Abstract
ATP-binding cassette transporters from several rhizobia and Salmonella enterica serovar Typhimurium, but not secondarily coupled systems, were inhibited by high concentrations (100 to 500 mM) of various osmolytes, an effect reversed by the removal of the osmolyte. ABC systems were also inactivated in isolated pea bacteroids, probably due to the obligatory use of high-osmolarity isolation media. Measurement of nutrient cycling in isolated pea bacteroids is impeded by this effect.
Amino acid uptake by Rhizobium leguminosarum is dominated by two ABC transporters, the general amino acid permease (Aap) and the branched-chain amino acid permease (Bra) (9, 21). These systems are essential for productive nitrogen fixation in pea nodules and have been proposed to participate in an amino acid cycle between the bacteroid and the plant (14). This was demonstrated by isolating double mutants with mutations in the aap/bra genes which resulted in yellow plants with dry weights similar to those of uninoculated controls grown on nitrogen-free medium. While investigating the effect of the osmotic strength of the medium on rates of transport, we noted a rapid inhibition of uptake by ABC (including Aap/Bra) but not other, non-ABC transporters, which has important implications for experiments on legume nodule physiology. Furthermore, the effect appears to be general for rhizobia and other gram-negative bacteria.
Uptake by ABC transporters.
Rhizobium leguminosarum bv. viciae strains 3841 (12) and A34 (6), Sinorhizobium meliloti 1021 (16), and Mesorhizobium loti 303099 (13) were grown overnight in acid minimal salts (AMS) (17) with glucose (10 mM) and NH4 (10 mM), washed in minimal salts, and prepared for transport assays by the rapid filtration method as previously described (18). The only change was that for S. meliloti the concentration of EDTA in AMS was lowered from 40 μΜ to 1 μΜ. Salmonella enterica serovar Typhimurium strain NCIMB 10249 was grown overnight on LB with IPTG (isopropyl-β-d-thiogalactopyranoside; 40 μg/ml) and tetracycline (10 μg/ml), and the next morning the culture was diluted 100× in fresh LB medium with IPTG (40 μg/ml) and tetracycline (10 μg/ml) and grown to an optical density at 600 nm of 0.4 to 0.6 before being harvested as described for rhizobial strains.
Ten seconds before 14C-labeled compounds (0.125 μCi at 25 μM) were introduced to cells of R. leguminosarum 3841, sucrose was added up to a final concentration of 500 mM (Fig. 1A). 2-Aminoisobutyric acid (AIB) was initially chosen as the solute to be transported as it is not metabolized by Rhizobium leguminosarum 3841. Previous work has shown that only Aap and Bra transport AIB at significant rates under these assay conditions (9, 21).
FIG. 1.
Uptake of AIB by R. leguminosarum exposed to different concentrations of various osmolytes. A. AIB uptake in cells exposed to increasing concentrations of sucrose (circles), NaCl (triangles), or mannitol (squares) immediately before assay. B. AIB uptake in cells exposed to 200 mM of the compound indicated on the x axis immediately before assay. Abbreviations: Suc, sucrose; Man, mannitol; Gly, glycerol; Glc, glucose; PEG 200, polyethylene glycol 200. Results are the mean uptake per minute over 4 minutes of at least three independent replicates shown plus and minus the standard error of the mean.
AIB uptake was decreased by 88% on the addition of 200 mM sucrose 10 seconds before the start of the assay. NaCl and mannitol were added in 100 mM increments 10 seconds before the assay instead of sucrose; mannitol caused an 86% decrease at 200 mM, while NaCl showed a complete inhibition at the same concentration (Fig. 1A). As 200 mM appeared to be the minimum concentration required for an inhibition of 80% or more, other osmolytes (potassium chloride, glycerol, glucose, and polyethylene glycol 200) were tested in the same manner (Fig. 1B). All of the compounds tested caused a decrease of at least 70% in AIB uptake when added at 200 mM 10 seconds before assays began. Prolonged incubation or overnight growth in the presence of sucrose (100 mM) resulted in even greater inhibition than when an agent was added 10 seconds before the start of an assay (data not shown).
