Abstract
Degradation of the cyanobacterial light-harvesting antenna, the phycobilisome, is a general acclimation response that is observed under various stress conditions. In this study we identified a novel mutant of Synechococcus elongatus PCC 7942 that exhibits impaired phycobilisome degradation specifically during nitrogen starvation, unlike previously described mutants, which exhibit aberrant degradation under nitrogen, sulfur, and phosphorus starvation conditions. The phenotype of the new mutant, AldΩ, results from inactivation of ald (encoding alanine dehydrogenase). AldΩ is deficient in transcription induction of a number of genes during nitrogen starvation. These genes include the “general nutrient stress-related” genes, nblA and nblC, the products of which are essential for phycobilisome degradation. Furthermore, transcripts of several specific nitrogen-responsive genes accumulate at lower levels in AldΩ than in the wild-type strain. In contrast, ald inactivation did not decrease the accumulation of transcripts during sulfur starvation. Transcription of ald is induced upon nitrogen starvation, which is consistent with the ability of wild-type cells to maintain a low cellular content of alanine under these conditions. Unlike wild-type cells, AldΩ accumulates alanine upon nitrogen starvation. Our analyses suggest that alanine dehydrogenase activity is necessary for an adequate cellular response to nitrogen starvation. Decomposition of alanine may be required to provide a sufficient amount of ammonia. Furthermore, the accumulated alanine, or a related metabolite, may interfere with the cues that modulate acclimation during nitrogen starvation. Taken together, our results provide novel information regarding cellular responses to nitrogen starvation and suggest that mechanisms related to nitrogen-specific responses are involved in modulation of a general acclimation process.
Cyanobacteria are photosynthetic prokaryotes which are very abundant in both terrestrial and aquatic habitats and contribute significantly to global carbon fixation. These organisms frequently encounter nutrient starvation and have evolved sophisticated mechanisms that allow them to survive under stress conditions (3, 32, 33, 36). Synechococcus elongatus PCC 7942, a commonly used model cyanobacterium, is nondiazotrophic; that is, it cannot fix dinitrogen. Nevertheless, S. elongatus PCC 7942 and other nondiazotrophic cyanobacteria are capable of surviving prolonged periods of nitrogen starvation (9, 20, 25, 29, 31).
S. elongatus PCC 7942 undergoes substantial morphological, biochemical, and physiological changes in response to nutrient limitation. The acclimation responses may be classified as specific responses and general (or global) responses (33, 34). The former allow efficient acquisition and utilization of the limiting nutrient, while the latter are the responses that occur with each of a number of nutrient starvation conditions or other growth-reducing environmental parameters. These responses help the cells survive the stress by modulating the cellular metabolism to match the ambient conditions. A prominent general acclimation response is degradation of the cyanobacterial light-harvesting complex, the phycobilisome (6, 12, 33). Modulation of light harvesting during nutrient limitation is essential to minimize surplus excitation of the photosynthetic apparatus and to prevent the possible oxidative damage resulting from such excitation. Several components essential for degradation of phycobilisomes during nutrient starvation have been identified using mutants in which this process is impaired (5, 8, 34, 35, 39). These mutants (designated nonbleaching [nbl] mutants) retain their blue-green pigmentation during starvation, unlike wild-type cells, which appear yellowish or bleached. Two components of the nbl pathway, NblA (5) and NblB (8), are specifically involved in the degradation process. The genes for these components are induced upon salt stress (23), emphasizing the role of the nbl pathway as a general response mechanism. Additionally, three regulatory components have been found; NblS (39) and NblR (34) are apparently a sensor and a response regulator, respectively, of a two-component signal transduction pathway, whereas NblC (35) is an apparent anti-sigma factor. The regulatory components are required for induction of nblA upon nutrient starvation. This gene encodes a polypeptide with a molecular mass of about 6 kDa, which is a key component in the degradation process. A recent study determined the crystal structure of NblA of Anabaena sp. strain PCC 7120 and revealed interactions of NblA with specific regions of the α subunits of phycocyanin and phycoerthrocyanin, which are apoproteins of the phycobilisome (4). Information gleaned from this study allowed a great step forward in our understanding of NblA function; however, the detailed mechanisms of phycobilisome degradation and the involvement of NblA in this process have not been determined yet.
The signal that triggers phycobilisome degradation in response to nutrient starvation is obscure. Principally, cells may respond directly to the decreasing level of the nutrient in their surroundings. Alternatively, the initial physiological or metabolic consequences of starvation may elicit the responses that allow cells to cope with the stress conditions. An example of the latter type of regulation is the modulation of the global regulators of nitrogen metabolism, PII and NtcA, by 2-oxoglutarate (11, 26, 33).
In this study we identified a novel mutant in which phycobilisome degradation was impaired only during nitrogen starvation. This phenotype, which is a result of inactivation of ald, the gene encoding alanine dehydrogenase (Ald), is different from the phenotype of mutants with mutations in the nbl pathway, which is expressed as aberrant phycobilisome degradation under nitrogen, sulfur, and phosphorus starvation conditions. Our comparative analyses of the ald mutant and wild-type strains suggested that mechanisms that control nitrogen-specific responses are involved in the modulation of an apparent general acclimation response. Additionally, this study provided further insight into modulation of nitrogen starvation responses by the metabolic status of the cell.
MATERIALS AND METHODS
Strains, culture conditions, and isolation of mutants with impaired phycobilisome degradation.
