Abstract
Brucella periplasmic cyclic β-1,2-glucan plays an important role during bacterium-host interaction. Nuclear magnetic resonance spectrometry analysis, thin-layer chromatography, and DEAE-Sephadex chromatography were used to characterize Brucella abortus cyclic glucan. In the present study, we report that a fraction of B. abortus cyclic β-1,2-glucan is substituted with succinyl residues, which confer anionic character on the cyclic β-1,2-glucan. The oligosaccharide backbone is substituted at C-6 positions with an average of two succinyl residues per glucan molecule. This O-ester-linked succinyl residue is the only substituent of Brucella cyclic glucan. A B. abortus open reading frame (BAB1_1718) homologous to Rhodobacter sphaeroides glucan succinyltransferase (OpgC) was identified as the gene encoding the enzyme responsible for cyclic glucan modification. This gene was named cgm for cyclic glucan modifier and is highly conserved in Brucella melitensis and Brucella suis. Nucleotide sequencing revealed that B. abortus cgm consists of a 1,182-bp open reading frame coding for a predicted membrane protein of 393 amino acid residues (42.7 kDa) 39% identical to Rhodobacter sphaeroides succinyltransferase. cgm null mutants in B. abortus strains 2308 and S19 produced neutral glucans without succinyl residues, confirming the identity of this protein as the cyclic-glucan succinyltransferase enzyme. In this study, we demonstrate that succinyl substituents of cyclic β-1,2-glucan of B. abortus are necessary for hypo-osmotic adaptation. On the other hand, intracellular multiplication and mouse spleen colonization are not affected in cgm mutants, indicating that cyclic-β-1,2-glucan succinylation is not required for virulence and suggesting that no low-osmotic stress conditions must be overcome during infection.
Brucella spp. are gram-negative, facultative intracellular bacteria that cause a chronic zoonotic disease known worldwide as brucellosis. Brucella abortus, the etiological agent of bovine brucellosis, causes abortion and infertility in cattle and undulant fever in humans (17). The human disease is also caused by Brucella melitensis, Brucella suis, and Brucella canis. Brucella spp. are intracellular pathogens that invade and proliferate within host cells; virulence is associated with the ability to multiply inside professional and nonprofessional phagocytic cells (40).
Cyclic glucans appear to be important as intrinsic components of gram-negative bacterial cell envelopes (7). Beyond their common features, glucans show an unexpected structural diversity (4). The role of cyclic glucan in gram-negative bacterial cell envelopes is still controversial. Cyclic-glucan mutants have a highly pleiotropic phenotype, suggesting an overall alteration of their cell envelope properties. Mutants deficient in cyclic-glucan production were found to be attenuated or avirulent compared to their wild-type parent strains, suggesting that the cyclic glucan is an important virulence attribute for the bacteria in their interaction with eukaryotic hosts.
In different bacterial species, glucans are substituted with different nonglycosidic residues, such as phosphoglycerol, phosphoethanolamine, phosphocholine, acetyl, succinyl, and methylmalonyl. The degree of substitution varies among species; for example, cyclic glucans produced by the broad-host-range Rhizobium sp. strain GRH2 and by certain strains of Rhizobium leguminosarum contain no anionic substituents (30, 45). Bradyrhizobium japonicum cyclic β-1,6-β-1,3-glucans are neutral but substituted with the zwitterionic phosphocholine (35). On the other hand, more than 90% of Sinorhizobium meliloti glucan and the broad-host-range Rhizobium sp. strain NGR234 cyclic β-1,2-glucan fraction are anionic (3, 32).
The stage of growth also influences the degree of substitution of cyclic β-1,2-glucans; for example, Geiger et al. (23) have shown that in S. meliloti 1021 the conversion of neutral glucans into anionic glucans occurs mostly during the exponential phase of growth. Thus, depending on the bacterial strain and growth conditions, glucans can be nonsubstituted or substituted with neutral or anionic substitutents.
Surprisingly, there is no correlation between the structure of the glucan and the nature of the substituents. Cyclic glucans of Ralstonia solanacearum (43) and Xanthomonas campestris (43, 44) and the linear glucan of Pseudomonas syringae (42) are devoid of substituents. Cyclic glucans of Azospirillum brasilense (1) are substituted only with succinyl residues, and cyclic glucans of B. japonicum are substituted only with phosphocholine (35). Cyclic glucan from S. meliloti and Agrobacterium tumefaciens are substituted with both succinyl and phosphoglycerol residues (7), while the cyclic glucan of Rhodobacter sphaeroides (41) and linear glucans of Erwinia chrysanthemi (15) are substituted with succinyl and acetate residues. Osmoregulated periplasmic glucans (OPG) originating from Escherichia coli (linear glucans) are the only ones known to be substituted with three different substituents, phosphoglycerol, phosphoethanolamine, and succinyl residues (27).
The transfer of phosphoglycerol has been shown to occur in the periplasmic compartment both in E. coli (5) and in S. meliloti (8). Succinyl transfer was also shown to occur in the periplasmic compartment in S. meliloti (6). In E. coli and R. sphaeroides, the genes mdoC and opgC, respectively, encode predicted membrane proteins required for wild-type succinyl modification of OPG (14, 29). E. coli has two phosphoglycerol transferases of glucans (I and II), one membrane bound and the second periplasmic. The mdoB gene encodes phosphoglycerol transferase I. In S. meliloti, the cgmB gene encodes a soluble protein required for phosphoglycerol modification of cyclic glucan (6). Nothing has been reported about the transfer of the other substituents to glucans.
Brucella spp. produce periplasmic cyclic glucans. They consist of a cyclic backbone with a degree of polymerization ranging from 17 to 25, in which all the glucose units are linked by β-1,2 linkages (11). In our laboratory, we previously cloned, sequenced, and disrupted the gene coding for the cyclic β-1,2-glucan synthetase (cgs) (9, 24) and cyclic β-1,2-glucan transporter (cgt) (36) responsible for the synthesis and transport to the periplasm of cyclic β-1,2-glucan, respectively. Both cgs and cgt mutants showed reduced survival in BALB/c mouse spleen tissues and impeded intracellular multiplication (9, 36), indicating that the presence of cyclic glucan in the periplasm is required for full B. abortus virulence. Furthermore, Arellano-Reynoso et al. (2) recently determined that Brucella cyclic glucan acts on host cell membranes at the levels of lipid rafts controlling vacuole maturation, avoiding lysosome fusion and allowing Brucella to reach its replicative niche.
