receptor-mediated entry of HSV.
HSV envelope glycoprotein gD binds to all known alphaherpesvirus receptors (8, 53), and this interaction is essential for entry (25). Fusion of bound virus with host membranes is thought to be triggered by the interaction with gD receptors. Studies of HSV-infected cells at early times postinfection using electron microscopy (EM) have detected virions fusing with the plasma membrane (16, 17, 34, 40, 50, 51), as well as virions inside membrane-bound vesicles (6, 11, 13, 17, 23, 40, 51). On the basis of morphological analyses alone, it is not possible to assess whether any given virion is engaging a pathway that could lead to infection. Virions are often detected in vesicles during nonproductive infections in which either the cells do not support virus penetration (6) or the virus itself is entry defective (61). In such instances, it is thought that the internalized virions are targeted ultimately for degradation. EM, coupled with treatments with a neutralizing antibody, has been used to demonstrate that infectious entry of HSV into Vero and HEp-2 cells could occur via fusion at the plasma membrane (16, 17). However, successful penetration of HSV from an intracellular compartment following endocytosis has not been excluded.
Weak bases, such as chloroquine, that block endosomal acidification and entry of viruses that require a low pH were shown to have little effect on early events in HSV infection of HEp-2 and Vero cells (60). Largely on the basis of the study of these cell types, it is thought that HSV cell entry does not require a low pH.
The pathway utilized by HSV for entry into two other common cultured cell lines was investigated. The conclusions obtained were different from those of earlier studies. On the basis of several experimental approaches, evidence is provided that CHO-K1 cells expressing HSV receptors and HeLa cells support an endocytic, pH-dependent route for HSV entry. Thus, HSV infection can be initiated by more than one pathway in a cell-specific manner.
MATERIALS AND METHODS
Cells and viruses.
HeLa and Vero cells (American Type Culture Collection, Manassas, Va.) were propagated in Dulbecco's modified Eagle's medium (Life Technologies, Grand Island, N.Y.) supplemented with 10% fetal calf serum (Life Technologies). CHO-K1 cells stably transformed with the Escherichia coli lacZ gene under the control of the HSV ICP4 promoter are designated CHO IEβ8 (39). CHO IEβ8 cells stably transformed to express HveA, nectin-1, or nectin-2 (18, 42, 58) (provided by G. Cohen and R. Eisenberg, University of Pennsylvania) were propagated in Ham's F12 medium (Life Technologies) supplemented with 10% fetal calf serum, 150 μg of puromycin (Sigma, St. Louis, Mo.) per ml, and 250 μg of Geneticin (Life Technologies) per ml. Cells were subcultured in nonselective medium prior to use in all experiments.
HSV-1 strains KOS and KOS 7134, which has the E. coli lacZ gene in place of the immediate-early ICP0 gene (5), were obtained from P. Schaffer (Harvard University). HSV-1 strain KOS-rid1 (obtained from P. Spear, Northwestern University) is a KOS derivative with a Q27P mutation in gD (12). HSV-1 strain KOS-rid1-tk12 contains the lacZ gene under control of the ICP4 promoter (39). HSV-1 strain MP (22) and HSV-2 strain G were obtained from the American Type Culture Collection. Virus stocks were grown and their titers were determined on Vero cells.
Electron microscopy.
HSV-1 strain KOS (multiplicity of infection [MOI] of 50) was added to cells and incubated for 2 h at 4°C to allow attachment. Cells were washed thrice with phosphate-buffered saline (PBS) and then incubated at 37°C for 20 min. Infected cells were washed with PBS and then fixed in 0.1 M sodium cacodylate buffer containing 2% glutaraldehyde (pH. 7.4) for 2 h. The cell pellet was washed three times in 0.1 M sodium cacodylate and then fixed in 1% osmium in the same buffer by incubation for 1 h at room temperature. The cell pellet was dehydrated in graded ethanol (35, 50, 75, 95, and 100%), followed by 100% propylene oxide, and infiltrated in a 1:1 mixture of propylene oxide and epoxy resin overnight. The pellet was embedded in a Beem capsule filled with epoxy resin and cured at 60°C for 48 h. The cured block was thin sectioned and stained in uranyl acetate and lead citrate. Samples were examined and photographed with a Hitachi H7000 electron microscope (National Cancer Institute, Frederick, Md.) operated at 75 kV.
HSV entry assay.
Confluent cell monolayers grown in 96-well dishes were infected with HSV-1 (strain KOS for CHO cells or strain KOS 7134 for HeLa or Vero cells) at an MOI of 1 and incubated at 37°C for 6 h. Cell lysates were prepared with 0.5% Nonidet P-40, chlorophenol red-β-d-galactopyranoside (Roche Diagnostic, Indianapolis, Ind.) was added, and β-galactosidase activity was read at 570 nm with a microtiter plate reader (Dynatech, Chantilly, Va.). Mean results and standard deviations were calculated for four replicate samples.
Inhibition of uptake from the cell surface.
HSV was prebound to cells at 4°C for 2 h and then treated with medium containing 0.3 M sucrose (hypertonic); glucose-free medium containing 2% bovine serum albumin (BSA), 0.3% 2-deoxy-d-glucose, and 0.05% sodium azide (energy depletion); or control medium for 30 min at 37°C. Cells were washed with PBS, and virions that had not penetrated cells were inactivated by washing for 2 min at 37°C with medium buffered to pH 4.7. Cells were incubated in normal medium for 6 h, after which virus entry was assessed.
Treatments with lysosomotropic agents.
