Abstract
The SOS response in Escherichia coli results in the coordinately induced expression of more than 40 genes which occurs when cells are treated with DNA-damaging agents. This response is dependent on RecA (coprotease), LexA (repressor), and the presence of single-stranded DNA (ssDNA). A prerequisite for SOS induction is the formation of a RecA-ssDNA filament. Depending on the DNA substrate, the RecA-ssDNA filament is produced by either RecBCD, RecFOR, or a hybrid recombination mechansim with specific enzyme activities, including helicase, exonuclease, and RecA loading. In this study we examined the role of RecA loading activity in SOS induction after UV irradiation. We performed a genetic analysis of SOS induction in strains with a mutation which eliminates RecA loading activity in the RecBCD enzyme (recB1080 allele). We found that RecA loading activity is essential for SOS induction. In the recB1080 mutant RecQ helicase is not important, whereas RecJ nuclease slightly decreases SOS induction after UV irradiation. In addition, we found that the recB1080 mutant exhibited constitutive expression of the SOS regulon. Surprisingly, this constitutive SOS expression was dependent on the RecJ protein but not on RecFOR, implying that there is a different mechanism of RecA loading for constitutive SOS expression.
The SOS response in Escherichia coli results in the simultaneously induced expression of more than 40 genes which occurs when cells are treated with DNA-damaging agents (7; for a review see reference 8). The elevated expression of these genes increases the capacity of cells for DNA repair, damage tolerance, DNA replication, and mutagenesis (29, 39). Historically, it is known that the SOS response is dependent on recA and lexA gene products, as well as on the presence of single-stranded DNA (ssDNA). The LexA protein is the repressor of the SOS regulon which binds to specific sequences, called SOS boxes, that are present in the promoter regions of SOS genes (15, 16, 17). LexA is responsible for the very weak basal expression of the SOS genes under normal physiological conditions. SOS induction occurs when activated RecA protein causes the self-cleavage of the LexA repressor (for reviews see references 23 and 36). The amounts of the RecA, LexA, RecN, DNA Pol II, UvrABC, RuvABC, UmuDC, and SfiA proteins, among other proteins, increase during SOS induction and influence various aspects of DNA metabolism.
A critical step in the SOS signaling mechanism is the production of a RecA-ssDNA filament, which is important for activation of the RecA protein. The RecA-ssDNA filament can be used for homologous recombination and SOS induction (for a review see reference 23). There are three essential enzymatic activities that are required for the formation of a RecA-ssDNA filament from double-stranded DNA (dsDNA) ends: helicase, 5′-3′ exonuclease, and RecA loading onto 3′-ssDNA. These activities are linked to the proteins of the two main recombination mechanisms in E. coli, RecBCD and RecFOR. The RecBCD enzyme has all three enzymatic activities, whereas in the RecFOR mechanism there are different proteins for different activities, including RecQ (helicase), RecJ (nuclease), and the RecFOR complex (RecA loading). The RecFOR recombination mechanism can process dsDNA ends only in a recBC sbcBC(D) multiple mutant, which contains an inactivated RecBCD enzyme, exonuclease I, and SbcCD nuclease (for a review see reference 14). In wild-type (wt) cells the RecFOR mechanism is involved in processing of single-strand gaps (SSGs) which occur in daughter strands after reinitiation of DNA replication downstream from a noncoding lesion (9, 14). The most important role in this process is that of the RecFOR complex itself, which replaces the single-strand DNA binding (SSB) protein complexed with ssDNA with the RecA protein (25). Additional elements of the RecFOR pathway (RecQ helicase and RecJ nuclease) may help in the production of more ssDNA from the original SSG to produce a longer filament.
Analogous to recombination, there are two pathways for SOS induction, the RecBCD and RecFOR pathways, which exhibit substrate specificity for dsDNA ends (or breaks) and SSGs, respectively (4, 21, 23). In wt cells, SOS induction immediately after UV irradiation is dependent on RecFOR proteins. However, when dsDNA ends appear later, as a result of nucleotide excision repair and replication fork collapse, SOS induction requires the RecBCD enzyme as well. This is reflected in the fact that SOS induction after UV irradiation in recFOR mutants is not completely eliminated but is delayed (10, 35, 38) and is dependent on the RecBCD enzyme (30).
The requirement for both RecBCD and RecFOR in SOS induction was revealed using null mutants whose mutations which completely eliminated the functions of the mutated gene products. In this study we focused on the RecA loading activity of the RecBCD enzyme in vivo and its role in the SOS signaling mechanism. We expected that elimination of RecA loading activity by appropriate mutations would have serious implications for SOS induction. To test the effect of a mutation which specifically eliminated RecA loading ability, we used the recB1080 allele (13, 40). The point mutation in this allele is located in the nuclease domain of the RecBCD enzyme (RecBnuc), which is also involved in RecA binding (33). Consequently, the RecB1080CD form of the enzyme is nuclease deficient and is unable to load RecA protein onto ssDNA, but it still has functional helicase activity (2, 37, 40).
In this study we compared the levels of SOS induction after UV irradiation in strains in which one or both mechanisms of RecA loading activity were inactivated. We found that RecA loading activity is essential for SOS induction. SOS induction in the recB1080 mutant is independent of the RecQ helicase and is partially dependent on the RecJ nuclease. We also found that the recB1080 mutant exhibits constitutive expression of the SOS regulon, which is eliminated by recJ and recD mutations. Surprisingly, recFOR mutations have no effect on constitutive SOS expression.
MATERIALS AND METHODS
Bacterial strains and bacteriophages.
The bacterial strains used in this study are shown in Table 1. The N5071, TRM452, and TRM387 strains were kindly provided by R. G. Lloyd, University of Nottingham, Nottingham, England. Transduction was carried out as described by Miller (24).
TABLE 1.
