Abstract
Hepatitis delta virus (HDV) particles are coated with the large (L), middle (M), and small (S) hepatitis B virus envelope proteins. In the present study, we constructed glycosylation-defective envelope protein mutants and evaluated their capacity to assist in the maturation of infectious HDV in vitro. We observed that the removal of N-linked carbohydrates on the S, M, and L proteins was tolerated for the assembly of subviral hepatitis B virus (HBV) particles but was partially inhibitory for the formation of HDV virions. However, when assayed on primary cultures of human hepatocytes, virions coated with S, M, and L proteins lacking N-linked glycans were infectious. Furthermore, in the absence of M, HDV particles coated with nonglycosylated S and L proteins retained infectivity. These results indicate that carbohydrates on the HBV envelope proteins are not essential for the in vitro infectivity of HDV.
Hepatitis delta virus (HDV), in association with the helper hepatitis B virus (HBV), causes acute and chronic infections which may eventually develop into cirrhosis and liver cancer in humans (6, 30). HDV is considered a satellite of HBV because it depends on the latter for the supply of envelope proteins that are essential for virion assembly (4). The HDV genome is a single-stranded circular RNA that encodes the small (p24) and large (p27) forms of the HDV antigen (HDAg) protein, but it lacks the coding capacity for envelope proteins. The HDV particle consists of an outer envelope of HBV origin and an inner ribonucleoprotein (RNP) made of the genomic HDV RNA and the HDV-encoded p24 and p27 delta proteins. Similar to that of HBV, the HDV envelope consists of a lipid membrane in which multiple copies of the three HBV surface proteins, designated large (L), middle (M), and small (S), are anchored. L, M, and S are encoded by a single open reading frame on the HBV genome, and they are translated from different in-frame start codons to a common stop codon (24). The L protein contains three distinct regions: the N-terminal pre-S1, the central pre-S2, and the C-terminal S regions. The M protein includes the pre-S2 and S regions, and S consists of the S domain only, but it is the most abundantly expressed (Fig. 1).
FIG. 1.
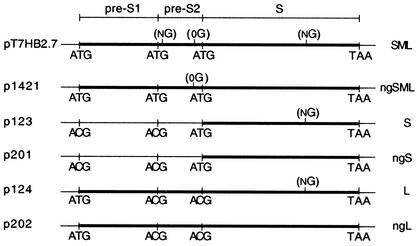
Schematic representations of plasmid DNA constructs. Plasmids pT7HB2.7, p1421, p123, p201, p124, and p202 direct the synthesis of wt SML (SML), ngSML, wt S (S), ngS, wt L (L), and ngL, respectively. Start codons (ATG) for S, M, and L open reading frames and start codons mutated to threonine codons (ACG) are indicated. The thick bars represent wt and unglycosylated envelope proteins. NG and 0G indicate N and O glycosylation sites, respectively.
A peculiar feature of the S protein is its ability to assemble empty (subviral) particles, which are secreted in large excess compared with the number of mature virions. Synthesis occurs at the endoplasmic reticulum membrane, and particles are formed by the budding of envelope protein aggregates into the lumen of a postendoplasmic reticulum/pre-Golgi cellular compartment (16). Transport to the extracellular space is thought to follow the constitutive secretion pathway. In addition to their capacity for subviral particle formation, singly expressed S proteins can envelop the HDV RNPs, leading to the formation of particles that are structurally identical to mature HDV but are functionally impaired (noninfectious) in the absence of L (31, 32, 34). In contrast, the HBV nucleocapsid envelopment requires the presence of L (but not M) in addition to S (5). Thus, L appears to be multifunctional by acting as a key element for HBV assembly and as a receptor-binding polypeptide for the infectivity of both HBV and HDV (22).
The three HBV envelope proteins appear as glycosylated and nonglycosylated isomers. N-linked carbohydrates are found at Asn-4 of the pre-S2 domain on the M protein and at Asn-146 of the S domains of S, M, and L proteins, but approximately half of these molecules remain unglycosylated at these sites. M is also O glycosylated at Thr-37 in its pre-S2 domain (36), but M-specific carbohydrates (N and O linked) are not crucial to the morphogenesis of infectious HBV or HDV virions, since M itself is dispensable for this process (5, 11). However, when present in the HBV envelope, M proteins lacking N-linked carbohydrates inhibit virion secretion (2, 23). N-linked glycans at Asn-146 are not required for the secretion of subviral particles, but unglycosylated S proteins are impaired in their capacity for HDV RNP envelopment (35).