Stock solutions of ammonium chloride (1.5 M) were prepared immediately prior to use. Monensin (75 mM; Sigma) and bafilomycin A1 (100 mM; Sigma) stock solutions were prepared in ethanol and dimethyl sulfoxide, respectively, and stored at −20°C. The pHs of all solutions were adjusted to 7.4 prior to use. Treatment of isolated virions with agents did not affect entry activity. Growth medium was removed from cells and replaced with medium containing drugs or medium containing control concentrations of ethanol or dimethyl sulfoxide, and the mixture was incubated for 30 min at 37°C. Virus was added, and cells were incubated in the constant presence of drugs for 2.5 h for microscopy experiments or for 6 h for gene expression assays.
Low-pH treatment of virions.
Purified HSV was diluted in cell culture medium buffered with 5 mM HEPES (Life Technologies), 5 mM 2-(N-morpholino)ethanesulfonic acid (MES; Sigma), and 5 mM succinate (Sigma) containing 0.2% BSA to achieve final pHs ranging from 7.4 to 3.0 and incubated at 4, 26, or 37°C for various durations. pH values measured at 4°C varied less than 0.05 U from those measured at 37°C. Prior to addition to cells, virus samples were neutralized by addition of pretitrated amounts of 0.05 N NaOH. Control samples were treated with distilled water in place of acid or base. Negatively stained preparations of virions treated at pH 4.7 appeared intact when examined by EM.
Immunofluorescence microscopy.
Cells were grown on glass coverslips overnight. Cultures were then chilled on ice, HSV-1 (KOS) was added at an MOI of 10, and the mixture was incubated for 2 h on ice. Cells were washed three times with PBS, and then warmed medium containing the indicated drugs was added. Infection proceeded for 2.5 h in the presence of cycloheximide. Cells were washed three times with PBS and fixed in methanol. Coverslips were air dried and blocked with PBS containing 1% BSA for 1 h. Virion capsids were visualized by staining with 2 μg of mouse monoclonal antibody against HSV-1 VP5 (ABI Technologies, Rockville, Md.) per ml, followed by an Alexa 488-labeled goat-anti-mouse secondary antibody (Molecular Probes, Eugene, Oreg.). Nuclei were counterstained with 25 ng of 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI; Roche) per ml. Coverslips were washed with PBS, mounted with Fluoromount G (Electron Microscopy Sciences), and viewed with a Zeiss Axioplan 2 imaging microscope equipped with a 100× oil immersion objective (Zeiss). Digital images were captured with a Zeiss AxioCam using Openlab v3.1 (Improvision, Lexington, Mass.) and processed with Adobe Photoshop v6.0.
RESULTS
Ultrastructural analysis of HSV entry.
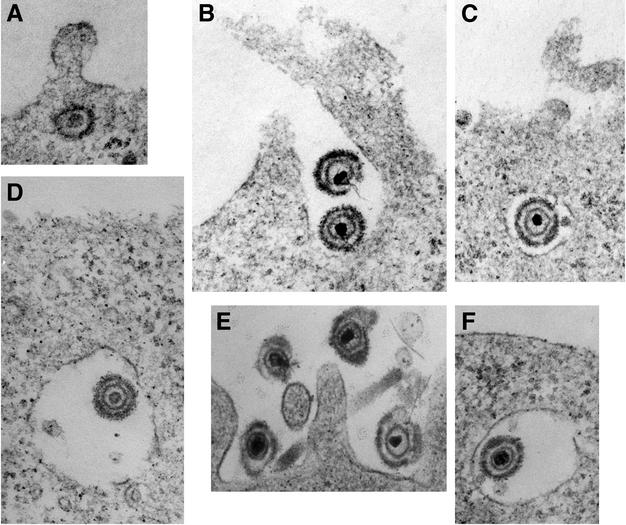
Cells infected with HSV-1 strain KOS for 20 min were analyzed by transmission EM to discern the physical features and localization of entering virions. Images of naked, or unenveloped, nucleocapsids in the cytosol adjacent to the plasma membrane were obtained with infected Vero cells (Fig. 1A) as previously reported (16, 17, 34, 40, 51). In contrast, EM analysis of infected CHO cells expressing the receptor nectin-1 (Fig. 1B) or HeLa cells (Fig. 1E) yielded images of enveloped virions surrounded by invaginations of the plasma membrane. Virions present in Vero cell surface invaginations were much more rare. Intact, enveloped virions in membrane-bound vesicles ranging from 300 to 800 nm in diameter were detected near the plasma membrane of both HeLa cells and nectin-1-expressing CHO cells (Fig. 1C, D, and F). Enveloped virions in Vero cell vesicles were seen much less frequently. Moreover, in HeLa cells and receptor-expressing CHO cells, particles that appeared to fuse with the cell surface to deposit naked nucleocapsids into the cytoplasm were not detected. This stands in contrast to results obtained with cell types such as Vero and HEp-2, in which abundant fusion with the cell surface is detected by EM (Fig. 1A) (16, 17, 51). Subsequent experiments were designed to address the possibility that HSV engages an endocytic pathway for successful entry into the HeLa and CHO cell lines.
FIG. 1.
Electron microscopic analysis of HSV-1 uptake into cells. HSV-1 strain KOS was bound to Vero (A), nectin-1-expressing CHO (B to D), or HeLa (E and F) cells for 2 h at 4°C. This was followed by a shift to 37°C for 20 min, and then the cells were processed for electron microscopy. Enveloped virions are 150 to 200 nm in diameter. Magnification, ×90,000.
Inhibitors of uptake from the cell surface impair HSV entry into HeLa and CHO cells.