S. elongatus PCC 7942 and all strains resulting from molecular manipulations of this wild-type strain were cultured as previously described (34). Nonbleaching mutants were isolated as described elsewhere (27). Briefly, S. elongatus PCC 7942 cells were mutagenized by transformation with a transposon-based inactivation library. The library was obtained by in vitro transposition of a genomic library using an EZ::TN KAN-2 insertion kit (Epicenter). Following growth of transformants in liquid growth medium in the presence of 25 μg/ml kanamycin, the cultures were starved for either sulfur or nitrogen, and cells exhibiting relatively high fluorescence were selected by fluorescence-activated cell sorting and plated. A second screen for nonbleaching phenotypes was performed by restreaking colonies on solid medium to which no sulfur or nitrogen source had been added. Genomic DNA of nonbleaching strains was analyzed by PCR to identify clones that had unaltered copies of the known genes of the nbl pathway. The following sets of primers were used to detect these genes: for nblA, ACCCGAGGGGGATCTGTG and ATCGCTGGGTCAACAACG; for nblB, ATGACTCCGGCGGAAATA and CTTCTAGACCATCGCCCC; for nblC, TTGACGCCGATGTATCTG and TAGCCACAATACCTGCTG; for nblR, CTGCGGAGTGCTATTCAG and GAATCGGGAACCCATGAC; and for nblS, GTTGCTGTAATCGCCTCA and CTAGAATGGCACGATGCA.
The mutants were created by random transposon insertion, and therefore, an inactivated gene was expected to yield a PCR fragment whose molecular weight was higher than that of its wild-type homolog. Mutant M7 contained unaltered copies of the previously identified genes of the nbl pathway, as PCR products that were identical sizes were produced from genomic DNA of the wild type and the mutant (not shown).
Cloning of the genomic fragment bearing the transposon.
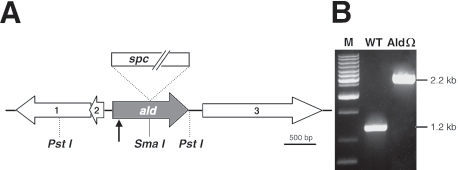
The transposon insertion site in mutant M7 was identified essentially as described previously for the NblC mutant (35). Briefly, genomic DNA was digested with PstI and ligated to pBluescript KS. Transformants of Escherichia coli DH5α were selected in the presence of kanamycin; only the desired clones were resistant to the antibiotic due to nptII included in the transposon. The sequence of the genomic regions around the transposon was determined using the transposon-specific primers provided with the EZ::TN insertion kit. The transposon was found to be inserted into ald at codon 31 (Fig. 1A).
FIG. 1.
(A) Physical map of the genomic region bearing ald in S. elongatus PCC 7942 (encoding alanine dehydrogenase). PstI, restriction sites used for cloning the relevant genomic region. The SmaI site was used for insertional inactivation of ald by a spectinomycin (spc) cassette. The arrow indicates the transposon insertion site in the original mutant, M7. The numbers indicate open reading frames neighboring ald, as follows: 1, predicted open reading frame (containing Fe-S cluster); 2, high-light-inducible protein-related open reading frame; 3, hypothetical protein open reading frame. (B) PCR analysis confirming complete chromosome segregation in the alanine dehydrogenase mutant AldΩ. Lane WT, S. elongatus PCC 7942; lane M, molecular weight markers. See Materials and Methods for details.
Insertional inactivation of ald and complementation of the ald mutant.
A DNA fragment containing ald and its flanking regions was obtained by PCR performed with genomic DNA of S. elongatus PCC 7942 and primers TGCCGCGTGAGCTCAAGGAT and CCTGAAAGGGTAGGGGCGAT. This fragment was cloned into the pGEM-T Easy vector (Promega), and a spectinomycin resistance cassette was inserted into the SmaI site. The resulting construct was transformed into S. elongatus PCC 7942, and clones resistant to spectinomycin (25 μg/ml) were selected. PCR with genomic DNA of one transformant, AldΩ, confirmed that there was double homologous recombination and complete chromosome segregation (Fig. 1B). A physical map of the inactivated ald gene and the neighboring genomic region is shown in Fig. 1A.
Cloning of ald in a neutral site was performed essentially as described earlier for nblC (35). A DNA fragment (1,874 bp) containing ald and its putative promoter region was obtained by PCR performed with genomic DNA of S. elongatus PCC 7942 and the primers ATCGCAGGCCATGGCACAAC and CCTGAAAGGGTAGGGGCGAT.
Nucleic acid isolation, Northern analysis, β-glucoronidase assay, and spectral measurements.
RNA was isolated as previously described (38). A riboprobe for nblA was made by in vitro transcription using MEGAscript T7 (Ambion).
Double-stranded DNA probes for nblA and nblC for Northern hybridization were obtained by performing PCR with the primers mentioned above for analysis of these genes in nonbleaching mutants, whereas the probe for ald was made by using the primers used for inactivation of the gene (see above). Additional fragments used as probes were made by performing PCR with the following primers: TGCTTTCACCAAGGTGGTGG and GCAACTGCAGCAGCAGCTTT for cpcB; CATCAATGGCTCGGGCAA and TCTTTGAGCACCGGCAGA for glnN; TCTGCCTGAAGATACGCTGA and GAGGTTCATCGTCGCCAGCA for icd; CCTGGCTTAAGGAGAGTTCC and CGACGCTGACTTAGATTGCG for glnB; TTCGCTTCGATCAGGCTGAG and GGCTGATCGGAATCACTTCG for glnA; and GCCAAAAGGCGTTCCTAG and CGGGACCAGTTTCAACCG for rhdA.