In this study, we used a site-directed mutagenesis approach to eliminate N-linked glycosylation codons on the three HBV envelope proteins and we analyzed the effects of the removal of N glycans on (i) subviral HBV particle formation, (ii) HDV assembly, and (iii) HDV infectivity. Production of HDV particles was carried out in the HuH-7 human hepatoma cell line as previously described (32), after cotransfection of the cells with plasmid pSVLD3 (a gift from J. Taylor, Fox Chase Cancer Center, Philadelphia, Pa.) for expression of the HDV RNPs and plasmid pT7HB2.7 or its derivatives for expression of the wild-type (wt) or mutant HBV envelope proteins (S, M, and L). We constructed a series of HBV envelope protein expression vectors (Fig. 1) where N-linked glycosylation sites had been mutated. Plasmid p1421 was a derivative of pT7HB2.7 in which the Asn codons (AAT) at positions 4 in the pre-S2 domain and 146 in the S domain had been mutated to Thr codons (ACT) to prevent glycosylation at this site. Plasmid p123 was a derivative of pT7HB2.7 in which the start codons (ATG) for the L and M proteins were changed to ACG to inhibit the expression of L and M and to direct the expression of S only. Plasmid p124 was also a derivative of pT7HB2.7 in which the ATG start codons for the S and M proteins were changed to ACG for expression of the L protein only. Plasmids p201 and p202 were derivatives of p123 and p124, respectively, in which the Asn codon (AAT) at position 146 in the S domain had been mutated to a Thr codon (ACT) to prevent N-linked glycosylation. In vitro mutagenesis was performed by using the PCR technique with two complementary mutagenic oligonucleotides by following the overlap extension method (15). PCR-generated fragments were inserted in pT7HB2.7, and the resulting mutant plasmids were sequenced by the dideoxy-sequencing method on double-stranded DNA templates with Sequenase (Amersham).
HDV particles were produced by the transfection of HuH-7 cells (106/well) with a mixture of plasmid pSVLD3 (1 μg) and plasmid pT7HB2.7 or its derivatives (2 μg) (32). Transfection was carried out by using the Fugene-6 reagent according to the instructions of the manufacturer (Roche, Inc.).
Effects of N-linked-glycosylation-defective S, M, and L mutants on the assembly of HDV particles.
HuH-7 cells were cotransfected with the HDV expression vector pSVLD3 and different combinations of HBV envelope protein expression vectors, including (i) p123 for the expression of wt S protein alone; (ii) p123 and p124 for the expression of wt S and L proteins (SL); (iii) pT7HB2.7 for the expression of wt S, M, and L proteins (SML); (iv) p201 for the expression of S lacking the N-linked glycosylation site (mutated at Asn-146) (ngS); (v) p201 and p202 for the expression of mutated S and L proteins lacking the N-linked glycosylation sites (ngSL); and (vi) p1421 for the expression of S, M, and L proteins lacking N-linked glycosylation sites (ngSML).
Culture fluids were harvested on days 6, 9, and 12 after transfection, and they were clarified by centrifugation at 5,000 × g at 4°C for 1 h. Viral particles from one-third of the clarified medium were subjected to sedimentation by centrifugation at 25,000 rpm in an SW41 rotor (Beckman) on 2 ml of a 30% sucrose cushion in 10 mM Tris-HCl (pH 7.4) containing 150 mM NaCl and 1 mM EDTA (TNE). After centrifugation, the pellet was disrupted by boiling it for 5 min in 50 mM Tris-HCl (pH 6.8) containing 2% sodium dodecyl sulfate, 0.1% bromophenol blue, 10% glycerol, and 2% β-mercaptoethanol, and the proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were transferred to polyvinylidene difluoride membranes (Bio-Rad), and the membranes were then blocked by incubation in TNE-1% casein for 1 h at room temperature. Following blocking, the membranes were incubated in a 1:1,000 dilution of rabbit anti-S (R247) antibodies (19) or a 1:200 dilution of rabbit anti-HDAg antibodies in a solution containing TNE, 1% casein, and 0.3% Tween 20 for 2 h at room temperature. After being washed in TNE-0.3% Tween 20, the membranes were incubated in a 1:5,000 dilution of mouse anti-rabbit antibodies coupled to horseradish peroxidase in TNE-1% casein-0.3% Tween 20 for 1 h at room temperature. After being washed extensively in TNE-0.3% Tween 20, the immunoblots were developed by using ECL Western blotting detection reagents (Amersham Pharmacia Biotech), and they were exposed to Kodak film for detection of light emission.