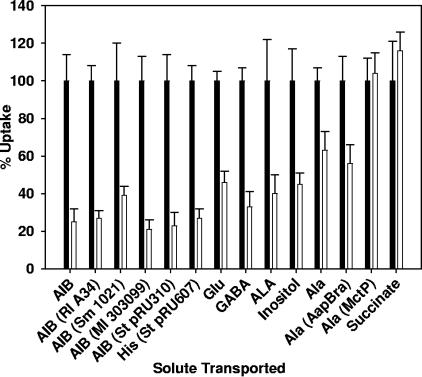
As a wide range of compounds of different sizes and charges were tested, it is unlikely that this effect is solute specific. It is also unlikely that so many different compounds would cause direct transport inhibition either by blocking the membrane components of the permeases or by binding to solute binding proteins (SBPs). As mentioned above, AIB is known to be transported into R. leguminosarum by only Aap and Bra, and therefore other solutes that utilize these two systems were tested. Glutamate is transported via Aap and Bra, while γ-amino-n-butyric acid is transported into cells only via Bra (9). These compounds show a pattern of inhibition by high osmolarity similar to that observed for AIB (Fig. 2).
FIG. 2.
Inhibition of uptake of different solutes by 200 mM sucrose in R. leguminosarum. Uptake rates in the presence of sucrose (200 mM) (open bars) are given as a percentage compared to control cultures (solid black bars). Strain 3841 was used except where indicated: Rl A34, R. leguminosarum A34; Sm 1021, S. meliloti 1021; Ml 303099, M. loti 303099; St pRU310, S. enterica serovar Typhimurium NCIMB 10249 containing plasmid pRU310 (aapJQMP); St pRU607, Salmonella enterica serovar Typhimurium NCIMB 10249 containing pRU607 (hisJQMP). Abbreviations: His, histidine; Ala (MctP), alanine uptake (500 μM) via MctP in strain RU1722; Ala (AapBra), alanine uptake (25 μM) via Aap and Bra in strain RU1180; GABA, γ-amino-n-butyric acid; ALA, δ-aminolevulinic acid; Ala, alanine. Results are the means of at least four independent replicates plus and minus the standard errors of the means.
The investigation was extended to include other ABC and non-ABC systems to determine the range of the effect. Alanine is transported at high affinity into strain 3841 via Aap and Bra and by a low-affinity secondary transporter, the monocarboxylate transport permease (MctP) (11). Strain RU1180 is mutated in MctP, so this was used for measurement of alanine uptake via Aap/Bra. myo-Inositol and δ-aminolevulinic acid are transported into 3841 by ABC systems: Int (8) and Dpp (5), respectively. Overnight cultures of either 3841 or RU1180 were grown in AMS (10 mM glucose, 10 mM NH4), except for the myo-inositol uptake assay, where cultures were grown in AMS (10 mM myo-inositol, 10 mM NH4). The data for high-affinity alanine, myo-inositol, and δ-aminolevulinic acid uptake also show that the addition of 200 mM sucrose 10 seconds before assays significantly reduced uptake (Fig. 2). However, while reduced (37%), the effect on alanine uptake via Aap/Bra was not as great as that for other solutes. Differences in uptake rates between solutes may be due to the number of systems capable of transporting a particular solute and/or the specific binding affinities between each SBP and each membrane transport complex.
Secondary transporters are not inhibited by osmotic upshift.
Strain RU1722, which is mutated in both aap and bra, was used to measure uptake of alanine via the secondarily coupled MctP. As MctP has a Km for alanine of 560 nM (11), the alanine concentration in transport assays was increased from 25 μM to 500 μM. Unlike ABC transport system-mediated uptake, no significant loss of alanine uptake via MctP occurred after osmotic upshift (Fig. 2). To test whether this is a general property of secondary transporters, uptake via the dicarboxylate transport (DCT) system was also investigated in cultures grown overnight in AMS (10 mM succinate, 10 mM NH4) (Fig. 2). No significant loss of uptake of succinate occurred in cells subjected to osmotic upshift.