To determine transcript abundance, scanned autoradiographs were subjected to image analysis (by ImageJ), and signals were normalized to rRNA. For accurate quantitation, we selected autoradiographs with subsaturating signals to avoid the limitations of the film chemistry.
Fusion of nblA promoter to β-glucoronidase as a reporter gene has been described previously (34). The β-glucoronidase assay was performed essentially as described by Jefferson et al. (16).
Absorbance spectra were obtained for cultures diluted with growth medium to obtain an optical density at 750 nm of 0.01. Spectra were obtained with a Cary 100 spectrophotometer equipped with a Labsphere DRA-CA-30I diffuse reflectance accessory. Phycocyanin quantitation was based on absorbance maxima at 620 nm.
Analysis of the alanine level.
Free amino acids were extracted from frozen cyanobacterial cell pellets with 80% ethanol and were assayed by high-performance liquid chromatography after derivatization with o-phthaldialdehyde, as described by Hagemann et al. (13).
The data below are from a single representative experiment, and at least three independent biological repetitions were performed.
RESULTS AND DISCUSSION
Alanine dehydrogenase is required for efficient degradation of phycobilisomes only during nitrogen starvation.
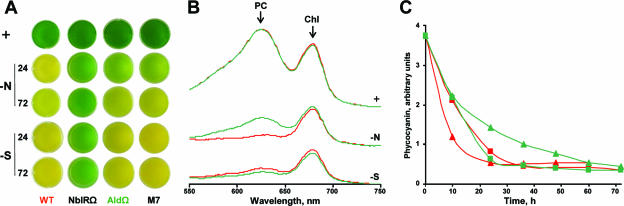
To elucidate the mechanism underlying phycobilisome degradation, we isolated mutants in which there was aberrant modulation of the pigments during nutrient starvation. Mutagenized cell populations starved for either sulfur or nitrogen were screened by fluorescence-activated cell sorting, and cells that maintained relatively high fluorescence were sorted, plated, and analyzed to determine the pigment degradation in response to nutrient starvation (27). One of the mutants isolated, M7, exhibited aberrant phycobilisome degradation only under nitrogen starvation conditions (Fig. 2A), in contrast to nonbleaching mutants with mutations in the nbl pathway (e.g., NblRΩ), in which phycobilisome degradation is impaired under nitrogen, sulfur, and phosphorus starvation conditions (33). Furthermore, mutant M7 contained unaltered copies of the previously isolated genes of the nbl pathway (see Materials and Methods). To identify the lesion in mutant M7, the genomic region bearing the transposon was cloned, and the nucleotide sequence flanking the transposon was determined. The transposon was found to be inserted into the coding region of ald (Fig. 1A). This gene encodes a protein exhibiting homology to l-alanine dehydrogenase (l-alanine:NAD+ oxidoreductase; EC 1.4.1.1; referred to as alanine dehydrogenase or Ald below), an enzyme that catalyzes the reversible conversion of alanine to pyruvate and ammonia. S. elongatus PCC 7942 Ald exhibited high levels of homology to other alanine dehydrogenases (for example, 54% identity and 71% similarity to the enzyme of Bacillus subtilis). Furthermore, S. elongatus PCC 7942 Ald is characterized by an NAD(H) binding domain typical of alanine dehydrogenases (7). Direct inactivation of ald by insertion of a spectinomycin resistance cassette (see Material and Methods) (Fig. 1A) resulted in a mutant, AldΩ, in which phycobilisome degradation was specifically impaired under nitrogen starvation conditions, similar to the original mutant, M7 (Fig. 2A).
FIG. 2.
Phenotype of AldΩ impaired in alanine dehydrogenase. (A) Cultures of S. elongatus PCC 7942 (WT), alanine dehydrogenase mutants AldΩ and M7, and NblR mutant NblRΩ grown in complete medium (+) or under nitrogen starvation conditions (−N) or sulfur starvation conditions (−S) for 24 and 72 h. (B) Absorbance spectra of the wild type (red) and AldΩ (green) grown in complete medium or starved for 24 h. The absorbance maxima of phycocyanin (PC), the major pigment of the phycobilisome, and chlorophyll a (Chl) are indicated. Absorbance spectra were obtained for cultures diluted with growth medium to obtain an optical density at 750 nm of 0.01. Groups of spectra were shifted along the y axis for clarity. (C) Amount of phycocyanin as a function of time in nitrogen-starved cells (triangles) or sulfur-starved cells (squares). The different colors represent different strains as described above. Wild-type and AldΩ cultures normalized to the same optical density at 750 nm contained the same number of cells; hence, the amount of phycocyanin reflects the pigment level per cell.
AldΩ exhibited attenuated phycobilisome degradation during nitrogen starvation; following 10 and 24 h of nitrogen starvation, the cellular content of phycocyanin, the major component of the phycobilisome in S. elongatus PCC 7942, was twofold higher in this mutant than in the wild type (Fig. 2C). A longer starvation time resulted in further loss of phycocyanin in the mutant, which following 72 h of starvation exhibited pigmentation comparable to that of wild-type cells (Fig. 2A) and, accordingly, had phycocyanin levels similar to those of wild-type cells (Fig. 2C). For comparison, the pigmentation of the nonbleaching mutant NblRΩ is shown in Fig. 2A. Notably, inactivation of ald did not affect phycobilisome degradation during sulfur starvation (Fig. 2A and B) or phosphorus starvation (not shown), unlike the case of mutants having mutations in the nbl pathway. Furthermore, impairment of ald did not influence the phycocyanin content or the chlorophyll content of cells grown in complete medium (Fig. 2B).