Particles from one-third of the clarified medium were precipitated by the addition of polyethylene glycol 8000 (PEG 8000; Sigma) to a final concentration of 10%. After incubation at 4°C for 1 h, the precipitates were collected by centrifugation at 10,000 × g for 20 min at 4°C. They were resuspended in RNABle (Eurobio) for RNA extraction by the guanidine thiocyanate-acid phenol method (7). The RNA samples were subjected to electrophoresis through a 2.2 M formaldehyde-1.5% agarose gel and transferred to a nylon membrane (Roche) before hybridization to an HDV-specific RNA probe. Strand-specific 32P-labeled riboprobes were synthesized for the separate detection of genomic or antigenomic HDV RNA. Labeled full-length HDV RNA probes were produced by in vitro transcription by using T7 or SP6 RNA polymerase (Promega).
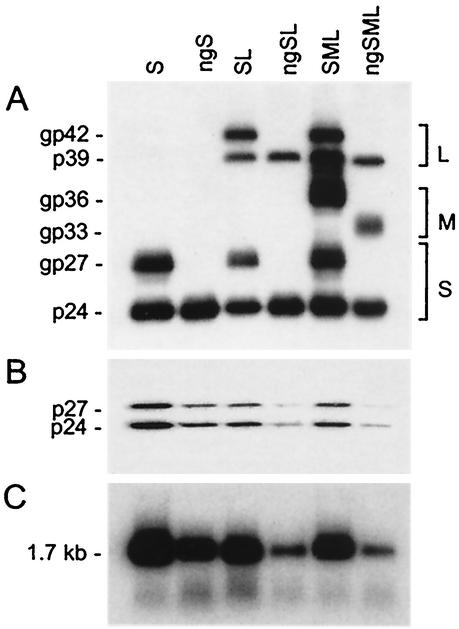
As shown in Fig. 2A and as expected, the wt HBV envelope proteins (S and L) were detected as unglycosylated (p24 and p39, respectively) and glycosylated (gp27 and gp42, respectively) forms. The wt M protein was detected only as a glycosylated gp36 form, since M contains N-linked glycosylation sites at position 4 in the pre-S2 domain and at position 146 in the S domain and an O-linked glycosylation site at position 37 in the pre-S2 domain. As expected, the ngS and ngL proteins, lacking glycosylation sites at position 146, were detected as unglycosylated p24 and p39 forms, respectively, whereas coexpression of the three N-linked glycosylation-defective proteins (ngSML) led to the expression of the p24, gp33, and p39 forms only. The gp33 glycoform of the M protein lacks N-linked glycans but retains O-linked carbohydrates at position 37 in the pre-S2 domain (36).
FIG. 2.