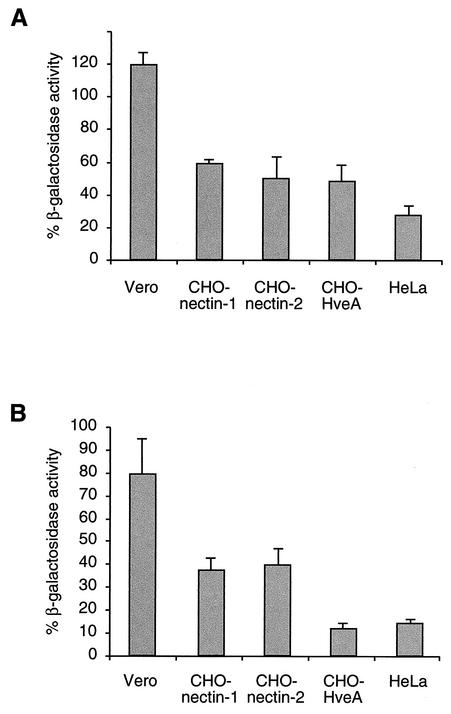
Since endocytosis is an energy-dependent process, uptake of material from the cell surface can be blocked with inhibitors of ATP synthesis (37). Therefore, HSV entry into cells treated for 30 min with energy depletion medium containing sodium azide and 2-deoxy-d-glucose was assessed (Fig. 2A). In this assay, the lacZ gene is present in either the virus or the cell and is driven by an HSV immediate-early gene promoter. β-Galactosidase expression is measured at 6 h postinfection as an indication of successful entry of virions. CHO cells expressing a single HSV receptor (HveA, nectin-1, or nectin-2) or HeLa cells treated with energy depletion medium were relatively refractory to virus entry. In contrast, when Vero cells were treated with energy depletion medium, no inhibitory effect on HSV entry was evident. This suggests that an early step in infection of HeLa and CHO cells is dependent on cellular energy, whereas a similar step in Vero cell entry is not.
FIG. 2.
Effects of inhibitors of endocytosis on HSV entry into cells. HSV-1 was bound to cells for 2 h at 4°C. The virus strains used were HSV-1 strain KOS (for nectin-1- and HveA-expressing CHO cells), HSV-1 strain KOS-rid1 (for nectin-2-expressing CHO cells), and lacZ+ HSV-1 strain KOS 7134 (for Vero and HeLa cells). Normal culture medium was replaced with glucose-free medium containing 2-deoxy-d-glucose and sodium azide (A) or hypertonic medium containing 0.3 M sucrose (B), and the cells were incubated for 30 min at 37°C. Treated and mock-treated cells were washed with PBS, and virus that had not penetrated the cells was inactivated by acid treatment. Infection proceeded for an additional 6 h, after which β-galactosidase activity was measured at A570 as an indication of virus entry. The activity in mock-treated cells was defined as 100%.
Treatment of cells with hypertonic medium has been demonstrated to inhibit receptor-mediated endocytosis (20). The effect of hypertonic treatment of different cells on HSV entry was assessed. Again, inhibition of endocytosis distinguished the two groupings of cells. Treatment of Vero cells with hypertonic medium had less of an effect on HSV entry than did treatment of the HeLa or the receptor-expressing CHO cell lines (Fig. 2B), supporting the importance of active endocytosis for HSV entry into the latter cells. Treatment of HeLa cells or receptor-expressing CHO cells with energy depletion or hypertonic medium did not completely abolish entry. This is likely due to incomplete inhibition of uptake from the cell surface under the conditions used here.
Lysosomotropic agents inhibit HSV infection of HeLa and CHO cells.
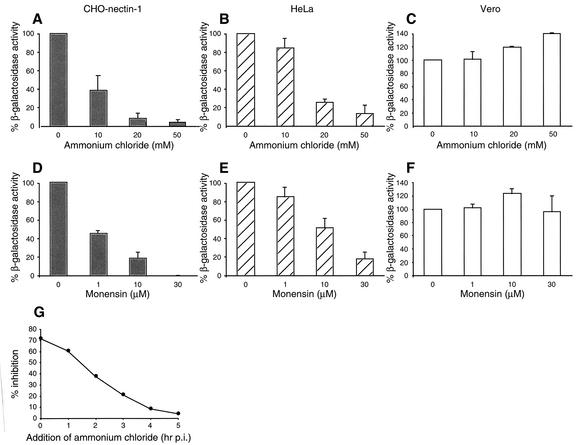
Following endocytosis, successful penetration and infection by some viruses require that particles be delivered to an acidic compartment (49). Ammonium chloride is a weak base that buffers the pH of acidic cellular compartments and characteristically inhibits entry of viruses that require a low pH for entry. Monensin is a carboxylic ionophore that blocks endosomal acidification. To determine the role of a low pH in endocytic entry of HSV, cells were treated with these agents and β-galactosidase expression was again used to indicate successful penetration and entry. Ammonium chloride or monensin was present in the medium at noncytotoxic concentrations for the duration of the infection. When nectin-1-expressing CHO cells or HeLa cells were treated with increasing concentrations of ammonium chloride, there was a dose-dependent inhibition of entry of HSV-1 strain KOS (Fig. 3A and B). In contrast, at all of the concentrations tested, ammonium chloride had no inhibitory effects on HSV entry into Vero (Fig. 3C) or HEp-2 (data not shown) cells, which is consistent with the results obtained with chloroquine (60).
FIG. 3.
Entry of HSV into cells treated with lysosomotropic agents. Nectin-1-expressing CHO cells (A, D, and G), HeLa cells (B and E), or Vero cells (C and F) were treated with the indicated concentrations of ammonium chloride (A to C) or monensin (D to F) for 30 min. Cells were infected with HSV-1 strain KOS (for nectin-1-expressing CHO cells) or KOS lacZ+ (for HeLa and Vero cells) at an MOI of 1 for 6 h in the presence of either lysosomotropic agent. (G) HSV-1 strain KOS was bound to cells by incubation for 2 h at 4°C and then washed with PBS. Cultures were warmed to 37°C, and at various times postinfection (p.i.), with the exception of the zero time point, noninternalized virus was inactivated by acid treatment. Medium containing 50 mM ammonium chloride was then added for the remainder of the 6-h total incubation time. The level of activity was calculated as a percentage relative to that obtained in the absence of a drug.