E. coli K-12 strains used in this study
| Strain | Relevant genotype | Reference or source |
|---|---|---|
| Bacterial strains related to AB1157 | ||
| AB1157 | F−thr-1 leuB6 Δ(gpt-proA)62 hisG4 thi-1 argE3 lacY1 galK2 ara-14 xyl-5 mtl-1 tsx-33 supE44 rpsL31 kdgK51 rfbD1 mgl-51 λ−rac | 3 |
| AM208 | AB1157 recR256::Tn5 | R. G. Loyd |
| N5170 | AB1157 thr+leu+ Δ(pro-lac) sfiA::Mud (Ap lac MuB::Tn9) | R. G. Loyd |
| N3071 | AB1157 recB268::Tn10 | R. G. Loyd |
| N3072 | AB1157 recA269::Tn10 | R. G. Loyd |
| RIK174 | AB1157 recB1080 | 13 |
| RIK123 | AB1157 recB1067 | 13 |
| RIK144 | AB1157 recD1903::Tn10d(Tet) | 13 |
| RIK155 | AB1157 recD1903::Tn10d(Tet) recB1067 | 13 |
| IRB103 | AB1157 recO1504::Tn5 | 27 |
| IIB360 | AB1157 (recB1080) argA::Tn10 | P1.N5071 × RIK174 |
| IIB361 | AB1157 (recB1067) argA::Tn10 | P1.N5071 × RIK123 |
| IIB290 | AB1157 recB1080 recD1903::Tn10d(Tet) | P1.RIK144 × RIK174 |
| IIB294 | AB1157 recF400::Tn5 | P1.WA576 × AB1157 |
| LMM1032 | AB1157 recJ2052::Tn10kan | D. Zahradka |
| LMM1215 | AB1157 ΔrecQ::kan | D. Zahradka |
| WA576 | AB1157 recF400::Tn5 | W. Wackernagel |
| Bacterial strains related to MG1655 | ||
| MG1655 | F−rec+ (wt) | |
| N5071 | MG1655 argA::Tn10 | R. G. Lloyd |
| TRM452 | Δlac ΔattB::PBADI-SceI | R. G. Lloyd |
| TRM387 | ΔargE::I-SceIcs::cat ΔattB::PBADI-SceI | 22 |
| IIB385 | Δlac ΔattB::PBADI-SceI ΔargE::I-SceIcs::cat | P1.TRM387 × TRM452 |
| IIB386 | Δlac ΔattB::PBADI-SceI ΔargE::I-SceIcs::cat sfiA::Mud (Ap lac MuB::Tn9) | P1.5170 × IIB385 |
| IIB388 | As IIB386, recB1080 argA::Tn10 | P1.IIB360 × IIB386 |
| IIB389 | As IIB386, recB1067 argA::Tn10 | P1.IIB361 × IIB386 |
| IIB390 | As IIB386, recB1080 recD1903::Tn10d(Tet) | P1.IIB290 × IIB386 |
| IIB391 | As IIB386, recB1067 recD1903::Tn10d(Tet) | P1.RIK155 × IIB386 |
| IIB392 | As IIB386, recO1504::Tn5 | P1.IRB103 × IIB386 |
| IIB393 | As IIB386, recB1080 recD1903::Tn10d(Tet) recO1504::Tn5 | P1.IRB103 × IIB390 |
| IIB394 | As IIB386, recB1067 recD1903::Tn10d(Tet) recO1504::Tn5 | P1.IRB103 × IIB391 |
| IIB395 | As IIB386, recD1903::Tn10d(Tet) | P1.RIK144 × IIB386 |
| IIB401 | As IIB386, recB1080 recO1504::Tn5 | P1.IRB103 × IIB388 |
| IIB402 | As IIB386, recB1067 recO1504::Tn5 | P1.IRB103 × IIB389 |
| IIB407 | As IIB386, ΔrecQ::kan | P1.LMM1215 × IIB386 |
| IIB408 | As IIB386, recB1080 ΔrecQ::kan | P1.LMM1215 × IIB388 |
| IIB409 | As IIB386, recJ2052::Tn10kan | P1.LMM1032 × IIB386 |
| IIB410 | As IIB386, recB1080 recJ2052::Tn10kan | P1.LMM1032 × IIB388 |
| IIB420 | As IIB386, recB268::Tn10 | P1.N3071 × IIB386 |
| IIB421 | As IIB386, recB268::Tn10 ΔrecQ::kan | P1.LMM1215 × IIB420 |
| IIB422 | As IIB386, recB268::Tn10 recO1504::Tn5 | P1.IRB103 × IIB420 |
| IIB423 | As IIB386, recB268::Tn10 recJ2052::Tn10kan | P1.LMM1032 × IIB420 |
| IIB424 | As IIB386, recA269::Tn10 | P1.N3072 × IIB386 |
| IIB570 | As IIB386, recR256::Tn10kan | P1.AM208 × IIB386 |
| IIB571 | As IIB386, recF400::Tn5 | P1.IIB294 × IIB386 |
| IIB572 | As IIB386, recB1080 recR256::Tn10kan | P1.AM208 × IIB388 |
| IIB573 | As IIB386, recB1080 recF400::Tn5 | P1.IIB294 × IIB388 |
| IIB574 | As IIB386, recB1080 recD1903::Tn10d(Tet) recR256::Tn10kan | P1.AM208 × IIB390 |
| IIB575 | As IIB386, recB1080 recD1903::Tn10d(Tet) recF400::Tn5 | P1.IIB294 × IIB390 |
| IIB576 | As IIB386, recB268::Tn10 recR256::Tn10kan | P1.AM208 × IIB420 |
| IIB577 | As IIB386, recB268::Tn10 recF400::Tn5 | P1.IIB294 × IIB420 |
Media and growth conditions.
Bacteria were grown in high-salt Luria broth at 37°C with aeration to the early log phase (optical density at 600 nm, ∼0.2) and then used for UV irradiation and β-galactosidase assays.
UV irradiation.
