Abstract
RpoS, the sigma factor of enteric bacteria that responds to stress and stationary phase, is subject to complex regulation acting at multiple levels, including transcription, translation, and proteolysis. Increased translation of rpoS mRNA during growth at low temperature, after osmotic challenge, or with a constitutively activated Rcs phosphorelay depends on two trans-acting small regulatory RNAs (sRNAs) in Escherichia coli. The DsrA and RprA sRNAs are both highly conserved in Salmonella enterica, as is their target, an inhibitory antisense element within the rpoS untranslated leader. Analysis of dsrA and rprA deletion mutants indicates that while the increased translation of RpoS in response to osmotic challenge is conserved in S. enterica, dependence on these two sRNA regulators is much reduced. Furthermore, low-temperature growth or constitutive RcsC activation had only modest effects on RpoS expression, and these increases were, respectively, independent of dsrA or rprA function. This lack of conservation of sRNA function suggests surprising flexibility in RpoS regulation.
RpoS, the general stress and stationary-phase (SP) sigma factor, is highly conserved among Escherichia coli, Salmonella enterica, and other related enteric bacteria. The diverse and often harsh conditions encountered by these bacteria, whether residing as pathogens in the gut or as saprophytes in the environment, require the ability to integrate multiple stress signals and initiate the appropriate cellular responses in order to survive. RpoS serves in this capacity as the master regulator of the general stress response. Its levels increase in response to a number of stress signals, including osmotic shock, nutrient depletion, low temperature, and growth into stationary phase (reviewed in reference 19). As RpoS becomes more abundant, it effectively competes with the vegetative sigma factor in binding to core RNA polymerase, leading to increased transcription of genes necessary for mediating the stress response (49).
Regulation of RpoS is complex, with a large posttranscriptional component, and involves trans-acting factors (19). These factors include several small regulatory RNAs (28, 39) which target a cis-acting antisense element within the rpoS mRNA untranslated leader (7). In E. coli, two such small RNAs (sRNAs), DsrA and RprA, activate rpoS translation by binding to and inhibiting the antisense element (reviewed in reference 30). DsrA is necessary for activation of rpoS translation in response to low temperature and osmotic shock (27), while RprA increases RpoS both in response to osmotic shock (29) and in response to a constitutively active rcsC allele, indicating a role in cell envelope stress (15, 29).
These sRNAs were initially discovered and characterized in E. coli, and their gene sequences are ≈90% identical in S. enterica. The high degree of sequence conservation shared by E. coli and S. enterica, in both rpoS and the sRNAs, suggested that their regulatory functions are likely to be conserved as well. Here we describe the results of experiments undertaken to characterize the roles of DsrA and RprA in S. enterica, specifically their effects on rpoS regulation. Our findings strongly suggest that, under the conditions tested, neither of the two sRNAs is required for optimal RpoS synthesis in S. enterica. Mutational analysis of the rpoS antisense element in S. enterica was also performed to further characterize the role of this element in RpoS synthesis. The results of this analysis are broadly comparable to those in E. coli.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. The katE-lac operon [op] fusion used in this work has been described previously and is a reporter of RpoS activity (6, 9, 21). Isolation of Mud insertions and construction of site-specific mutations is described below. The high-frequency generalized transducing bacteriophage P22 mutant HT105/1 int-201 was used for transduction in S. enterica by standard methods (12). Phage P1 vir was used for transduction in E. coli, also by standard methods (41). Bacteria were grown in media and at temperatures which are described for each individual experiment. LB was used as rich medium (41), and the minimal medium was morpholinepropanesulfonic acid (34) modified as described elsewhere (4). Plates were prepared by using nutrient agar (Difco) with 5 g of NaCl per liter. Antibiotics were added to rich medium to final concentrations as follows: 20 μg of tetracycline hydrochloride per ml, 20 μg of chloramphenicol per ml, 50 μg of kanamycin sulfate per ml, 200 μg of streptomycin sulfate per ml, and either 100 μg of sodium ampicillin per ml for high-copy-number plasmids or 30 μg per ml for low-copy-number plasmids. In minimal medium, kanamycin sulfate was added to a final concentration of 100 μg per ml.
TABLE 1.
Strains
| Straina | Description | Source or reference |
|---|---|---|
| S. enterica strains | ||
| TE6134 | hfq-1::Mud-Cam | 6 |
| TE6153 | putPA1303::Kanr-katE-lac [op] | 6 |
| TE6266 | hfq-1::Mud-Cam putPA1303::Kanr-rpoS-lac [pr] | 7 |
| TE6369-2 | hfq-1::Mud-Cam putPA1303::Kanr-rpoS-lac [pr] (C470G) | 7 |
| TE6369-3 | hfq-1::Mud-Cam putPA1303::Kanr-rpoS-lac [pr] (G550C) | 7 |
| TE6382 | hfq-1::Mud-Cam putPA1303::Kanr-rpoS-lac [pr] (C470G) (G550C) | 7 |
| TE6557 | hfq-1::Mud-Cam putPA1303::Kanr-rpoS-lac [pr] (C469G) | 7 |
| TE6558 | hfq-1::Mud-Cam putPA1303::Kanr-rpoS-lac [pr] (G551C) | 7 |
| TE6590 | hfq-1::Mud-Cam putPA1303::Kanr-rpoS-lac [pr] (C469G) (G551C) | 7 |
| TE6850 | clpX1::Tn10d-Cam | 10 |
| TE6851 | mviA22::Tn10d-Cam | 10 |
| TE8007 | JF3490 dksA4::Tn10d-Tet | J. Foster |
| TE8012 | TE8007 LT2A putPA1303::Kanr-katE-lac [op] | |
| TE8544 | putPA::katE-lac [op] rpoS1074::tetAR (A.S., deletes nt 461-464 of rpoS leader) | |
| TE8546 | putPA::katE-lac [op] rpoS1076::cat (deletes from bp 110 upstream to 469 of rpoS leader) | |
| TE8566 | putPA::katE-lac [op] ΔdsrA::tetAR | |
| TE8567 | putPA::katE-lac [op] ΔrprA::tetAR | |
| TE8587 | putPA::katE-lac [op] ΔdsrA::cat | |
| TE8588 | putPA::katE-lac [op] ΔcysC::tetAR | |
| TE8589 | putPA::katE-lac [op] rpoS1084::tetAR (AGGA, deletes nt 554-557 of rpoS leader) | |
| TE8607 | ΔcysC::tetAR | |
| TE8608 | putPA1303::Kanr-katE-lac [op] ΔdsrA::cam | |
| TE8610 | putPA1303::Kanr-katE-lac [op] ΔrprA::tetAR | |
| TE8613 | putPA1303::Kanr-katE-lac [op] ΔdsrA::cam ΔrprA::tetAR | |
| TE8622 | ΔcysC::tetAR rpoS1076::cat (deletes from bp 110 upstream to 469 of rpoS leader) | |
| TE8701 | putPA::katE-lac [op] rpoS1080::tetAR (ΔA.S.-AGGA, deletes nt 461-557) | |
| TE8737 | rpoS1082::MudJ (codon 66) | |
| TE8794 | rpoS1083::MudJ (codon 222) | |
| TE8804 | nlpD::MudJ (nlpD codon 213, nt 9 of rpoS leader) | |
| TE8805 | rpoS1079::MudK (codon 187) | |
| TE8807 | rpoS1077::MudK (codon 22) | |
| TE8808 | rpoS1078::MudK (codon 216) | |
| TE8810 | rpoS1081::MudK (codon 250) | |
| TE8815 | rpoS1078::MudK (codon 216) hfq-1::Mud-Cam | |
| TE8852 | TE8815 rpoS (C469G) | |
| TE8853 | TE8815 rpoS (C470G) | |
| TE8854 | TE8815 rpoS (G471C) | |
| TE8855 | TE8815 rpoS (C549G) | |
| TE8856 | TE8815 rpoS (G550C) | |
| TE8857 | TE8815 rpoS (G551C) | |
| TE8858 | TE8815 rpoS (C469G) (G551C) | |
| TE8859 | TE8815 rpoS (C470G) (G550C) | |
| TE8860 | TE8815 rpoS (G471C) (C549G) | |
| TE8935 | nlpD::MudJ (nlpD codon 302, nt 276 of rpoS leader) | |