We tested whether ammonium chloride acts at an early or late step during the 6-h assay. At different times postinfection, noninternalized virus was inactivated; this was followed by agent addition. This also enabled an assessment of whether the inhibitory effect on entry was due to an effect on virus interaction with the cell surface. The later the ammonium chloride was added to infected nectin-1-expressing CHO cells, the less inhibition of entry was observed (Fig. 3G). This suggests that the chemical acts at an early, postbinding step in entry on virions that have already been internalized.
Monensin treatment also blocked HSV entry into nectin-1-expressing CHO cells or HeLa cells in a dose-dependent manner (Fig. 3D and E). Monensin had little inhibitory effect on entry into Vero cells (Fig. 3F). Similar results were obtained when cells were treated with the weak base methylamine (data not shown).
In nectin-1-expressing CHO cells and HeLa cells, greater than 80% of the entry activity measured was inhibited by lysosomotropic agents. This suggests that, in these cells, the majority of virions that succeed in commencing viral gene expression utilize a pH-dependent entry pathway. That alteration of the organellar pH blocks HSV entry is consistent with the role of endocytosis in entry into these cells.
The effect of lysosomotropic agents on the entry of several HSV strains mediated by other receptors was determined to verify that the above-described observations are neither strain nor receptor dependent. Entry of HSV-1 strains KOS and MP and HSV-2 strain G into CHO cells mediated by HveA was blocked by ammonium chloride and monensin (Table 1). Likewise, entry of HSV-1 strain KOS-rid1 mediated by either nectin-2 or nectin-1 was inhibited by both lysosomotropic agents. Entry of strain KOS-rid1 into Vero cells was not inhibited (Table 1).
TABLE 1.
Effects of lysosomotropic agents on HSV entry
| Virus | Cell line | % β-Galactosidase expression with:
|
|
|---|---|---|---|
| NH4CI (50 mM) | Monensin (30 μM) | ||
| HSV-1 strain KOS-rid1 | Vero | 119.4 ± 19.1 | 93.8 ± 3.0 |
| CHO expressing nectin-1 | 1.0 ± 0.9 | 0 | |
| CHO expressing nectin-2 | 0 | 0 | |
| HeLa | 9.9 ± 3.0 | 6.6 ± 1.2 | |
| HSV-1 strain KOS | CHO expressing HveA | 4.0 ± 0.7 | 0 |
| HSV-1 strain MP | CHO expressing nectin-1 | 12.5 ± 3.9 | 9.7 ± 1.9 |
| HSV-2 strain G | CHO expressing nectin-1 | 0 | 10.5 ± 3.2 |
| CHO expressing HveA | 1.7 ± 1.2 | 7.0 ± 2.7 | |
Effect of a mildly acidic pH on the entry activity of virions.
Inactivation of virions by acid pretreatment is a hallmark of viruses that can utilize a pH-activated entry pathway. When viruses such as Semliki Forest virus are treated with buffers at pHs 4.8 to 6.5, their fusion glycoproteins are prematurely activated, so when the acid-treated virus is added to cells, it is incompetent for entry (3). As regards HSV, it has been known for some time that penetration of cell-bound virions is inhibited by exposure to sodium citrate buffered to the highly acidic pH of 3 (21, 24, 46).
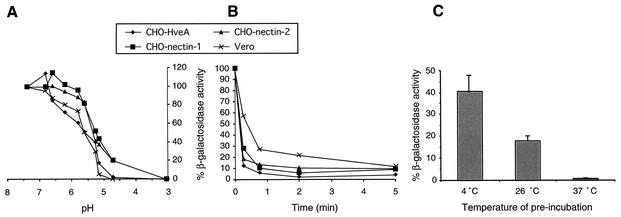
In light of these data, and now the apparent requirement of a low pH for entry of HSV into certain cell types, we characterized the effects of pH on the infectivity of isolated HSV particles. Aliquots of purified virus were incubated at various pHs, neutralized, and then assayed for successful entry into cells. Efficient entry of HSV-1 strain KOS into CHO cells expressing HveA or nectin-1 and entry of KOS-rid1 into CHO cells expressing nectin-2 declined when virions were treated with buffers set at pH ≤6.0 prior to their addition to cells (Fig. 4A). Specifically, 50% of entry activity was abolished when virions were incubated with buffers between pHs 5.0 and 5.5. Entry was completely inactivated at pH ≤4.7. Under these experimental conditions, the effects of pH on the cell, including cell surface receptors, can be ruled out. Thus, treatment with pH <6.0 directly affects the entry activity of virions.
FIG. 4.
Effect of a mildly acidic pH on entry activity of HSV virions. (A) Aliquots of cell-free HSV-1 (strain KOS or KOS-rid1, in the case of nectin-2-expressing CHO cells) were adjusted to a range of pHs, as shown, incubated at 37°C for 10 min, and then neutralized to pH 7.4. Treated virions were incubated with cells, and β-galactosidase activity was measured as an indication of virus entry. (B) Time course of low-pH inactivation of HSV virions. Samples of HSV-1 strain KOS or KOS-rid1 were incubated in pH 4.7 buffer at 37°C for the indicated times, immediately neutralized to pH 7.4, and assayed for HSV entry. (C) Effect of temperature on low-pH inactivation of HSV virions. HSV-1 strain KOS was adjusted to pH 4.7 for 10 min at 4, 26, or 37°C, neutralized, added to nectin-1-expressing CHO cells, and then assayed for entry. The activity obtained from samples that were maintained at pH 7.4 for the duration of the experiment was defined as 100%.