Production of N-linked-glycan-defective HDV particles. Culture fluids from HuH-7 cells were harvested on days 6, 9, and 12 after transfection of 106 cells with a mixture of 1 μg of HDV recombinant pSVLD3 plasmid DNA and 2 μg of pT7HB2.7 or mutant plasmid DNA coding for wt or unglycosylated HBV envelope proteins, respectively. Particles from the culture fluids were concentrated and assayed for the presence of HBV envelope proteins (A) or delta proteins (B) after electrophoresis on a 12% acrylamide gel, transfer to a polyvinylidene difluoride membrane, and immunodetection with a rabbit anti-HBsAg antibody (1:1,000 dilution) or rabbit anti-HDAg antibody (1:200 dilution). Rabbit immunoglobulin G was then probed with horseradish-labeled mouse anti-rabbit antibodies and a chemiluminescent substrate. Light emission was detected by exposure to Biomax ML film (Kodak). The molecular masses of the S, M, and L HBV envelope proteins and delta proteins are indicated at the left side of each panel. The size markers were prestained proteins (Amersham), measured in kilodaltons (e.g., gp42, glycoprotein with a molecular size of 42 kDa). Sedimented particles from the culture medium were also assayed for the presence of HDV RNA after RNA extraction, gel electrophoresis, and Northern blot hybridization by using a genomic strand-specific 32P-labeled HDV RNA probe (C). The size in kilobases of HDV genomic RNA is indicated.
Measurements of HDV RNA by Northern blot analysis were conducted by comparison of viral RNA in transfected cells or culture medium to known amounts of synthetic HDV RNA (20). Measurements of envelope protein signals by immunoblot analysis were conducted by comparison of S proteins in transfected cells or culture medium to known amounts of purified HBV envelope protein preparations (20). Serial dilution (one-half) was made for each sample and standard (RNA or protein), and autoradiogram signal measurements were performed by using a densitometer (data not shown). As shown in Fig. 2 and Table 1, it was estimated that culture medium from cells producing S-coated particles contained approximately 100 pg of HDV RNA (108 genome equivalents) and 80 ng of S proteins (2 × 1010 empty particle equivalents) per ml. For each of the HDV mutants, the amounts of HDV RNA in the PEG precipitates were normalized to those of S proteins (Table 1), and the normalized numbers reflected the efficiency of S for HDV assembly and/or stability. When particles were analyzed for the presence of the p24 and p27 delta proteins, we found a good correlation between HDV protein and HDV RNA expression levels (Fig. 2B and C). To ascertain that the differences in the types of packaging of HDV RNA were not due to variation in the efficiencies of transfection, we verified that equivalent levels of intracellular HDV RNA were present at day 9 posttransfection in all of the transfected cells (data not shown). We observed a reduction (up to fourfold in the present study) in the ability of glycosylation-defective S proteins to assist in HDV maturation in comparison to that of wt S, as evidenced by the relative amounts of both HDV proteins (p24 and p27) and HDV RNA in the HDV particles coated with the S protein only (S-HDV particles) versus the ngS-HDV particles. In addition, when N-glycosylation-defective S mutants were expressed in the presence of mutated L as ngSL-HDV or ngSML-HDV, the efficiency of virion assembly was further reduced, to 12% of that of the wt SL-HDV or SML-HDV particles, respectively (Fig. 2; Table 1). The expression of M proteins lacking the Asn-4 and Asn-146 codons had no impact on the secretion of HDV, since the level of HDV RNA in both ngSL-HDV and ngSML-HDV particles was at 12% of that of SL-HDV and SML-HDV, respectively (Table 1). Overall, these results indicate that carbohydrates are instrumental in the assembly and/or stability of HDV virions in the presence of the three HBV envelope proteins. This is in contrast to their function in subviral and HBV particle formation, and it clearly suggests that morphogenesis of HDV and HBV virions occurs through different cellular pathways.
TABLE 1.