After the cultures reached the early log phase, 10 ml of each culture was centrifuged, and the cells were resuspended in 10 ml of M/15 phosphate buffer. UV irradiation was performed by using a 50-J/m2 dose from a Hoefer UVC 500 UV cross-linker (Amersham Biosciences). After irradiation the cells were centrifuged, resuspended in 10 ml of high-salt Luria broth, and then incubated for 180 min at 37°C. The bacteria were irradiated at room temperature.
Measurement of β-galactosidase activity.
In the bacterial strains used for the β-galactosidase assay lacZ was fused to the sfiA regulatory region, and induction of the SOS response was assayed by determining the β-galactosidase activity as described by Miller (24); the results were expressed in Miller units. SOS induction was also described by using the induction factor (IF), which is the ratio of the β-galactosidase activity in UV-irradiated cells to the β-galactosidase activity in the control cells at a given time.
RESULTS
RecA loading activity is essential for SOS induction.
To test specifically whether RecA loading activity is required for SOS induction, we used strains with separately inactivated RecFOR-mediated RecA loading and RecBCD-mediated RecA loading, as well as strains in which both mechanisms of RecA loading were inactivated. All strains had a lacZ insertion downstream of the regulatory region of sfiA, a gene which belongs to the SOS regulon. The levels of lacZ expression were assayed by determining the β-galactosidase activity (expressed in Miller units); this activity was proportional to the level of SOS induction. Another measure of SOS induction is the induction factor, which is the ratio of the lacZ expression in cells treated with UV irradiation to the lacZ expression in cells not treated with UV irradiation.
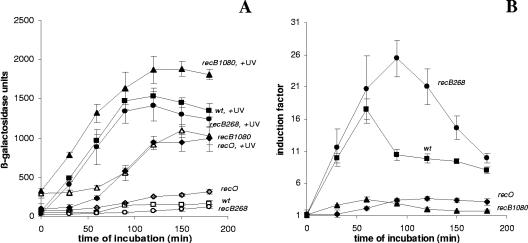
Figure 1 shows the levels of SOS expression in the wt strain and recB268, recB1080, and recO mutants. The results are expressed in Miller units (β-galactosidase units) for preparations that received and did not receive UV treatment (Fig. 1A) and as IFs (Fig. 1B). The highest level of SOS expression after UV irradiation was that of the recB1080 mutant, and the maximum value was more than 1,900 (β-galactosidase units). The level of SOS expression after UV irradiation in wt cells was lower (maximum value, ∼1,600 β-galactosidase units), but it was comparable to that of the recB1080 mutant. On the other hand, the levels of SOS expression after UV irradiation in the recB268 (maximum value, ∼1,400 β-galactosidase units) and recO (maximum value, ∼1,000 β-galactosidase units) mutants were lower than the levels of SOS expression in the wt strain and the recB1080 mutant.
FIG. 1.
(A) Induced levels of SOS expression (in β-galactosidase units) for the wt strain (strain IIB386) (▪) and recO (strain IIB392) (⧫), recB1080 (strain IIB388) (▴), and recB268 (strain IIB420) (•) mutants after UV irradiation during a 180-min incubation and basal levels of SOS expression (in β-galactosidase units) for the wt strain (strain IIB386) (□) and recO (strain IIB392) (⋄), recB1080 (strain IIB388) (▵), and recB268 (strain IIB420) (○) mutants during a 180-min incubation. (B) IFs (see Materials and Methods) for the wt strain (strain IIB386) (▪) and recO (strain IIB392) (▴), recB1080 (strain IIB388) (▴), and recB268 (strain IIB420) (•) mutants after UV irradiation during a 180-min incubation. The symbols indicate means of at least three independent experiments, and the error bars indicate standard deviations.
An important feature of the recB1080 mutant was that it had an increased basal level of SOS expression (maximum value, ∼1,100 β-galactosidase units) which was considerably higher than the basal levels of SOS expression in the wt strain (maximum value, ∼170 β-galactosidase units), the recO mutant (maximum value, ∼300 β-galactosidase units), and the recB268 mutant (maximum value, ∼150 β-galactosidase units) (Fig. 1A). However, the IFs were greatest for the recB268 (recB null) mutant (IF, ∼26) and wt cells (IF, ∼18), in which the SOS induction started immediately after UV irradiation (Fig. 1B). The reason for greater IFs for the recB268 mutant and wt cells was that the recB1080 single mutant had constitutive subinduction (high level of SOS expression without UV irradiation). In contrast, the recO single mutant showed a typical delayed SOS response that resulted in a maximum IF of around 3.5. The recB1080 single mutant showed levels of SOS induction (IF, around 3.5) similar to those of the recO mutant, but induction started immediately after UV irradiation (Fig. 1B).