| TE8936 | rpoS:::MudJ (codon 36) | |
| TE8983 | rpoS1077::MudK (codon 22) ΔdsrA::cat | |
| TE9049 | nlpD::[FRT-lacZY pKG137 Kanr] (nlpD codon 302, nt 276 of rpoS leader) | |
| TE9050 | rpoS::[FRT-lacZY pKG137 Kanr] (rpoS codon 36) | |
| TE9051 | rpoS::[FRT-lacZY pKG137 Kanr] (rpoS codon 222) | |
| TE9052 | nlpD::[FRT-lacZY pKG137 Kanr] (nlpD codon 213, nt 9 of rpoS leader) | |
| TE9053 | rpoS::[FRT-lacZY pKG137 Kanr] (rpoS codon 4) | |
| TE9160 | rpoS1081::MudK (codon 250) (TTG→ATG start codon) | |
| TE9179 | MS1868 StrA1 rpoS::[tetAR rpsL+] (AGGA) | S. Maloy |
| TE9213 | rpoS1081::MudK (codon 250) (TTG→ATG start codon) ΔdsrA::cat | |
| TE9219 | rpoS1081::MudK (codon 250) (TTG→ATG start codon) ΔdsrA::cat ΔrprA::tetAR | |
| TE9236 | TE8815 rpoS (G461C) | |
| TE9237 | TE8815 rpoS (G461A) | |
| TE9238 | TE8815 rpoS (G461T) | |
| TE9239 | TE8815 rpoS (G462C) | |
| TE9240 | TE8815 rpoS (G462A) | |
| TE9241 | TE8815 rpoS (G462T) | |
| TE9242 | TE8815 rpoS (G463C) | |
| TE9243 | TE8815 rpoS (G463A) | |
| TE9244 | TE8815 rpoS (G463T) | |
| TE9245 | TE8815 rpoS (G464C) | |
| TE9246 | TE8815 rpoS (G464A) | |
| TE9247 | TE8815 rpoS (G464T) | |
| TE9316 | rpoS1077::MudK (codon 22) zef-6829::tetAR rcsC55 (T903A) | |
| TE9317 | rpoS1077::MudK (codon 22) zef-6829::tetAR rcsC+ | |
| TE9318 | rpoS1077::MudK (codon 22) zef-6829::tetAR rcsC64 (F473I) | |
| TE9333 | gmm-21::MudJ zef-6829::tetAR rcsC55 (T903A) | |
| TE9334 | gmm-21::MudJ zef-6829::tetAR rcsC+ | |
| TE9352 | rpoS1077::MudK (codon 22) zef-6829::tetAR rcsC55 (T903A) ΔrprA::cam | |
| TE9353 | rpoS1077::MudK (codon 22) zef-6829::tetAR rcsC+ ?rprA::cam | |
| TE9354 | rpoS1077::MudK (codon 22) zef-6829::tetAR rcsC64 (F473I) ΔrprA::cam | |
| TE9368 | gmm-21::MudJ zef-6829::tetAR rcsC64 (F473I) | |
| TE9394 | gmm-21::MudJ zef-6829::tetAR rcsC+ ?rprA::cam | |
| TE9395 | gmm-21::MudJ zef-6829::tetAR rcsC55 (T903A) ΔrprA::cam | |
| TE9396 | gmm-21::MudJ zef-6829::tetAR rcsC64 (F473I) ΔrprA::cam | . |
| TE9426 | TE8807/pNM12 (vector) | |
| TE9427 | TE8983/pNM12 (vector) | |
| TE9428 | TE8983/pNM3 (PBAD-E. coli dsrA+) | |
| TE9429 | TE8807/pBAD18 (vector) | |
| TE9430 | TE8983/pBAD18 (vector) | |
| TE9431 | TE8983/pTE780 (PBAD-S. enterica dsrA+) | |
| TE9452 | TE8815 rpoS (T468C) | |
| TE9453 | TE8815 rpoS (C476A) (C477A) | |
| E. coli strains | ||
| SG22182 | MC4100 ara mal::lacIQ | D. Sledjeski |
| DDS1365 | MC4100 ara mal::lacIQdsrA1::cat | D. Sledjeski |
| TE6897 | MC4100 ara mal::lacIQtrpDC700:: putPA1303::Kanr-katE-lac [op] | |
| TE6898 | MC4100 ara mal::lacIQtrpDC700:: putPA1303::Kanr-rpoS-lac [pr] | |
| TE6913 | MC4100 ara mal::lacIQtrpDC700:: putPA1303::Kanr-katE-lac [op] dsrA1::cat | |
| TE6914 | MC4100 ara mal::lacIQtrpDC700:: putPA1303::Kanr-rpoS-lac [pr] dsrA1::cat | |
| TE8096 | BW26678 E. coli K-12/pKD46 [AmpR, pSC101 rep (Ts) araC+ PBAD-λ red] | B. Wanner |
| TE9062 | zgd::tetAR (just upstream of rpsL+) | |
| TE9416 | TE6898/pBAD18 (vector) | |
| TE9418 | TE6914/pBAD18 (vector) | |
| TE9419 | TE6914/pTE780 (PBAD-S. enterica dsrA+) | |
| TE9422 | TE6898/pNM12 (vector) | D. Sledjeski |
| TE9424 | TE6914/pNM12 (vector) | |
| TE9425 | TE6914/pNM3 (PBAD-E. coli dsrA+) | D. Sledjeski |
Numbering corresponds to the position of the last nucleotide retained from the rpoS leader or the codon within which the fusion occurs (i.e., the last intact codon is one previous).
Isolation and analysis of rpoS-lac fusions formed by insertion of Mud transposons.
Insertions of MudJ (MudI 1734) to form operon fusions and MudK to form protein fusions (1, 8) were obtained by screening large pools of insertions (>105 clones) for linkage to the rpoS region. Since rpoS is very close to cysC (6 kb separate the two genes), a phage P22-transducing lysate grown on each insertion pool was used to transduce TE8607 (Δ cysC::tet) to Kanr Cys+ on plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). Blue colonies were picked, purified once on selective medium, patched onto NB agar containing kanamycin, and tested for an rpoS mutant phenotype by scoring formation of bubbles when spotted with 5 μl of 3% hydrogen peroxide. Insertions were mapped by PCR, and the precise joint with the Mud element was located by DNA sequencing. The starting insertion pools were isolated by mutagenesis of S. enterica LT2 using the method of Hughes and Roth (22). The original host for MudK insertions carried a plasmid unrelated to the current project (pRF1) (14); therefore, backcrossed candidate insertions were checked to confirm that this plasmid had been lost as expected.
Preliminary characterization showed both expected and unexpected properties for strains carrying these fusions. Fusions were recovered within rpoS but also in the upstream region encoding the C-terminal part of nlpD. Polarity of the insertions lying within this part of nlpD on expression of rpoS is consistent with the location of the major rpoS promoter roughly in the middle of the nlpD gene (21, 23, 46). Also as expected, all lac protein fusions to rpoS tested were found to be substantially (≈4- to 5-fold) dependent on function of the hfq and dksA genes, known from previous studies to affect rpoS translation but not transcription (6, 33, 48). Sensitivity to clpX and mviA mutations was tested in the LT2A background (10). The effect of mutations blocking the protein turnover pathway was variable, depending on the location of the insertion site within rpoS. The MudK insertion at rpoS codon 22 was unaffected by loss of clpX or mviA, whereas expression of the insertion at codon 250 was increased five- to sixfold in both clpX and mviA mutant backgrounds during exponential phase in LB medium. This behavior is consistent with identification of K173 as a critical residue in the RpoS “turnover element” in E. coli (2). In stationary phase, expression of sensitive rpoS-lac protein fusions became independent of clpX and mviA. Other results in S. enterica (21) show that this behavior is not characteristic of the RpoS protein itself or of RpoS-dependent reporters. We can explain this result by postulating that the rpoS dependence of mviA (rssB/sprE) expression during stationary phase found in E. coli (40) is conserved in S. enterica.
One unexpected result was that several MudK insertions in rpoS (screened as dark blue Lac+ colonies on X-Gal plates) were found to be out of frame but expressed lac as strongly as in-frame fusions. Remarkably, an insertion in the +1 frame at codon 36 expressed lac at a threefold-higher level during exponential phase than any in-frame fusion recovered. Several high-expressing out-of-frame fusions were confirmed to have the predicted sequence across Mu and into the first 300 bp of lacZ. The explanation for this unusual situation is likely that: (i) exponential-phase expression of in-frame rpoS-lac protein fusions is quite low (≈10-fold lower than any rpoS-lac operon fusion), due at least in part to the action of the antisense element; and (ii) the sequence at the joint with MudK consists of the sequence XTG, where X is contributed by rpoS and TG is from Mu. This XTG codon is in frame with lacZ. The sequences of all high-expressing out-of-frame insertions contained a plausible ribosome-binding site (RBS) upstream from an initiation codon. This suggests that the high relative expression of out-of-frame fusions is an artifact due to the novel sequence at the insertion joint.