Taken together, the pigment analyses of the ald mutant suggested that Ald plays a role in the modulation of phycobilisome degradation specifically during nitrogen starvation.
Expression of genes associated with phycobilisome modulation.
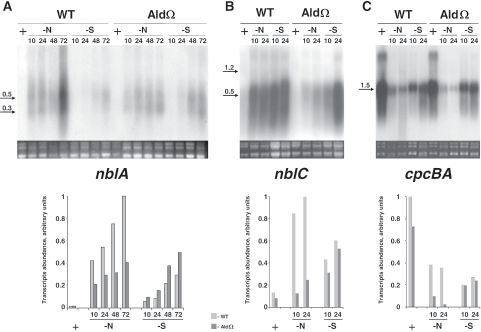
NblA is essential for phycobilisome degradation, and transcription of its gene, nblA, is highly induced upon nutrient starvation (1, 2, 5, 18, 21, 22, 28). We therefore compared the abundance of nblA transcripts in the ald mutant to the abundance in the wild-type strain. Northern analysis revealed lower levels of nblA transcripts in AldΩ during nitrogen starvation (Fig. 3A). Quantitative analysis indicated that the mutant exhibited 40 to 60% of the transcript level present in nitrogen-starved wild-type cells (Fig. 3A). In contrast, nblA transcript accumulation during sulfur starvation was not reduced in AldΩ (Fig. 3A). These data were supported by an assessment of the activity of the nblA promoter using β-glucoronidase as a reporter; the activity of the nblA promoter in AldΩ was lower during nitrogen starvation but not during sulfur starvation (see Fig. S1 in the supplemental material).
FIG. 3.
Expression of genes affecting the phycocyanin level in S. elongatus PCC 7942 (WT) and alanine dehydrogenase mutant AldΩ: Northern analysis of nblA (A), nblC (B) and cpcBA (C). Cultures were grown in complete medium (+) or were starved for nitrogen (−N) or sulfur (−S) for the times indicated above the lanes (in hours). The bar graphs are quantitative representations of the hybridization results.
The recently identified gene nblC, a new member of the nbl pathway (35), is induced in wild-type cells upon nitrogen and sulfur starvation (Fig. 3B). Accumulation of nblC transcripts in the ald mutant was impaired under nitrogen starvation conditions, whereas comparable levels were observed for the wild-type and AldΩ strains under sulfur starvation conditions (Fig. 3B). Quantitative analysis indicated that the levels of the nblC transcript were four- to sevenfold lower in the mutant than in the wild-type cells (Fig. 3B). Taken together, the results of Northern analyses of nblA and nblC indicated that there was reduced transcript accumulation during nitrogen starvation but not during sulfur starvation, which is consistent with the aberrant phycobilisome degradation observed specifically during nitrogen starvation.
The level of phycocyanin, the major component of the phycobilisome during starvation, reflects the balance between the synthesis and degradation rates of this antenna pigment system. Northern analysis of the cpcBA operon (encoding the α and β subunits of phycocyanin) indicated that AldΩ did not exhibit elevated transcript levels during starvation; in fact, transcription repression of this operon seemed to occur even faster in the mutant than in wild-type cells (Fig. 3C). Therefore, we concluded that the aberrant pigmentation of AldΩ is due to impaired pigment degradation.
Examination of ald transcripts and alanine levels during starvation.
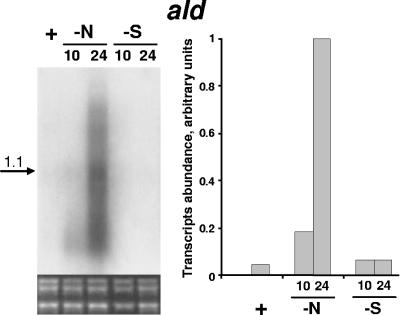
To obtain further insight into the function of Ald during starvation, we examined the transcript abundance of ald, as well as the alanine levels, as a function of starvation. The level of ald transcripts gradually increased under nitrogen starvation conditions, and in 24 h the levels were 20-fold higher in starved wild-type cells than in nonstarved wild-type cells (Fig. 4). However, up to 24 h of sulfur starvation did not result in accumulation of ald transcripts (Fig. 4).
FIG. 4.
Expression of ald in S. elongatus PCC 7942. For details see the legend to Fig. 3.
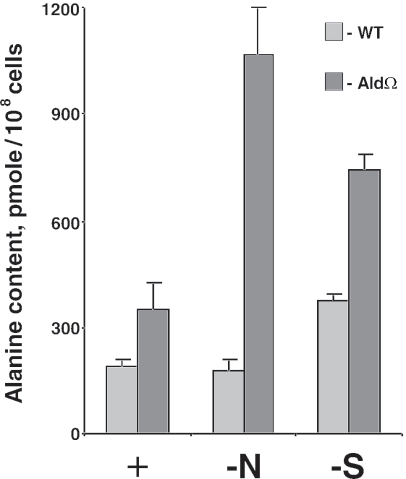
Analysis of alanine levels revealed differences between starved cultures of the wild type and AldΩ (Fig. 5). The size of the alanine pool increased fivefold in response to 24 h of nitrogen starvation in the mutant, while the alanine levels in nutrient-replete and nitrogen-starved wild-type cells were the same (Fig. 5). Sulfur starvation resulted in accumulation of alanine by both strains; however, this occurred to a greater extent in the mutant (Fig. 5). The alanine contents observed indicated that Ald has an important role in alanine degradation during starvation. The higher alanine levels in the nitrogen-starved mutant than in the nitrogen-starved wild-type cells may be explained by induction of ald in the wild type upon nitrogen starvation (Fig. 4). Presumably, increased activity of Ald resulting from induction of the ald gene allows the efficient decomposition of alanine obtained following protein degradation during nitrogen starvation. This scenario is supported by the alanine accumulation observed in wild-type cells starved for sulfur for 24 h, since these cells lacked ald induction at this time during starvation (Fig. 4). Taken together, the results of the comparative analysis of the alanine levels in the wild-type and AldΩ strains, as well as the results of the Northern analysis of ald, suggest that Ald has an important role in mobilization of nitrogen resources under starvation conditions.