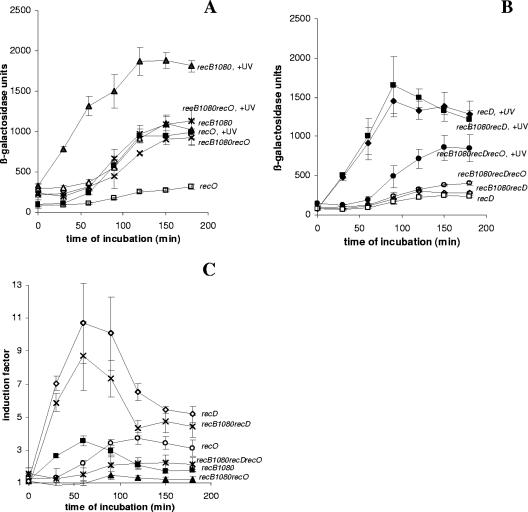
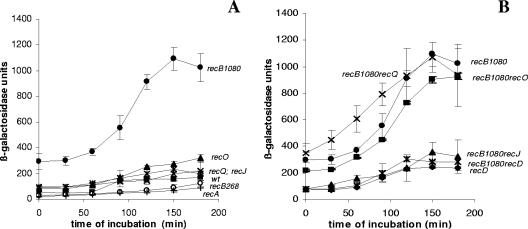
Figure 2A shows the SOS induction (expressed in β-galactosidase units) in a recB1080 recO strain in which both mechanisms of RecA loading were eliminated. The recO mutation reduced SOS induction in the recB1080 genetic background to the basal level of SOS expression in the recB1080 single mutant. This was consistent with our expectation that RecA loading activity is crucial for SOS induction. This decrease in SOS expression was due to the effect of UV irradiation rather than to the effect on constitutive SOS expression. Similar results were obtained with recB1080 recF and recB1080 recR double mutants (data not shown). To further test whether RecA loading activity is essential for the SOS signaling mechanism, we monitored SOS induction in a recB1080 recD double mutant and in a recB1080 recD recO triple mutant. It is known that RecB1080C(D−), an enzyme produced by recB1080 recD cells, possesses RecA loading activity due to inactivation of the RecD subunit, which is an inhibitor of RecA loading (1). On the basis of this knowledge, we expected that inactivation of recO in a recB1080 recD genetic background would allow delayed SOS induction to occur. The SOS induction in the recB1080 recD recO triple mutant was less than that in the recB1080 recD double mutant and was similar to the SOS expression in a recB1080 recO double mutant. It should be emphasized that the recD mutation reduced the basal level of SOS expression in a recB1080 background (Fig. 2B). However, the level of β-galactosidase activity after UV irradiation was considerably higher than the basal level, suggesting that the recB1080 recD recO strain was able to induce an SOS response. The results shown in Fig. 2A and B are expressed as IFs in Fig. 2C. When both mechanisms of RecA loading were eliminated (recB1080 recO double mutant), there was almost no SOS induction and the IF was around 1 (Fig. 2C). This was consistent with the requirement of RecA loading activity for SOS induction after UV irradiation. In agreement with this, the recB1080 recD recO triple mutant showed SOS induction (IF, ∼2.5) which was comparable to the SOS induction in recO (IF, ∼3.5) and recB1080 (IF, ∼3.5) single mutants (Fig. 2C). Although the triple mutant exhibited a smaller effect on SOS induction, the SOS induction curve still differs from the SOS induction curve for the recB1080 recO double mutant, in which there was almost no SOS induction (Fig. 2C). Similar results were obtained with recB1080 recD recF and recB1080 recD recR triple mutants (data not shown), as well as with the recB1067 allele (another mutation in the gene encoding the nuclease center of RecB) (13) instead of recB1080 (data not shown). All these data strongly suggest that RecA loading activity is essential for SOS induction.
FIG. 2.
(A) Induced levels of SOS expression (in β-galactosidase units) for recB1080 (strain IIB388) (▴), recB1080 recO (strain IIB401) (*), and recO (strain IIB392) (▪) mutants after UV irradiation during a 180-min incubation and basal levels of SOS expression (in β-galactosidase units) for recB1080 (strain IIB388) (▴), recB1080 recO (strain IIB401) (×), and recO (strain IIB392) (□) mutants during a 180-min incubation. (B) Induced levels of SOS expression (in β-galactosidase units) for recD (strain IIB395) (▪), recB1080 recD (strain IIB390) (▴), and recB1080 recD recO (strain IIB393) (•) mutants after UV irradiation during a 180-min incubation and basal levels of SOS expression (in β-galactosidase units) for recD (strain IIB395) (□), recB1080 recD (strain IIB390) (⋄), and recB1080 recD recO (strain IIB393) (○) mutants during a 180-min incubation. (C) IFs (see Materials and Methods) for recO (strain IIB392) (○), recB1080 (strain IIB388) (▪), recB1080 recO (strain IIB401) (▴), recB1080 recD (strain IIB390) (×), recB1080 recD recO (strain IIB393) (*), and recD (strain IIB395) (⋄) mutants after UV irradiation during a 180-min incubation. The symbols indicate means of at least three independent experiments, and the error bars indicate standard deviations.
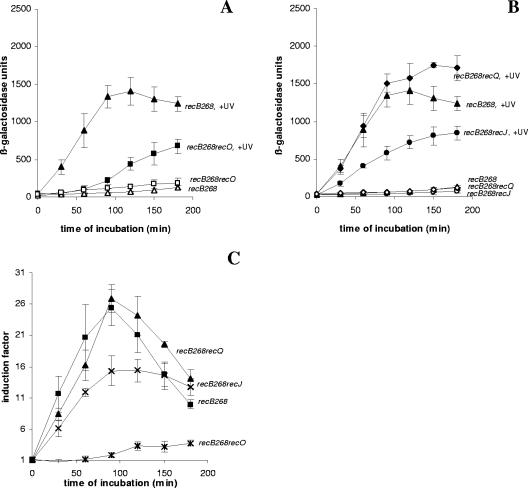
We also looked for SOS induction in a recB268 recO double mutant (Fig. 3). The recB268 allele is a null allele which inactivates all of the functions of the RecBCD enzyme, including RecA loading ability. This double mutant was also deficient in both known mechanisms of RecA loading, and its SOS induction was much lower than that of the recB268 single mutant (Fig. 3A) and wt cells (Fig. 1A). Unexpectedly, the low level of SOS induction was observed in this strain regardless of the inactivation of both known mechanisms of RecA loading. In Fig. 3C the data are expressed as IFs. Although the recB268 recO double mutant showed greater SOS induction (IF, ∼3.7) than the recB1080 recO double mutant (IF, ∼1) and the induction was comparable to the induction of the recO single mutant (IF, ∼4), the absolute level of expression of β-galactosidase after UV irradiation in the recB268 recO double mutant was considerably lower than that in the recB1080 recO double mutant and that in the recO single mutant.
FIG. 3.