A second puzzling and cautionary result comes from comparison of MudJ (operon) fusions upstream and downstream of the rpoS initiation codon. We found that two early fusions, one within nlpD at +9 with respect to the rpoS transcriptional start and the other at +276 showed no SP induction in LB medium, whereas later fusions at codon 36 and codon 222 showed normal induction. This behavior indicated that normal transcriptional regulation depends on sequences substantially downstream of the promoter. However, leader-dependent regulation is not consistent with our previous study of SP induction (21), which showed that a short segment surrounding the promoter region displayed the full range of SP regulation of transcription.
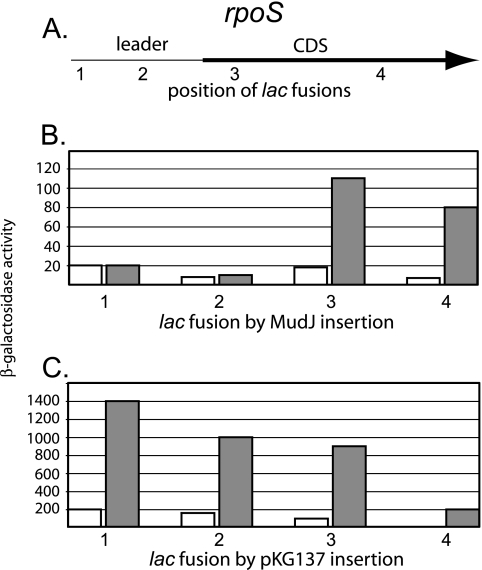
To resolve this issue and map the apparent discontinuity, a number of precisely targeted lac operon fusions were constructed using the method of Ellermeier et al. (13). In this method, the λ Red recombination system is used to direct insertion of a drug resistance marker flanked by directly repeated FLP recombination target (FRT) sites into the bacterial chromosome. The insertions are then resolved to leave a single unmarked FRT site, which is targeted for Flp/FRT-mediated integration of a replication-defective Kanr plasmid to provide the lac genes and form either an operon or a protein fusion. The lac fusions used here were constructed with pKG137 (M. Garsha and J. Slauch, personal communication), a plasmid that forms lac operon fusions including a strong RBS for lacZ. As shown in Fig. 1, all the fusions made by this method were regulated, including those at +9 and +276 with respect to the transcriptional start, in contrast to the behavior of MudJ fusions inserted at exactly the same sites. The different behavior of MudJ insertions is currently unexplained but presumably depends on the nature of the extra sequences present at the attR end of MudJ, including about 400 bp from Mu as well as a substantial segment of the E. coli trp operon.
FIG. 1.
Comparison of the regulation of lac operon fusions formed by MudJ insertion or plasmid integration. A. The rpoS coding sequence (bold line) and upstream leader are indicated, with labels indicating four sites at which fusions were isolated or constructed as described in the text. These positions are: 1 (+9 of the leader), 2 (+276 of the leader), 3 (codon 36 of rpoS), and 4 (codon 222 of rpoS). B. Analysis of lac expression from MudJ insertions at sites 1 to 4. Open bars indicate exponential-phase cultures, and filled bars indicate SP cultures, as defined in the text. SP induction of rpoS transcription is measured as the ratio of the value from the filled bars to open bars. C. The same experiment as in panel B, except that each fusion was made by integration of plasmid pKG137, as described in Materials and Methods. Strains for panel B were TE8804, TE8935, TE8936, and TE8794; strains for panel C were TE9052, TE9049, TE9050, and TE9051. Each bar represents the average of at least three experiments. Standard deviations were within 15% of the mean.
Plasmid pTE780 (PBAD-S. enterica dsrA) was constructed by PCR using the following primers: 5′-GCGGGATCCTACCTGACGCTTTTTATCGCAACTCTCTACTGTTTCTCCATCACATCAGATTTCCTGGTGT-3′ and 5′-GCGTCTAGAACCGTTAAAAAGGCCGAAA-3′ on LT2 DNA as template. Sequences from dsrA are shown in italics. The PCR product was purified, digested with BamHI and XbaI, and cloned into pBAD18 (18).
Construction of insertion and point mutations using λ Red recombination.
Other point mutations and insertion/deletions were made by direct transformation of S. enterica, either with oligonucleotides or with DNA segments amplified by PCR, utilizing the λ Red recombination system as provided on plasmid pKD46 (11). Exponential-phase recipient cells carrying pKD46, grown at 30°C with selection for ampicillin resistance (Ampr), were induced by treatment with 0.2% arabinose for 1 hour before electroporation, after which transformants were grown out in liquid medium before plating and selection at 37°C. A few experiments utilized pSIM5, a plasmid for mutant construction based on the method of Yu et al. (51) and obtained from D. Court.
Most of the unmarked point mutations were obtained as follows. First, an insertion of tetAR near or at the target site was isolated. Subsequently, a recipient bearing that insertion and induced for λ Red was transformed with a mutated oligo and selection applied for loss of Tetr (Bochner selection [5, 31]). For unknown reasons, this selection has a high background for insertions at certain sites. For insertion within the rpoS RBS, it was important that the recipient strain also contained katE-lac [op], an RpoS-dependent reporter fusion. Replacement of the tetAR cassette restored a Lac+ phenotype, visualized by subsequent single colony isolation on MacConkey lactose agar. Some double mutants with lesions affecting both the antisense element and the RBS region were constructed by an iterative procedure in which a tet insertion derivative of an existing point mutant was constructed as an intermediate. A second, more rapid method used a singly mutant DNA template (lesion in the RBS) for PCR to introduce the second mutation, which was recovered by transformation of a recipient deleted for the region between the antisense element and the RBS. This deletion, marked with tetAR, was from strain TE8701 (ΔrpoS1080::tetAR).
A mutation changing the rpoS TTG start codon to ATG was obtained by oligo transformation as described above, but a second mutation changing the TTG to TCG could not be screened in the same way since it does not confer a Lac+ phenotype. To make this change, we designed and constructed a different counterselectable insertion in the RBS region of rpoS, based on the known dominance of wild-type rpsL (Strs) in merodiploids containing one Strs and one Strr allele, as exploited by others (42). First, tetAR was inserted just upstream of the rpsL+ gene of an Strs E. coli strain. Chromosomal DNA from this strain (TE9062) was used as template to amplify a tetAR-rpsL+ cassette, and this DNA segment was then inserted at the RBS of S. enterica rpoS. TE9179, carrying both the rpoS::[tetAR-rpsL+] insertion and the strA1 (Strr) allele, was found to be Strs, as expected. For unknown reasons the strain forms small colonies on NB agar lacking streptomycin, but this slow growth phenotype appears to be stable. Transformants were easily obtained by selecting Strr in this background, again using λ Red recombination. The vast majority of these were Tets when mutated oligonucleotide DNA was added to the transformation mixture; three candidate transformants were sequenced, and all contained the TTG-to-TCG change.
Point mutations were backcrossed by transduction of recipient strain TE8607 (ΔcysC::tetAR ΔrpoS1076::cat), selecting Cys+ and screening for loss of the Camr marker in the rpoS leader. Double mutants carrying both a lac fusion and linked point mutation were constructed by transduction using a donor strain carrying the lac fusion (marked with Kanr) and the ΔrpoS1084::tetAR insertion into a recipient bearing the desired point mutation. The desired class of transductants was Kanr Lac+ Tets. The rpoS leader genotype of each recombinant strain was confirmed by DNA sequencing.
Targeted insertion/deletions of several genes were made by the λ Red method. The extent of deletion for each construct was as follows: ΔcysC deletes 11 bp of the leader including the RBS precisely to the stop codon; ΔdsrA deletes from the −35 hexamer of the promoter through the terminal poly(U) sequence (2068766 to 2068651 of GenBank NC_003197); ΔrprA also deletes from the −35 hexamer of the promoter through the terminal poly(U) sequence (1444972 to 1444822 of GenBank NC_003197). For reference, the rpoS leader extends from 3067051 to 3066487 of GenBank NC_003197.
β-Galactosidase assays.