In this report, we describe how a fraction of B. abortus cyclic β-1,2-glucan is substituted with O-succinyl residues, which confer on the cyclic β-1,2-glucans an anionic character. We describe the molecular and functional characterization of a new gene (cgm) coding for the enzyme named cyclic glucan modifier that catalyzes the succinylation of B. abortus cyclic β-1,2-glucan.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used are listed in Table 1. E. coli strains were grown on Luria broth (LB) (37). B. abortus strains were grown in brucella broth (BB) (Difco Laboratories, Detroit, Mich). If necessary, media were supplemented with the appropriate antibiotics at the following concentrations: kanamycin (Km), 50 μg/ml; ampicillin (Amp), 100 μg/ml; and nalidixic acid (Nal), 5 μg/ml. Merodiploid strains for complementation analysis were obtained by biparental mating as previously described (21).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Phenotype or genotypea | Reference or source |
|---|---|---|
| Strains | ||
| E. coli XL1-blue MRF | Δ(mcrA)183 Δ(mcrB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac[F′ proAB lacIqZΔM15 Tn10(Tetr)] | 26 |
| E. coli S17.1(λpir) | λ lysogenic S17.1 derivative producing π protein for replication of plasmids carrying oriR6K (Nals) | K. N. Timmis |
| B. abortus | ||
| 2308 | Virulent; field isolated; wild type; Nalr erythritolr | 38 |
| Cgm08 | B. abortus 2308 cgm mutant; cgm::Kmr | This study |
| Cgm08(pBB4cgm) | B. abortus 2308 cgm mutant with plasmid pBB4cgm; Kmr Ampr | This study |
| B. abortus | ||
| S19 | Vaccine strain; Nalr erythritols; naturally occcuring derivative of B. abortus 2308 | 38 |
| Cgm19 | B. abortus S19 cgm mutant; cgm::Kmr | This study |
| Cgm19(pBB4cgm) | B. abortus S19 cgm mutant with plasmid pBB4cgm; Kmr Ampr | This study |
| Plasmids | ||
| pBBR1MCS-4 | Broad-host-range cloning vector (Ampr) | 28 |
| pBB4cgm | pBBR1MCS-4 containing B. abortus cgm gene; Ampr | This study |
| pvTcgm | T-easy vector containing B. abortus cgm gene; Ampr | This study |
Ampr, ampicillin resistance: Cbr, carbenicillin resistance: Kmr, kanamycin resistance, Nalr, nalidixic acid resistance.
All the protocols using live brucellae were performed in a biosafety level 3 laboratory facility.
Cloning and DNA sequencing.
The putative B. abortus cgm gene was amplified from B. abortus S2308 genomic DNA by PCR using the primers cgm-fw. (5′-TCATTTTCGGTGCGTGTTTT-3′) and cgm-rev. (5′-CATAAAAAAAGAGCCGCAAG-3′), designed according to the sequence of a B. abortus gene highly homologous to the R. sphaeroides OpgC gene (12, 14).
The amplified products were ligated to T-easy vector (Promega). The resulting plasmid containing a 1.8-kb fragment was named pvTcgm. DNA sequencing was carried out by the dideoxy method with an automated model 373 DNA sequencer (Perkin-Elmer Applied Biosystems Division, Foster City, CA) according to the manufacturer's instructions.
Isolation of cyclic β-1,2-glucan from cells.
Cells from 100 ml of stationary-phase cultures (optical density at 600 nm [OD600], 3.2) grown for 48 h at 37°C (250 rpm) were harvested by centrifugation at 10,000 × g for 10 min. The pellets were extracted with ethanol (70% ethanol; 1 h at 37°C). The ethanolic extracts were centrifuged, and the supernatants were dried in a speed-vac centrifuge and subjected to gel filtration on Bio-Gel P4 columns (78 by 1.8 cm; Bio-Rad Laboratories, Richmond, Calif.). The glucans were eluted with 0.1 M pyridine-acetate buffer (pH 5.5). Fractions of 1.5 ml were collected, and carbohydrates were detected by the anthrone-sulfuric acid method (20).
For preparative purposes, 6-liter cultures of B. abortus S19 were extracted with 70% ethanol, and the cyclic β-1,2-glucans were subjected to Bio-Gel P4 gel filtration as described above. The glucans were eluted with acetic acid (5%). The anthrone-sulfuric-acid-positive material was collected from six columns. Fractions of a compound with elution volumes corresponding to the cyclic β-1,2-glucans were pooled and concentrated. The glucans were further purified by high-performance liquid chromatography (HPLC).
HPLC.
Products recovered from the preparative Bio-Gel P4 column were subjected to HPLC on a C18 ODS2 Pharmacia column (25 cm by 4 mm). After application of each sample, the column was washed with water (25 ml) and the glucans were eluted in isocratic form with 40 ml of 20% methanol at a flow rate of 0.5 ml min−1 at room temperature. Fractions of 1 ml were collected. The glucans eluted in fractions 4 and 5.
DEAE-Sephadex chromatography.
Materials applied to DEAE-Sephadex A25 columns were batch eluted with 6 ml of water and with 6 ml each of 50, 100, 250, and 500 mM NaCl. Fractions of 1.5 ml were collected, and carbohydrates were detected by the anthrone-sulfuric acid method.
Chemical treatments.
Cyclic β-1,2-glucan recovered from Bio-Gel P4 columns was successively treated with HCl (10 mM) at 100°C for 90 min and with NaOH (0.1 N) at 37°C for 30 min as previously described for removing polysaccharide nonsugar substituents (25).
TLC of cyclic β-1,2-glucan.
Cells from cultures grown for 48 h were harvested by centrifugation at 10,000 × g for 10 min. Cyclic β-1,2-glucans were extracted from the cell pellets with ethanol (70% ethanol; 1 h at 37°C). The ethanolic extracts were centrifuged in an Eppendorf centrifuge, and the supernatants were dried in a speed-vac centrifuge. The extracted glucans were dissolved in 70% ethanol and subjected to thin-layer chromatography (TLC) as described previously (10). The TLC plates were developed by spraying them with 5% sulfuric acid in ethanol and heating them for 10 min at 120°C.
NMR analysis.
Glucans were purified by Bio-Gel P4 chromatography and HPLC on a C18 silica column as described previously. 1H, 13C, and 31P nuclear magnetic resonance (NMR) spectroscopy was performed with a Bruker AM 500-MHz spectrometer at 30°C.The samples were dissolved in D2O. Chemical shifts were measured relative to the external 1,4-dioxane standard.
Construction of a B. abortus β-1,2-glucan succinyltransferase mutant.
An MscI DNA fragment (675 bp) of cgm was digested from pvTcgm. The deleted plasmid was ligated to a 1.3-kb HincII fragment containing a kanamycin resistance cassette (33). The recombinant suicide plasmid was electroporated into B. abortus S19 and B. abortus S2308 strains. Transconjugants were selected in BB agar with Km (50 μg/ml). Double-crossover events were selected by streaking colonies in duplicate on BB agar with Km (50 μg/ml) and on BB agar with Amp (100 μg/ml). Km-resistant, Amp-sensitive clones were selected as possible double recombinants. Putative double recombinants were confirmed by colony PCR using the primers cgm-fw. (5′-TCATTTTCGGTGCGTGTTTT-3′) and cgm-rev. (5′-CATAAAAAAAGAGCCGCAAG-3′). Genetic complementation of the cgm mutants was carried out with plasmid pBB4cgm, containing a wild-type copy of the B. abortus cgm gene. Plasmid pBB4cgm was introduced into the mutants by biparental mating using E. coli S17.1 as the donor strain (9, 16).
Effects of osmolarity on growth in liquid and on solid media.