(A) Induced levels of SOS expression (in β-galactosidase units) for recB268 (strain IIB420) (▴) and recB268 recO (strain IIB422) (▪) mutants after UV irradiation during a 180-min incubation and basal levels of SOS expression (in β-galactosidase units) for recB268 (strain IIB420) (▵) and recB268 recO (strain IIB422) (□) mutants during a 180-min incubation. (B) Induced levels of SOS expression (in β-galactosidase units) for recB268 recQ (strain IIB421) (⧫), recB268 (strain IIB420) (▴), and recB268 recJ (strain IIB423) (•) mutants after UV irradiation during a 180-min incubation and basal level of SOS expression (in β-galactosidase units) for recB268 recQ (strain IIB421) (⋄), recB268 (strain IIB420) (▵), and recB268 recJ (strain IIB423) (○) mutants during a 180-min incubation. (C) IFs (see Materials and Methods) for recB268 (strain IIB420) (▪), recB268 recO (strain IIB422) (*), recB268 recJ (strain IIB423) (×), and recB268 recQ (strain IIB421) (▴) mutants after UV irradiation during a 180-min incubation. The symbols indicate means of at least three independent experiments, and the error bars indicate standard deviations.
Effects of RecQ helicase and RecJ nuclease on SOS induction after UV irradiation.
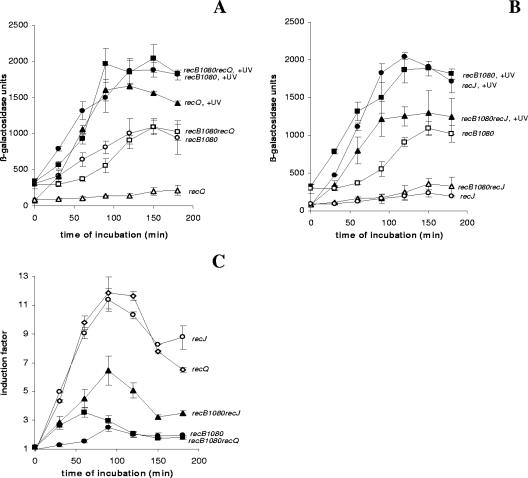
From the results described above, it is clear that RecA loading activity plays an important role in the SOS signaling mechanism after UV irradiation. We also wanted to test whether the RecQ helicase and the RecJ nuclease are important for SOS induction after UV irradiation. We compared SOS induction in the recB1080 single mutant with SOS induction in the recB1080 recQ and recB1080 recJ double mutants (Fig. 4). In Fig. 4A the results are expressed in β-galactosidase units. The recB1080 recQ double mutant exhibited SOS induction after UV irradiation similar to that of the recB1080 single mutant, implying that RecQ is not important for SOS induction in the recB1080 background (Fig. 4A). When SOS induction was expressed as an IF, the level of SOS induction of the recB1080 recQ strain (IF ∼2.5) was somewhat lower than the level of SOS induction of the recB1080 single mutant (IF, ∼3.5) (Fig. 4C). This can be explained by the fact that the recB1080 recQ double mutant had a slightly higher basal level of expression of the SOS response than the recB1080 single mutant had (Fig. 4A). On the other hand, the recJ mutation moderately reduced SOS induction in a recB1080 background (Fig. 4B). The decrease in SOS induction was caused by the effect of the recJ mutation on the basal level of SOS expression in the recB1080 background (compare the basal levels of SOS expression in the recB1080 single mutant and the recB1080 recJ double mutant in Fig. 4B). This is discussed below (see Fig. 5). When the results were expressed as IFs, the recB1080 recJ double mutant had a higher level of SOS induction (IF, ∼7) than the recB1080 single mutant (Fig. 4C) because the recB1080 recJ double mutant had a much lower basal level of expression of the SOS response. These results support the conclusion that the RecQ helicase is not required for SOS induction after UV irradiation in a recB1080 background and the conclusion that the RecJ nuclease is partially required due to its effect on basal SOS expression. We also examined the effects of recQ and recJ mutations in a recB268 background (Fig. 3B). The recB268 recQ mutant had a somewhat higher level of SOS induction expressed in β-galactosidase units, whereas the recB268 recJ double mutant had a lower level of SOS induction than the recB268 single mutant (Fig. 3B). Similar results were obtained when the data were expressed as IFs (Fig. 3C).
FIG. 4.
(A) Induced levels of SOS expression (in β-galactosidase units) for recB1080 (strain IIB388) (•), recB1080 recQ (strain IIB408) (▪), and recQ (strain IIB407) (▴) mutants after UV irradiation during a 180-min incubation and basal levels of SOS expression (in β-galactosidase units) for recB1080 (strain IIB388) (○), recB1080 recQ (strain IIB408) (□), and recQ (strain IIB407) (▵) mutants during a 180-min incubation. (B) Induced levels of SOS expression (in β-galactosidase units) for recB1080 (strain IIB388) (▪), recB1080 recJ (strain IIB410) (▴), and recJ (strain IIB409) (•) mutants after UV irradiation during a 180-min incubation and basal levels of SOS expression (in β-galactosidase units) for recB1080 (strain IIB388) (□), recB1080 recJ (strain IIB410) (▵), and recJ (strain IIB409) (○) mutants during a 180-min incubation. (C) IFs (see Materials and Methods) for recB1080 (strain IIB388) (▪), recB1080 recQ (strain IIB408) (•), recB1080 recJ (strain IIB410) (▴), recJ (strain IIB409) (○), and recQ (strain IIB407) (⋄) mutants after UV irradiation during a 180-min incubation. The symbols indicate means of at least three independent experiments, and the error bars indicate standard deviations.
FIG. 5.
(A) Basal levels of SOS expression (in β-galactosidase units) for recB1080 (strain IIB388) (•), wt (strain IIB386) (▪), recO (strain IIB392) (▴), recQ (strain IIB407) (×), recJ (strain IIB409) (*), recB268 (strain IIB420) (○), and recA (strain IIB424) (+) strains during a 180-min incubation. (B) Basal levels of SOS expression (in β-galactosidase units) for recB1080 (strain IIB388) (•), recB1080 recO (strain IIB401) (▪), recB1080 recJ (strain IIB410) (▴), recB1080 recQ (strain IIB408) (×), recD (strain IIB395) (⧫), and recB1080 recD (strain IIB390) (*) mutants during a180-min incubation. The symbols indicate means of at least three independent experiments, and the error bars indicate standard deviations.
