Abstract
Recently, cell-based replicon systems for hepatitis C virus (HCV), in which the nonstructural proteins stably replicate subgenomic viral RNA in Huh7 cells, were developed. To date, one limitation of using these replicon systems to advance drug discovery is the inability of other genotypic derivatives, beyond those of two distinct strains of genotype 1b (HCV-N and Con1), to stably replicate in Huh7 cells. In this report, we evaluated a series of replicon genotype 1a-1b chimeras, as well as a complete genotype 1a replicon clone. A subgenomic replicon construct containing only type 1a sequences failed to generate stable colonies in Huh7 cells even after repeated attempts. Furthermore, addition of an NS5A adaptive mutation (S2204I) which enhances type 1b replicon efficiency was insufficient to confer replication to the wild-type 1a replicon. This subgenomic replicon was subsequently found to be inefficiently translated in Huh7 cells compared to a type 1b replicon, and the attenuation of translation mapped to the N-terminal region of NS3. Therefore, to ensure efficient translation and thereby support replication of the 1a genome, the coding sequence for first 75 residues from type 1a were replaced with the type 1b (strain Con 1) NS3 coding sequence. Although nonstructural proteins were expressed at lower levels with this replicon than with type 1b and although the amount of viral RNA was also severalfold lower (150 copies of positive-strand RNA per cell), the replicon stably replicated in Huh7 cells. Notwithstanding this difference, the ratio of positive- to negative-strand RNA of 26 was similar to that found with the type 1b replicon. Similar results were found for a 1b replicon expressing the type 1a RNA-dependent RNA polymerase. These 1a hybrid replicons maintained sensitivity to alpha interferon (IFN-α), albeit with an eightfold-higher 50% inhibitory concentration than type 1b replicons. Evidence is provided herein to confirm that this differential response to IFN-α may be attributed directly to the type 1a polymerase.
Hepatitis C virus (HCV) is the primary cause of non-A, non-B transfusion-associated hepatitis and accounts for more than 200 million hepatitis cases worldwide (8, 14). Few infections are symptomatic, and this virus readily establishes a chronic infection in up to 80% of infected individuals which persists for decades (1). Significant progress in understanding HCV replication has been made by using recombinant polymerases or cell-based subgenomic replicon systems; however in the absence of a virus propagation system, detailed analyses of viral replication remain limited. Despite advances in the establishment of stable subgenomic HCV replication systems in Huh7 cells (2, 9, 13), these replicons contain only genotype 1b genome sequences.
Enhanced replicons, those harboring adaptive mutations within the viral coding region, result in higher copy numbers of subgenomic RNA upon selection, However, only type 1b genome sequences, from two distinct strains, have demonstrated functionality (2, 7, 9, 10, 12). To date, extension of replicon systems to other HCV genotypes, such as type 1a (strain H77) (17), or additional type 1b strains (HC-J4) (18) has not been reported as successful.
Herein, we describe chimeric subgenomic replicons containing sequences from the Con1 strain (type 1b) and H77 strain (type 1a). Two constructs, one containing a contiguous unit of nonstructural genes from type 1a, except for the NS3 coding sequence for the N-terminal 75 residues from type 1b, and one containing a contiguous unit of nonstructural genes from type 1b, except for the NS5B coding sequence from type 1a, are presented herein.
MATERIALS AND METHODS
Cell culture and media.
Huh7 cell lines were cultured in Dulbecco's modified Eagle medium (Invitrogen) containing 10% fetal calf serum (JRH Biosciences), 1% penicillin-streptomycin, 1% nonessential amino acids, and 0.5 mg of Geneticin/ml. Cells were grown to 75% confluence prior to splitting.
Plasmid construction.
Using PCR, a DNA fragment (F1) containing the coding sequence for NS3 (from amino acid 76), NS4A, NS4B, and the N terminus of NS5A (148 amino acids) was amplified from pCV-H77C DNA (kind gift from Charlie Rice). PCR mixtures contained 1× Pfu PCR buffer (Stratagene), 0.25 μM BG1000 primer (5′CATCCAGATGTACACCAATGTGGAC3′), 0.25 μM BG1002 primer (5′CAATTCTGTGAAGAATTCGGGCGATG3′), 10 ng of pCV-H77C DNA, and 2 U of Turbo Pfu (Stratagene). The BG1000 primer contained a single T-to-C nucleotide change (underlined) from the pCV-H77C DNA to create a BsrGI restriction site for subcloning. The BG1002 primer contained a single A-to-G nucleotide change (underlined) to create an EcoRI restriction site. The amplification conditions were 95°C for 30 s, 60°C for 30 s, and 72°C for 3 min 30 s for 25 cycles and then 72°C for 10 min. The DNA product was digested with the BsrGI and EcoRI restriction enzymes and purified from agarose gels with a gel purification kit (Qiagen) according to the manufacturer's protocol. After purification, the DNA fragment was ligated into pHCVrep1b (BB7; kind gift from Charlie Rice) vector DNA at the BsrGI and EcoRI sites. The recombinant plasmid pBB7-F1 was sequenced to confirm the authentic type 1a H77 HCV sequence. To generate the DNA fragment (F2) encoding a sequence extending from within NS5A to amino acid 132 of NS5B, PCR amplification was performed using pCV-H77C DNA as the templates and the primer pair BG1001 (5′CATCGCCCGAATTCTTCACAGAATTG3′) and BG1004 (5′GATGGTAGTGTCAATTGGTGTTAC3′). The primer BG1001 contained a single nucleotide change, T to C (underlined), to create an EcoRI restriction site. Similarly, a T-to-A change (underlined) was added in primer BG1004 to create an MfeI restriction site. The 1.3-kb PCR product was digested with EcoRI and MfeI and ligated into pLitmus38 (New England BioLabs), resulting in pF2-Lit38. To incorporate the S2204I (referred to as S1179I in reference 2) amino acid change in NS5A, the pF2-Lit38 construct was subjected to site-directed mutagenesis (underlined) using oligonucleotides HCV 1a S9844I forward oligo (5′CTTCTATGGCCAGCTCCTCGGCTATCCAGCTGTCCGCTCCATCTC3′) and HCV 1a S9844I reverse oligo (5′GAGATGGAGCGGACAGCTGGATAGCCGAGGAGCTGGCCATAGAAG3′), as outlined in the manufacturer's protocol for the QuikChange site-directed mutagenesis kit (Stratagene). Both wild-type and mutagenized F2 fragments were subsequently cloned into pBB7-F1 at the EcoRI and MfeI sites to generate pBB7-F1/F2 and pBB7-F1/F2/S/I. The recombinant plasmids were sequenced to confirm the authentic type 1a H77 HCV sequence as well as the S2204I amino acid change in NS5A.