Although studies with lysosomotropic agents show that exposure to a low intracellular pH is not required for infection of Vero cells (Fig. 3; Table 1) (29, 60), acid pretreatment did impair HSV infectivity for Vero cells (Fig. 4A and B). A possible explanation of these data is that a pH of 4.7 to 6.0 disrupts virion functions required for entry via either pathway, whereas HSV entry into only specific cell types, such as HeLa, has a physiological requirement for a mildly acidic pH. Low- pH treatment apparently did not globally alter virus structure. Treatment of virions or recombinant soluble gD with pH 4.7 did not grossly affect the reactivity of a panel of anti-gD conformation-dependent antibodies, including those that recognize epitopes important for receptor binding (data not shown). Also, acid treatment of radiolabeled HSV did not grossly alter binding to HveA or nectin-1 on the cell surface, as measured in heparin-resistant binding assays, and treatment of gD with pH 4.7 had no effect on binding to recombinant HveA or nectin-1 immobilized on an enzyme-linked immunosorbent assay plate (data not shown).
The inactivation of HSV by a low pH in this assay was irreversible. Entry activity, as measured by β-galactosidase expression at 6 h postinfection, was not restored by neutralization of the treated virus preparation (Fig. 4A). To test whether virions treated at a low pH could regain infectivity in a longer-term assay, virions were incubated at a pH of 4.7, neutralized, and added to Vero cell monolayers. At 48 h postinfection, plaque formation was still inhibited by >90%, indicating that entry activity was not restored (data not shown).
Low-pH treatment rapidly impairs the entry activity of HSV virions. To demonstrate this, virions were treated with a low pH for various lengths of time, neutralized, and then assayed for entry. With all of the virus-cell combinations tested, more than 70% of entry activity was lost within 15 to 45 s of exposure to pH 4.7 (Fig. 4B). Maximal inactivation was achieved within 2 to 5 min. Inactivation of HSV by a low pH is also temperature dependent. Virions were exposed to a low pH for 10 min at different temperatures, neutralized, and then added to nectin-1-expressing CHO cells. Entry was inactivated by >50% following preincubation at 4°C (Fig. 4C); however, maximum inactivation was only seen when virions were preincubated at temperatures of >26°C. Similar temperature dependence of inactivation was seen when acid-treated HSV was assayed on HveA- or nectin-2-expressing CHO cells or on Vero cells (data not shown).
Lysosomotropic agents alter capsid transport during HSV entry into HeLa and CHO cells.
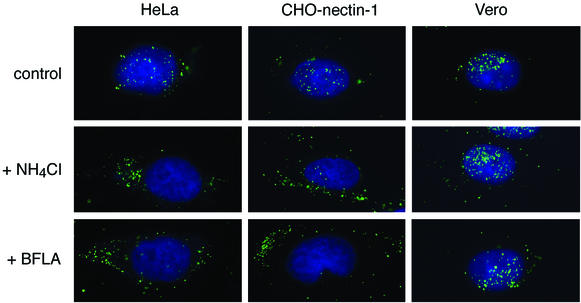
During successful HSV infections, nucleocapsids bind to the nucleus and release their viral genomes (2, 34, 51). Since treatment with lysosomotropic agents often leads to nonspecific disruptions in the cell, examination of their effects on an early entry event, such as capsid transport, will help to rule out possible pleiotropic effects on later processes, such as viral gene expression. Entry of HSV was analyzed by fluorescence microscopy by using an antibody directed against major capsid protein VP5. At 2.5 h postinfection of HeLa, nectin-1-expressing CHO, or Vero cells, a discretely punctate pattern around the nucleus was observed (Fig. 5; control). On the basis of serial confocal microscopy sections, the majority of the punctate fluorescence signals were most likely individual virus particles (data not shown). When HeLa cells or nectin-1-expressing CHO cells were infected in the presence of ammonium chloride, the majority of fluorescent particles were distributed in the cytoplasm (Fig. 5). In contrast, in treated Vero cells, the majority of virions were distributed around the nucleus, as in the untreated control cells (Fig. 5).
FIG. 5.
Effects of lysosomotropic agents on the distribution of HSV capsids during entry. HSV-1 strain KOS was bound to cells for 2 h at 4°C. Cells were washed with PBS, and then warmed medium containing no agent (control), 25 mM ammonium chloride (+ NH4Cl), or 50 nM bafilomycin A1 (+ BFLA) was added. Infection proceeded for 2.5 h in the presence of cycloheximide. Cells were washed with PBS, fixed, and then probed with anti-VP5 antibody to detect capsids (green). Nuclei were counterstained with DAPI (blue). Cells were viewed with a 100× oil immersion objective. The results shown are representative of two independent experiments.
Bafilomycin A1 inhibits the vacuolar H+-ATPase in the endosomal membrane that is responsible for acidification (43). Treatment with bafilomycin A1 blocked delivery of HSV capsids to the nuclei of nectin-1-expressing CHO cells and HeLa cells (Fig. 5). By contrast, transport to the nuclei of Vero cells was unaffected by bafilomycin A1 (Fig. 5), even at concentrations as high as 200 nM (data not shown). The inhibitory effects of lysosomotropic agents on the targeting of HSV capsids to the nucleus, as well as on viral immediate-early gene expression (Fig. 3; Table 1), suggest that exposure to a low intracellular pH is required for successful entry into HeLa cells and receptor-expressing CHO cells.