The inhibition of ABC transporters occurs in a wide range of gram-negative bacteria.
AIB uptake was also inhibited in R. leguminosarum A34 on addition of 200 mM sucrose 10 seconds before assays (Fig. 2), demonstrating that this effect is not specific to strain 3841. Furthermore, AIB uptake by S. meliloti 1021 and M. loti 303099 was also inhibited by the addition of sucrose (Fig. 2). To determine whether this effect is restricted to rhizobia or is a general effect for gram-negative bacteria, transport of AIB and histidine was measured in Salmonella enterica serovar Typhimurium NCIMB 10249. AIB is not transported by Salmonella serovar Typhimurium NCIMB 10249, so the R. leguminosarum 3841 aapJQMP genes were expressed from a lacZ promoter in plasmid pRU310 (10). Just as for strain 3841, AIB uptake via Aap in S. enterica serovar Typhimurium was severely inhibited by sucrose (Fig. 2). To ensure that the inhibition is not specific to rhizobial ABC systems, the native S. enterica serovar Typhimurium histidine transport operon (hisJQMP) was expressed from a lacZ promoter in plasmid pRU607 (10), and once again transport was inhibited (Fig. 2). The His system of S. enterica serovar Typhimurium is the most intensively studied of all bacterial ABC transporters, suggesting that this effect occurs in all gram-negative bacteria.
Inhibition of ABC transporters is reversible.
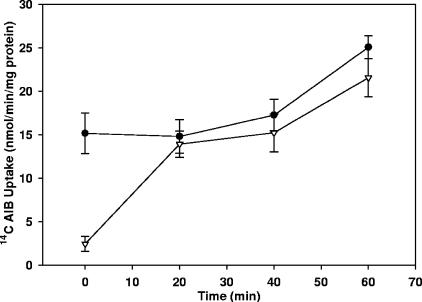
It is possible that the osmotic upshift causes permanent damage to the outer membrane, as occurs in cold osmotic down-shock. However, no detectable periplasmic proteins were released to the medium by osmotic up-shock (data not shown). This suggested that permanent damage does not occur, and this was tested by incubating cells in 200 mM sucrose as described for Fig. 1 but then removing the sucrose by centrifugation. AIB uptake was restored almost immediately when the sucrose was removed, indicating that the inhibition of uptake via ABC systems caused by osmotic upshift is reversible (Fig. 3).
FIG. 3.
Uptake of [14C]AIB by R. leguminosarum after osmotic upshift and subsequent downshift. Open triangles indicate the culture that was exposed to 200 mM sucrose; filled circles indicate the control culture. Osmotic upshift was present only in the 0-min assay and was removed for subsequent assays. Results are the mean uptake rate over 4 min from at least four independent replicates plus and minus the standard error of the mean.
Measurement of transport by bacteroids.
Previous work has shown little amino acid uptake in isolated rhizobial bacteroids via ABC systems, although it is known that these transporters must be expressed and active in nodules (7, 14, 15). The exception to this is the transport and oxidation of glutamate by isolated soybean bacteroids (3, 20). However, while the transport system for glutamate is not characterized in Bradyrhizobium japonicum, the Km is 800 nM, which compares to 81 nM for Aap (18, 20), suggesting that it may be a secondarily coupled system. Isolated pea bacteroids have been incubated in various combinations of amino acids, and while it has been concluded that the addition of glutamate stimulates the secretion of aspartate and alanine, uptake rates for glutamate were not determined (1, 2, 19). Furthermore, the types of intermediates and the rate of their secretion were very variable. These data are consistent with some form of amino acid cycling occurring in bacteroids, but direct measurement of active uptake of amino acids by pea bacteroids is lacking. However, bacteroids have a very fragile outer membrane, and to our knowledge all bacteroid experiments for the last 40 years have been conducted in high-osmolarity medium, typically containing around 300 mM sucrose or mannitol (1, 4, 22). The work described in this paper has shown that these are conditions that selectively inhibit uptake.