All strains were grown in LB with 170 mM NaCl (LB-NaCl) until stationary phase. Cultures were harvested by centrifugation (12,500 × g for 5 min) and washed twice with phosphate-buffered saline (PBS), and the cells were suspended in LB or LB-NaCl at an OD600 of 0.90. For studies in liquid media, cell suspensions were diluted 1/100 in LB or LB-NaCl and incubated at 37°C with continuous stirring (250 rpm); samples were taken at different times, and CFU/ml were determined after appropriate dilution on BB agar plates. For studies on solid media, after being washed with PBS, the cells were suspended to an OD600 of 0.9 and serially diluted in PBS, and 10 μl of the dilutions was spotted on LB agar or LB agar with one of the following additions: 170 mM NaCl, 170 mM KCl, 110 mM K2SO4, or 340 mM sucrose. The plates were incubated at 37°C for 5 days before being read.
Cell culture and infection assay.
HeLa cells were cultured at 37°C in a 5% CO2 atmosphere in minimal essential medium (Gibco, Paisley, Scotland) supplemented with 2 mM glutamine and 5% fetal calf serum without antibiotics (cell culture medium). Infection of cells with different Brucella strains was performed at a multiplicity of infection of 100 as previously described (39). The murine-macrophage-like J774 cell line was used to test phagocytic cells. Cells at 105/well were infected with a bacterial suspension prepared as described above for HeLa cells except for the following changes: the culture medium was RPMI 1640 (Gibco, Paisley, Scotland) supplemented with 5% fetal bovine serum (Gibco), and the multiplicity of infection was 50.
Virulence in mice.
Virulence was determined by quantitating the survival of the strains in mouse spleens at different times postinfection, as previously described (16). Groups of 9-week-old female BALB/c mice were injected intraperitoneally with 104 CFU of B. abortus 2308, B. abortus S19, and their isogenic mutants B. abortus Cgm08 and B. abortus Cgm19 in 0.2 ml of sterile PBS. At 2, 4, and 12 weeks postinfection, the animals were sacrificed, and the spleens were removed, weighed, homogenized in sterile 150 mM NaCl solution, serially diluted, and plated onto BB agar with the appropriate antibiotics to determine the number of CFU per spleen.
Nucleotide sequence accession number.
The sequence of the B. abortus cyclic-β-1,2-glucan succinyltransferase gene (cgm) has been assigned GenBank accession number AY553720.
RESULTS
Brucella sp. cyclic β-1,2-glucans are partially modified with O-ester-linked anionic substituents.
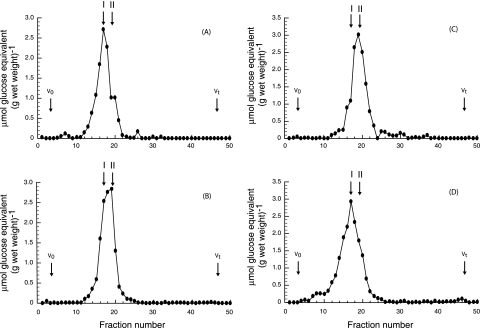
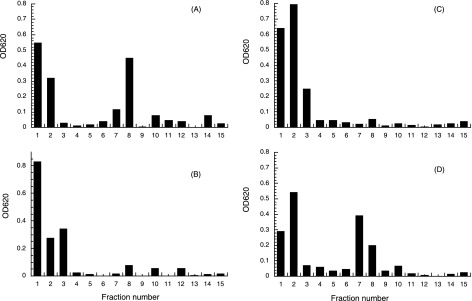
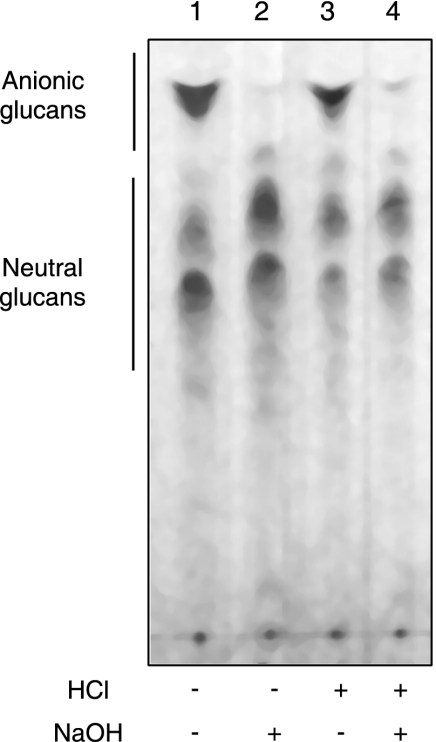
Cells of B. abortus 2308 grown in BB medium were extracted with 70% ethanol. The elution profile from gel filtration chromatography using a Bio-Gel P4 column showed that under these conditions, one main sugar-containing compound was extracted (Fig. 1A). The elution volume of this material corresponds to the previously characterized cyclic β-1,2-glucans of B. abortus (10). Cellular β-1,2-glucans recovered from the Bio-Gel P4 column (fractions 13 to 22) were subjected to DEAE-Sephadex chromatography (Fig. 2). A fraction (approximately 50%) percolated through the column, indicating that the glycan was neutral, while the rest of the sample eluted as negatively charged glucans (Fig. 2A). In order to determine the nature of the linkage between cyclic β-1,2-glucans and the anionic substituent, the 70% ethanol extracts were subjected to different treatments and TLC analysis. As shown in Fig. 3, mild alkali treatment completely abolished the presence of the anionic fraction, and no effect was observed after acidic treatment. After the alkali treatment, the elution volume of the main peak was increased (Fig. 1A and B) and became neutral according to DEAE-Sephadex chromatography (Fig. 2B). Since all the anionic glucans were converted to neutral after this treatment, we concluded that B. abortus cyclic β-1,2-glucans contain only O-ester-linked anionic substituents. The same results were obtained with cyclic β-1,2-glucans obtained from B. abortus strain S19, B. melitensis, B.suis, B. ovis, and B. canis. Furthermore, the anionic cyclic glucans of B. abortus recovered from TLC and alkali treatment had degrees of polymerization identical to those of the neutral glucans, suggesting that there is not a preference for a particular degree of polymerization for anionic modification (data not shown).
FIG. 1.
Bio-Gel P4 chromatography of cyclic β-1,2-glucans accumulated in vivo by wild-type and mutant strains. Cells from 100-ml cultures were harvested and extracted with 70% ethanol. The extracts were subjected to chromatography on Bio-Gel P4 columns (78 by 1.8 cm). The glucans eluted with 0.1 M pyridine-acetate buffer (pH 5.5). Fractions of 1.5 ml were collected, and carbohydrates were expressed as μmol glucose equivalents (g wet weight)−1. (A) B. abortus 2308 cellular glucans. (B) B. abortus 2308 cellular glucans after mild alkali treatment (0.1 N NaOH for 30 min at 37°C). (C) B. abortus Cgm08 cellular glucans. (D) B. abortus Cgm(pBB4cgm) cellular glucans. V0, void volume; Vt, total volume; I, anionic glucan; II, neutral glucan.
FIG. 2.
DEAE-Sephadex chromatography of cellular glucans accumulated by wild-type and mutant strains. Glucans were recovered from Bio-Gel P4 columns (fractions 13 to 22) and subjected to chromatography on DEAE-Sephadex columns (0.25 by 3 cm). The glucans were eluted with 1.5 ml water (fractions 1 to 3), 1.5 ml 0.05 M NaCl (fractions 4 to 6), 1.5 ml 0.1 M NaCl (fractions 7 to 9), 1.5 ml 0.25 M NaCl (fractions 10 to 12), and 0.5 M NaCl (fractions 13 to 15). Fractions of 0.5 ml were collected, and carbohydrates were measured by the anthrone-sulfuric acid method (20). (A) B. abortus 2308 cellular glucans. (B) B. abortus 2308 cellular glucans after mild alkali treatment (0.1 N NaOH for 30 min at 37°C). (C) B. abortus Cgm08 cellular glucans. (D) B. abortus Cgm08(pBB4cgm) cellular glucans.