RecJ nuclease is required for a constitutive level of SOS expression in the recB1080 mutant.
We found that the recB1080 mutant had a level of constitutive SOS expression response (about 1,100 β-galactosidase units) which was much higher than the levels of expression in the wt strain (∼170 β-galactosidase units) and in recO (∼300 β-galactosidase units), recQ (∼200 β-galactosidase units), recJ (∼200 β-galactosidase units), recB268 (∼150 β-galactosidase units), and recA (∼80 β-galactosidase units) mutants (Fig. 1A and 5A). A comparison of the abilities of strains to achieve basal levels of SOS expression is summarized in Fig. 5B. The basal level of SOS expression in the recB1080 recO double mutant was a little lower but comparable to the basal level of SOS expression in the recB1080 single mutant, implying that RecFOR-dependent RecA loading is not important for constitutive SOS expression in the recB1080 mutant. Similar results were obtained with recB1080 recF and recB1080 recR double mutants (data not shown). We also examined whether the RecJ nuclease and the RecQ helicase have any effect on the level of constitutive SOS expression in a recB1080 mutant. The basal level of SOS expression in a recB1080 recJ double mutant (∼350 β-galactosidase units) was considerably lower than the basal levels of SOS expression in recB1080 single mutants (∼1,100 β-galactosidase units), implying that RecJ is required for constitutive SOS expression (Fig. 5B). In contrast, RecQ is not important for constitutive SOS expression since the constitutive SOS expression in the recB1080 recQ double mutant was comparable to the constitutive SOS expression in the recB1080 single mutant (Fig. 5B). In addition, the level of constitutive SOS expression was also considerably lower in a recB1080 recD double mutant (∼300 β-galactosidase units), implying that the basal level of SOS expression in a recB1080 strain is also recD dependent.
DISCUSSION
In this study we examined the effect of specific inactivation of RecA loading activity of the RecBCD enzyme on the SOS signaling mechanism. The effect of any mutation on SOS induction, expressed in β-galactosidase units, can be examined at two levels. One level is the effect on SOS induction due to DNA-damaging agents (external DNA damage, expressed by the IF), and the other level is the effect on basal SOS expression (internal DNA damage). For example, from our results it is obvious that the effects of recFOR mutations in a recB1080 background are at the level of SOS induction due to external agents (in this case UV irradiation) (Fig. 2), whereas the effect of a recJ mutation is at the level of basal SOS expression (Fig. 4 and 5). We show here that when both mechanisms of RecA-ssDNA filament formation were inactivated, SOS induction was drastically reduced (see the curves for the recB1080 recO and recB268 recO double mutants in Fig. 2 and 3) compared to the SOS induction in the controls (recB1080, recB268, and wt strains). When either the RecBCD or RecFOR pathway is intact, SOS induction occurs, but the kinetics are different (Fig. 1). These results strongly support the hypothesis that active RecA loading onto ssDNA is important as part of the SOS signaling mechanism caused by an external agent (UV irradiation).
The main DNA damage after UV irradiation is intrastrand cross-links, and pyrimidine dimers are the most abundant type of DNA damage (8). Since pyrimidine dimers are noncoding lesions, DNA replication stops at the site of the DNA lesion and reinitiates downstream from the noncoding lesion (11). This results in production of an SSG to which the SSB protein binds, producing SSGs complexed with SSB (20). These complexes are the substrates for the RecFOR machinery which specifically replaces the SSB with RecA, producing the RecA-ssDNA filament on SSG (14, 20, 25). Then the RecA protein is activated and can catalyze the self-cleavage of the LexA repressor. Since SSGs are formed before the dsDNA ends, the SOS induction at an early time after UV irradiation is dependent on the RecFOR system (see the SOS induction in recB1080 and recB268 mutants shown Fig. 1, 2, and 3). In contrast, later, when dsDNA ends are formed as the result of replication fork collapse, SOS induction is dependent on the RecBCD enzyme (see the SOS induction in the recO mutant and in the recB1080 recD recO triple mutant shown Fig. 1 and 2). However, our results (see the presence of some SOS induction in the recB268 recO double mutant shown in Fig. 3A and C) also suggest that some additional RecA loading mechanism (active or passive) could exist in the cell.
In the recB1080 mutant recombination and dsDNA end repair are performed by a hybrid recombination mechanism in which helicase activity is provided by the RecB1080CD enzyme, 5′→3′ exonuclease is provided by RecJ, and RecA loading is provided by RecFOR proteins (12). Our results show that the absence of RecJ nuclease has a moderate effect on SOS induction after UV irradiation in a recB1080 genetic background (Fig. 4B). This effect is not as serious as the effect on recombination (12). Also, the absence of RecJ has a moderate effect on SOS induction in a recB268 background (Fig. 3B). Our results also show that the RecQ helicase is not required for SOS induction in the recB1080 (Fig. 4) and recB268 backgrounds (Fig. 3). In recB1080 and recB268 mutants RecA loading activity is dependent on the RecFOR function. Obviously, the most important substrates for SOS induction after UV irradiation are SSGs which are responsible for the high level of SOS induction before the appearance of dsDNA ends. Since SSGs are already regions of ssDNA, they do not need as much nuclease activity for the formation of ssDNA as is required for the processing of dsDNA ends. After UV irradiation, the RecJ nuclease together with the RecQ helicase could only extend the region of ssDNA within SSG, but these activities do not seem to be important for the further increase in SOS induction, as implied by our results. Possibly, the shorter RecA-ssDNA filaments are sufficient for SOS induction but not for recombination. This may be the explanation for the different genetic requirements for recombination and SOS induction with respect to a recJ mutation in a recB1080 background.