Cells were centrifuged, resuspended in Z buffer (100 mM KPO4 [pH 7.0], 10 mM KCl, 1 mM MgSO4), and then permeabilized by treatment with sodium dodecyl sulfate (SDS) and chloroform (32). Assays were performed in Z buffer containing 50 mM β-mercaptoethanol. Usually, reactions were carried out in microtiter (96-well) plates and read in a Molecular Devices plate reader. Activities (change in optical density at 420 nm [OD420] per minute) were normalized to cell density (OD650) and were always compared with activities of appropriate controls assayed at the same time. For experiments involving cultures grown to different densities, the number of cells harvested was adjusted to provide approximately equal cell densities in the assay. One unit of activity, as determined by this method, is equivalent to ≈23 units as measured by the Miller assay (32). The latter assay is much more sensitive and was employed in selected experiments for this reason.
Immunological detection of proteins.
Cultures were grown as described in the figure legends. Exponential-phase samples were taken at an OD600 of ≈0.13, and SP samples were taken after 24 h or 48 h for cultures growing at 37°C or 18°C, respectively. Protein samples were prepared from 1-ml culture volumes by centrifugation and resuspension in 100 μl Tris-SDS buffer (5 mM Tris-HCl, 2% SDS). Samples were vortexed, then boiled for 10 min, and centrifuged for 30 min, and the supernatants were collected and stored at −20°C. The total protein concentration of each sample was determined using a Lowry-based protein assay (Bio-Rad) according to the manufacturer's protocol. For each gel, protein samples were diluted in sample buffer to an equal concentration and then boiled for 5 min prior to loading onto 10 or 12% polyacrylamide mini-gels. Gels were loaded with either 10 μg of total protein per well (SP protein samples) or 50 μg per well (exponential-phase samples). Electrophoresis was carried out at 75 V for 2.5 h, and resolved proteins were transferred to a Sequi-blot polyvinylidene difluoride membrane (Bio-Rad) at 100 V for 50 min using Bio-Rad's Mini Trans-Blot transfer cell. Subsequent steps and RpoS detection, using the R12 monoclonal antibody (6), were carried out as previously described (21) with the following exception. Membranes prepared from stationary-phase protein samples, containing relatively higher levels of RpoS, were incubated for 2 to 3 h with a secondary antibody directly conjugated to horseradish peroxidase.
Northern blot assays.
To detect DsrA, a probe (designated SL1) specific for the first stem-loop of DsrA, was designed based on a probe used to study DsrA in E. coli (28). The SL1 probe is a single-stranded biotinylated DNA oligo and has the following sequence: 5′-biotin-AATCGTTACACCAGGAAATCTGATGTG.
Cultures were grown overnight, diluted 1:1,000 into LB containing ampicillin and 0.02% l-arabinose, and incubated at either 18 or 32°C. Total RNA was isolated from 1 ml of culture grown to mid-log phase using an RNeasy mini kit (QIAGEN) according to the manufacturer's protocol. RNA samples were diluted to 1 μg in a final volume of 15 μl in glyoxal load dye (Ambion) and incubated at 50 to 55°C for at least 1 hour prior to loading onto 6% urea-polyacrylamide mini-gels. Electrophoresis was carried out at 100 V for 80 min in 1× Tris-borate-EDTA (TBE) buffer.
Gels were assembled into a Bio-Rad Mini Trans-Blot unit, and RNA was transferred to a BrightStar membrane (Ambion) at 100 V for 50 min in 1× TBE. The RNA was cross-linked with a UV cross-linker (Stratagene) using the autocrosslink function. Cross-linked membranes were incubated at 37°C in 10 ml of Ultrahyb-Oligo buffer (Ambion) for 2 to 4 h and then with the same buffer containing the SL1 probe at a concentration of 185 ng/ml. Hybridization was carried out overnight at 37°C. The blots were washed and developed using the BrightStar Biodetect nonisotopic kit (Ambion) according to the manufacturer's protocol.
RESULTS
Conservation of dsrA and rprA gene sequences between E. coli and S. enterica.
As noted previously by the authors who first reported the functions of the dsrA and rprA genes in E. coli (28, 29), these genes are highly conserved between E. coli K-12 and S. enterica LT2 as well as in certain other enteric species. In fact, conservation of sequence is thought to have predictive value in the search for new small regulatory RNA genes (16, 47). Both the dsrA and rprA genes reside at similar positions in the two bacterial chromosomes. Each gene is flanked by the same neighboring genes. The primary sequence of S. enterica dsrA shows eight substitutions and three missing nucleotides compared to its E. coli counterpart (≈90% identity), and these changes can be accommodated with limited effect on the folded structure (24, 43). Furthermore, the nucleotides of DsrA RNA that are predicted to pair with rpoS mRNA are completely conserved, as shown in Fig. 2. The S. enterica rprA gene shows even higher identity to its E. coli counterpart (three substitutions in 107 nucleotides [nt]), and its interface with rpoS mRNA is completely conserved as well. The promoter sequences of the two genes are also nearly identical.
FIG. 2.
A. RNA sequence of a segment of the 565-nt S. enterica rpoS leader RNA, starting at nt 456 (110 nt upstream from the start codon), and folded to show pairing between the antisense element and the rpoS RBS region (stems I, II, and III). Paired regions flank the Shine-Dalgarno (S.D.) complementarity to 16S rRNA and extend to the start codon (UUG in S. enterica, AUG in E. coli). Nucleotides that differ between S. enterica and E. coli are marked by filled circles. The antisense element and the RBS region are connected by 63 nt, which are not shown but are indicated by the oval. B. Pairing is shown between the antisense element (extended by an additional 18 nt on the upstream side) and two different sRNA regulators of RpoS: DsrA and RprA. Paired nucleotides are indicated by vertical lines, and spaces have been introduced where needed to facilitate the alignment. The stems of the antisense element are overlined for reference. The gray boxes indicate a hexameric sequence whose complementarity with the target RpoS RNA is essential for sRNA function, as shown for E. coli (28, 29). The DsrA sequence shown starts at +1, and the RprA sequence starts at +28.
This conservation of sequence suggests that the functions of these two genes are important and also implies these functions should be conserved between the two species. Therefore, we investigated the role of these two sRNAs in regulation of S. enterica rpoS under three conditions shown to activate rpoS expression in E. coli: low temperature (DsrA), osmotic shock (DsrA and RprA), and activation by RcsC (RprA).
Effect of low temperature.
Sledjeski et al. reported that growth at low temperature (20°C) has a dramatic effect on rpoS in E. coli. Expression in exponential phase was reported to increase by ≈100-fold compared to 42°C (44). This increase specifically required DsrA (44) and was a posttranscriptional effect on synthesis, consistent with the known mode of action of overexpressed DsrA on RpoS at 37°C (28). The effect of a dsrA mutation was less dramatic in stationary phase but still significant (8- to 10-fold).
To study the role of dsrA and rprA in S. enterica, we characterized the effect of the deletion of each gene separately, or both genes together, on expression of the RpoS-dependent reporter, katE-lac [op]. This reporter was used because the level of expression of rpoS-lac [pr] fusions is very low in exponential phase, particularly since the rpoS gene has a TTG start codon in S. enterica (see below). Results obtained by assay of katE-lac were confirmed by Western blotting of RpoS itself. As a positive control, we used the katE-lac reporter fusion in an E. coli MC4100 derivative that was either wild type for dsrA or carried the dsrA1::cat mutation (44). As expected, little effect of dsrA loss was seen for cells in exponential phase at 37°C, either in E. coli or in S. enterica (Fig. 3A). For dsrA+ E. coli cells in exponential phase at 18°C, expression of katE-lac [op] was about 10-fold higher than at 37°C (compare Fig. 3A and B; note different scale). The E. coli dsrA mutant showed ≈6.5-fold-lower expression compared to wild type at 18°C. A modest increase in reporter expression at low temperature was also seen in S. enterica (≈2-fold), but there was no effect of deleting dsrA or rprA, either singly or together. Western blot analysis using a monoclonal antibody specific for RpoS confirmed this finding (Fig. 3C and D). A dsrA mutation has a large effect on both RpoS levels and expression of katE-lac [op] at 18°C in E. coli, but not in S. enterica. Additionally, the effect of dsrA1::cat on rpoS-lacZ [pr] activity was assessed in E. coli as performed previously by Sledjeski et al. (44). In exponential phase at 18°C, the rpoS-lacZ activity of the dsrA+ cells was ≈12-fold higher than at 37°C, and the activity of the dsrA1::cat cells was ≈14-fold less than wild type at 18°C (data not shown).