Characteristics of HDV particles coated with wild-type or N-linked-glycosylation-defective HBV envelope proteins
| HDV particle designation | S protein concn (ng/ml of culture medium) | HDV RNA concn (pg/ml of culture medium) | Amt of HDV RNA (pg normalized to 80 ng of S protein) |
|---|---|---|---|
| S-HDV | 80 | 100 | 100 |
| ngS-HDV | 80 | 25 | 25 |
| SL-HDV | 40 | 50 | 100 |
| ngSL-HDV | 40 | 6 | 12 |
| SML-HDV | 80 | 50 | 50 |
| ngSML-HDV | 40 | 3 | 6 |
How could the absence of N-linked glycans at Asn-146 be detrimental to the ability of the S protein to assist in HDV assembly? One possible explanation is that removal of glycans may affect the affinity between S and HDV RNPs as previously suggested (35). Protein-protein interactions between S and the p27 HDV protein are thought to provide the molecular basis for virion assembly, which is mediated, at least in part, by the farnesyl chain of p27 through a mechanism that has not been fully elucidated (6, 13, 17). The N-linked carbohydrates, which are exposed luminally in cellular organelles during synthesis, are not likely to be involved in a direct binding to the p27 component of the RNP, considering the role of S in HDV RNP envelopment. In fact, the removal of carbohydrates on the HBV envelope proteins may have various consequences, including a modification in their interaction with molecular chaperones and lectins, resulting in an alteration of their intracellular trafficking (1, 26). However, in the case of ngS, we did not observe any inhibition of secretion as measured by metabolic labeling and pulse-chase analysis (data not shown); the kinetic of ngS secretion as subviral particles was equivalent to that of the wt. One hypothesis to explain the selective effect of S deglycosylation on HDV assembly, and not on subviral-particle formation, is that carbohydrates may contribute to the stabilization of HDV virions. It is assumed that lateral S-S interactions direct the assembly of subviral HBV particles and that the contribution, if any, of N-linked carbohydrates in this process is at most marginal. In contrast, S proteins at the surface of an HDV particle (which is different in size from a subviral particle) may present a suboptimal level of lateral interactions in order to accommodate an RNP. The role of S carbohydrates may then become critical for the stability of mature HDV. Whatever the mechanism may be by which carbohydrates act in the maturation of HDV, inhibitors of glycosylation processing, which have been shown to block the assembly of HBV through their effect on the M protein (3), are likely to be active in HDV formation by preventing S glycosylation.
The assembly of progeny virions coated with envelope proteins lacking N-linked glycans was clearly inefficient in HuH-7 cells, but we managed to produce sufficient amounts of glycosylation-defective HDV virions to carry out in vitro infectivity assays.
Effects of glycosylation-defective S, M, and L mutants on the infectivity of HDV particles.
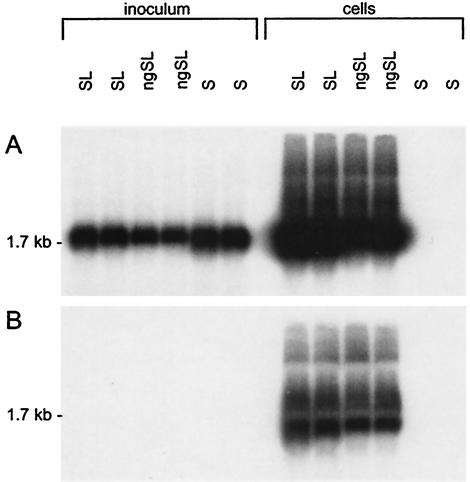
Because the M protein is dispensable for the in vitro infectivity of HDV virions (31), we chose to ignore the role of M-specific glycans (N or O linked) in this process (11). Thus, experiments were conducted with HDV virions coated only with the S and L proteins in the presence (SL-HDV) or the absence (ngSL-HDV) of the Asn-146 glycosylation site. For infection assays, primary human hepatocyte cultures were used as susceptible cells after their isolation from residual human liver tissue that was not usable for liver transplant (donor patients were free of any HBV or HDV markers, including HDV RNA and anti-HDAg antibodies in their sera) (12, 33). For the preparation of the inocula, culture fluids collected from HuH-7 cells at days 6, 9, and 12 posttransfection were pooled and clarified by centrifugation at 5,000 × g for 1 h at 4°C and viral particles were concentrated by precipitation in the presence of 10% PEG as described above. Inocula were adjusted to 108 genome equivalents/ml in serum-free medium, resulting in a 2× concentration of HuH-7 supernatant for the SL-HDV particles and a 17× concentration of that for the ngSL-HDV particles. Primary hepatocytes (2 × 106 cells/35-mm-diameter well) were exposed to wt SL-HDV or ngSL-HDV in duplicate cultures for 16 h on day 3 postseeding in the presence of 4% PEG 8000 (14). Cells were harvested at day 9 postexposure for measurement of intracellular genomic and antigenomic HDV RNA. The antigenomic HDV RNA (a replicative intermediate of the viral genome), which is absent in extracellular virions, served as a marker of infection (31). As previously described (33), in cases of successful infection, the level of intracellular HDV RNA is undetectable prior to day 6 postinoculation and reaches a maximum level at day 9. Because infections are abortive in the absence of HBV, the intensity of the intracellular HDV RNA signal at day 9 is considered proportional to the infectivity of the inoculum. As illustrated in Fig. 3, both intracellular genomic and antigenomic HDV RNAs were detected in hepatocytes exposed to SL-HDV or ngSL-HDV particles whereas only the genomic form was present in the inoculum. This clearly indicated that ngSL-HDV particles were infectious in vitro. Although the relative infectivity of ngSL-HDV could not be estimated precisely, these results were reproducible with different preparations of virions or hepatocyte cultures and in the absence of PEG in the inoculum. As expected, S-HDV particles were not infectious, as evidenced by the absence of intracellular HDV RNA at day 9 postexposure.