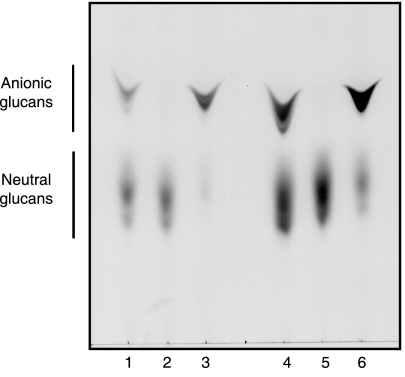
FIG. 3.
Thin-layer chromatographic analysis of ethanol extracts of B. abortus 2308. Ethanolic extracts were either directly applied to thin-layer chromatography plates (lane 1) or first subjected to mild alkali treatment (lane 2), acidic treatment (lane 3), or both treatments (lane 4). Alkali treatment resulted in selective removal of O-ester linkages.
B. abortus anionic cyclic β-1,2-glucans contain succinyl substituents.
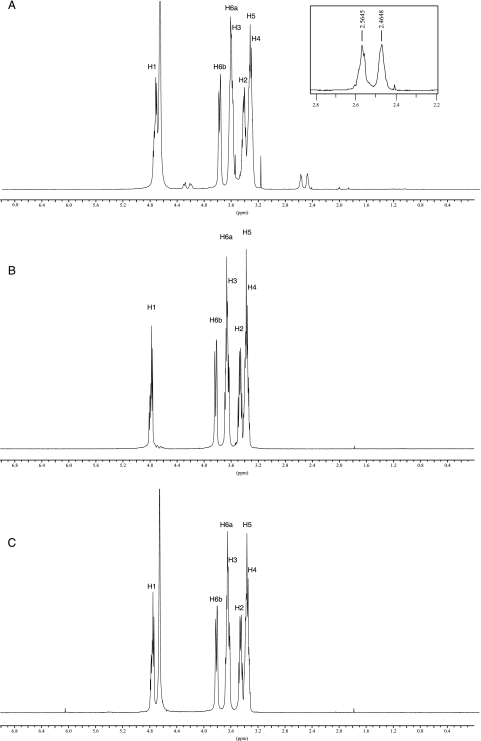
In order to determine the chemical nature of the anionic substituent of B. abortus cyclic β-1,2-glucan, cell-associated glucans were purified by Bio-Gel P4 column and C18 silica chromatography, eluting with 20% methanol. Purified glucans in D2O were subjected to NMR spectroscopy at room temperature using a Bruker AM-500 spectrometer. The 1H-NMR spectrum of the glycan sample prepared from the wild-type strain showed the presence of two clear and prominent signals between 2.4 and 2.6 ppm (Fig. 4A) that could be identified as succinyl residues. These signals corresponded unambiguously to the methylene protons of succinate. Furthermore, the signals between 4.25 and 4.35 ppm were attributed to H-6 and H-6′, respectively, of glucose residues, with succinate linked to C-6 via an ester bond (31). In contrast, in the 1H-NMR spectrum of the glycan sample that was treated with alkali, chemical shifts between 2.4 and 2.6 and between 4.25 and 4.35 that were indicative of succinyl residues were not detected (Fig. 4B). Therefore, based on the above-mentioned evidence from NMR analysis, the wild-type cyclic glucan contains succinyl substituents. In the anomeric region of the 1H-NMR spectra, overlapping doublets at 4.8 ppm were observed. The shift values were characteristic of β configuration of all glucose residues. Signals between 3.38 and 3.44 ppm correspond to the H-2 protons involved in β-1,2 linkage. Chemical shifts attributed to other protons in the glucan molecule are listed in Table 2. From the area of the peaks corresponding to succinyl residues, we estimated that the cyclic β-1,2-glucans of B. abortus have on average two succinyl residues per glucan molecule.
FIG. 4.
1H-NMR spectra of purified cyclic β-1,2-glucans from B. abortus. (A) B. abortus S19. (B) B. abortus S19 treated with alkali. (C) B. abortus Cgm19. The two signals between 2.4 and 2.6 ppm correspond to the methylene protons of the succinic acid substituents. In panel A, the resonance at 3.16 is derived from methanol. The resonance at 4.7 is H2O. Chemical shifts are expressed relative to external 1,4-dioxane.
TABLE 2.
13C and 1H chemical shifts and assignments of B. abortus cyclic-β-1,2-glucan resonances
| Proton | Chemical shifts (ppm)a |
|---|---|
| C1 | 102.4-102.5-102.8-102.9 |
| C-2 | 82.4-82.6-82.9-83.2 |
| C-3 | 76.1-76.2-76.3 |
| C-4 | 69.4-69.5-69.6 |
| C-5 | 77.0 |
| C-6 | 61.36-61.40 |
| H-1 | 4.70-4.72 |
| H-2 | 3.38-3.40-3.42-3.44 |
| H-3 | 3.60 |
| H-4 | 3.30 |
| H-5 | 3.32 |
| H-6a | 3.61 |
| H-6b | 3.76-3.78 |
Chemical shifts were measured at 30°C and referenced relative to external 1% (vol/vol) 1,4-dioxane.
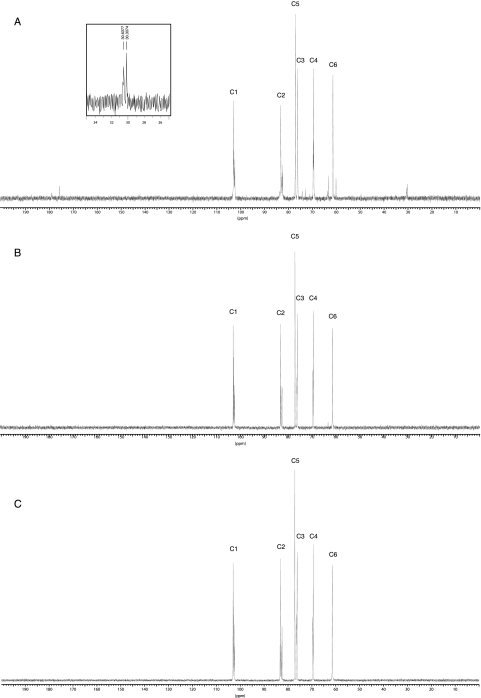
13C-NMR analysis of the periplasmic glucans revealed a peak cluster of C-1 resonance at 102.8 and 102.9 ppm (Fig. 5A). The presence of signals with a chemical shift above 102 ppm indicates β configuration of glucose. No signals at 92 to 96 ppm, characteristic of the C-1 resonances of reducing glucose residues, could be observed. These results confirm the previous observation that the glucan molecules of B. abortus are cyclic and all glucose residues are β linked (11). Resonances below 60 ppm indicate nonsugar substituents. In the 13C-NMR spectra, two CH2 signals of succinate at 30.3 and 30.6 ppm were present (31). These signals were missing in extracts after mild alkali treatment, confirming that the anionic character of B. abortus cyclic β-1,2-glucan is determined by the presence of succinyl residues (Fig. 5B). The signals at 74.2, 72.8, 63.7, 63.2, and 60.1 ppm (Fig. 5A) could be attributed to the chemical shift of other carbons depending on substitution at C-6 with succinyl residues, since these signals were also missing after mild alkali treatment. The resonances at 82 to 83 ppm were assigned to C-2 implicated in β-1,2-linkages (Table 2). The absence of a signal at 73 to 74 ppm indicates that all C-2 carbons are involved in glycosidic bonds (18). The spectra from this analysis did not reveal chemical-shift signals indicative of substitutions by other additional components. Furthermore, phosphoryl substituents were not detected on the cyclic β-1,2-glucans of B. abortus by 31P-NMR spectroscopy (data not shown).