FIG. 5.
Alanine levels in S. elongatus PCC 7942 (WT) and alanine dehydrogenase mutant AldΩ. Cultures were grown in nutrient-sufficient medium (+) or under nitrogen starvation conditions (−N) or sulfur starvation conditions (−S) for 24 h. The data are averages and standard deviations of three samples. Differences between wild-type and mutant cells were statistically significant under nitrogen and sulfur starvation conditions (P = 0.0004 and P = 0.0002, respectively). No significant difference (P = 0.02) was observed in replete medium (as determined by a t test, α = 0.01).
Notably, in addition to the dominant 1.1-kb transcript (presumably the monocistronic transcript of ald), the Northern analysis indicated that there was induction of high-molecular-weight transcripts (Fig. 4). This suggests that polycistronic transcripts, including ald and ORF3, may be produced. To examine the possibility of a polar effect of the DNA cassettes inserted in ald on downstream genes, a DNA fragment (about 1.9 kb) bearing the coding region of ald and its putative promoter region was inserted into a neutral site within the genome of AldΩ. The resulting transformant exhibited pigment degradation under nitrogen starvation the was similar to that of the wild-type strain (data not shown), and therefore, a polar effect may be excluded.
The growth of AldΩ in nutrient-sufficient medium is similar to the growth of a wild-type culture (not shown). Apparently (Fig. 5), a sufficient level of alanine is produced in the mutant despite the impairment of ald. Alanine dehydrogenases from various bacteria exhibit relatively high degrees of homology (see above). Aside from ald, the S. elongatus PCC 7942 genome does not contain an ortholog encoding known alanine dehydrogenases. Therefore, unless there is a unique type of alanine dehydrogenase, we should consider the possibility that there is a metabolic route, aside from amination of pyruvate, which produces alanine in AldΩ. Possibly, the sequential action of a branched amino acid aminotransferase (IlvE) and valine-pyruvate aminotransferase (AvtA) leads to production of alanine from 2-keto-isovalerate. Additionally, alanine may be produced by the activity of serine-pyruvate aminotransferase, which yields hydroxypyruvate and alanine from serine and pyruvate. The genes encoding the enzymes mentioned above are present in the S. elongatus PCC 7942 genome, supporting the suggestion that alanine may be produced via these enzymatic reactions.
In addition to changes in alanine levels, an analysis of 18 amino acids (see Materials and Methods) indicated that there were significant and reproducible changes in the cellular levels of serine, glycine, and threonine. A comparison of AldΩ with wild-type cells under nitrogen starvation conditions revealed that in the mutant the pools of serine and glycine were 2- and 3.5-fold larger, respectively, whereas the threonine level was 3-fold higher in nitrogen-starved AldΩ (not shown). The reflection of the elevated level of alanine in the higher levels of the three additional amino acids may be explained by the activity of serine-pyruvate aminotransferase. This enzyme catalyzes the reversible conversion of serine and pyruvate to hydroxypyruvate and alanine and thus provides a direct link between alanine and the metabolic route of serine, glycine, and threonine.
Effect of inactivation of ald on nitrogen- and sulfur-specific responses.
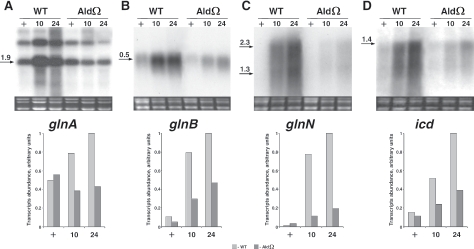
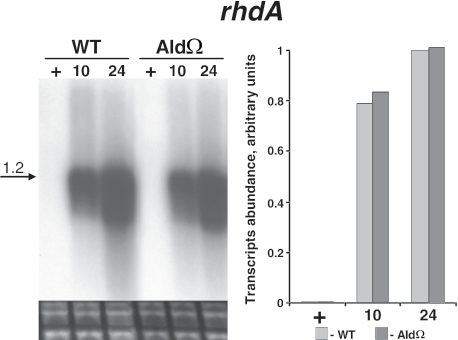
To further investigate the consequences of inactivation of ald on gene expression during nitrogen starvation, we examined glnA, glnB, glnN, and icd. These genes, which are known to be induced upon nitrogen starvation, encode a type I glutamine synthetase, a global regulator of nitrogen starvation responses (PII), a type III glutamine synthetase and isocitrate dehydrogenase, respectively (25). When starved for nitrogen, the ald mutant exhibited a lower level of transcripts of each of the genes mentioned above compared to the level in starved wild-type cells (Fig. 6A to D). For example, the levels of glnA, glnB, glnN, and icd transcripts were 2.5-, 2-, 5-, and 2.5-fold lower, respectively, in 24-h nitrogen-starved mutant cells than in wild-type cells. In contrast to this impairment in transcript accumulation upon nitrogen starvation, rhdA, which encodes a thiosulfate:cyanide sulfurtransferase-like protein, was induced normally in sulfur-starved mutant cells (Fig. 7).