DISCUSSION
Here we show that HSV can enter and successfully infect cells via endocytosis. We also demonstrate for the first time that, following virus internalization, the intracellular pH is critical for the entry process. Thus, in addition to the well-characterized pH-independent pathway, HSV utilizes a distinct pH-dependent pathway. The requirements for endocytosis and a low pH were found to be cell specific.
Herpesviruses and retroviruses are frequently cited as examples of viruses that enter cells by pH-independent fusion of the virion envelope with a cell membrane, often the plasma membrane (19, 35, 53). Here, we demonstrate that HSV, the prototypical alphaherpesvirus, can also enter cells and initiate gene expression by an endocytic mechanism. Endocytic vesicles mediate entry of HSV into HeLa and CHO cells. The human epidermoid HeLa cell line is commonly used to investigate many facets of HSV infection, including entry. CHO cells have been instrumental in identifying and studying the role of receptors in HSV entry. In these cell types, exposure to an acidic environment appears to be required for successful penetration and infection by HSV. This stands in contrast to cell lines such as Vero and HEp-2, in which HSV entry is achieved primarily by plasma membrane fusion at a neutral pH. Infectious yields of HSV from Vero and HeLa cells are similar. Direct comparisons indicate that yields of progeny virus differ by less than sixfold in these two cell types (45, 57). Receptor-expressing CHO cells infected with HSV-1 strain KOS produce 10,000-fold more progeny virus than do control CHO cells but 10-fold to 100-fold less than do HeLa cells (39). Thus, HSV entry into HeLa cells and receptor-expressing CHO cells via the pH-dependent pathway leads to productive infection.
Several different cellular molecules can function in HSV entry. HSV primarily uses heparan sulfate for initial attachment, but other glycosaminoglycans, such as dextran or dermatan sulfate, can substitute in its absence (48). The essential gD-binding receptors include a diverse array of molecules, including protein members of the immunoglobulin and tumor necrosis factor receptor families, as well as modified forms of heparan sulfate (8, 53). We provide evidence that, following binding to one or more receptors, HSV can also utilize more than one route of entry.
Once HSV has bound to the cell surface, the cellular factors that determine whether it fuses directly or enters via endocytosis are not known. A functional HveA homolog has been identified in Vero cells (15), and nectins have been detected on the Vero and HeLa cell surfaces (10, 30). Antibodies against nectin-1 have been shown to effectively block virus entry into Vero, HeLa, and nectin-1-expressing CHO cells (10, 30). Binding of virion gD to the receptor nectin-1 or HveA is required for entry into the corresponding receptor-expressing CHO cell line (31, 42). Thus, the gD-receptor interaction is an initial step in the entry process that leads to both entry via fusion at the cell surface and entry via endocytosis.
Inhibitors of uptake from the cell surface had little effect on HSV entry into Vero cells, but they diminished entry into HeLa and receptor-expressing CHO cells (Fig. 2). Specific internalization mechanisms utilized by HSV might include clathrin-mediated endocytosis or non-clathrin-dependent processes, such as macropinocytosis (49).
In some studies, lysosomotropic agents have been shown to impede HSV infection. However, these effects have been attributed to actions against later steps in the replicative cycle, such as glycoprotein assembly (26, 28), and not against entry events. On the basis of fluorescence microscopy and a viral immediate-early gene reporter assay, we report that entry of HSV into HeLa cells and receptor-expressing CHO cells is blocked by agents that elevate the endosomal pH (Fig. 3 and 5; Table 1). By 2.5 h postinfection, the bulk of particles arrive at the nuclei of untreated cells, regardless of the entry pathway. Thus, in the appropriate cell type, HSV entry via either the pH-dependent or the pH-independent pathway appears to be efficient. In HeLa cells and receptor-expressing CHO cells, entry of the majority of infectious particles can be inhibited by lysosomotropic agents. This suggests that the predominant route of entry into these cells requires a low pH.
We propose that successful penetration of some cells by HSV requires trafficking of the virus to an acidic intracellular compartment or endosome. One candidate compartment is the early endosome, the major sorting station of endocytosed material and site of penetration of some pH-dependent viruses. Further studies are required to determine whether a low pH triggers fusion of the HSV envelope with the limiting membrane of HeLa or CHO cell endosomes.
In addition to inhibition by lysosomotropic agents, viruses that have pH requirements for penetration often have other features in common, such as induction of membrane fusion activity by a low pH and inactivation of virions by acid pretreatment. When cells are either infected with a low-pH-activated virus or express pH-dependent fusion glycoproteins and are then bathed in acidic buffer, they typically fuse with surrounding cells to form polykaryocytes or syncytia (14, 59). It should be noted, however, that HSV syncytium formation and cell-cell fusion are not strictly analogous to virus-cell fusion during entry. Nonetheless, with few exceptions (1, 4), the bulk of the evidence indicates that HSV-induced fusion of cultured cells does not require an acid pH (7, 41, 44, 52, 56). In this regard, HSV is similar to viruses such as HIV.
HSV virion inactivation occurred at pHs 4.7 to 6.0 in a rapid, temperature-dependent, and irreversible manner (Fig. 4). Similar characteristics of inactivation have been noted for influenza virus and Semliki Forest virus (3, 54). Notably, viruses that enter exclusively via a pH-independent mechanism, such as Sendai virus (9) and HIV (36), are typically not inactivated by acidification. The mechanism of HSV inactivation by a low pH is not known. However, since acidification has little effect on gD conformation, including epitopes that are important for receptor binding, and no effect on the ability of gD to engage receptors (data not shown), we propose that low-pH inactivation of HSV entry occurs at a step subsequent to the gD-receptor interaction, namely, the virus-cell fusion step. Possible targets of inactivation include the HSV glycoproteins gB, gH, and gL, all of which, together with gD, are required for fusion (41, 44, 52, 56).