FIG. 5.
13C-NMR spectra of purified cyclic β-1,2-glucans from B. abortus. (A) B. abortus S19. (B) B. abortus S19 treated with alkali. (C) B. abortus Cgm19. Chemical shifts are expressed relative to external 1,4-dioxane (δ 67.4 ppm).
Identification, cloning, and nucleotide sequence of B. abortus cyclic β-1,2-glucan succinyltransferase.
A gene with high homology to the opgC gene of Rhodobacter sphaeroides was identified in the B. abortus 2308 genome (12, 14). The gene is highly conserved in B. melitensis and B. suis (19, 34). A DNA fragment of approximately 1.8 kb was cloned in the T-easy vector and sequenced. Analysis of the sequence revealed that the 1.8-kb DNA fragment contained an open reading frame of 1,182 bp coding for a protein of 393 amino acids which is 39% identical to OpgC of R. sphaeroides and 56% identical to a hypothetical cyclic glucan succinyltransferase of S. meliloti. Accordingly, the gene was named cgm for cyclic glucan modifier. Two inverted repeats of 8 bp (AGAGCCGCCCTTGCGGCTCT) followed by TTTTTTATG are found 157 nucleotides downstream from the stop codon of cgm. This could be an intrinsic transcription terminator, as no sizeable open reading frame starts before this point. Complete genome sequencing of the B. abortus, B. melitensis, and B. suis genomes revealed that the cgs (BAB1_0108), cgt (BAB1_1017), and cgm (BAB1_1718) genes—involved in synthesis, transport, and modification of cyclic β-1,2-glucans, respectively—are not contiguous in B. abortus chromosome 1 (12).
The opgC gene of R. sphaeroides and the mdoC gene of E. coli encode glucan succinyltransferases (14, 29). Cgm, OpgC, and MdoC have similar sizes (393, 399, and 385 amino acids, respectively) and exhibit stretches of hydrophobic amino acids over their entire lengths. For MdoC (29), 10 transmembrane segments (TMS) have been predicted by the TopPredII program (13). The same program predicted 11 TMS and 9 TMS for OpgC and Cgm, respectively. Nevertheless, no amino acid similarities between MdoC and Cgm/OpgC were found.
Cyclic β-1,2-glucans of the cgm mutant are not succinylated.
Insertional mutagenesis of cgm in B. abortus 2308 and S19 was carried out by deleting an MscI fragment (675 bp) of the intragenic region of cgm and replacing it with a kanamycin resistence cassette. The resulting Kmr mutants, named B. abortus Cgm08 and Cgm19, were mapped by PCR.
Cyclic β-1,2-glucans recovered from the wild type and cgm mutants were analyzed by TLC. As is shown in Fig. 6, lanes 2 and 5, cgm mutants accumulated no anionic cyclic β-1,2-glucan. As expected, when plasmid pBB4cgm was introduced, the mutant strains recovered the synthesis of the anionic fraction (Fig. 6, lanes 3 and 6). Alkali-treated wild-type cyclic β-1,2-glucans (Fig. 3A, lane 2) exhibit the same migration pattern as cgm mutants (Fig. 6, lanes 2 and 5), thus confirming that in the cgm mutants, cyclic glucans are not substituted with anionic residues.
FIG. 6.
TLC of cyclic β-1,2-glucans formed by B. abortus strains. Total cellular glucans of B. abortus strains were extracted and subjected to TLC as described in Materials and Methods. B. abortus S19 (lanes 1 to 3): lane 1, wild type; lane 2, Cgm19; lane 3, Cgm19(pBB4cgm). B. abortus 2308 (lanes 4 to 6): lane 4, wild-type; lane 5, Cgm08; lane 6, Cgm08(pBB4cgm).
Cyclic β-1,2-glucans from B. abortus wild-type and cgm mutant strains were further analyzed by Bio-Gel P4 columns and DEAE-Sephadex chromatography. Cyclic β-1,2-glucans recovered from a cgm mutant exhibited the same elution profile in Bio-Gel P4 and on a DEAE-Sephadex column (Fig. 1B and C and 2B and C, respectively) as alkali-treated wild type cyclic β-1,2-glucans, showing their neutral character. When the plasmid pBB4cgm harboring B. abortus cgm was introduced into the mutant strain, the elution profiles of the glycan by gel filtration and anion-exchange chromatography were the same as that of the glycan from the wild type (Fig. 1D and 2D).
1H-NMR and 13C-NMR analyses revealed that the cyclic β-1,2-glucan from the cgm mutant lacked succinyl residues, since the signals between 2.4 and 2.6 ppm and between 4.25 and 4.35 ppm were not present in the 1H-NMR spectra (Fig. 4C). The signals at 30.3 and 30.6 ppm corresponding to succinyl substituents were also absent in the 13C-NMR spectra (Fig. 5C). These results confirmed that B. abortus cgm is a cyclic β-1,2-glucan succinyltransferase responsible for succinylation of cyclic β-1,2 glucan.
Moreover, as no other differences between the cyclic β-1,2-glucan wild-type and mutant spectra existed, the mutation on the cgm cyclic β-1,2-glucan succinyltransferase gene did not induce other substitutions by any additional components or any other change of the glucan structure.
Succinylation of B. abortus cyclic β-1,2-glucan is not required for virulence in mice and intracellular multiplication but is essential for survival under hypo-osmotic conditions.