A third DNA fragment [F3(c)], containing the H77 HCV 1a sequence from the codon for amino acid 133 in NS5b to the 3′ end of the HCV genome was amplified from pCV-H77C DNA by PCR using the primers BG1003 (GTAACACCAATTGACACTACCATC) and RB8000 (5′GCACTAGTACTTGATCTGCAGAGAGGCCAGTATCAGCACTCTCTGCAGTCAAGCGG). BG1003 had an MfeI site, and RB8000 had SpeI and ScaI restriction sites. The ScaI site denotes the terminal end of the HCV genome to allow production of the authentic 3′ end by in vitro transcription. The oligonucleotide contains two changes from the H77 sequence, an A-to-T change (underlined) at the −3 position and T-to-A change (underlined) at the −44 position of the HCV 3′ nontranslated region (NTR). The changes reflected the DNA sequence in pBB7 and maintained the base pairing structure of the 3′ X tail (19). The F3(c) fragment was digested with MfeI and SpeI and purified from an agarose gel as described above and was ligated into pBB7-F1/F2 and pBB7-F1/F2/S/I to generate pH771b75 and pH771b75S/I, respectively. Both recombinant plasmids were sequenced to confirm the authentic type 1a H77 HCV sequence.
To generate the full-length H77 replicon constructs, two DNA fragments were amplified by PCR. The encephalomyocarditis virus (EMCV) internal ribosome entry site (IRES) fragment was created from the pBB7 template by using the following primer combinations: BG13 (5′GTTCTTCTGAGTTTAAACAGACCACAACGG3′) and IRES/NS31a3′ (5′GTACGCCGTGATGGGCGCCATGGTATTATCGTGTTTTTCA3′). The type 1a NS3 fragment was made from the pCV-H77C template by using primers IRES/NS31a5′ (5′TGAAAAACACGATAATACCATGGCGCCCATCACGGCGTA3′) and BG100R (5′GTCCACATTGGTGTACATCTGGATGAC3′). The primer BG1000R contains an A-to-G nucleotide change (underlined) in order to create a BsrGI site. Both fragments were agarose gel purified as previously described. The purified fragments were added to the PCR mixture in equimolar ratios of DNA totaling 10 ng. Primers BG13 and BG100R were used to generate a fused 1-kb product from the two individual fragments. The 1-kb DNA was digested with PmeI and BsrGI, agarose gel purified, and ligated to the pH771b75 vector to produce pH77. The same fragment was ligated to pH771b75S/I to generate pH77S/I. The recombinant plasmids pH77 and pH77S/I were sequenced to confirm the authentic type 1a H77 HCV sequence.
To create pBB71a5B, which contains the H77 type 1a NS5B coding sequence replacing the NS5B coding sequence in pHCVrep1b, PCRs were carried out to amplify the H77 type 1a NS5B gene by using pCV-H77C DNA and the primers 1A 10501 (CCTGGACAGGCGCACTGATCACC) and RB7801 3′ (CTCCCCCAACCGATGAACGGGTACGTAAACACTCCAGGCCAATAG). The 1A 10501 primer had two nucleotide changes (italicized) from the 1a sequence to create a BclI restriction site. The RB7801 3′ primer had two nucleotide changes (underlined) to create a SnaBI site 3′ of the NS5B gene. The DNA product was digested with BclI and SnaBI and cloned into pBB7-SN, which is a BB7 replicon derivative with a SnaBI restriction site 3′ to the NS5B stop codon.
In vitro transcription.
Replicon plasmid DNA was linearized with ScaI at 1 U per μg of DNA for 1 h at 37°C. The linearization of the plasmid generates the authentic 3′ end of the HCV 3′ NTR and provides the template to generate the appropriate RNA transcript from the upstream T7 promoter. The DNA was subsequently purified in two phenol-chloroform extractions, followed by ethanol precipitation. The linearized DNA pellet was dried and resuspended in nuclease-free water for in vitro transcription with the Megascript T7 kit (Ambion). A total of 1 μg of linearized DNA in 1× reaction buffer, 1× enzyme mixture, and 7.5 mM ATP, UTP, CTP, and GTP was incubated at 37°C for 4 h. Next, 1 U of RQ1 DNase enzyme (Promega) was added to the reaction mixture and the mixture was incubated at 37°C for 2 h. The newly synthesized RNA was purified with the RNasy kit (Qiagen) and further purified from DNA by an additional digestion with 1 U of RNase-free DNase (Qiagen) for 1 h at 25°C in accordance with the manufacturer's protocol. The RNA was eluted in nuclease-free water and stored at −80°C.
RNA electroporation.