Bacteroids of R. leguminosarum strain A34 were isolated anaerobically as previously described (1) and held in phosphate buffer (10 mM, pH 7.2, 2 mM MgCl2) with sucrose (300 mM). Succinate was transported into bacteroids at 38 nmol min−1 mg protein−1, only slightly lower than in free-living cells. However, no glutamate transport, which occurs through Aap and Bra, was detected in any of the bacteroid isolates. From what we now know about the osmotic inhibition of ABC uptake systems, the results with bacteroids are not surprising.
A high osmotic concentration may affect a number of steps in the transport cycle of ABC uptake systems, including binding of solute to the SBP, docking of the SBP to the membrane complex, or possibly the coupling of ATP hydrolysis to solute movement. While this study does not reveal the mechanism behind the inhibition of ABC transporters by high osmolarity, the effect is clear and the implications for understanding rhizobial bacteroid physiology very important. Effectively, all experiments on isolated bacteroids have been done with severe inhibition of ABC uptake systems. Given the demonstration by genetic means that ABC uptake systems are essential for productive nitrogen fixation, this may explain why labeling studies of isolated bacteroids are so variable. Labeling studies with isolated bacteroids will be completely biased toward compounds using secondarily coupled systems for uptake, with the effective “knockout” of all ABC uptake systems. Studies with isolated bacteroids can still be useful if we appreciate this limitation. It emphasizes the need for an integrated approach, where whole-plant studies, as well as nuclear magnetic resonance of nodules and metabolic profiling of fractionated nodule, are combined to understand nodule function.
Acknowledgments
P.S.P. acknowledges financial support from the Biotechnology and Biological Sciences Research Council (United Kingdom).
REFERENCES
- 1.Allaway, D., E. Lodwig, L. A. Crompton, M. Wood, T. R. Parsons, T. Wheeler, and P. S. Poole. 2000. Identification of alanine dehydrogenase and its role in mixed secretion of ammonium and alanine by pea bacteroids. Mol. Microbiol. 36:508-515. [DOI] [PubMed] [Google Scholar]
- 2.Appels, M. A., and H. Haaker. 1991. Glutamate oxaloacetate transaminase in pea root nodules—participation in a malate/aspartate shuttle between plant and bacteroid. Plant Physiol. 95:740-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergersen, F. J., and G. L. Turner. 1988. Glutamate as a carbon source for N2-fixing bacteroids prepared from soybean root nodules. J. Gen. Microbiol. 134:2441-2448. [Google Scholar]
- 4.Bergersen, F. J., and G. L. Turner. 1967. Nitrogen fixation by the bacteroid fraction of breis of soybean root nodules. Biochim. Biophys. Acta 141:507-515. [DOI] [PubMed] [Google Scholar]
- 5.Carter, R. A., K. H. Yeoman, A. Klein, A. H. F. Hosie, G. Sawers, P. S. Poole, and A. W. B. Johnston. 2002. dpp genes of Rhizobium leguminosarum specify uptake of delta-aminolevulinic acid. Mol. Plant-Microbe Interact. 15:69-74. [DOI] [PubMed] [Google Scholar]
- 6.Downie, J. A., Q. S. Ma, C. D. Knight, G. Hombrecher, and A. W. B. Johnston. 1983. Cloning of the symbiotic region of Rhizobium leguminosarum—the nodulation genes are between the nitrogenase genes and a nifA-like gene. EMBO J. 2:947-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dupont, L., I. Garcia, M. C. Poggi, G. Alloing, K. Mandon, and D. Le Rudulier. 2004. The Sinorhizobium meliloti ABC transporter Cho is highly specific for choline and expressed in bacteroids from Medicago sativa nodules. J. Bacteriol. 186:5988-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fry, J., M. Wood, and P. S. Poole. 2001. Investigation of myo-inositol catabolism in Rhizobium leguminosarum bv. viciae and its effect on nodulation competitiveness. Mol. Plant-Microbe Interact. 14:1016-1025. [DOI] [PubMed] [Google Scholar]