In this study we also found that the recB1080 mutant exhibited constitutive expression of the SOS regulon (Fig. 1A and 5). This phenotype is characteristic of some mutants that have mutations in genes with defects in replication, recombination, and repair, including rep, priA, dnaQ, recG, recN, dam, uvrD, etc. (5, 6, 18, 19, 26, 28, 31, 32, 34). Constitutive SOS expression is caused by endogenous DNA damage which can lead to ssDNA and to replication fork collapse (19). The level of constitutive SOS expression (as well as the level of SOS induction) depends on two processes. One of these processes is the ability of the bacterial strain to obtain SOS expression by particular gene functions which are involved in the SOS signaling mechanism, and the other is the ability to repair DNA damage (time of persistence of DNA damage within the cell). The recB1080 mutant is relatively resistant to UV irradiation, but it requires more time to complete repair, which is reflected in the lower viability of this mutant (12, 13). Consequently, in a recB1080 mutant DNA lesions are likely to persist for a longer time, and this can explain the constitutive SOS expression in a recB1080 mutant.
The genetic analysis of constitutive SOS expression in the recB1080 mutant produced unexpected results. First, we found that RecFOR-mediated RecA-ssDNA filament formation is not important for constitutive SOS expression (see the constitutive SOS expression in a recB1080 recO double mutant shown Fig. 5). This result implies that some additional RecA loading mechanism independent of RecBCD and RecFOR could be present in recB1080 cells. If the assumption that shorter RecA-ssDNA filaments are sufficient for SOS expression is correct, then this type of RecA loading could be a passive process. Second, we found that constitutive SOS expression in the recB1080 mutant is dependent on the RecJ nuclease and that it is not present in a recB1080 recD double mutant (Fig. 5). This is the opposite of what we found for SOS induction due to an external agent (UV irradiation). It is possible that when the number of DNA lesions is small (basal level of endogenous DNA damage), the role of the RecJ nuclease and constitutive RecA loading activity independent of Chi (recB1080 recD double mutant) is more critical than the role after UV irradiation, when the number of lesions and SSGs is much higher. Perhaps collapsed replication forks are the main endogenous DNA lesions, and the nuclease activities of RecJ and RecBCD could be important for the processing of these forks. An alternative explanation for the importance of RecJ in constitutive SOS expression is that RecJ is somehow involved in RecA loading independent of RecBCD and RecFOR (13).
Concluding remarks.
In conclusion, we found that active RecA loading either by RecFOR or by RecBCD is necessary for SOS induction after UV irradiation. We also found that the absence of RecJ nuclease has a moderate effect on SOS induction in a recB1080 mutant, whereas the RecQ helicase is not important. In addition, we found that RecJ (but not RecFOR and RecQ) is important for the constitutive SOS expression in a recB1080 mutant, and this could be the explanation for the moderate effect of a recJ mutation on SOS induction caused by UV irradiation. The question which remains to be answered is the mechanism of RecA loading responsible for constitutive SOS induction in the recB1080 mutant.
Acknowledgments
We are grateful to Mary Sopta (Ruðer Bošković Institute) for critical reading of the manuscript.
This work was supported by the Croatian Ministry of Science (grant 0098 070).
REFERENCES
- 1.Amundsen, S. K., A. F. Taylor, and G. R. Smith. 2000. The RecD subunit of the Escherichia coli RecBCD enzyme inhibits RecA loading, homologous recombination, and DNA repair. Proc. Natl. Acad. Sci. USA 97:7399-7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, D. G., J. J. Churchill, and S. C. Kowalczykowski. 1999. A single mutation, recBD1080A, eliminates RecA protein loading but not Chi recognition by RecBCD enzyme. J. Biol. Chem. 274:27139-27144. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann, B. J. 1996. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, p. 2460-2488. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 4.Chaudhury, A. M., and G. R. Smith. 1985. Role of Escherichia coli RecBC enzyme in SOS induction. Mol. Gen. Genet. 201:525-528. [DOI] [PubMed] [Google Scholar]
- 5.Chua, K. L., Y. K. Mak, and P. Oliver. 1993. Expression of the recA gene in recombination-deficient (rec−) strains of Escherichia coli. Biochimie 75:775-783. [DOI] [PubMed] [Google Scholar]
- 6.Dunman, P. M., L. Ren, M. S. Rahman, V. A. Palejwala, H. S. Murphy, M. R. Volkert, and M. Z. Humayun. 2000. Escherichia coli cells defective for the recN gene display constitutive elevation of mutagenesis at 3,N(4)-ethenocytosine via an SOS-induced mechanism. Mol. Microbiol. 37:680-686. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez de Henestrosa, A. R., T. Ogi, S. Aoyagi, D. Chafin, J. J. Hayes, H. Ohmori, and R. Woodgate. 2000. Identification of additional genes belonging to the LexA regulon in Escherichia coli. Mol. Microbiol. 35:1560-1572. [DOI] [PubMed] [Google Scholar]
- 8.Friedberg, E. C., G. C. Walker, and W. Siede. 1995. DNA repair and mutagenesis, p. 407-464. ASM Press, Washington, D.C.