Huh7 cells were passaged 2 days prior to electroporation and grown to 75% confluence. Cells were split and resuspended to a final concentration of 107 cells/ml in Opti-MEM medium (Invitrogen). RNA was aliquoted into a prechilled 0.2-mm-gap cuvette (Bio-Rad) on ice. A 0.2-ml aliquot of the cell suspension was then added to the cuvette, which contained 5 μg of RNA. The RNA was immediately electroporated into the Huh7 cells by using the Gene Pulser (Bio-Rad), adjusted to deliver one pulse at 0.2 kV, 100 Ω, and 960 μF. The cuvettes were then chilled on ice for 10 min to allow the cells to recover, followed by plating onto a 100-mm-diameter tissue culture plate in complete medium. Selection medium containing 0.5 mg of G418 (Invitrogen)/ml was added to the cells 24 to 48 h postelectroporation.
Western blot analysis.
Total-cell lysates from 104 cells were harvested from replicon cells in 1× LDS buffer (Invitrogen). The lysates were heated at 70°C for 10 min in the presence of dithiothreitol before electrophoresis on a 10% BIS-Tris NuPage polyacrylamide gel (Invitrogen) in 1× MOPS (morpholinepropanesulfonic acid) buffer. The protein was transferred to a polyvinylidene difluoride (Invitrogen) membrane by using a SemiDry Trans Blot system (Bio-Rad). Following the transfer, the membrane was rinsed once with phosphate-buffered saline (PBS) containing 0.5% Tween 20 (PBS-Tween) and blocked in PBS-Tween containing 5% nonfat milk for 1 h. After being washed with PBS-Tween, the membrane was incubated with the primary antibody at a 1:2,500 dilution for 1 h at 25°C, either anti-NS5A (generated from peptide sequence PPLEGEPGDPDLSDGS) or anti-NS5B (generated from peptide sequence TRDPTTPLARAAWETARHT). Prior to incubation with a horseradish peroxidase-conjugated anti-rabbit immunoglobulin G secondary antibody (Amersham) diluted 1:5,000, the blot was washed in PBS-Tween. Following the incubation with the secondary antibody, the blot was washed again and treated with Super Signal chemiluminescent reagent (Pierce) according to the manufacturer's protocol and exposed to X-ray film.
Genomic DNA PCRs.
Genomic DNA was isolated by using the DNeasy tissue kit (Qiagen) according to the manufacturer's protocol. Primers Neo forward (5′TCAAGACCGACCTGTCCGGTGCCC) and Neo reverse (5′CTTGAGCCTGGCGAACAGTTCGGC) were used to amplify a 380-bp product with 100 ng of genomic DNA. All PCRs were performed with 2.5 U of Pfu Turbo polymerase (Stratagene) per 100 μl of reaction mixture; the mixture contained primer and deoxynucleoside triphosphate at final concentrations of 250 nM and 100 μM, respectively, as well as 20 mM Tris-HCl (pH 8.8), 2 mM MgSO4, 10 mM KCl, 10 mM (NH4)2SO4, 0.1% Triton X-100, and 0.1 mg of nuclease-free bovine serum albumin/ml. Control primers GAPDH forward (5′ACCACAGTCCATGCCATCAC) and GAPDH reverse (5′TCCACCACCCTGTTGCTGTA) were used to demonstrate the quality of the intact genomic DNA in each PCR.
Northern blotting.
Total cellular RNA was extracted by using the RNeasy kit (Qiagen). Northern blot analysis was done according to the protocol of Guo et al. (7). Briefly, 5 μg of total RNA was electrophoresed through a 1.0% agarose gel containing 2.2 M formaldehyde, transferred to a nylon membrane, and immobilized by UV cross-linking (Stratagene). Hybridization was carried out with [α-32P]UTP-labeled neomycin RNA in a solution containing 50% deionized formamide, 5× SSC (750 mM sodium chloride, 75 mM sodium citrate), Denhardt's solution, 0.02 M sodium phosphate (pH 6.8), 0.2% sodium dodecyl sulfate (SDS), 100 μg of sheared denatured salmon sperm DNA/ml, and 100 μg of yeast RNA/ml for 16 h at 58°C. The membranes were washed once in 2× SSC-0.1% SDS for 30 min at room temperature and twice in 0.1× SSC-0.1% SDS for 30 min at 68°C. Membranes were exposed to X-ray film.
Taqman analysis.
TaqMan PCR and compound analysis were performed as described previously (5).
Negative-strand replicon RNA detection.
To achieve strand-specific detection, tagged primer 5′ACATGCGCGGCATCTAGACCGGCTACCTGCCCATTC3′ containing HCV RNA sequences and a 15-base tag of nonrelated sequence at the 5′ end was used for reverse transcription (RT) with the Thermoscript RT-PCR system (Invitrogen). This Taqman assay with a tagged primer was shown to be specific for negative-strand HCV RNA in a linear range of over 7 orders of magnitude (between 102 and 109 copies/reaction), and the details of the method development will be published elsewhere. Briefly, 9 μl of total cell RNA and 1 μl of 10 μM primer was incubated with reverse transcriptase at 60°C for 1 h. Following incubation, 2 μl of cDNA product containing the 5′ tag was amplified for TaqMan quantification by using 48 μl of TaqMan Universal Master Mix (Applied Biosystems) as well as primers neo-tag (5′ACATGCGCGGCATCTAGA3′), neo reverse (5′CCGGCTACCTGCCCATTC3′), and neo probe (5′FAM-ACATCGCATCGAGCGAGCACGTAC-TAMRA3′). Samples were mixed briefly and placed in an ABI7700 (Applied Biosystems) at 50°C for 2 min and then 95°C for 10 min, with cycling parameters set to 94°C for 15 s and 55°C for 1 min for 40 cycles. The negative-strand copy number in each reaction was determined by linear regression analysis based on the slope and intercept generated with a negative-strand copy standard curve. The number of negative-strand copies per cell was determined by dividing the total number of negative-strand copies per reaction by the total number of cells per reaction.
RT-PCR and DNA sequencing.