FIG. 3.
Effect of low temperature on RpoS. Cells carrying the RpoS-dependent reporter katE-lac [op] were grown in LB medium at either 37°C (A) or 18°C (B) to early exponential phase (OD600 of 0.18) and assayed for β-galactosidase activity. The S. enterica strains used were wild type (TE6153), ΔdsrA (TE8608), ΔrprA (TE8610), and ΔdsrA ΔrprA (TE8613). The E. coli strains were wild type (TE6897) and dsrA1::cat (TE6913). The indicated strains were also analyzed for RpoS protein by Western blotting as described in Materials and Methods. Cells were grown to exponential phase (C) or stationary phase (D) in LB medium at 18°C. (E) A lac operon fusion to +11 of the S. enterica dsrA gene was constructed as described in Materials and Methods, and its expression was assayed by measuring β-galactosidase activity after growth to exponential phase in LB medium at the indicated temperatures.
The temperature response in E. coli results from a combination of ≈6-fold-increased expression from the dsrA promoter at 25°C compared to 37°C, as well as stabilization of the RNA at low temperature (37). A lac operon fusion to the chromosomal S. enterica dsrA gene was constructed to test whether there was a response similar to that of E. coli. We observed 2.5- to 3-fold-higher expression at 18°C compared to 37°C (Fig. 3E).
TTG start codon for rpoS.
Expression of S. enterica rpoS is particularly low because the gene starts with a TTG initiation codon (26), compared to the ATG start found in E. coli. In strain LT2, this is partially compensated at the level of the RpoS protein and reporters such as katE-lac [op] by the mviA V102G mutation, which eliminates regulated RpoS protein turnover (3, 10). We are not aware of any protein sequence analysis that would confirm the assignment of the initiation codon in either E. coli or S. enterica.
The origin of this difference between E. coli and S. enterica lineages is not clear. The DNA sequences of several LT2 isolates (stored frozen for many years) all show the TTG start (unpublished data). On the other hand, rpoS from a virulent Salmonella strain (ATCC 14028s) has an ATG start (50). Since it has recently been found that N-terminal deletions of rpoS may retain substantial function (17, 36), we tested the function of the S. enterica TTG initiation codon. Site-directed mutations changing the start codon were substituted at the native rpoS locus in the bacterial chromosome as described in Materials and Methods. Substitution of TTG with ATG increased expression of an rpoS-lac protein fusion (codon 250) by ≈10-fold, whereas substitution of TTG by TCG decreased expression by more than 50-fold. This genetic test confirms that the predicted TTG start codon carries out this role for rpoS in S. enterica.
Osmotic shock.
Sucrose challenge experiments were carried out following the protocol described by Majdalani et al. (27). Cells with a single-copy rpoS-lac [pr] fusion were grown in LB medium at 30°C and challenged with sucrose during early exponential phase. The lac fusion used for this experiment was formed by insertion of MudK at codon 250 of the rpoS gene. In other backgrounds, this fusion can be subject to the protein turnover control exerted by mviA/rssB/sprE and the ClpXP protease (data not shown), but the “wild-type” LT2 strain employed for this experiment is defective for this pathway (3, 10). The signal from this lac fusion was increased by substituting an ATG start codon, as found in E. coli. This change should not affect pairing with the antisense element, and it also did not affect induction of rpoS by osmotic shock (data not shown).
Addition of sucrose causes cells to plasmolyse, reducing their cross-section for light scattering and thereby resulting in decreased turbidity. Even without a change in lac expression, this decrease in turbidity would result in an artifactual increase in β-galactosidase activity if it were normalized to OD650 as is typically done. For this reason, following Majdalani et al., we plotted the total β-galactosidase activity from a fixed volume of cells versus the amount of protein in the same sample, as determined at various times after challenge. In Fig. 4A, the results from a representative experiment are given for osmotic challenge of a dsrA single mutant, compared to wild type. In panel B, the same experiment was carried out with a dsrA rprA double mutant, also compared to wild type. It can be seen that the response to sucrose was reduced in the dsrA single mutant but did not appear to be further reduced in the dsrA rprA double mutant. Growth of the double mutant was apparently sensitive to sucrose, based on decreased protein accumulation in the challenged culture, and this observation was reproducible.
FIG. 4.
Effect of osmotic challenge on rpoS-lac. The lac protein fusion employed was formed by insertion of the transposon MudK at codon 250 of the S. enterica rpoS gene (see Materials and Methods for details). Cultures were grown in LB medium at 30°C to early exponential phase, split into duplicate cultures, and grown to an OD600 of 0.12. Cultures were challenged with prewarmed aliquots of either sucrose dissolved in LB medium (addition of ≈1/5 volume of 2 M sucrose to give 0.464 M final concentration; ≈16%) or LB alone. Samples were removed at time zero and at 15, 30, and 45 min after challenge. Cells were concentrated and assayed for total protein and for β-galactosidase activity. (A) Comparison of wild-type cells (squares) and a dsrA mutant (circles); sucrose-challenged cells are represented by filled symbols. (B) Comparison of the wild type to the ΔdsrA ΔrprA double mutant under the same conditions. (C) Data from a set of such experiments. Each bar represents the ratio of enzyme activities from the 45-min and time-zero samples, where both values have been normalized to the corresponding protein concentration. Dark bars represent sucrose-challenged cultures, and light bars are untreated controls. The strains used were wild type (TE9160), ΔdsrA (TE9213), and ΔdsrA ΔrprA (TE9219).
The incomplete effect in the S. enterica double mutant contrasts with the results obtained for E. coli. There, a dsrA knockout reduced basal rpoS-lac expression by about sevenfold (at 32°C), but the relative induction of rpoS by osmotic shock was still nearly as high as in the wild type (28). In contrast, induction was almost eliminated for the dsrA rprA double mutant (calculated from Fig. 6 of reference 27). Combined data from a set of trials in S. enterica are shown in Fig. 4C. Each bar represents the ratio of enzyme activity normalized to protein, comparing values from 45 min postchallenge to time zero. It can be seen that the double mutant retained substantial induction by osmotic shock, although it was noticeably decreased from the wild-type level. The normalization to protein levels employed here is technically demanding because of limited sensitivity of the small-scale protein assay as well as high background (data not shown) and because the comparison made is between ratios of ratios (activity divided by protein, at two different times). Therefore, we also analyzed the data in a simpler fashion, by calculating the ratio of total β-galactosidase activity in sucrose-challenged and control cultures at 45 min postchallenge. This alternative approach confirms that the double mutant retained about two-thirds the induction seen in the wild type (data not shown). Since growth of this mutant appears somewhat more sensitive to osmotic shock than the wild type, the value of two-thirds is actually a lower limit for the relative inducibility of the mutant. In summary, our results indicate that in S. enterica osmotic induction of rpoS was decreased by about one-third in a dsrA rprA double mutant, while the defect in the E. coli double mutant was reported to be nearly complete (27).
FIG. 6.
Expression of dsrA from PBAD. A. Strains of E. coli or S. enterica with an rpoS-lac [pr] fusion in the bacterial chromosome, deleted for dsrA, and also carrying a plasmid PBAD-dsrA construct from the species indicated, were induced with 0.02% arabinose and grown overnight to stationary phase in LB-Amp medium at 32°C. Activity of β-galactosidase is plotted for each strain, normalized to the expression seen with a vector control. B and C. Northern analysis of DsrA accumulation in E. coli and S. enterica strains carrying plasmids expressing either S. enterica dsrA (B) or E. coli dsrA (C) under the control of the PBAD promoter. Strains carrying the appropriate empty vector were used as controls. Culture conditions, RNA purification, and Northern blotting were carried out as described in Materials and Methods. Strains were TE9416, TE9418, TE9419, TE9422, TE9424, TE9425, TE9426, TE9427, TE9428, TE9429, TE9430, and TE9431.
Effect of constitutive RcsC.