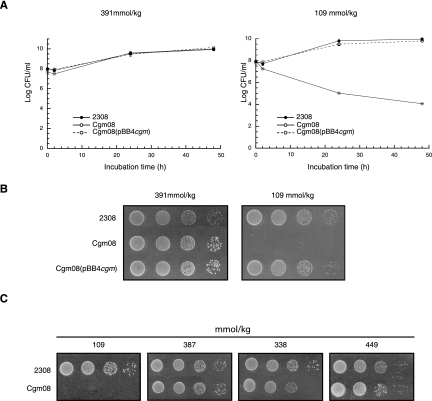
The presence of a substituted form of the cyclic β-1,2-glucans may have significant implications for the functions of these molecules in the Brucella infection process. To evaluate the importance of cyclic-glucan substitution in the infectivity of B. abortus, intracellular survival and experimental infection in mouse assays were carried out. No significant differences were observed between the wild-type and cgm mutant strains on intracellular multiplication in HeLa or J774 macrophage cell lines and on clearance from the spleens of infected mice (data not shown). These results indicated that B. abortus cyclic β-1,2-glucan succinylation is not required for virulence. It has been widely reported that alphaproteobacterial periplasmic glucans play a central role during hypo-osmotic adaptation. In order to define the role of B. abortus cyclic β-1,2-glucan succinylation on hypo-osmotic adaptation, the wild-type and cgm mutant strains were subjected to hypo-osmotic stress conditions. As is shown in Table 3, when the B. abortus 2308 wild-type strain was subjected to decreasing osmotic pressure (from 391 to 109 mmol/kg), the total amount of cyclic β-1,2-glucans recovered from the cells did not change. However, the anionic/neutral-glucan ratio was significantly increased from 1.08 at normal osmolarity (391mmol/kg) to 2.42 at low osmolarity (109 mmol/kg). The addition of other salts or of a sugar, such as sucrose, restored the anionic/neutral-glucan ratio to the level obtained with LB-NaCl 170 mM. This effect was independent of the chemical nature of the solute, and thus, the increase observed in anionic glucans at 109 mmol/kg appears to be dependent solely on the decreased osmolarity of the medium. These results show that in B. abortus, as was suggested for E. coli (7), the anionic cyclic β-1,2-glucans may play a role in hypo-osmotic adaptation, being more necessary at low than at high osmolarity. Accordingly, it might be speculated that the cgm mutant, which is unable to produce anionic glucans, must be less efficient at surviving and or multiplying at low osmotic pressure. As is shown in Fig. 7A, the B. abortus cgm mutant did not multiply in liquid medium with an osmotic pressure of 109 mmol/kg (LB with no addition of NaCl). Growth was restored by complementation with a plasmid containing cgm (Fig. 7A) or by restoring the osmotic pressure to 391 mmol/kg with the addition of 170 mM of NaCl (Fig. 7A). We concluded that modification of cyclic β-1,2-glucans with succinyl residues through the action of Cgm is required for wild-type growth at low osmotic pressure. The same effect was observed on solid LB medium (Fig. 7B), and growth was restored upon the addition of different osmolites (Fig. 7C).
TABLE 3.
Osmolarity affects the substitution of B. abortus cyclic β-1,2-glucana
| Solute (mM) | Osmolarity (mmol/kg)b | Total glucansc | Neutral glucansd | Anionic glucanse | Anionic/neutral glucans |
|---|---|---|---|---|---|
| 0 NaCl | 109 | 19.05 | 29.28 | 70.72 | 2.42 |
| 170 NaCl | 391 | 20.23 | 48.13 | 51.87 | 1.08 |
| 170 KCl | 387 | 19.41 | 48.21 | 51.79 | 1.07 |
| 110 K2SO4 | 338 | 19.20 | 48.32 | 51.68 | 1.07 |
| 340 sucrose | 441 | 20.15 | 49.01 | 50.99 | 1.04 |
B. abortus strain 2308 was cultured in 100 ml of LB or LB with the addition of 170 mM NaCl, 170 mM KCl, 110 mM K2SO4, and 340 mM sucrose at 37°C for 48 h. The cell pellets were extracted with 70% ethanol, and the neutral- and anionic-cyclic-β-1,2-glucan contents were determined after Bio-Gel P4 chromatography and DEAE-Sephadex chromatography as described in Materials and Methods.
The osmolarity of the medium was measured on a Vapro-Vapor Pressure Osmometer (model 5520; WESCOR).
The total cellular-glucan content is expressed as milligrams of glucose equivalent per liter of culture.
The neutral-glucan content is expressed as a percentage of the total cellular-glucan content.
The anionic-glucan content is expressed as a percentage of the total cellular-glucan content.
FIG. 7.
Effects of osmolarity on growth of different strains of B. abortus. (A) Studies in liquid media. B. abortus 2308 wild type, Cgm08, and the complemented strain Cgm08(pBB4cgm) were grown in LB-NaCl or LB as described in Materials and Methods. The number of viable bacteria at each time point was determined by making serial dilutions in PBS and plating aliquots on brucella broth plates. (B and C) Studies on solid media. For studies on solid media, 10 μl of the dilutions were spotted on LB agar or LB agar with the addition of 170 mM NaCl (B) or on LB agar or LB agar with one of the following additions: 170 mM KCl, 110 mM K2SO4, or 340 mM sucrose (C). The plates were incubated at 37°C for 5 days before being read.
DISCUSSION
Large amounts of OPG are found in the periplasmic space of Proteobacteria. Depending on the species considered, OPG could be modified to various extents by a variety of substituents; however, the role attributed to these substituents remains unclear. We have discovered in our laboratory that B. abortus produces cyclic β-1,2-glucans, which are required for full expression of virulence (2, 9, 36). As far as we know, nothing has been reported about the presence and role of nonglycosidic substituents modifying cyclic β-1,2-glucans of Brucella spp.
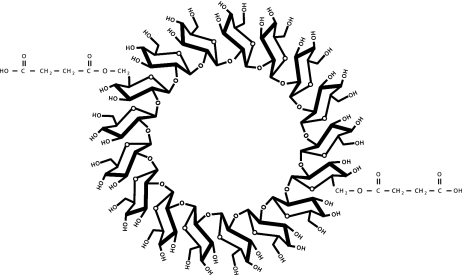
In this report, we demonstrated that Brucella spp. accumulated cyclic β-1,2-anionic substituted glucans. Physical evidence of the presence of anionic cyclic β-1,2-glucans with novel succinyl substituents was provided by several methods, including NMR spectroscopy, TLC, and DEAE-Sephadex anion-exchange chromatography. Our 1H-NMR and 13C-NMR analyses confirmed the previous observation that B. abortus glucans are cyclic, with all the glucose residues β linked (11); moreover, we identified in both profiles signals corresponding to succinyl residues. We estimated that B. abortus cyclic β-1,2-glucans are modified on average with two succinyl residues per molecule of glucan at the C-6 positions of the glucose residues (Fig. 8). The signals corresponding to succinyl residues were no longer present after mild alkali treatment, indicating that they are O-ester linked. Moreover, since after this treatment all glucan molecules became neutral and no other signals were detected in the 1H-NMR and 13C-NMR spectra, it can be concluded that the anionic character is solely determined by the presence of succinyl residues. On the other hand, mild acidic treatment did not alter TLC migration of the anionic cyclic glucan, indicating that no phosphodiester-linked substituents were present. Moreover, the use of 31P-NMR spectroscopy did not reveal the presence of phosphoryl substituents. These assays rule out the presence of phosphoglycerol, as was demonstrated in S. meliloti, A. tumefaciens, and E. coli glucans (7, 27), and zwitterionic residues, like phosphocholine or phosphoethanolamine, present in B. japonicum and E. coli glucans, respectively (27, 35).
FIG. 8.
Schematic representation of the structure of the succinyl-substituted cyclic β-1,2-glucan (degree of polymerization, 17) synthesized by B. abortus.