One to 2 μg of total RNA and primer BG1018 (5′CTGCAGAGAGGCCAGTATCAGC) or UP25 (5′GAGTAGGCAAAACCAGAACCAGCG) were used for cDNA synthesis with Thermoscript reverse transcriptase (Invitrogen) according to the manufacturer's recommendations. Next, 1 μl of the cDNA reaction mixture was used for PCR to generate four overlapping DNA fragments by using primer BG1014 (5′GAACCGGCTAATAGCCTTCGCC3′) paired with UP3 (5′CAAGTCGTATTCTGGTTGTGGG3′), BG1000 (5′CATCCAGATGTACACCAATGTGGAC3′) paired with BG1002 (5′CAATTCTGTGAAGAATTCGGGCGATG3′), UP31 (5′GACAGACTGCAAGTTCTGGACAGC3′) paired with UP3 (5′CAAGTCGTATTCTGGTTGTGGG3′), and UP16 (5′CAATTCAAGGGGGGAAAACTGCGG3′) paired with BG1005 (5′ACTAGTACTTGATCTGCAGAGAGGC3′). The PCR products were purified by agarose gel electrophoresis and ligated into the PCR cloning vector pCRBlunt-TOPO (Invitrogen). Up to 17 clones of each fragment were sequenced to confirm integrity.
Indirect immunofluorescence.
Chimeric type 1a replicon or parental Huh7 cells were grown on chamber slides at 106 cells/well. The cells were washed once with PBS and fixed in 2% paraformaldehyde-PBS solution for 10 min at 25°C. After the monolayer was rinsed once with PBS, 1 ml of 0.2% Triton X-100-PBS solution was applied for 20 min at 4°C. NS3 (Biogenesis), NS5B (Biogenesis), or NS5A (peptide sequence mentioned previously) antisera were diluted 1:100 in Tris-buffered saline (TBS) (25 mM Tris-HCl [pH 7.4], 200 mM NaCl) and added to humidified chamber slides for 1 h at 37°C. The cells were washed three times with TBS for 5 min. A fluorescein isothiocyanate-labeled secondary antibody was diluted 1:100 in TBS and applied to the cells for 1 h at 37°C, followed by three Tris-saline washes of 5 min each. Fluorescence was examined with a confocal microscope.
Cell-free translation.
Cell-free translation was carried out as described previously (6) by using template RNA from pH 77S/I and pH 771b75S/I.
RESULTS
H77 subgenomic replicons.
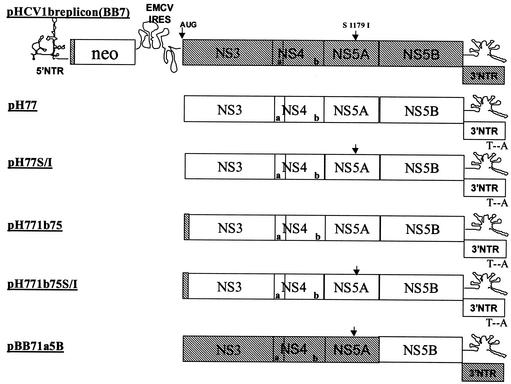
Several subgenomic replicons containing sequences from genotype 1a were constructed (Fig. 1): (i) a replicon entirely of nonstructural coding sequence from strain H77 (pH77), (ii) the pH77 replicon encoding an adaptive mutation in NS5A, S2204I, which was shown previously to enhance type 1b replicon stability (2) (pH77S/I), (iii) the pH77 replicon expressing 75 N-terminal type 1b residues of NS3 (pH771b75), (iv) the pH771b75 replicon encoding the S2204I (referred to as S1179I in reference 2) adaptive mutation in NS5A (pH771b75S/I), and (v) a type 1b replicon (strain BB7) chimera expressing the type 1a NS5B (pBB71a5B). These replicons all contain the 5′ NTR element from the HCV strain Con1, since it was shown previously to be capable of supporting the replication of a heterologous type 1b genome (strain N) (7).
FIG. 1.
Schematic representation of the construction of a chimeric type 1a replicon. The pHCV1b replicon (BB7) structure is shown at the top, with the Con1 type 1b sequence shown in hatched boxes with protein names. Open boxes, type 1a HCV pCV-H77C sequence. The NS3 gene starting codon, AUG, and the NS5A adaptive mutation S2204I are shown above the genome (arrows). The 5′ NTR, the 3′ NTR, and the EMCV IRES structures are schematically shown.
Several changes were also introduced into the replicons to facilitate subcloning. Specifically, three restriction sites were created: a BsrGI site at nucleotide (nt) 226, an EcoRI site at nt 3286, and an MfeI site at nt 4584 (nucleotide numbering starts from the first codon for NS3). Although these changes did not alter amino acid coding sequences, they may play some role in replication and/or translation efficiency. Furthermore, an A-to-T transversion was made at the −44 position of the H77 3′ NTR to maintain the proper T-A base pairing between nt −44 and −3 and thereby the putative secondary structure within the 3′ X tail (15, 16).
Subgenomic replicons pH77 and pH77S/I, representing the wild-type H77 and an H77 replicon with an adaptive mutation in NS5A, respectively, were unable to support stable replication after RNA electroporation into Huh7 cells with selection with 0.5 mg of G418/ml. In contrast, a parallel electroporation using wild-type 1b replicon BB7 RNA resulted in high transduction efficiency, with >10,000 colonies per 5 μg of RNA.
Previously, the N-terminal region from type 1a NS3 was shown to decrease translation in a transient assay when placed downstream of the EMCV IRES (7). To evaluate whether this 1a sequence precluded the ability to select for stable 1a replicons in our study, two additional type 1a chimera replicons, pH771b75 and pH771b75S/I, encoding the first 75 amino acids of type 1b NS3 (strain BB7) were generated. Electroporation of these hybrid replicons into Huh7 cells resulted in enhanced protein synthesis 4 h posttransfection, compared to that with the wild-type 1a replicon RNA control (data not shown). Attempts to select for stable replication of pH771b75, in the presence of G418, failed to result in colony formation. However, pH771b75S/I readily formed stable colonies, albeit at a 1,000-fold lower frequency than wild-type 1b replicon RNA, around 10 to 50 colonies per 5 μg of RNA.