The third characterized small RNA-mediated regulation of rpoS in E. coli involves induction of RprA via the Rcs phosphorelay system, which also regulates capsule synthesis. As described by Majdalani et al. (29), in E. coli RprA RNA levels are increased about 50-fold by the constitutive rcsC137 allele of the gene encoding the transmembrane sensor kinase RcsC. The recessive nature of this allele (A904V) suggests that it affects a negative regulatory activity contributed by the response regulator domain of this hybrid sensor kinase. RpoS-LacZ levels are increased more than 20-fold by the same mutation, and most of this increase is rprA dependent. We tested two similar constitutively active alleles of rcsC in S. enterica, rcsC55 (T903A) and rcsC64 (F473I), described by Garcia-Calderon et al. (15). Otherwise-isogenic strains were constructed carrying an rpoS::MudK insertion (lac protein fusion at codon 22 of rpoS) and were either wild type for rcsC or carried one of the two constitutive rcsC alleles. Subsequently, a deletion of rprA was introduced into each strain. Expression of rpoS-lac was elevated 2.5- to 3-fold by both rcsC alleles (Fig. 5); however, deletion of rprA had almost no effect on the activation. As a positive control for the effect of constitutive rcsC* mutations, similar strains were constructed by substituting the lac fusion with an insertion of MudJ (forms operon fusions) in the gmm/wcaH gene to monitor transcription of the capsule biosynthesis cluster (15). Activation of wcaH by the mutant alleles of rcsC was at least 50-fold (expression in the wild-type rcsC+ strain was below the limit of detection for this assay). Although RprA was not previously observed to regulate capsule synthesis, introduction of the rprA deletion reduced expression of wcaH-lac to about 60% of the level seen with wild-type rprA. In summary, we found ≈3-fold activation of rpoS expression by both of these constitutive rcsC alleles, but there is no evidence for involvement of the RprA RNA in this response.
FIG. 5.
Effect of activated rcsC on rpoS-lac. In the left half of the figure, the lac protein fusion was formed by insertion of the transposon MudK at codon 22 of the S. enterica rpoS gene. In the right half, the lac fusion was the gmm-21::MudJ insertion (also called wcaH) (15). Two activated alleles of rcsC were compared to wild type, and each set consisted of strains either wild type or mutant for rprA. Cells were grown overnight to stationary phase in LB medium at room temperature (23 to 25°C) and assayed for β-galactosidase activity, normalized to the OD600 as described in Materials and Methods. Results shown are the averages and standard deviations for at least seven independent experiments. Strains for rpoS-lac were rcsC+ rprA+ (TE9317), rcsC+ ΔrprA (TE9353), rcsC55 rprA+ (TE9316), rcsC55 ΔrprA (TE9352), rcsC64 rprA+ (TE9318), and rcsC64 ΔrprA (TE9354). The corresponding strains with the gmm-21::MudJ insertion were TE9334, TE9394, TE9333, TE9395, TE9368, and TE9396.
Testing the effect of overexpression of DsrA on rpoS-lac.
The experiments presented in this paper do not support a significant role for the DsrA and RprA RNAs in the regulation of rpoS in S. enterica. One possible explanation is that the few sequence differences between the dsrA and rprA genes of S. enterica and E. coli are responsible for this unexpected result. Therefore, we tested the function of S. enterica dsrA more extensively by using the overexpression phenotype described by Majdalani et al. (28). The dsrA gene from S. enterica was expressed from the pBAD promoter in plasmid pTE780, and a similar plasmid, pNM3, carrying the E. coli dsrA gene was tested in parallel (28). These plasmids together with vector controls were introduced into E. coli and S. enterica strains bearing appropriate rpoS-lac [pr] fusions. Cultures were grown overnight at 32°C in LB medium with ampicillin, then diluted into the same medium containing arabinose as the inducer, and grown overnight to stationary phase. Preliminary experiments testing various levels of inducer showed that at high levels (0.2% arabinose) the empty vector controls displayed a significant negative effect on rpoS expression. Because of this, the experiments described here were performed with intermediate levels of arabinose (0.02%).
The results are shown in Fig. 6A. Expression of either E. coli or S. enterica dsrA in E. coli resulted in significant induction of rpoS. There was an ≈8-fold increase in activity of rpoS-lac [pr] with overexpression of the E. coli dsrA gene; for S. enterica dsrA the increase was slightly less, ≈6-fold. In contrast, neither gene was able to activate rpoS expression in the S. enterica strain background. This negative result in S. enterica, obtained using a MudK insertion in the native rpoS locus as the reporter, was confirmed using a fusion construct identical to the reporter tested in E. coli: E. coli rpoS fused to lac (6) (data not shown). These results show that S. enterica dsrA is capable of activating rpoS expression in E. coli.
Northern blot analysis of RNA purified from induced exponential cultures showed that DsrA RNA expressed from PBAD was clearly visible in E. coli, but no signal could be detected in S. enterica (Fig. 6B and C). DsrA expressed from the chromosome was also detected in the E. coli vector control strains at 18°C, but not at 32°C (Fig. 6B and C). The pattern of rRNA in all samples was normal (data not shown) (35), and it was determined that the plasmids were maintained in all strains under these conditions (data not shown). Furthermore, arabinose induction of a PBAD-lacZ construct was observed in S. enterica, to approximately the level of a fully induced single-copy lac operon (data not shown). Failure to overexpress DsrA RNA of either type in S. enterica suggests that the defect lies with DsrA RNA turnover, but it allows the formal possibility that S. enterica rpoS also fails to respond.
Mutations affecting the antisense element and its RBS target in S. enterica rpoS.
All previous studies on function of the antisense element employed a lac fusion to the E. coli rpoS gene and its upstream sequences (6). The effects of point mutations on rpoS expression were studied primarily in the S. enterica background (7), while the effects of deletion and overexpression of small RNA genes have been studied in E. coli (28, 29, 44, 52). Therefore, we tested the effects of mutations changing either the antisense element or the rpoS RBS in S. enterica. As described above, the sequence of the rpoS leader is highly conserved in S. enterica compared to E. coli, particularly in the region between the antisense element and the start codon (Fig. 2). Even in the 63-nt connector region, which is predicted to form two stem-loops (20), the eight substitutions found in S. enterica would affect loop nucleotides rather than paired stem nucleotides (data not shown).
A number of point mutations were constructed directly on the bacterial chromosome using the λ Red recombination system and selection for Tets transformants as described in Materials and Methods and combined with a MudK insertion in the rpoS gene to measure their effects on expression. Mutations in the rpoS leader were studied in a strain carrying an insertion in the hfq gene for several reasons. First, an hfq mutation is known to sensitize E. coli to the effects of similar mutations (7). This may be due to the existence of other, as-yet-uncharacterized Hfq-dependent activating sRNAs, or it may be due to far-upstream elements of the rpoS leader which also have Hfq-dependent effects (9). A second reason to use an hfq mutant is that interactions with other molecules might confound the interpretation of phenotypes for strains with compensatory mutations.
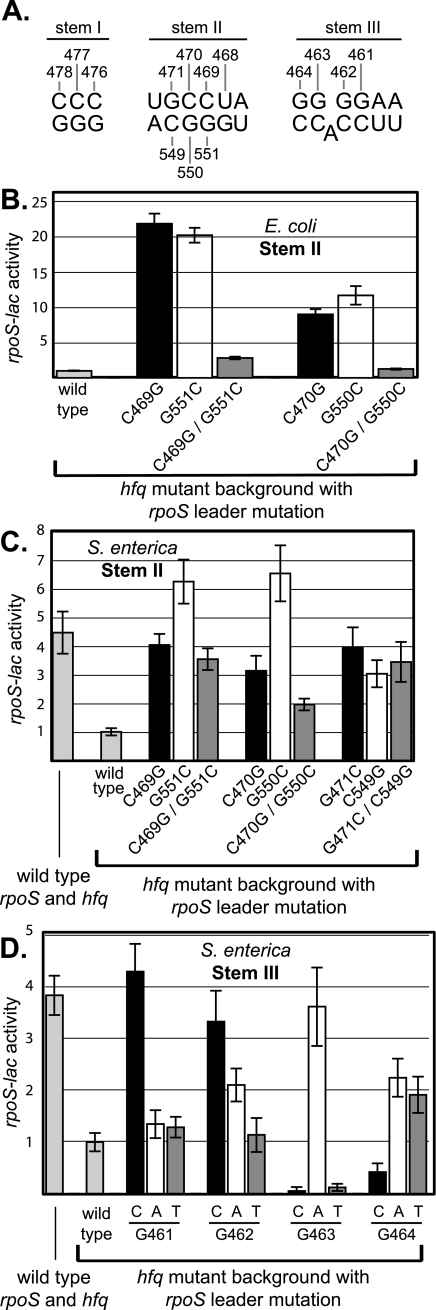
The results of these experiments are shown in Fig. 7. The effects observed for mutations in S. enterica are broadly, but not completely, compatible with what was reported previously in E. coli. For three positions of stem II lying within the antisense element and the corresponding three positions in the RBS region, each mutation tested showed a mutant phenotype with elevated rpoS expression (Fig. 7C). Two differences from E. coli were noted. First, the magnitude of the effect on expression in the mutants was somewhat lower in S. enterica than in E. coli. Second, the effect of compensatory mutations was not as complete or dramatic as observed in E. coli and, for one position, compensation was not observed at all (compare panels C and B).