Targets of HSV infection in the human host include cells of epithelial and neuronal origin. HSV also successfully infects many types of cultured cells from many species. Perhaps HSV has achieved a wide host range by utilizing multiple receptors, as well as multiple pathways, for entry. The possibility remains that more than one entry pathway is active in a single cell type, but here we demonstrate that the predominant route of HSV entry into HeLa cells and receptor-expressing CHO cells is an endocytic, low-pH one.
Acknowledgments
We thank Gary Cohen, Roselyn Eisenberg, Priscilla Schaffer, and Pat Spear for reagents used in this study and Jeff Cohen, Qing Li, Pat Spear, and Gary Whittaker for critical reading of the manuscript and helpful discussions.
REFERENCES
- 1.Ali, M. A., M. Butcher, and H. P. Ghosh. 1987. Expression and nuclear envelope localization of biologically active fusion glycoprotein gB of herpes simplex virus in mammalian cells using cloned DNA. Proc. Natl. Acad. Sci. USA 84:5675-5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batterson, W., D. Furlong, and B. Roizman. 1983. Molecular genetics of herpes simplex virus. VIII. further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of the viral reproductive cycle. J. Virol. 45:397-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bron, R., J. M. Wahlberg, H. Garoff, and J. Wilschut. 1993. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J. 12:693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butcher, M., K. Raviprakash, and H. P. Ghosh. 1990. Acid pH-induced fusion of cells by herpes simplex virus glycoproteins gB and gD. J. Biol. Chem. 265:5862-5868. [PubMed] [Google Scholar]
- 5.Cai, W., and P. A. Schaffer. 1991. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J. Virol. 65:4078-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campadelli-Fiume, G., M. Arsenakis, F. Farabegoli, and B. Roizman. 1988. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J. Virol. 62:159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campadelli-Fiume, G., E. Avitabile, S. Fini, D. Stirpe, M. Arsenakis, and B. Roizman. 1988. Herpes simplex virus glycoprotein D is sufficient to induce spontaneous pH-independent fusion in a cell line that constitutively expresses the glycoprotein. Virology 166:598-602. [DOI] [PubMed] [Google Scholar]
- 8.Campadelli-Fiume, G., F. Cocchi, L. Menotti, and M. Lopez. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 10:305-319. [DOI] [PubMed] [Google Scholar]
- 9.Chejanovsky, N., Y. I. Henis, and A. Loyter. 1986. Fusion of fluorescently labeled Sendai virus envelopes with living cultured cells as monitored by fluorescence dequenching. Exp. Cell Res. 164:353-365. [DOI] [PubMed] [Google Scholar]
- 10.Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 72:9992-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dales, S., and H. Silverberg. 1969. Viropexis of herpes simplex virus by HeLa cells. Virology 37:475-480. [DOI] [PubMed] [Google Scholar]
- 12.Dean, H. J., S. S. Terhune, M. T. Shieh, N. Susmarski, and P. G. Spear. 1994. Single amino acid substitutions in gD of herpes simplex virus 1 confer resistance to gD-mediated interference and cause cell-type-dependent alterations in infectivity. Virology 199:67-80. [DOI] [PubMed] [Google Scholar]
- 13.Epstein, M. A., K. Hummeler, and A. Berkaloff. 1964. The entry and distribution of herpes virus and colloidal gold in HeLa cells after contact in suspension. J. Exp. Med. 119:291-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Florkiewicz, R. Z., and J. K. Rose. 1984. A cell line expressing vesicular stomatitis virus glycoprotein fuses at low pH. Science 225:721-723. [DOI] [PubMed] [Google Scholar]
- 15.Foster, T. P., V. N. Chouljenko, and K. G. Kousoulas. 1999. Functional characterization of the HveA homolog specified by African green monkey kidney cells with a herpes simplex virus expressing the green fluorescence protein. Virology 258:365-374. [DOI] [PubMed] [Google Scholar]
- 16.Fuller, A. O., R. E. Santos, and P. G. Spear. 1989. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J. Virol. 63:3435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller, A. O., and P. G. Spear. 1987. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc. Natl. Acad. Sci. USA 84:5454-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 20.Heuser, J. E., and R. G. Anderson. 1989. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Highlander, S. L., S. L. Sutherland, P. J. Gage, D. C. Johnson, M. Levine, and J. C. Glorioso. 1987. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J. Virol. 61:3356-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoggan, M. D., and B. Roizman. 1959. The isolation and properties of a variant of herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am. J. Hyg. 70:208-219. [DOI] [PubMed] [Google Scholar]
- 23.Holmes, I. H., and D. H. Watson. 1963. An electron microscope study of the attachment and penetration of herpes virus in BHK21 cells. Virology 21:112-123. [DOI] [PubMed] [Google Scholar]
- 24.Huang, A. S., and R. R. Wagner. 1964. Penetration of herpes simplex virus into human epidermoid cells. Prog. Soc. Exp. Biol. Med. 116:863-869. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, D. C., and M. W. Ligas. 1988. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J. Virol. 62:4605-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, D. C., and P. G. Spear. 1982. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J. Virol. 43:1102-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kock, J., E. M. Borst, and H. J. Schlicht. 1996. Uptake of duck hepatitis B virus into hepatocytes occurs by endocytosis but does not require passage of the virus through an acidic intracellular compartment. J. Virol. 70:5827-5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyama, A. H., and T. Uchida. 1984. Inhibition of multiplication of herpes simplex virus type 1 by ammonium chloride and chloroquine. Virology 138:332-335. [DOI] [PubMed] [Google Scholar]