Replicon RNA from pH77S/I and pH771b75S/I was evaluated for translational efficiency in Huh7 cell extracts by using the system of Grassman et al. (6). As expected, pH77S/I exhibited approximately a four- to fivefold-lower rate of translation than pH771b75S/I (Fig. 2).
FIG. 2.
Rate of translation of H77 and H771B75 replicon RNAs. The translation assay was carried out as described by Grassmann et al. (6) by using the full-length replicon RNAs and cytoplasmic S10 extracts of Huh7 cells. The positions of the major translation products, i.e., of the neomycin phosphotransferase and of NS3, are indicated. The rate of translation of the NS3 protein was determined by phosphorimage analysis of the protein band from three independent experiments, after normalization for the translation of neomycin. Lanes are labeled at the top of the gel. C, control lysate, no RNA.
H77 replicon validation.
pH771b75S/I cells (clone 1 cells) showed sensitivity to trypsin treatment and cell passage. Many clonal cells failed to survive the initial cell passage from the cloning cylinder. Nevertheless, several clonal colonies were successfully amplified with Huh7 cell-conditioned media and once established had a doubling time of around 30 h. Cell viability and replicon RNA copy number remained stable throughout long-term passage. For the work presented herein, several clonal pH771b75S/I-expressing cell lines were evaluated; all showed similar profiles (data not shown). Data are shown for clone 1 cells.
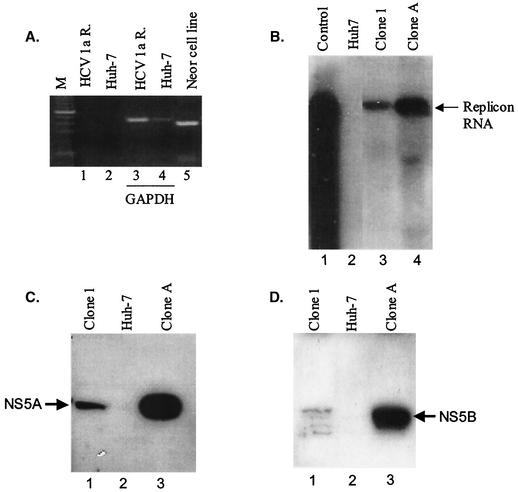
That authenticity of clone 1 cells to stably express the HCV replicon was first confirmed by showing the absence of neomycin resistance gene DNA integration within the cellular genome (Fig. 3A). Next, the presence of full-length subgenomic RNA was analyzed by Northern blotting. As expected, an 8-kb RNA transcript was identified (Fig. 3B). Notably, the abundance of the replicon RNA was considerably lower than that found previously with the type 1b replicon (clone A cells). Clone A cells constitute a cloned-replicon cell line generated from pHCVrep1b and have RNA copy numbers similar to those of other reported replicon cells. Western blot and indirect immunofluorescence assays were utilized to confirm the presence of NS5A and NS5B proteins in total-cell lysates. Clearly, both NS5A and NS5B proteins were expressed in clone 1 replicon cells and were absent from naive parental Huh7 cell extracts (Fig. 3C and D). A single polyprotein product with cross-reactivity to antipeptide sera specific for NS5A was detected; however, the hyperphosphorylation state of this protein was not assessed. Use of antisera against NS5B resulted in the detection of multiple bands on a Western blot, most likely due to protein stability (Fig. 3D). The relative levels of NS5A and NS5B proteins were approximately 5- to 10-fold lower in the clone 1 replicon cells than in clone A cells, and this may account for the differences in replication efficiency and replicon RNA copy number among cell lines. The antisera reacting with purified type 1a and type 1b proteins had similar efficiencies.
FIG. 3.
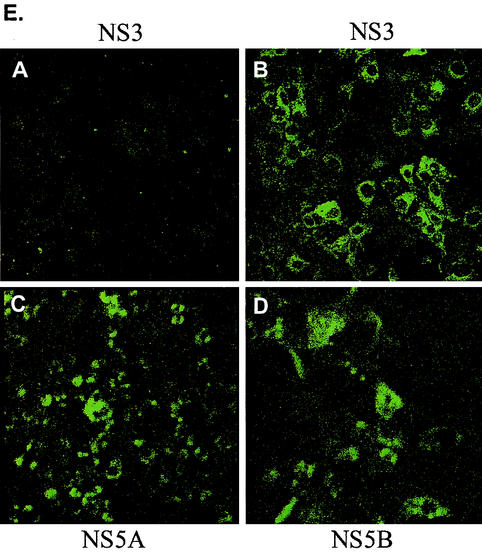
Replication of a chimeric H77 type 1a subgenomic replicon in Huh7 cells. (A) PCR analysis of the chimeric type 1a replicon cell genomic DNA. Genomic DNA was isolated from chimeric type 1a replicon cells (lanes 1 and 3), parental Huh7 cells (lanes 2 and 4), and a G418-resistant cell line (lane 5) and was used in PCRs with primer pairs that amplify a 380-bp neoR fragment (lanes 1, 2, and 5) or primer pairs that amplify a 400-bp GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene fragment (lanes 3 and 4). (B) Northern blot analysis of total RNA extracted from chimeric type 1a replicon cells. Total RNA (5 μg) was electrophoresed through denaturing formaldehyde agarose gel. The blot was probed with in vitro-transcribed replicon RNA. Lane 1, in vitro-transcribed chimeric replicon RNA; lane 2, RNA from parental Huh7 cells; lane 3, RNA from chimeric type 1a replicon cells; lane 4, RNA from type 1b replicon cells. (C and D) Western blot analysis of NS protein expression in chimeric type 1a replicon cells. Chimeric type 1a cells (lanes 1), parental Huh7 cells (lanes 2), and type 1b replicon cells (lanes 3) (104 cells each) were lysed and separated by SDS-polyacrylamide gel electrophoresis, and the blot was probed for NS5A and NS5B proteins by using antibodies against NS5A and NS5B peptides. (E) Indirect immunofluorescence detection of NS3 (section B), NS5A (section C), and NS5B (section D) proteins in chimeric type 1a replicons. Section A shows NS3 staining for parental Huh7 cells. Similar backgrounds were seen with NS5A and NS5B antibodies.