- 9.Ganesan, A. K., and P. C. Seawell. 1975. The effect of lexA and recF mutations on post-replication repair and DNA synthesis in Escherichia coli K-12. Mol. Gen. Genet. 141:189-205. [DOI] [PubMed] [Google Scholar]
- 10.Hegde, S., J. S. Sandler, A. J. Clark, and M. V. Madiraju. 1995. recO and recR mutations delay induction of the SOS response in Escherichia coli. Mol. Gen. Genet. 246:254-258. [DOI] [PubMed] [Google Scholar]
- 11.Heller, R. C., and K. J. Marians. 2006. Replication fork reactivation downstream of a blocked nascent leading strand. Nature 439:557-562. [DOI] [PubMed] [Google Scholar]
- 12.Ivančić-Baće, I., P. Peharec, S. Moslavac, N. Škrobot, E. Salaj-Šmic, and K. Brčić-Kostić. 2003. RecFOR function is required for DNA repair and recombination in a RecA loading-deficient recB mutant of Escherichia coli. Genetics 163:485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jockovich, M. E., and R. S. Myers. 2001. Nuclease activity is essential for RecBCD recombination in Escherichia coli. Mol. Microbiol. 41:949-962. [DOI] [PubMed] [Google Scholar]
- 14.Kuzminov, A. 1999. Recombinational repair of DNA damage in Escherichia coli and bacteriophage lambda. Microbiol. Mol. Biol. Rev. 63:751-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis, L. K., G. R. Harlow, L. A. Greg-Jolly, and D. W. Mount. 1994. Identification of high affinity binding sites for LexA which define new DNA damage-inducible genes in Escherichia coli. J. Mol. Biol. 241:507-523. [DOI] [PubMed] [Google Scholar]
- 16.Little, J. W., D. W. Mount, and C. R. Yanisch-Perron. 1981. Purified LexA protein is a repressor of the recA and lexA genes. Proc. Natl. Acad. Sci. USA 78:4199-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little, J. W., and D. W. Mount. 1982. The SOS regulatory system of Escherichia coli. Cell 29:11-22. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd, R. G., and C. Buckman. 1991. Genetic analysis of the recG locus of Escherichia coli K-12 and of its role in recombination and DNA repair. J. Bacteriol. 173:1004-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCool, J. D., E. Long, J. F. Petrosino, H. A. Sandler, S. M. Rosenberg, and S. J. Sandler. 2004. Measurement of SOS expression in individual Escherichia coli K-12 cells using fluorescence microscopy. Mol. Microbiol. 53:1343-1357. [DOI] [PubMed] [Google Scholar]
- 20.McGlynn, P. 2004. Links between DNA replication and recombination in prokaryotes. Curr. Opin. Genet. Dev. 14:107-112. [DOI] [PubMed] [Google Scholar]
- 21.McPartland, A., L. Green, and H. Echols. 1980. Control of recA gene RNA in E. coli: regulatory and signal genes. Cell 20:731-737. [DOI] [PubMed] [Google Scholar]
- 22.Meddows, T. R., A. P. Savory, and R. G. Lloyd. 2004. RecG helicase promotes DNA double-strand break repair. Mol. Microbiol. 52:119-132. [DOI] [PubMed] [Google Scholar]
- 23.Michel, B. 2005. After 30 years of study, the bacterial SOS response still surprises us. PloS Biol. 3:e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, J. H. 1992. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Morimatsu, K., and S. C. Kowalczykowski. 2003. RecFOR proteins load RecA protein onto gapped DNA to accelerate DNA strand exchange: a universal step of recombinational repair. Mol. Cell 11:1337-1347. [DOI] [PubMed] [Google Scholar]
- 26.O'Reilly, E. K., and K. N. Kreuzer. 2004. Isolation of SOS constitutive mutants of Escherichia coli. J. Bacteriol. 186:7149-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paškvan, I., E. Salaj-Šmic, I. Ivančić-Baće, K. Zahradka, Ž. Trgovčević, and K. Brčić-Kostić. 2001. The genetic dependence of RecBCD-Gam mediated double strand end repair in Escherichia coli. FEMS Microbiol. Lett. 205:299-303. [DOI] [PubMed] [Google Scholar]
- 28.Peterson, K. R., K. F. Wertman, D. W. Mount, and M. G. Marinus. 1985. Viability of Escherichia coli K-12 DNA adenine methylase (dam) mutants requires increased expression of specific genes in the SOS regulon. Mol. Gen. Genet. 201:14-19. [DOI] [PubMed] [Google Scholar]
- 29.Radman, M. 1975. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci. 5A:355-367. [DOI] [PubMed] [Google Scholar]
- 30.Renzette, N., N. Gumlaw, J. T. Nordman, M. Krieger, S.-P. Yeh, E. Long, R. Centore, R. Boonsombat, and S. J. Sandler. 2005. Localization of RecA in Escherichia coli K-12 using RecA-GFP. Mol. Microbiol. 57:1074-1085. [DOI] [PubMed] [Google Scholar]
- 31.SaiSree, L., M. Reddy, and J. Gowrishankar. 2000. lon incompatibility associated with mutations causing SOS induction: null uvrD alleles induce an SOS response in Escherichia coli. J. Bacteriol. 182:3151-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simić, D., B. Vuković-Gačić, A. Ajanović, and J. Knežević-Vukčević. 1991. Activation of RecA protein in recombination-deficient strains of Escherichia coli following DNA-damaging treatments. Mutat. Res. 254:255-262. [DOI] [PubMed] [Google Scholar]
- 33.Spies, M., and S. C. Kowalczykowski. 2006. The RecA binding locus of RecBCD is a general domain for recruitment of DNA strand exchange proteins. Mol. Cell 21:573-580. [DOI] [PubMed] [Google Scholar]
- 34.Uzest, M., S. D. Ehrlich, and B. Michel. 1995. Lethality of rep recB and rep recC double mutants of Escherichia coli. Mol. Microbiol. 17:1177-1188. [DOI] [PubMed] [Google Scholar]
- 35.Thoms, B., and W. Wackernagel. 1987. Regulatory role of recF in the SOS response of Escherichia coli: impaired induction of SOS genes by UV irradiation and nalidixic acid in a recF mutant. J. Bacteriol. 169:1731-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker, G. C. 1984. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol. Rev. 48:60-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, J., R. Chen, and D. A. Julin. 2000. A single nuclease active site of the Escherichia coli RecBCD enzyme catalyzes single-stranded DNA degradation in both directions. J. Biol. Chem. 275:507-513. [DOI] [PubMed] [Google Scholar]
- 38.Whitby, M. C., and R. G. Lloyd. 1995. Altered SOS induction associated with mutations in recF, recO and recR. Mol. Gen. Genet. 246:174-179. [DOI] [PubMed] [Google Scholar]
- 39.Witkin, E. M. 1976. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol. Rev. 40:867-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu, M., J. Souaya, and D. A. Julin. 1998. Identification of the nuclease active site in the multifunctional RecBCD enzyme by creation of a chimeric enzyme. J. Mol. Biol. 283:797-808. [DOI] [PubMed] [Google Scholar]