FIG. 3.
Infectivity of HDV particles coated with N-glycan-defective HBV envelope proteins based on RNA blot hybridization analysis of HDV RNA extracted from primary hepatocytes exposed to S-HDV, SL-HDV, and ngSL-HDV particles. In this experiment, 2 × 106 cells were exposed to approximately 108 genome equivalents of wt or mutant HDV particles. Infection assays were performed in duplicate cultures. Five micrograms (1:5) of total cellular RNA extracted from 106 cells harvested 12 days after inoculation was analyzed for the presence of genomic (A) or antigenomic (B) HDV RNA. RNA extracted from 0.5 ml of inoculum was analyzed under the same conditions. RNA was separated on a 1.5% agarose-2.2 M formaldehyde gel, transferred to nylon membranes, and hybridized to strand-specific 32P-labeled HDV RNA probes. Following hybridization, the filters were washed, dried, and autoradiographed at −70°C for 12 h with an intensifying screen. The size in kilobases of HDV genomic and antigenomic RNA is indicated.
Our results demonstrate that N-linked glycans on HBV envelope proteins are not required for HDV entry in vitro, and only the presence of unglycosylated S and L proteins at the virion surface is sufficient for infectivity. It is noteworthy that the Duck hepatitis B virus, the avian member of the Hepadnaviridae family, lacks carbohydrates at the virion surface, as the viral envelope consists of only two (S and L) unglycosylated proteins (27). In contrast, S and L HBV envelope proteins appear invariably under glycosylated and unglycosylated forms. The strict conservation of the N glycosylation sites among all of the HBV genotypes therefore suggests an important role for the envelope protein carbohydrates in the HBV life cycle in vivo (25). Previous studies had demonstrated that N-linked glycosylation at Asn-146 in the S domain was not necessary for the secretion of subviral or mature HBV particles, although virion secretion may be affected by the absence of glycans on the M protein (2, 3, 23). This finding was somewhat surprising, since HBV may be assembled and secreted in the absence of M and the resulting SL-coated virions were infectious in vitro (5, 11). It was then proposed that glycosylation of the M protein could function as a regulator of HBV secretion. However, a precise understanding of the N- or O-linked glycans in the HBV replication cycle still awaits further investigation, but one can speculate that glycosylation of HBV envelope proteins is dispensable in vitro yet essential in vivo in ensuring the stability of circulating virions. Carbohydrates at the surfaces of enveloped animal viruses are often instrumental at one or several steps of the replication cycle, including infectivity (8-10, 18, 21, 28). For instance, they can protect from antibody-mediated immune recognition by hiding a specific epitope that is either involved in binding to the viral receptor or, more generally, susceptible to neutralizing antibodies (29). As for HDV, carbohydrates of the envelope proteins are functional for virion morphogenesis as nonessential regulators but nonfunctional for in vitro infectivity. Under the reasonable assumption that HBV and HDV utilize the same receptor at the human hepatocyte surface, envelope protein glycans are not expected to be necessary for the in vitro infectivity of HBV either. This expectation, however, remains to be proven. The possibility that carbohydrates contribute to the infectivity of HBV while being dispensable for that of HDV cannot be excluded, since the selective pressure that has led to their conservation among all of the HBV genotypes has not been exerted on HDV. Glycosylation may be just one more property of the HBV envelope proteins that HDV does not fully require for its own survival.