Consistent with the Western blot data, perinuclear expression of the nonstructural proteins NS3, NS5A, and NS5B was confirmed by indirect immunofluorescence (Fig. 3E). Although qualitative, the relative fluorescence signals were lower than those from wild-type 1b replicon cells (data not shown).
Replication dynamics.
To begin to understand the kinetics of RNA synthesis in the hybrid H77 replicon system, the positive-strand viral RNA copy number was analyzed by using TaqMan. Specifically, clone 1 cells contained approximately 150 replicon RNA copies per cell (Table 1). Consistent with this result, other clonal cell lines containing pH771b75S/I and a pooled cell line from approximately 50 pH771b75S/I-expressing colonies were found to have similar viral RNA levels (data not shown). Although type 1b replicon cells can have variability in copy number between systems (2, 13), most type 1b clones reported to date generally appear capable of supporting higher levels of viral RNA than the type 1a replicon cells reported herein. Furthermore, this difference cannot be attributed to the use of different parental Huh7 sources since the clone 1 cells were generated by using the same parental cell line as that used for clone A cells.
TABLE 1.
Replicon RNA copy numbers per cell
| Replicon clone | cpca for:
|
+/− ratiob | |
|---|---|---|---|
| + strand | − strand | ||
| A (BB7) | 1,498 | 39 | 39 |
| 1 (pH771b75S/I) | 153 | 6 | 26 |
| 2 (pBB71a5B) | 150 | NDc | ND |
cpc, copy numbers per cell.
+/− ratio, ratio of + strand cpc to − strand cpc.
ND, not determined.
An additional stable clonal replicon, the type 1b replicon expressing type 1a NS5B (strain H77), was also generated (clone 2 cells) from pBB71a5B. The colony-forming efficiency for this construct was similar to that for pH771b75S/I. No phenotypic differences between clone 1 or clone 2 cell lines were apparent (data not shown). Moreover, consistent with the observation of low viral RNA levels expressed in clone 1 cells, clone 2 cells also contained approximately a 10-fold-smaller amount of positive-strand RNA than cells with type 1b replicons (Table 1). Thus, an overall lower replication and/or translation efficiency for type 1a sequences may account for this differential. Similar to those in clone A cells, viral RNA levels in clone 1 and clone 2 cells decrease severalfold upon cellular confluence, suggesting similar relationships with respect to cell density or cell cycling parameters in the control of viral RNA replication (data not shown).
Based on liver tissue samples, negative-strand HCV RNA levels are often 10- to 100-fold lower than positive-strand RNA levels (4). To assess whether the same differential in HCV RNA is conserved with the HCV replicon system, the ratio of positive to negative message was determined by using a negative-strand-specific TaqMan assay. Clone 1 cells contained an average of 6 copies of negative-strand replicon RNA per cell compared to 40 copies per cell for clone A cells (Table 1). However, the ratios of positive-strand RNA to negative-strand RNA for all replicons were similar, 26 for clone 1 cells and 39 for clone A cells, and were consistent with the reduced efficiency of replication for type 1a.
Adaptation mutations.
Adaptive mutations in replicons are thought to be required for efficient type 1b replicon replication (2, 11, 12), although the HCV-N subgenomic replicon contained no such mutations (9). The inability to select for replication of type 1a sequences in the absence of an adaptive mutation (pH77 and pH771b75) suggests that type 1a replicon sequences may be less fit to establish or support replication in the subgenomic context. In fact, testing with pH77S/I RNA, pH771b75S/I RNA, and pBB71a5B RNA confirmed that only RNA from the last two were capable of forming negative-strand RNA after transient transfection (data not shown).
Evaluation of replicon sequences from clone 1 cells for additional adaptive mutations resulted in the identification of a total of four residue changes across NS3, NS5A, and NS5B (Table 2). No nucleotide changes were present within the NS4A or NS4B coding region. The two mutations found within NS3 (S1358P and K1609E) were reflected in the coding sequences of all plasmid clones examined, whereas the E1986V mutation within NS5A and the V2599I change within NS5B were each reflected in the coding sequences of 9 of 17 plasmid clones. All nine plasmid clones encoding the E1986V change also encoded the V2599I mutation. Last, two silent mutations within the NS5B coding sequence, a C to A at nt 5134 and a G-to-A conversion at nt 5554 (starting from the NS3 coding sequence), were present in all plasmid clones. Examination of replicon sequences from clone 2 cells showed one conserved mutation (A2768S) in the type 1a NS5B (Table 2). No additional changes were found across the entire 1b nonstructural coding sequence.
TABLE 2.
Conserved mutations in H77 type 1a chimeric replicons
| Clone and protein | Nucleotide change(s)a | Amino acid change(s)b |
|---|---|---|
| 1 (pH771b75S/I) | ||
| NS3 | T995C; A1747G | S1358P (9/9); K1609E (9/9) |
| NS5A | A2879T | E1986V (10/18) |
| NS5B | G4718A; G5554A; C5134A | V2599I (9/17); silent; silent |
| 2 (pBB71a5B) | ||
| NS5B | G5224T | A2768S |
Numbering starts from the first nucleotide of the NS3 gene.