FIG. 7.
Effects of point mutations in the antisense element and RBS region on expression of rpoS in E. coli and S. enterica. S. enterica strains with mutations in the rpoS leader on the bacterial chromosome were constructed as described in Materials and Methods. The rpoS::MudK (codon 216) fusion and an hfq::Mud-Cam insertion were introduced by P22 transduction. E. coli fusion strains have been described elsewhere or were made in the same way (7). Cultures were grown overnight in LB medium at 23 to 25°C to SP and assayed for β-galactosidase activity. A. Locations of point mutations within the antisense element. B. Results with strains TE6266, TE6557, TE6558, TE6590, TE6369-2, TE6369-3, and TE6382. C. Results with strains TE8808, TE8815, and TE8852 to TE8860. D. Results for strains TE8808, TE8815, and TE9236 to TE9247.
A comprehensive set of mutations in the top strand of stem III was also constructed (Fig. 7D). The pattern for this set is complex: of 12 mutations, 3 have a strong mutant phenotype with elevated expression, 3 are weakly mutant with elevated expression, and 3 are mutant but show decreased expression (as much as 10-fold decreased), while 3 are wild type (Fig. 7D). We do not currently have an explanation for this complex pattern. However, we emphasize that precisely the same pattern was observed in a similar panel of mutations affecting E. coli rpoS at positions 461 to 464 (C. Cunning and T. Elliott, unpublished data). The sole exception was G461A, which is mutant in E. coli and normal in S. enterica.
The conservation of all nucleotides involved in the folded structure as well as the phenotypes of single mutations affecting paired nucleotides are both consistent with a similar function for the antisense element in S. enterica as in E. coli. As a final support for this interpretation, we sought and obtained additional mutations, including some that have a down phenotype with decreased expression of rpoS. These include a double mutation in stem I (C476G C477G, sevenfold elevated) and T468C (fourfold decreased). Phenotypes for both of these mutants are consistent with the model.
DISCUSSION
High overall sequence conservation of the DsrA and RprA small regulatory RNAs in S. enterica and E. coli, as well as complete conservation of their interface with the target in the rpoS leader, suggests conservation of their function. Working with S. enterica, we tested the role played by these two sRNAs in rpoS expression under three specific conditions: growth at low temperature, after osmotic shock, and with constitutive activation of the Rcs phosphorelay by an RcsC* mutation. Only osmotic shock showed a specific requirement for the two RNAs, and even this dependence was partial, whereas in E. coli it is complete (27).
The intrinsic competence of S. enterica DsrA for rpoS regulation was tested in an E. coli host, where it was found to be nearly as effective as E. coli DsrA. Conservation of the ability to stimulate rpoS expression is not surprising, given the sequence identity. The reciprocal test, of E. coli DsrA in an S. enterica host, showed no stimulation of rpoS but was inconclusive, since Northern blot assays did not show any accumulation of the sRNA.
Mutations affecting the antisense element of the S. enterica rpoS leader region have phenotypes generally consistent with those seen in E. coli, except that predicted compensatory double mutants in stem II are not clearly wild type. The reason for this difference from E. coli is not known. Although suppression in double stem II mutants of E. coli was a striking finding in support of the antisense model, weak or absent suppression in S. enterica is not necessarily a strong argument against the model. To explain this, we can invoke additional (as-yet-unknown) factors which interact (or fail to interact) to give elevated expression even with the paired structure present. There is abundant additional evidence that function of the antisense element is important in S. enterica. First, the single top-strand (antisense element) mutants do have a mutant phenotype. Second, for the 469/551 and 470/550 pairs, the double mutant is reduced over expression in the bottom strand mutant alone. So, there is some suppression, but it is weak. Third, we carried out an extensive genetic analysis of the top strand of stem III, and although an explanation for the detailed expression pattern is not available, it is clear that in this respect S. enterica does look almost exactly like E. coli.
In summary, DsrA and RprA functions in regulation of rpoS are not conserved, despite sequence conservation of the sRNA regulators and no change in the sRNA-target interface. The most likely explanation for this is RNA instability in S. enterica, though this was not demonstrated directly.
It does not seem too surprising that the sRNAs are not important for S. enterica rpoS, since rpoS induction by low-temperature growth and activated RcsC* are both much reduced compared to E. coli. The dsrA promoter remains modestly sensitive to temperature in S. enterica, albeit less so than in E. coli (38). The E. coli response is reported to be largely dependent on an unusual −10 sequence (TAAGGT) and an AT-rich 17-bp spacer between the −35 and −10 hexamers (38). The −10 sequence as well as the length and AT-rich motif of the spacer are completely conserved in S. enterica. A primary difference between the two promoters is found between −10 and +1. In E. coli, replacement of the dsrA promoter's native start site with that of lac UV5p also decreased the temperature response. Although mutant dsrA promoter constructs were not assessed in the context of rpoS translation activation, this difference may explain the less dramatic effect of temperature on the dsrA promoter in S. enterica. Regulation of the response to osmotic shock is also clearly different in the two species. The magnitude of the response is similar, but dependence on both sRNAs is much reduced in S. enterica.
Despite partial conservation of thermoregulation for the dsrA promoter and strong expression of the dsrA-lac [op] fusion, DsrA does not accumulate in S. enterica to a level detectable by Northern blotting even when overproduced from the PBAD promoter. Why would S. enterica transcribe DsrA only to degrade it? It might be that this sRNA is more susceptible to RNase activity in S. enterica or has lower affinity for Hfq, which stabilizes DsrA in E. coli (45). A more interesting possibility would be that, since the action of sRNAs is often coupled with degradation (16), there might exist additional or more competitive targets for DsrA action in S. enterica.
How can the nonconservation of DsrA and RprA functions with respect to rpoS regulation be reconciled with high sequence conservation of these genes in E. coli, S. enterica, and other enteric bacteria? For the sRNAs, the simplest explanation is that they have additional targets where their function is adaptive. Some evidence has been presented for additional DsrA functions in E. coli (25, 43), although these studies have relied on overexpression of DsrA and thus may not reflect normal physiology. There is no obvious sign that these sequences have been recently exchanged between the two species. Conservation of the antisense element might be explained by the presence of additional sRNAs, like those described in E. coli (47), or upstream leader sequences with Hfq-dependent action on this target (9). To our knowledge, a search for unique sRNAs in S. enterica has not been made.
Alternatively, activities of other regulatory elements may be tuned to the presence of the antisense element in the same way as they rely on a strong promoter and RBS region. However, this argument is somewhat less persuasive, given the counterexample of the change to a TTG start codon in S. enterica and loss of the protein turnover pathway in many strains of the LT2 lineage.
Acknowledgments
We are grateful to individuals cited in the text for bacterial strains.
This study was supported by Public Health Service grant GM63616.