FIG. 6.
Expression of nitrogen starvation-responsive genes in S. elongatus PCC 7942 (WT) and alanine dehydrogenase mutant AldΩ. For details see the legend to Fig. 3.
FIG. 7.
Expression of rhdA, a sulfur starvation-responsive gene, in S. elongatus PCC 7942 (WT) and alanine dehydrogenase mutant AldΩ. For details see the legend to Fig. 3.
Role of alanine dehydrogenase during nitrogen starvation.
It should be pointed out that the genes examined (nblA, glnA, glnN, glnB, and icd) are known to be controlled by NtcA, the transcriptional regulator that modulates gene expression during nitrogen starvation (for reviews see references 9 to 11 and 33), and nblC has a potential NtcA binding site (not shown). We suggest that impaired accumulation of transcripts of NtcA-dependent genes in AldΩ results from a lower cellular level of 2-oxoglutarate; binding of this metabolite activates NtcA as a transcriptional modulator (25, 26, 33). Furthermore, the mutant may contain lower levels of NtcA. Additionally, since mutual interactions of the two global nitrogen regulators, PII and NtcA, are essential for an adequate cellular response to nitrogen starvation, we suggest that there is improper phosphorylation and hence lower activation of PII in AldΩ.
It is noteworthy that an NtcA mutant exhibits attenuated phycobilisome degradation specifically during nitrogen starvation (30), similar to AldΩ. The phenotype of the ntcA mutant may be attributed to the involvement of NtcA in transcription induction of nblA (22). Therefore, this mutant provides support for the cross talk between mechanisms related to nitrogen-specific and general acclimation responses (33), as suggested for AldΩ (see below).
Northern analysis of nblA (Fig. 3), as well as nblC, glnB, glnN, and icd (not shown), following 72 h of nitrogen starvation indicated that the transcript levels were lower in AldΩ than in wild-type cells. This result indicates that ald inactivation affects not only the kinetics of transcript accumulation during the first 24 h of nitrogen starvation but also has a longer-term impact.
A global comparison of gene expression during starvation in the wild type and the mutant is required to comprehensively evaluate the consequences of inactivation of ald.
We suggest the following hypothesis to explain the requirement for Ald activity for adequate acclimation to nitrogen starvation conditions. This hypothesis assigns importance to decomposition of the alanine resulting from protein degradation during nitrogen starvation. Generally, protein degradation is an important aspect of acclimation to nutrient limitation as it allows recycling of cellular components and acquisition of amino acids for de novo protein synthesis during starvation. The amino acid composition of the degraded proteins does not necessarily match the amino acids required for the newly produced proteins, and therefore further breakdown of amino acids is essential for adequate acclimation to the nutrient stress. For example, deamination of alanine by Ald, yielding ammonia and pyruvate, may provide the cells with a nitrogen source under the starvation conditions. Principally, various transaminases could help the cells exploit the alanine pool; however, alanine accumulation due to ald inactivation or a lack of induction (following 24 h of sulfur starvation) indicates that Ald has a central role in alanine decomposition. Improper alanine degradation may impair timely formation of specific proteins and therefore affect some of the nitrogen acclimation responses.
An additional explanation is that accumulation of alanine during nitrogen starvation, like the accumulation which occurs in AldΩ, interferes specifically with the cellular signaling processes associated with nitrogen starvation responses. An elevated alanine level may signal that sufficient protein degradation has occurred, and therefore, phycobilisome degradation is attenuated under nitrogen starvation conditions. With this in mind, it will be interesting to comprehensively compare protein degradation in the wild-type and mutant strains; alanine accumulation may affect degradation of cellular components other than the phycobilisomes. Furthermore, “metabolic control” is commonly used to modulate cellular responses to fit ambient conditions; namely, changes in the concentration of cellular metabolites trigger acclimation responses. An example of metabolic control is the response of cyanobacteria to nitrogen starvation; these organisms perceive nitrogen status by sensing the internal concentration of 2-oxoglutarate (17, 19, 24). In enterobacteria changes in cellular glutamine pools elicit nitrogen starvation responses (14, 15).
Interestingly, Ald was shown to be required for normal sporulation in B. subtilis (37). It has been suggested that pyruvate produced by Ald activity generates energy via the tricarboxylic acid cycle. This explanation was supported by partial rescue of the Bacillus ald mutation by addition of exogenous pyruvate. However, as complementation of the sporulation impairment was only partial, an additional role, plausibly regulatory, was assigned to the cellular alanine levels (37).
Although the mode of action of alanine is still not clear, we suggest that the elevated concentration of alanine, or a biochemical consequence of alanine accumulation, interferes with the cellular cues that modulate nitrogen acclimation responses. Apparently, alanine is accumulated following 24 h of sulfur starvation; therefore, alanine accumulation (or an elevated level of alanine per se) is not sufficient to affect the cellular sulfur acclimation responses.