Number starts from the beginning of the HCV polyprotein. Changes E1986V and V2599I were consistently identified together on the same clone. Ratios of the numbers of clones in which changes were found to the numbers of clones tested are in parentheses.
Thus, conserved mutations indeed occurred and may have directly contributed to the stability of replication, although it was unclear which mutation or combination of mutations was responsible for augmenting RNA translation or replication. Several replicon RNA constructs were generated in pH771b75, each individually encoding one of the mutations identified in the clone 1 cells, namely, K1609E, E1986V, or V2599I. In repeated electroporation experiments, no colonies formed from any of these RNAs, while RNA from pH771b75S/I generated colonies.
Drug discovery applications.
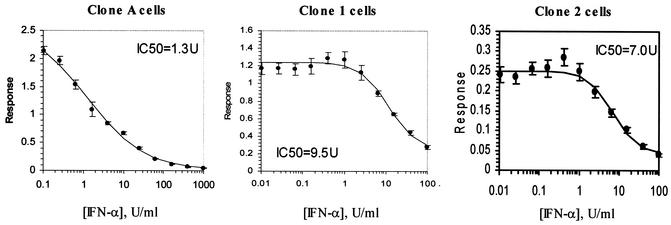
To study the utility of the type 1a replicon cells in antiviral assays, the susceptibility to alpha interferon (IFN-α) was first examined. Replicon cells were treated with IFN-α at various concentrations for 40 h to determine a 50% inhibitory concentration (IC50). As with clone A, the type 1a replicon cells retained sensitivity to IFN-α in a dose-dependent manner; however, a sevenfold difference in IC50 between replicons was apparent, with an IC50 of 9.5 ± 1.5 U/ml for clone 1 cells and 1.3 ± 0.18 U/ml for clone A cells (Fig. 4). Furthermore, testing with clone 2 cells showed an IC50 of 7.0 ± 1.7 U/ml. Together, these data indicate that replicons expressing type 1a RNA-dependent RNA polymerase are less sensitive to IFN-α, although cell passage or growth characteristics may also partially influence susceptibility. Interestingly, similar decreases in susceptibility to IFN-β for clone 1 and clone 2 were apparent, while comparable susceptibilities to IFN-γ for type 1b and the hybrid 1a replicon cells were observed.
FIG. 4.
IFN response curves for type 1b replicon (clone A) and chimeric type 1a cells (clone 1 and clone 2 cells). IFN-α was titrated by using a threefold serial dilution, and cells were treated for 48 h before RNA harvest. RNA levels were quantified by Taqman analysis, and data represent averages from four experiments. y axis, relative response of viral RNA normalized to cellular RNA.
DISCUSSION
In this report, it is shown that chimeric subgenomic replicons between HCV type 1a (strain H77) and type 1b (strain Con1) sequences are competent to replicate in Huh7 cells, albeit with reduced colony formation efficiency and viral RNA levels. Similar to what was found for type 1b replicons (2, 12), mutations were identified in the 1a replicon sequences upon replication in Huh7 cells. Our results extended the studies on two type 1b isolates, namely, HCV-N (9) and HCV Con1 (13), and indicate that sequences beyond those of the two reported clinical isolates can support replication in cell culture.
The demonstration of replication-competent replicons containing 5′ NTR sequences from type 1b and nonstructural genes (except for sequences encoding the first 75 residues of NS3) from type 1a indicate that functional interactions between viral termini and nonstructural proteins encoded by sequences from different genotypes can occur in Huh7 cells. The 98% sequence identity of 5′ NTRs between type 1a and type 1b genomes and the 80% sequence identity within the nonstructural genes may facilitate this interaction. However, the interaction of nonstructural genes and viral NTRs from different genotypes may be suboptimal, and this may partially account for the lower replication efficiency within these type 1a replicons. This result is in agreement with the in vivo studies using genotype 1b (strain J4) cDNA which contained the 5′ and 3′ untranslated regions of a genotype 1a strain (18). The infectivity of three full-length cDNA clones was tested by direct injection of RNA transcripts into the liver of a chimpanzee, and only one clone was found to be infectious. Interestingly, unlike the functionality of the 5′ NTR across genotypes, we found that the Con1 3′ NTR could not substitute for the type 1a H77 3′ NTR even though the sequences are greater than 86% identical (data not shown).
Our data and those of Guo et al. (7) indicate that the DNA sequence encoding the type 1b NS3 N-terminal 75 amino acids had an effect on the translation of the nonstructural proteins. However, since translation and replication are linked for bovine viral diarrhea virus and likely HCV (6), we cannot rule out the possibility that there is also a direct effect on replicase activity that contributes to the enhanced ability to select for stable replicons when the type 1b sequence is present.
Similar to what was found for type 1b replicons, conserved mutations occur within type 1a sequences. Interestingly, of the two mutations identified within NS3, both were located in the helicase domain, a preferred site for adaptive change (9, 12). In particular, the K1609E mutation was also found to occur in two independent Con 1 replicons (12). This mutation directly enhanced colony-forming efficiency fivefold and conferred additive effects on replication efficiency when combined with other NS3 mutations. Since this particular amino acid change was observed in several independent laboratories, it will be interesting to test whether K1609E in NS3 modulates helicase activity in in vitro assays. The other mutation found within NS3, S1358P, represents a change from the type 1a to the type 1b coding sequence for that position (amino acid 1358 is proline in Con1).
No mutations were detected in NS4A and NS4B. An E1986V change in NS5A and a V2599I mutation in NS5B were found in one-half of the subclones. Interestingly, all clones containing the change in NS5A also contained the corresponding mutation in NS5B, indicating a potential interaction between these proteins. Recently, NS5A was shown to locate in the endoplasmic reticulum membrane through an amphipathic alpha-helix anchor formed by the N-terminal 30 amino acids (3). Since the E1986V mutation occurred on the hydrophilic side of the amphipathic helix facing the cytoplasm, membrane association of NS5A was not likely affected.