REFERENCES
- 1.Altman, E., J. R. Roth, A. Hessel, and K. E. Sanderson. 1996. Transposons currently in use in genetic analysis of Salmonella species, p. 2613-2626. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 2. American Society for Microbiology, Washington, D.C. [Google Scholar]
- 2.Becker, G., E. Klauck, and R. Hengge-Aronis. 1999. Regulation of RpoS proteolysis in Escherichia coli: the response regulator RssB is a recognition factor that interacts with the turnover element in RpoS. Proc. Natl. Acad. Sci. USA 96:6439-6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin, W. H., Jr., X. Wu, and W. E. Swords. 1996. The predicted amino acid sequence of the Salmonella typhimurium virulence gene mviA+ strongly indicates that MviA is a regulator protein of a previously unknown S. typhimurium response regulator family. Infect. Immun. 64:2365-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bochner, B. R., and B. N. Ames. 1982. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J. Biol. Chem. 257:9759-9769. [PubMed] [Google Scholar]
- 5.Bochner, B. R., H. C. Huang, G. L. Schieven, and B. N. Ames. 1980. Positive selection for loss of tetracycline resistance. J. Bacteriol. 143:926-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, L., and T. Elliott. 1996. Efficient translation of the RpoS sigma factor in Salmonella typhimurium requires host factor I, an RNA-binding protein encoded by the hfq gene. J. Bacteriol. 178:3763-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, L. A. T. E. 1997. Mutations that increase expression of the rpoS gene and decrease its dependence on hfq function in Salmonella typhimurium. J. Bacteriol. 179:656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castilho, B. A., P. Olfson, and M. J. Casadaban. 1984. Plasmid insertion mutagenesis and lac gene fusion with mini-Mu bacteriophage transposons. J. Bacteriol. 158:488-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunning, C., L. Brown, and T. Elliott. 1998. Promoter substitution and deletion analysis of upstream region required for rpoS translational regulation. J. Bacteriol. 180:4564-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunning, C., and T. Elliott. 1999. RpoS synthesis is growth rate regulated in Salmonella typhimurium, but its turnover is not dependent on acetyl phosphate synthesis or PTS function. J. Bacteriol. 181:4853-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis, R. W., D. Botstein, and J. Roth. 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 13.Ellermeier, C. D., A. Janakiraman, and J. M. Slauch. 2002. Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene 290:153-161. [DOI] [PubMed] [Google Scholar]
- 14.Elliott, T., and X. Wang. 1991. Salmonella typhimurium prfA mutants defective in release factor 1. J. Bacteriol. 173:4144-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Calderon, C. B., M. Garcia-Quintanilla, J. Casadesus, and F. Ramos-Morales. 2005. Virulence attenuation in Salmonella enterica rcsC mutants with constitutive activation of the Rcs system. Microbiology 151:579-588. [DOI] [PubMed] [Google Scholar]
- 16.Gottesman, S. 2004. The small RNA regulators of Escherichia coli: roles and mechanisms. Annu. Rev. Microbiol. 58:303-328. [DOI] [PubMed] [Google Scholar]
- 17.Gowrishankar, J., K. Yamamoto, P. R. Subbarayan, and A. Ishihama. 2003. In vitro properties of RpoS σS mutants of Escherichia coli with postulated N-terminal subregion 1.1 or C-terminal region 4 deleted. J. Bacteriol. 185:2673-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hengge-Aronis, R. 2002. Signal transduction and regulatory mechanisms involved in control of the σS (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 66:373-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch, M., and T. Elliott. 2002. Role of ppGpp in rpoS stationary-phase regulation in Escherichia coli. J. Bacteriol. 184:5077-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch, M., and T. Elliott. 2005. Fis regulates transcriptional induction of RpoS in Salmonella enterica. J. Bacteriol. 187:1568-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes, K. T., and J. R. Roth. 1988. Transitory cis complementation: a method for providing transposition functions to defective transposons. Genetics 119:9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lange, R., D. Fischer, and R. Hengge-Aronis. 1995. Identification of transcriptional start sites and the role of ppGpp in the expression of rpoS, the structural gene for the sigma S subunit of RNA polymerase in Escherichia coli. J. Bacteriol. 177:4676-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lease, R. A., and M. Belfort. 2000. A trans-acting RNA as a control switch in Escherichia coli: DsrA modulates function by forming alternative structures. Proc. Natl. Acad. Sci. USA 97:9919-9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lease, R. A., D. Smith, K. McDonough, and M. Belfort. 2004. The small noncoding DsrA RNA is an acid resistance regulator in Escherichia coli. J. Bacteriol. 186:6179-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, I. S., J. Lin, H. K. Hall, B. Bearson, and J. W. Foster. 1995. The stationary-phase sigma factor sigma S (RpoS) is required for a sustained acid tolerance response in virulent Salmonella typhimurium. Mol. Microbiol. 17:155-167. [DOI] [PubMed] [Google Scholar]
- 27.Majdalani, N., S. Chen, J. Murrow, K. St. John, and S. Gottesman. 2001. Regulation of RpoS by a novel small RNA: the characterization of RprA. Mol. Microbiol. 39:1382-1394. [DOI] [PubMed] [Google Scholar]
- 28.Majdalani, N., C. Cunning, D. Sledjeski, T. Elliott, and S. Gottesman. 1998. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc. Natl. Acad. Sci. USA 95:12462-12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majdalani, N., D. Hernandez, and S. Gottesman. 2002. Regulation and mode of action of the second small RNA activator of RpoS translation, RprA. Mol. Microbiol. 46:813-826. [DOI] [PubMed] [Google Scholar]
- 30.Majdalani, N., C. K. Vanderpool, and S. Gottesman. 2005. Bacterial small RNA regulators. Crit. Rev. Biochem. Mol. Biol. 40:93-113. [DOI] [PubMed] [Google Scholar]
- 31.Maloy, S. R., and W. D. Nunn. 1981. Selection for loss of tetracycline resistance by Escherichia coli. J. Bacteriol. 145:1110-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, J. H. 1981. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Muffler, A., D. Fischer, and R. Hengge-Aronis. 1996. The RNA-binding protein HF-I, known as a host factor for phage Qbeta RNA replication, is essential for rpoS translation in Escherichia coli. Genes Dev. 10:1143-1151. [DOI] [PubMed] [Google Scholar]
- 34.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pronk, L. M., and K. E. Sanderson. 2001. Intervening sequences in rrl genes and fragmentation of 23S rRNA in genera of the family Enterobacteriaceae. J. Bacteriol. 183:5782-5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajkumari, K., and J. Gowrishankar. 2002. An N-terminally truncated RpoS σS protein in Escherichia coli is active in vivo and exhibits normal environmental regulation even in the absence of rpoS transcriptional and translational control signals. J. Bacteriol. 184:3167-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Repoila, F., and S. Gottesman. 2001. Signal transduction cascade for regulation of RpoS: temperature regulation of DsrA. J. Bacteriol. 183:4012-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Repoila, F., and S. Gottesman. 2003. Temperature sensing by the dsrA promoter. J. Bacteriol. 185:6609-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Repoila, F., N. Majdalani, and S. Gottesman. 2003. Small non-coding RNAs, co-ordinators of adaptation processes in Escherichia coli: the RpoS paradigm. Mol. Microbiol. 48:855-861. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz, N., C. N. Peterson, and T. J. Silhavy. 2001. RpoS-dependent transcriptional control of sprE: regulatory feedback loop. J. Bacteriol. 183:5974-5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Skorupski, K., and R. K. Taylor. 1996. Positive selection vectors for allelic exchange. Gene 169:47-52. [DOI] [PubMed] [Google Scholar]
- 43.Sledjeski, D., and S. Gottesman. 1995. A small RNA acts as an antisilencer of the H-NS-silenced rcsA gene of Escherichia coli. Proc. Natl. Acad. Sci. USA 92:2003-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sledjeski, D. D., A. Gupta, and S. Gottesman. 1996. The small RNA, DsrA, is essential for the low temperature expression of RpoS during exponential growth in Escherichia coli. EMBO J. 15:3993-4000. [PMC free article] [PubMed] [Google Scholar]
- 45.Sledjeski, D. D., C. Whitman, and A. Zhang. 2001. Hfq is necessary for regulation by the untranslated RNA DsrA. J. Bacteriol. 183:1997-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takayanagi, Y., K. Tanaka, and H. Takahashi. 1994. Structure of the 5′ upstream region and the regulation of the rpoS gene of Escherichia coli. Mol. Gen. Genet. 243:525-531. [DOI] [PubMed] [Google Scholar]
- 47.Wassarman, K. M., F. Repoila, C. Rosenow, G. Storz, and S. Gottesman. 2001. Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev. 15:1637-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Webb, C., M. Moreno, M. Wilmes-Riesenberg, R. Curtiss III, and J. W. Foster. 1999. Effects of DksA and ClpP protease on sigma S production and virulence in Salmonella typhimurium. Mol. Microbiol. 34:112-123. [DOI] [PubMed] [Google Scholar]
- 49.Weber, H., T. Polen, J. Heuveling, V. F. Wendisch, and R. Hengge. 2005. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 187:1591-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilmes-Riesenberg, M. R., J. W. Foster, and R. Curtiss III. 1997. An altered rpoS allele contributes to the avirulence of Salmonella typhimurium LT2. Infect. Immun. 65:203-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang, A., S. Altuvia, A. Tiwari, L. Argaman, R. Hengge-Aronis, and G. Storz. 1998. The OxyS regulatory RNA represses rpoS translation and binds the Hfq (HF-I) protein. EMBO J. 17:6061-6068. [DOI] [PMC free article] [PubMed] [Google Scholar]