In summary, analyses of AldΩ indicate that processes related to oxidative alanine consumption affect phycobilisome degradation, a general acclimation response observed under various stress conditions. The effect of ald inactivation on modulation of the “general stress-related” genes (nblA and nblC), however, is limited to nitrogen starvation conditions. Furthermore, AldΩ exhibits aberrant modulation of nitrogen-specific genes. Taken together, the results of this study provide novel information regarding modulation of cellular responses to nitrogen starvation and insight into the contribution of processes that regulate nitrogen-specific responses to the modulation of a cyanobacterial general acclimation response.
Supplementary Material
Acknowledgments
We thank Dalia Hacohen and Klaudia Michl for technical assistance.
This study was supported by the German Israel Foundation (grant I-729-27.9/2002) and by the Israel Science Foundation (grant 683/03-17.1).
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Baier, K., H. Lehmann, D. P. Stephan, and W. Lockau. 2004. NblA is essential for phycobilisome degradation in Anabaena sp. strain PCC 7120 but not for development of functional heterocysts. Microbiology 150:2739-2749. [DOI] [PubMed] [Google Scholar]
- 2.Baier, K., S. Nicklisch, C. Grundner, J. Reinecke, and W. Lockau. 2001. Expression of two nblA-homologous genes is required for phycobilisome degradation in nitrogen-starved Synechocystis sp. PCC6803. FEMS Microbiol. Lett. 195:35-39. [DOI] [PubMed] [Google Scholar]
- 3.Bhaya, D., R. Schwarz, and A. R. Grossman. 1997. Molecular responses to environmental stress, p. 397-442. In M. Potts and B. A. Whitton (ed.), Ecology of cyanobacteria: their diversity in time and space. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 4.Bienert, R., K. Baier, R. Volkmer, W. Lockau, and U. Heinemann. 2006. Crystal structure of NblA from Anabaena sp. PCC 7120, a small protein playing a key role in phycobilisome degradation. J. Biol. Chem. 281:5216-5223. [DOI] [PubMed] [Google Scholar]
- 5.Collier, J. L., and A. R. Grossman. 1994. A small polypeptide triggers complete degradation of light-harvesting phycobiliproteins in nutrient-deprived cyanobacteria. EMBO J. 13:1039-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collier, J. L., S. K. Herbert, D. C. Fork, and A. R. Grossman. 1994. Changes in the cyanobacterial photosynthetic apparatus during acclimation to macronutrient deprivation. Photosynth. Res. 42:173-183. [DOI] [PubMed] [Google Scholar]
- 7.Delforge, D., E. Depiereux, B. De, X., E. Feytmans, and J. Remacle. 1993. Similarities between alanine dehydrogenase and the N-terminal part of pyridine nucleotide transhydrogenase and their possible implication in the virulence mechanism of Mycobacterium tuberculosis. Biochem. Biophys. Res. Commun. 190:1073-1079. [DOI] [PubMed] [Google Scholar]
- 8.Dolganov, N., and A. R. Grossman. 1999. A polypeptide with similarity to phycocyanin alpha-subunit phycocyanobilin lyase involved in degradation of phycobilisomes. J. Bacteriol. 181:610-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores, E., J. E. Frias, L. M. Rubio, and A. Herrero. 2005. Photosynthetic nitrate assimilation in cyanobacteria. Photosynth. Res. 83:117-133. [DOI] [PubMed] [Google Scholar]
- 10.Flores, E., and A. Herrero. 2005. Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem. Soc. Trans. 33:164-167. [DOI] [PubMed] [Google Scholar]
- 11.Forchhammer, K. 2004. Global carbon/nitrogen control by PII signal transduction in cyanobacteria: from signals to targets. FEMS Microbiol. Rev. 28:319-333. [DOI] [PubMed] [Google Scholar]
- 12.Grossman, A. R., M. R. Schaefer, G. G. Chiang, and J. L. Collier. 1993. Environmental effects on the light-harvesting complex of cyanobacteria. J. Bacteriol. 175:575-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagemann, M., J. Vinnemeier, I. Oberpichler, R. Boldt, and H. Bauwe. 2005. The glycine decarboxylase complex is not essential for the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Biol. (Stuttgart) 7:15-22. [DOI] [PubMed] [Google Scholar]
- 14.Hu, P., T. Leighton, G. Ishkhanova, and S. Kustu. 1999. Sensing of nitrogen limitation by Bacillus subtilis: comparison to enteric bacteria. J. Bacteriol. 181:5042-5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda, T. P., A. E. Shauger, and S. Kustu. 1996. Salmonella typhimurium apparently perceives external nitrogen limitation as internal glutamine limitation. J. Mol. Biol. 259:589-607. [DOI] [PubMed] [Google Scholar]
- 16.Jefferson, R. A., T. A. Kavanagh, and M. W. Bevan. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6:3901-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent, S., H. Chen, S. Bedu, F. Ziarelli, L. Peng, and C. C. Zhang. 2005. Nonmetabolizable analogue of 2-oxoglutarate elicits heterocyst differentiation under repressive conditions in Anabaena sp. PCC 7120. Proc. Natl. Acad. Sci. USA 102:9907-9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, H., and L. A. Sherman. 2002. Characterization of Synechocystis sp. strain PCC 6803 and Δnbl mutants under nitrogen-deficient conditions. Arch. Microbiol. 178:256-266. [DOI] [PubMed] [Google Scholar]