Last, susceptibility testing with IFN-α and IFN-β, but not IFN-γ, showed distinct differences between type 1b and type 1a replicon cells. Additionally, a type 1b replicon expressing a type 1a NS5B also showed elevated IC50s for IFN-α and IFN β, therefore suggesting that susceptibility may be partially associated with replicon RNA levels, viral replication rate, or other cell-specific characteristics which are as yet undefined. Additional studies to assess this phenotype are ongoing. Although the replicons described in this paper were type 1a-type 1b chimeras, antiviral compounds against type 1a HCV NS5B can now be tested in a cell-based system.
ADDENDUM IN PROOF
While this article was being reviewed, a type 1a H77 replicon was described, using a highly permissive Huh7 cell line obtained by curing replicon cells with IFN-α treatment. Our results support the findings of Blight et al. (K. J. Blight, J. A. McKeating, J. Marcotrigiano, and C. M. Rice, J. Virol. 77: 3181-3190, 2003) and extend the type 1a replicons in regular Huh7 cells.
Acknowledgments
We thank C. Rice and J. Bukh for reagents and fellow members of the Virology Department at GSK Upper Providence for helpful discussions. We thank J.-T. Guo in Christoph Seeger's laboratory for expert help with Northern blot analysis.
REFERENCES
- 1.Alter, H. J., and L. B. Seeff. 2000. Recovery, persistence and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin. Liver Dis. 20:17-35. [DOI] [PubMed] [Google Scholar]
- 2.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. [DOI] [PubMed] [Google Scholar]
- 3.Brass, V., E. Bieck, R. Montserret, V. Wolk, J. A. Hellings, H. E. Blum, F. Penin, and D. Moradpour. 2002. An amino-terminal amphipathic alpha helix mediates membrane association of the hepatitis C virus nonstructural protein 5A. J. Biol. Chem. 277:8130-8139. [DOI] [PubMed] [Google Scholar]
- 4.Craggs, J., J. Ball, B. Thomson, W. Irving, and A. Grabowska. 2001. Development of a strand-specific RT-PCR based assay to detect the replicative form of hepatitis C virus RNA. J. Virol. Methods 94:111-120. [DOI] [PubMed] [Google Scholar]
- 5.Dhanak, D., K. Duffy, V. K. Johnston, J. Lin-Goerke, M. Darcy, A. N. G. B. Shaw, C. Silverman, A. T. Gates, D. L. Earnshaw, D. J. Casper, A. Kaura, A. Baker, C. Greenwood, L. L. Gutshall, D. Maley, A. DelVecchio, R. Macarron, G. A. Hofmann, Z. Alnoah, H.-Y. Cheng, G. Chan, S. Khandekar, R. M. Keenan, and R. T. Sarisky. 2002. Identification and biological characterization of heterocyclic inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 277:38322-38327. [DOI] [PubMed] [Google Scholar]
- 6.Grassmann, C. W., O. Isken, and S.-E. Behrens. 1999. Assignment of the multifunctional NS3 protein of bovine viral diarrhea virus during RNA replication: an in vivo and in vitro study. J. Virol. 73:9196-9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo, J., V. Bichko, and C. Seeger. 2001. Effect of alpha interferon on the hepatitis C virus replicon. J. Virol. 75:8516-8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houghton, M. 1996. Hepatitis C viruses, p. 1035-1058. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 9.Ikeda, M., M. Yi, K. Li, and S. M. Lemon. 2002. Selectable subgenomic and genome-length dicistronic RNAs derived from an infectious molecular clone of the HCV-N strain of hepatitis C virus replicate efficiently in cultured Huh7 cells. J. Virol. 76:2997-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kishine, H., K. Sugiyama, M. Hijikata, N. Kata, H. Takahashi, T. Noshi, Y. Nio, M. Hosaka, Y. Miyanari, and K. Shimotohno. 2002. Subgenomic replicon derived from a cell line infected with the hepatitis C virus. Biochem. Biophys. Res. Commun. 293:993-999. [DOI] [PubMed] [Google Scholar]
- 11.Krieger, N., V. Lohmann, and R. Bartenschlager. 2001. Enhancement of hepatitis C virus RNA replication by cell culture-adaptive mutations. J. Virol. 75:4614-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohmann, V., F. Korner, A. Dobierzewska, and R. Bartenschlager. 2001. Mutations in hepatitis C virus RNAs conferring cell culture adaptation. J. Virol. 75:1437-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohmann, V., F. Korner, J.-O. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 14.Rice, C. M. 1996. Flaviviridae: the viruses and their replication, p. 1035-1058. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 15.Tanaka, T., N. Kato, M. J. Cho, and K. Shimotohno. 1995. A novel sequence found at the 3′ terminus of hepatitis C virus genome. Biochem. Biophys. Res. Commun. 215:744-749. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka, T., N. Kato, M. J. Cho, K. Sugiyama, and K. Shimotohno. 1996. Structure of the 3′ terminus of the hepatitis C virus genome. J. Virol. 70:3307-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanagi, M., R. Purcell, S. Emerson, and J. Bukh. 1997. Transcripts from a single full length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc. Natl. Acad. Sci. USA 94:8738-8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanagi, M., M. St. Claire, M. Shapiro, S. Emerson, R. Purcell, and J. Bukh. 1998. Transcripts of a chimeric cDNA clone of hepatitis C virus genotype 1b are infectious in vivo. Virology 244:161-172. [DOI] [PubMed] [Google Scholar]
- 19.Yanagi, M., M. St. Claire, S. U. Emerson, R. H. Purcell, and J. Bukh. 1999. In vivo analysis of the 3′ untranslated region of the hepatitis C virus after in vivo mutagenesis of an infectious cDNA clone. Proc. Natl. Acad. Sci. USA 96:2291-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]