In this report, we have also identified, sequenced, and disrupted a B. abortus open reading frame that codes for a cyclic-glucan succinyltransferase that exhibits high homology to the cyclic-glucan succinyltransferase of R. sphaeroides (39% identity) (14). The gene was named B. abortus cgm for cyclic glucan modifier. Two cgm mutants (Cgm08 and Cgm19) were obtained from B. abortus 2308 and S19 strains, respectively. Analysis of cyclic glucans synthesized by cgm mutants has shown that they were identical to the cyclic glucans synthesized by parental strains, except for the fact that they completely lacked the succinyl modification. These results confirmed that B. abortus cgm encodes a cyclic β-1,2-glucan succinyltransferase responsible for succinylation of β-1,2-cyclic glucan and that succinyl residues are the only substituents of Brucella cyclic glucan. The mutants accumulate neutral cyclic β-1,2-glucans with a degree of polymerization identical to that of the neutral fraction synthesized by the parental wild-type strain, indicating that cyclization and substitution are independent processes.
Although other mutants of glucan substituent transferases have been described for E. coli, S. meliloti, and R. sphaeroides, those mutants contain increased levels of other substituents, such as phosphoethanolamine, succinyl, and acetyl substituents, respectively (6, 14, 22). B. abortus cgm mutants accumulated neutral cyclic β-1,2-glucans; the lack of succinyl residues did not promote any increment of other nonglycosidic substituents. Therefore, B. abortus is a good model that might shed light on the role that this substituent plays in the adaptation to osmotic stress and in pathogenicity. In this report, we demonstrated that succinyl modification of B. abortus glucans was essential for survival under hypo-osmotic conditions; moreover, the amounts of succinylated cellular glucans changed according to the osmolarity of the growth medium, being significantly increased at low osmotic pressure. On the other hand, contrary to what happens with B. abortus cgs and cgt mutants (9, 36), no effect on virulence and intracellular multiplication was apparent with the cgm mutant, indicating that succinyl substituents on the cyclic β-1,2-glucan are not required for full expression of virulence or intracellular multiplication and also suggesting that during intracellular trafficking and host colonization, brucellae do not encounter hypo-osmotic-stress conditions. Moreover, the presence of an alkali-sensitive anionic substituent in different Brucella species and the fact that the cgm genes are identical in B. abortus, B. suis, and B. melitensis suggest that substitution of cyclic glucan is not a determining factor for host specificity.
Acknowledgments
This work was supported by a grant from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, SECYT), Buenos Aires, Argentina (PICT 2000 no. 01-09194) and a grant from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Buenos Aires, Argentina (PIP 02346). M.S.R. and A.E.C. are fellows of CONICET. N.I.I. and R.A.U. are members of the research career of CONICET.
We thank J. J. Cazzulo for critical reading of the manuscript. We acknowledge Fabio Fraga and Pablo Briones, University of General San Martín, for technical assistance. We thank G. Burton from the Departamento de Química Orgánica, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, for his helpful assistance in the interpretation of 1H-NMR and 13C-NMR spectra.
REFERENCES
- 1.Altabe, S. G., P. Talaga, J. M. Wieruszeski, G. Lippens, R. Ugalde, and J. P. Bohin. 1998. Periplasmic glucans of Azospirillum brasilense, p. 390. In C. Elmerich, A. Kondorosi, and W. E. Newton (ed.), Biological nitrogen fixation for the 21st century. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 2.Arellano-Reynoso, B., N. Lapaque, S. Salcedo, G. Briones, A. E. Ciocchini, R. Ugalde, E. Moreno, I. Moriyon, and J. P. Gorvel. 2005. Cyclic beta-1,2-glucan is a Brucella virulence factor required for intracellular survival. Nat. Immunol. 6:618-625. [DOI] [PubMed] [Google Scholar]
- 3.Batley, M., J. W. Redmond, and A. J. Wicken. 1987. Nuclear magnetic resonance spectra of lipoteichoic acid. Biochim. Biophys. Acta 901:127-137. [DOI] [PubMed] [Google Scholar]
- 4.Bohin, J. P. 2000. Osmoregulated periplasmic glucans in Proteobacteria. FEMS Microbiol. Lett. 186:11-19. [DOI] [PubMed] [Google Scholar]
- 5.Bohin, J. P., and E. P. Kennedy. 1984. Regulation of the synthesis of membrane-derived oligosaccharides in Escherichia coli. Assay of phosphoglycerol transferase I in vivo. J. Biol. Chem. 259:8388-8393. [PubMed] [Google Scholar]
- 6.Breedveld, M. W., J. A. Hadley, and K. J. Miller. 1995. A novel cyclic beta-1,2-glucan mutant of Rhizobium meliloti. J. Bacteriol. 177:6346-6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breedveld, M. W., and K. J. Miller. 1994. Cyclic beta-glucans of members of the family Rhizobiaceae. Microbiol. Rev. 58:145-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breedveld, M. W., and K. J. Miller. 1995. Synthesis of glycerophosphorylated cyclic (1,2)-beta-glucans in Rhizobium meliloti strain 1021 after osmotic shock. Microbiology 141:583-588. [DOI] [PubMed] [Google Scholar]
- 9.Briones, G., N. Iñón de Iannino, M. Roset, A. Vigliocco, P. S. Paulo, and R. A. Ugalde. 2001. Brucella abortus cyclic beta-1,2-glucan mutants have reduced virulence in mice and are defective in intracellular replication in HeLa cells. Infect. Immun. 69:4528-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briones, G., N. Iñón de Iannino, M. Steinberg, and R. A. Ugalde. 1997. Periplasmic cyclic 1,2-beta-glucan in Brucella spp. is not osmoregulated. Microbiology 143:1115-1124. [DOI] [PubMed] [Google Scholar]
- 11.Bundle, D. R., J. W. Cherwonogrodzky, and M. B. Perry. 1988. Characterization of Brucella polysaccharide B. Infect. Immun. 56:1101-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chain, P. S., D. J. Comerci, M. E. Tolmasky, F. W. Larimer, S. A. Malfatti, L. M. Vergez, F. Aguero, M. L. Land, R. A. Ugalde, and E. Garcia. 2005. Whole-genome analyses of speciation events in pathogenic brucellae. Infect. Immun. 73:8353-8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claros, M. G., and G. von Heijne. 1994. TopPred II: an improved software for membrane protein structure predictions. Comput. Appl. Biosci. 10:685-686. [DOI] [PubMed] [Google Scholar]
- 14.Cogez, V., E. Gak, A. Puskas, S. Kaplan, and J. P. Bohin. 2002. The opgGIH and opgC genes of Rhodobacter sphaeroides form an operon that controls backbone synthesis and succinylation of osmoregulated periplasmic glucans. Eur. J. Biochem. 269:2473-2484. [DOI] [PubMed] [Google Scholar]