- 29.Koyama, A. H., and T. Uchida. 1987. The mode of entry of herpes simplex virus type 1 into Vero cells. Microbiol. Immunol. 31:123-130. [DOI] [PubMed] [Google Scholar]
- 30.Krummenacher, C., I. Baribaud, M. Ponce de Leon, J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2000. Localization of a binding site for herpes simplex virus glycoprotein D on herpesvirus entry mediator C by using antireceptor monoclonal antibodies. J. Virol. 74:10863-10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krummenacher, C., A. V. Nicola, J. C. Whitbeck, H. Lou, W. Hou, J. D. Lambris, R. J. Geraghty, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 72:7064-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez, M., F. Cocchi, L. Menotti, E. Avitabile, P. Dubreuil, and G. Campadelli-Fiume. 2000. Nectin2α (PRR2α or HveB) and nectin2δ are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D. J. Virol. 74:1267-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez, M., F. Eberle, M. G. Mattei, J. Gabert, F. Birg, F. Bardin, C. Maroc, and P. Dubreuil. 1995. Complementary DNA characterization and chromosomal localization of a human gene related to the poliovirus receptor-encoding gene. Gene 155:261-265. [DOI] [PubMed] [Google Scholar]
- 34.Lycke, E., B. Hamark, M. Johansson, A. Krotochwil, J. Lycke, and B. Svennerholm. 1988. Herpes simplex virus infection of the human sensory neuron: an electron microscopy study. Arch. Virol. 101:87-104. [DOI] [PubMed] [Google Scholar]
- 35.Marsh, M., and A. Helenius. 1989. Virus entry into animal cells. Adv. Virus Res. 36:107-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClure, M. O., M. Marsh, and R. A. Weiss. 1988. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 7:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12:575-625. [DOI] [PubMed] [Google Scholar]
- 38.Miller, N., and L. M. Hutt-Fletcher. 1992. Epstein-Barr virus enters B cells and epithelial cells by different routes. J. Virol. 66:3409-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 40.Morgan, C., H. M. Rose, and B. Mednis. 1968. Electron microscopy of herpes simplex virus. I. Entry. J. Virol. 2:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muggeridge, M. I. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J. Gen. Virol. 81:2017-2027. [DOI] [PubMed] [Google Scholar]
- 42.Nicola, A. V., M. Ponce de Leon, R. Xu, W. Hou, J. C. Whitbeck, C. Krummenacher, R. I. Montgomery, P. G. Spear, R. J. Eisenberg, and G. H. Cohen. 1998. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J. Virol. 72:3595-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez, L., and L. Carrasco. 1994. Involvement of the vacuolar H+-ATPase in animal virus entry. J. Gen. Virol. 75:2595-2606. [DOI] [PubMed] [Google Scholar]
- 44.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 45.Petrovskis, E. A., A. L. Meyer, and L. E. Post. 1988. Reduced yield of infectious pseudorabies virus and herpes simplex virus from cell lines producing viral glycoprotein gp50. J. Virol. 62:2196-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenthal, K. S., J. Killius, C. M. Hodnichak, T. M. Venetta, L. Gyurgyik, and K. Janiga. 1989. Mild acidic pH inhibition of the major pathway of herpes simplex virus entry into HEp-2 cells. J. Gen. Virol. 70:857-867. [DOI] [PubMed] [Google Scholar]
- 47.Shieh, M. T., D. WuDunn, R. I. Montgomery, J. D. Esko, and P. G. Spear. 1992. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J. Cell Biol. 116:1273-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shukla, D., and P. G. Spear. 2001. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Investig. 108:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535-1545. [DOI] [PubMed] [Google Scholar]
- 50.Smith, J. D., and E. de Harven. 1974. Herpes simplex virus and human cytomegalovirus replication in WI-38 cells. II. An ultrastructural study of viral penetration. J. Virol. 14:945-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136:1007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spear, P. G. 1993. Membrane fusion induced by herpes simplex virus, p. 201-232. In J. Bentz (ed.), Viral fusion mechanisms. CRC Press, Inc., Boca Raton, Fla.
- 53.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 54.Stegmann, T., F. P. Booy, and J. Wilschut. 1987. Effects of low pH on influenza virus: activation and inactivation of the membrane fusion capacity of the hemagglutinin. J. Biol. Chem. 262:17744-17749. [PubMed] [Google Scholar]
- 55.Takahashi, K., H. Nakanishi, M. Miyahara, K. Mandai, K. Satoh, A. Satoh, H. Nishioka, J. Aoki, A. Nomoto, A. Mizoguchi, and Y. Takai. 1999. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J. Cell Biol. 145:539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Visalli, R. J., and C. R. Brandt. 1991. The HSV-1 UL45 gene is not required for growth in Vero cells. Virology 185:419-423. [DOI] [PubMed] [Google Scholar]
- 58.Warner, M. S., R. J. Geraghty, W. M. Martinez, R. I. Montgomery, J. C. Whitbeck, R. Xu, R. J. Eisenberg, G. H. Cohen, and P. G. Spear. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246:179-189. [DOI] [PubMed] [Google Scholar]
- 59.White, J., K. Matlin, and A. Helenius. 1981. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J. Cell Biol. 89:674-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wittels, M., and P. G. Spear. 1991. Penetration of cells by herpes simplex virus does not require a low pH-dependent endocytic pathway. Virus Res. 18:271-290. [DOI] [PubMed] [Google Scholar]
- 61.Zhou, G., V. Galvan, G. Campadelli-Fiume, and B. Roizman. 2000. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J. Virol. 74:11782-11791. [DOI] [PMC free article] [PubMed] [Google Scholar]