- 19.Li, J. H., S. Laurent, V. Konde, S. Bedu, and C. C. Zhang. 2003. An increase in the level of 2-oxoglutarate promotes heterocyst development in the cyanobacterium Anabaena sp. strain PCC 7120. Microbiology 149:3257-3263. [DOI] [PubMed] [Google Scholar]
- 20.Lindell, D., D. Erdner, D. Marie, O. Prasil, M. Koblizek, F. Le Gall, R. Rippka, F. Partensky, D. J. Scanlan, and A. F. Post. 2002. Nitrogen stress response of Prochlorococcus strain PCC 9511 (oxyphotobacteria) involves contrasting regulation of ntcA and amt1. J. Phycol. 38:1113-1124. [Google Scholar]
- 21.Luque, I., J. A. Ochoa De Alda, C. Richaud, G. Zabulon, J. C. Thomas, and J. Houmard. 2003. The NblAI protein from the filamentous cyanobacterium Tolypothrix PCC 7601: regulation of its expression and interactions with phycobilisome components. Mol. Microbiol. 50:1043-1054. [DOI] [PubMed] [Google Scholar]
- 22.Luque, I., G. Zabulon, A. Contreras, and J. Houmard. 2001. Convergence of two global transcriptional regulators on nitrogen induction of the stress-acclimation gene nblA in the cyanobacterium Synechococcus sp. PCC 7942. Mol. Microbiol. 41:937-947. [DOI] [PubMed] [Google Scholar]
- 23.Marin, K., Y. Kanesaki, D. A. Los, N. Murata, I. Suzuki, and M. Hagemann. 2004. Gene expression profiling reflects physiological processes in salt acclimation of Synechocystis sp. strain PCC 6803. Plant Physiol. 136:3290-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muro-Pastor, M. I., J. C. Reyes, and F. J. Florencio. 2001. Cyanobacteria perceive nitrogen status by sensing intracellular 2-oxoglutarate levels. J. Biol. Chem. 276:38320-38328. [DOI] [PubMed] [Google Scholar]
- 25.Muro-Pastor, M. I., J. C. Reyes, and F. J. Florencio. 2005. Ammonium assimilation in cyanobacteria. Photosynth. Res. 83:135-150. [DOI] [PubMed] [Google Scholar]
- 26.Ninfa, A. J., and P. Jiang. 2005. PII signal transduction proteins: sensors of alpha-ketoglutarate that regulate nitrogen metabolism. Curr. Opin. Microbiol. 8:168-173. [DOI] [PubMed] [Google Scholar]
- 27.Perelman, A., J. Shaltiel, E. Sendersky, and R. Schwarz. 2004. Use of flow cytometry for efficient isolation of cyanobacterial mutants deficient in modulation of pigment level. BioTechniques 36:948-950, 952. [DOI] [PubMed] [Google Scholar]
- 28.Richaud, C., G. Zabulon, A. Joder, and J. C. Thomas. 2001. Nitrogen or sulfur starvation differentially affects phycobilisome degradation and expression of the nblA gene in Synechocystis strain PCC 6803. J. Bacteriol. 183:2989-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauer, J., U. Dirmeier, and K. Forchhammer. 2000. The Synechococcus strain PCC 7942 glnN product (glutamine synthetase III) helps recovery from prolonged nitrogen chlorosis. J. Bacteriol. 182:5615-5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sauer, J., M. Görl, and K. Forchhammer. 1999. Nitrogen starvation in Synechococcus PCC 7942: involvement of glutamine synthetase and NtcA in phycobiliprotein degradation and survival. Arch. Microbiol. 172:247-255. [DOI] [PubMed] [Google Scholar]
- 31.Sauer, J., U. Schreiber, R. Schmid, U. Volker, and K. Forchhammer. 2001. Nitrogen starvation-induced chlorosis in Synechococcus PCC 7942. Low-level photosynthesis as a mechanism of long-term survival. Plant Physiol. 126:233-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scanlan, D. J., and N. J. West. 2002. Molecular ecology of the marine cyanobacterial genera Prochlorococcus and Synechococcus. FEMS Microbiol. Ecol. 40:1-12. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz, R., and K. Forchhammer. 2005. Acclimation of unicellular cyanobacteria to macronutrient deficiency: emergence of a complex network of cellular responses. Microbiology 151:2503-2514. [DOI] [PubMed] [Google Scholar]
- 34.Schwarz, R., and A. R. Grossman. 1998. A response regulator of cyanobacteria integrates diverse environmental signals and is critical for survival under extreme conditions. Proc. Natl. Acad. Sci. USA 95:11008-11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sendersky, E., R. Lahmi, J. Shaltiel, A. Perelman, and R. Schwarz. 2005. NblC, a novel component required for pigment degradation during starvation in Synechococcus PCC 7942. Mol. Microbiol. 58:659-668. [DOI] [PubMed] [Google Scholar]
- 36.Singh, A. K., L. M. McIntyre, and L. A. Sherman. 2003. Microarray analysis of the genome-wide response to iron deficiency and iron reconstitution in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 132:1825-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siranosian, K. J., K. Ireton, and A. D. Grossman. 1993. Alanine dehydrogenase (Ald) is required for normal sporulation in Bacillus subtilis. J. Bacteriol. 175:6789-6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tu, C. J., J. Shrager, R. L. Burnap, B. L. Postier, and A. R. Grossman. 2004. Consequences of a deletion in dspA on transcript accumulation in Synechocystis sp. strain PCC6803. J. Bacteriol. 186:3889-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Waasbergen, L. G., N. Dolganov, and A. R. Grossman. 2002. nblS, a gene involved in controlling photosynthesis-related gene expression during high light and nutrient stress in Synechococcus elongatus PCC 7942. J. Bacteriol. 184:2481-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.