- 15.Cogez, V., P. Talaga, J. Lemoine, and J. P. Bohin. 2001. Osmoregulated periplasmic glucans of Erwinia chrysanthemi. J. Bacteriol. 183:3127-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comerci, D. J., G. D. Pollevick, A. M. Vigliocco, A. C. Frasch, and R. A. Ugalde. 1998. Vector development for the expression of foreign proteins in the vaccine strain Brucella abortus S19. Infect. Immun. 66:3862-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbel, J. M. 1997. Brucellosis: an overview. Emerg. Infect. Dis. 2:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dell, A., W. S. York, M. McNeil, A. G. Darvill, and P. Albersheim. 1983. The cyclic structure of beta-d-(1,2)-linked D-glucans secreted by Rhizobia and Agrobacteria. Carbohydr. Res. 117:185-200. [Google Scholar]
- 19.DelVecchio, V. G., V. Kapatral, R. J. Redkar, G. Patra, C. Mujer, T. Los, N. Ivanova, I. Anderson, A. Bhattacharyya, A. Lykidis, G. Reznik, L. Jablonski, N. Larsen, M. D'Souza, A. Bernal, M. Mazur, E. Goltsman, E. Selkov, P. H. Elzer, S. Hagius, D. O'Callaghan, J. J. Letesson, R. Haselkorn, N. Kyrpides, and R. Overbeek. 2002. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. USA 99:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dische, Z. 1962. General color reactions. Methods Carbohydr. Chem. 1:478-492. [Google Scholar]
- 21.Ditta, G., S. Stanfield, D. Corbin, and D. R. Helinski. 1980. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 77:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiedler, W., and H. Rotering. 1985. Characterization of an Escherichia coli mdoB mutant strain unable to transfer sn-1-phosphoglycerol to membrane-derived oligosaccharides. J. Biol. Chem. 260:4799-4806. [PubMed] [Google Scholar]
- 23.Geiger, O., A. C. Weissborn, and E. P. Kennedy. 1991. Biosynthesis and excretion of cyclic glucans by Rhizobium meliloti 1021. J. Bacteriol. 173:3021-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iñón de Iannino, N., G. Briones, M. Tolmasky, and R. A. Ugalde. 1998. Molecular cloning and characterization of cgs, the Brucella abortus cyclic beta(1-2) glucan synthetase gene: genetic complementation of Rhizobium meliloti ndvB and Agrobacterium tumefaciens chvB mutants. J. Bacteriol. 180:4392-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iñón de Iannino, N., and R. A. Ugalde. 1989. Biochemical characterization of avirulent Agrobacterium tumefaciens chvA mutants: synthesis and excretion of beta-(1-2)glucan. J. Bacteriol. 171:2842-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerpseth, B., A. Greener, J. M. Short, J. Viola, and P. L. Kretz. 1992. XL-1 Blue MRF′ E. coli cells: McrA-, McrB-, McrF-, Mrr-, HsdR-derivative of XL-1 Blue cells. Strat. Mol. Biol. 5:81-83. [Google Scholar]
- 27.Kennedy, E. P. 1996. Herman Moritz Kalckar—March 26, 1908-May 17, 1991. Biogr. Mem. Natl. Acad. Sci. 69:149-164. [PubMed] [Google Scholar]
- 28.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 29.Lacroix, J. M., E. Lanfroy, V. Cogez, Y. Lequette, A. Bohin, and J. P. Bohin. 1999. The mdoC gene of Escherichia coli encodes a membrane protein that is required for succinylation of osmoregulated periplasmic glucans. J. Bacteriol. 181:3626-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Lara, I. M., G. Orgambide, F. B. Dazzo, J. Olivares, and N. Toro. 1993. Characterization and symbiotic importance of acidic extracellular polysaccharides of Rhizobium sp. strain GRH2 isolated from acacia nodules. J. Bacteriol. 175:2826-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matulova, M., R. Toffanin, L. Navarini, R. Gilli, S. Paoletti, and A. Cesaro. 1994. NMR analysis of succinoglycans from different microbial sources: partial assignment of their 1H and 13C NMR spectra and location of the succinate and the acetate groups. Carbohydr. Res. 265:167-179. [DOI] [PubMed] [Google Scholar]
- 32.Miller, K. J., R. S. Gore, and A. J. Benesi. 1988. Phosphoglycerol substituents present on the cyclic beta-1,2-glucans of Rhizobium meliloti 1021 are derived from phosphatidylglycerol. J. Bacteriol. 170:4569-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oka, A., H. Sugisaki, and M. Takanami. 1981. Nucleotide sequence of the kanamycin resistance transposon Tn903. J. Mol. Biol. 147:217-226. [DOI] [PubMed] [Google Scholar]
- 34.Paulsen, I. T., R. Seshadri, K. E. Nelson, J. A. Eisen, J. F. Heidelberg, T. D. Read, R. J. Dodson, L. Umayam, L. M. Brinkac, M. J. Beanan, S. C. Daugherty, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, W. C. Nelson, B. Ayodeji, M. Kraul, J. Shetty, J. Malek, S. E. Van Aken, S. Riedmuller, H. Tettelin, S. R. Gill, O. White, S. L. Salzberg, D. L. Hoover, L. E. Lindler, S. M. Halling, S. M. Boyle, and C. M. Fraser. 2002. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc. Natl. Acad. Sci. USA 99:13148-13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rolin, D. B., P. E. Pfeffer, S. F. Osman, B. S. Szwergold, F. Kappler, and A. J. Benesi. 1992. Structural studies of a phosphocholine substituted beta-(1,3);(1,6) macrocyclic glucan from Bradyrhizobium japonicum USDA 110. Biochim. Biophys. Acta 1116:215-225. [DOI] [PubMed] [Google Scholar]
- 36.Roset, M. S., A. E. Ciocchini, R. A. Ugalde, and N. Iñón de Iannino. 2004. Molecular cloning and characterization of cgt, the Brucella abortus cyclic beta-1,2-glucan transporter gene, and its role in virulence. Infect. Immun. 72:2263-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Sangari, F. J., J. M. Garcia-Lobo, and J. Aguero. 1994. The Brucella abortus vaccine strain B19 carries a deletion in the erythritol catabolic genes. FEMS Microbiol. Lett. 121:337-342. [DOI] [PubMed] [Google Scholar]
- 39.Sieira, R., D. J. Comerci, D. O. Sanchez, and R. A. Ugalde. 2000. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. J. Bacteriol. 182:4849-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith, L. D., and T. A. Ficht. 1990. Pathogenesis of Brucella. Crit. Rev. Microbiol. 17:209-230. [DOI] [PubMed] [Google Scholar]
- 41.Talaga, P., V. Cogez, J. M. Wieruszeski, B. Stahl, J. Lemoine, G. Lippens, and J. P. Bohin. 2002. Osmoregulated periplasmic glucans of the free-living photosynthetic bacterium Rhodobacter sphaeroides. Eur. J. Biochem. 269:2464-2472. [DOI] [PubMed] [Google Scholar]
- 42.Talaga, P., B. Fournet, and J. P. Bohin. 1994. Periplasmic glucans of Pseudomonas syringae pv. syringae. J. Bacteriol. 176:6538-6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Talaga, P., B. Stahl, J. M. Wieruszeski, F. Hillenkamp, S. Tsuyumu, G. Lippens, and J. P. Bohin. 1996. Cell-associated glucans of Burkholderia solanacearum and Xanthomonas campestris pv. citri: a new family of periplasmic glucans. J. Bacteriol. 178:2263-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.York, W. S. 1995. A conformational model for cyclic beta-(1→2)-linked glucans based on NMR analysis of the beta-glucans produced by Xanthomonas campestris. Carbohydr. Res. 278:205-225. [DOI] [PubMed] [Google Scholar]
- 45.Zevenhuizen, L. P., A. van Veldhuizen, and R. H. Fokkens. 1990. Re-examination of cellular cyclic beta-1,2-glucans of Rhizobiaceae: distribution of ring sizes and degrees of glycerol-1-phosphate substitution. Antonie Leeuwenhoek 57:173-178. [DOI] [PubMed] [Google Scholar]