Acknowledgments
This work was supported in part by the INSERM, the CNRS, the Association pour la Recherche contre le Cancer (ARC), the Fondation pour la Recherche Médicale (FRM), La Ligue contre le Cancer, and the Institut National de la Transfusion Sanguine (INTS).
REFERENCES
- 1.Benham, A. M., and I. Braakman. 2000. Glycoprotein folding in the endoplasmic reticulum. Crit. Rev. Biochem. Mol. Biol. 35:433-473. [DOI] [PubMed] [Google Scholar]
- 2.Block, T. M., X. Lu, A. Mehta, J. Park, B. S. Blumberg, and R. Dwek. 1998. Role of glycan processing in hepatitis B virus envelope protein trafficking. Adv. Exp. Med. Biol. 435:207-216. [DOI] [PubMed] [Google Scholar]
- 3.Block, T. M., X. Lu, F. M. Platt, G. R. Foster, W. H. Gerlich, B. S. Blumberg, and R. A. Dwek. 1994. Secretion of human hepatitis B virus is inhibited by the imino sugar N-butyldeoxynojirimycin. Proc. Natl. Acad. Sci. USA 91:2235-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonino, F., K. H. Heermann, M. Rizzetto, and W. H. Gerlich. 1986. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J. Virol. 58:945-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruss, V., and D. Ganem. 1991. The role of envelope proteins in hepatitis B virus assembly. Proc. Natl. Acad. Sci. USA 88:1059-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey, J. L. 1998. Hepatitis delta virus: molecular biology, pathogenesis and immunology. Antivir. Ther. 3:37-42. [PubMed] [Google Scholar]
- 7.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 8.Dedera, D. A., R. L. Gu, and L. Ratner. 1992. Role of asparagine-linked glycosylation in human immunodeficiency virus type 1 transmembrane envelope function. Virology 187:377-382. [DOI] [PubMed] [Google Scholar]
- 9.Delos, S. E., M. J. Burdick, and J. M. White. 2002. A single glycosylation site within the receptor-binding domain of the avian sarcoma/leukosis virus glycoprotein is critical for receptor binding. Virology 294:354-363. [DOI] [PubMed] [Google Scholar]
- 10.Felkner, R. H., and M. J. Roth. 1992. Mutational analysis of the N-linked glycosylation sites of the SU envelope protein of Moloney murine leukemia virus. J. Virol. 66:4258-4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernholz, D., P. R. Galle, M. Stemler, M. Brunetto, F. Bonino, and H. Will. 1993. Infectious hepatitis B virus variant defective in pre-S2 protein expression in a chronic carrier. Virology 194:137-148. [DOI] [PubMed] [Google Scholar]
- 12.Ferrini, J. B., L. Pichard, J. Domergue, and P. Maurel. 1997. Long-term primary cultures of adult human hepatocytes. Chem. Biol. Interact. 107:31-45. [DOI] [PubMed] [Google Scholar]
- 13.Glenn, J. S., J. A. Watson, C. M. Havel, and J. M. White. 1992. Identification of a prenylation site in delta virus large antigen. Science 256:1331-1333. [DOI] [PubMed] [Google Scholar]
- 14.Gripon, P., C. Diot, and C. Guguen-Guillouzo. 1993. Reproducible high level infection of cultured adult human hepatocytes by hepatitis B virus: effect of polyethylene glycol on adsorption and penetration. Virology 192:534-540. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi, R., B. Krummel, and R. K. Saiki. 1988. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 16:7351-7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huovila, A. P., A. M. Eder, and S. D. Fuller. 1992. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J. Cell Biol. 118:1305-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang, S. B., and M. M. C. Lai. 1993. Isoprenylation mediates direct protein-protein interactions between hepatitis large delta antigen and hepatitis B virus surface antigen. J. Virol. 67:7659-7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarvis, D. L., L. Wills, G. Burow, and D. A. Bohlmeyer. 1998. Mutational analysis of the N-linked glycans on Autographa californica nucleopolyhedrovirus gp64. J. Virol. 72:9459-9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenna, S., and C. Sureau. 1998. Effect of mutations in the small envelope protein of hepatitis B virus on assembly and secretion of hepatitis delta virus. Virology 251:176-186. [DOI] [PubMed] [Google Scholar]