- 9.Hosie, A. H. F., D. Allaway, H. A. Dunsby, C. S. Galloway, and P. S. Poole. 2002. Rhizobium leguminosarum has a second general amino acid permease with unusually broad substrate specificity and high similarity to branched-chain amino acid transporters (Bra/LIV) of the ABC family. J. Bacteriol. 184:4071-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosie, A. H. F., D. Allaway, M. A. Jones, D. L. Walshaw, A. W. B. Johnston, and P. S. Poole. 2001. Solute-binding protein-dependent ABC transporters are responsible for solute efflux in addition to solute uptake. Mol. Microbiol. 40:1449-1459. [DOI] [PubMed] [Google Scholar]
- 11.Hosie, A. H. F., D. Allaway, and P. S. Poole. 2002. A monocarboxylate permease of Rhizobium leguminosarum is the first member of a new subfamily of transporters. J. Bacteriol. 184:5436-5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston, A. W. B., and J. E. Beringer. 1975. Identification of the Rhizobium strains in pea root nodules using genetic markers. J. Gen. Microbiol. 87:343-350. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe, K. Idesawa, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, Y. Mochizuki, S. Nakayama, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7:331-338. [DOI] [PubMed] [Google Scholar]
- 14.Lodwig, E. M., A. H. F. Hosie, A. Bourdes, K. Findlay, D. Allaway, R. Karunakaran, J. A. Downie, and P. S. Poole. 2003. Amino-acid cycling drives nitrogen fixation in the legume-Rhizobium symbiosis. Nature 422:722-726. [DOI] [PubMed] [Google Scholar]
- 15.McRae, D. G., R. W. Miller, W. B. Berndt, and K. Joy. 1989. Transport of C4-dicarboxylates and amino acids by Rhizobium meliloti bacteroids. Mol. Plant-Microbe Interact. 2:273-278. [Google Scholar]
- 16.Meade, H. M., S. R. Long, G. B. Ruvkun, S. E. Brown, and F. M. Ausubel. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 149:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole, P. S., A. Blyth, C. J. Reid, and K. Walters. 1994. myo-Inositol catabolism and catabolite regulation in Rhizobium leguminosarum bv viciae. Microbiology 140:2787-2795. [Google Scholar]
- 18.Poole, P. S., M. Franklin, A. R. Glenn, and M. J. Dilworth. 1985. The transport of l-glutamate by Rhizobium leguminosarum involves a common amino acid carrier. J. Gen. Microbiol. 131:1441-1448. [Google Scholar]
- 19.Rosendahl, L., M. J. Dilworth, and A. R. Glenn. 1992. Exchange of metabolites across the peribacteroid membrane in pea root nodules. J. Plant Physiol. 139:635-638. [Google Scholar]
- 20.Udvardi, M. K., C. L. Salom, and D. A. Day. 1988. Transport of l-glutamate across the bacteroid membrane but not the peribacteroid membrane from soybean root nodules. Mol. Plant-Microbe Interact. 1:250-254. [Google Scholar]
- 21.Walshaw, D. L., and P. S. Poole. 1996. The general l-amino acid permease of Rhizobium leguminosarum is an ABC uptake system that influences efflux of solutes. Mol. Microbiol. 21:1239-1252. [DOI] [PubMed] [Google Scholar]
- 22.Waters, J. K., B. L. Hughes, L. C. Purcell, K. O. Gerhardt, T. P. Mawhinney, and D. W. Emerich. 1998. Alanine, not ammonia, is excreted from N2-fixing soybean nodule bacteroids. Proc. Natl. Acad. Sci. USA 95:12038-12042. [DOI] [PMC free article] [PubMed] [Google Scholar]