- 20.Jenna, S., and C. Sureau. 1999. Mutations in the carboxyl-terminal domain of the small hepatitis B virus envelope protein impair the assembly of hepatitis delta virus particles. J. Virol. 73:3351-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kayman, S. C., R. Kopelman, S. Projan, D. M. Kinney, and A. Pinter. 1991. Mutational analysis of N-linked glycosylation sites of Friend murine leukemia virus envelope protein. J. Virol. 65:5323-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Seyec, J., P. Chouteau, I. Cannie, C. Guguen-Guillouzo, and P. Gripon. 1999. Infection process of the hepatitis B virus depends on the presence of a defined sequence in the pre-S1 domain. J. Virol. 73:2052-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta, A., X. Lu, T. M. Block, B. S. Blumberg, and R. A. Dwek. 1997. Hepatitis B virus (HBV) envelope glycoproteins vary drastically in their sensitivity to glycan processing: evidence that alteration of a single N-linked glycosylation site can regulate HBV secretion. Proc. Natl. Acad. Sci. USA 94:1822-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nassal, M. 1996. Hepatitis B virus morphogenesis. Curr. Top. Microbiol. Immunol. 214:297-337. [DOI] [PubMed] [Google Scholar]
- 25.Norder, H., A. M. Courouce, and L. O. Magnius. 1994. Complete genomes, phylogenetic relatedness, and structural proteins of six strains of the hepatitis B virus, four of which represent two new genotypes. Virology 198:489-503. [DOI] [PubMed] [Google Scholar]
- 26.Prange, R., M. Werr, and H. Loffler-Mary. 1999. Chaperones involved in hepatitis B virus morphogenesis. Biol. Chem. 380:305-314. [DOI] [PubMed] [Google Scholar]
- 27.Pugh, J. C., J. J. Sninsky, J. W. Summers, and E. Schaeffer. 1987. Characterization of a pre-S polypeptide on the surfaces of infectious avian hepadnavirus particles. J. Virol. 61:1384-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quiñones-Kochs, M. I., L. Buonocore, and J. K. Rose. 2002. Role of N-linked glycans in a human immunodeficiency virus envelope glycoprotein: effects on protein function and the neutralizing antibody response. J. Virol. 76:4199-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679-684. [DOI] [PubMed] [Google Scholar]
- 30.Rizzetto, M. 2000. Hepatitis D: virology, clinical and epidemiological aspects. Acta Gastro-Enterol. Belg. 63:221-224. [PubMed] [Google Scholar]
- 31.Sureau, C., B. Guerra, and R. E. Lanford. 1993. Role of the large hepatitis B virus envelope protein in infectivity of the hepatitis delta virion. J. Virol. 67:366-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sureau, C., B. Guerra, and H. Lee. 1994. The middle hepatitis B virus envelope protein is not necessary for infectivity of hepatitis delta virus. J. Virol. 68:4063-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sureau, C., J. R. Jacob, J. W. Eichberg, and R. E. Lanford. 1991. Tissue culture system for infection with human hepatitis delta virus. J. Virol. 65:3443-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, C.-J., P.-J. Chen, J.-C. Wu, D. Patel, and D.-S. Chen. 1991. Small-form hepatitis B surface antigen is sufficient to help in the assembly of hepatitis delta virus-like particles. J. Virol. 65:6630-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, C. J., S. Y. Sung, D. S. Chen, and P. J. Chen. 1996. N-linked glycosylation of hepatitis B surface antigens is involved but not essential in the assembly of hepatitis delta virus. Virology 220:28-36. [DOI] [PubMed] [Google Scholar]
- 36.Werr, M., and R. Prange. 1998. Role for calnexin and N-linked glycosylation in the assembly and secretion of hepatitis B virus middle envelope protein particles. J. Virol. 72:778-782. [DOI] [PMC free article] [PubMed] [Google Scholar]