Abstract
The adhesive holdfast is required for irreversible surface anchoring of Caulobacter crescentus cells. The holdfast is synthesized early during swarmer cell development and, together with pili and a functional flagellum, contributes to optimal attachment during cell differentiation. We present evidence that the timing of holdfast formation in swarmer cells is regulated posttranslationally and is dependent on the diguanylate cyclase PleD.
During Caulobacter crescentus development, surface adhesion is coupled to cell proliferation. Each cell division is asymmetric and generates a sessile stalked (ST) cell and a motile, flagellated swarmer (SW) cell. A single flagellum and adhesive pili are assembled at one pole during cell division (24, 30). SW cells are motile for a defined period (3) before differentiating into ST cells. During differentiation, the flagellum and pili are lost and are replaced by an adhesive holdfast and a stalk at the same pole (Fig. 1). While the pili and flagellum facilitate C. crescentus surface binding, an intact holdfast structure is required for irreversible surface anchoring (4, 8, 18, 26). A mutant screen for components involved in surface colonization revealed that a large fraction of mutations mapped to genes required for polar-organelle function or assembly, lending additional support for a critical role for these extracellular appendices in C. crescentus surface attachment (4; data not shown). Several mutants mapped to established and novel genes involved in holdfast synthesis or control and showed the strongest reduction in surface binding (6, 25; data not shown).
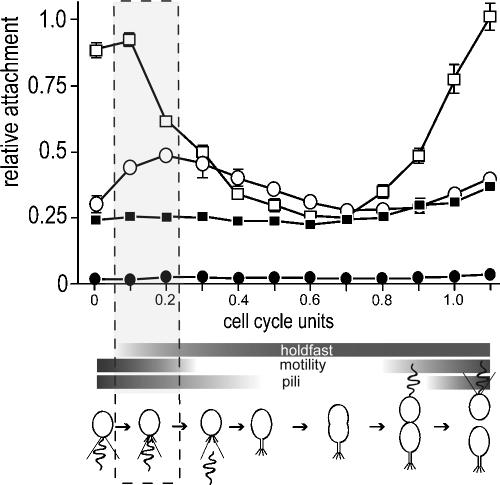
FIG. 1.
Surface attachment during the C. crescentus cell cycle. SW cells of the CB15 wild type (ATCC 19089) (open squares) and isogenic ΔpilA (open circles), ΔflgFG (closed squares), and ΔCC2277 (closed circles) mutants were purified and suspended in fresh peptone-yeast extract medium (per liter, 2.0 g of peptone and 1.0 g of yeast extract). Aliquots were removed from synchronized cultures throughout the cell cycle at 15-minute intervals, transferred to microtiter plates, and allowed to bind to the plastic surface for 15 min. Attachment was quantified by crystal violet staining according to the method of O'Toole and Kolter (19). The surface exposure and activity of polar organelles are indicated with horizontal bars below the time scale. Appearance and disappearance of pili were taken from Sommer and Newton (26). Motility was monitored microscopically throughout the cell cycle, and the presence of a polar holdfast was determined by fluorescent staining as described in the text. Cell cycle progression is indicated schematically below the graph. The time window of development during which flagellum, pili, and holdfast are exposed concomitantly at the same cell pole is boxed.
To analyze the contribution of polar organelles to C. crescentus surface binding during development, CB15 wild type (ATCC 19089) and isogenic mutants lacking flagellum, pili, or holdfast were analyzed in differentiating SW cells. Newborn SW cells were isolated by Ludox gradient centrifugation (27) and released into fresh medium. At the time points indicated, aliquots of cells were transferred to microtiter plates and allowed to bind to the plastic surface for 15 min before attachment was measured by crystal violet staining. Surprisingly, motile SW cells rather than holdfast-bearing ST cells showed the highest attachment activity. C. crescentus wild type attachment peaked 15 to 30 min after newborn SW cells were released into fresh medium, at a time when the cells were still fully motile (Fig. 1). Upon leaving the motile stage of development, attachment levels quickly dropped and reached their lowest level at the ST cell stage, only to increase again toward the end of the cell cycle (Fig. 1). A mutant lacking pili (pilA) showed a similar but reduced peak during SW cell differentiation (Fig. 1). In contrast, a mutant lacking the flagellum (flgFG) showed basal, ST cell-like attachment levels throughout the cell cycle (Fig. 1). The same attachment defect was observed for mutants with a paralyzed flagellum (data not shown), arguing that an active motor is required for the attachment peak during SW-to-ST cell transition. Cells that lacked a holdfast structure (ΔCC2277) completely failed to bind to plastic surfaces (Fig. 1). These experiments suggested that the temporal pattern of surface binding during C. crescentus development is primarily influenced by the exposure and activities of the flagellar motor and holdfast, while polar pili, possibly through their ability to retract (24), contribute to the efficiency of surface contacts.
Because the holdfast is critical for irreversible surface anchoring of cells, the finding that maximal attachment occurs early in SW cell development is at odds with the current belief that the holdfast is not present in SW cells and is synthesized at a late stage of the SW-to-ST cell transition after cells have ejected the flagellum (13). To assess the possibility that the holdfast is synthesized at an earlier, motile phase of the cell cycle, we developed an improved holdfast-staining method based on a mixture of Oregon Green-conjugated wheat germ agglutinin (0.2 mg/ml) and Calcofluor White (0.1 mg/ml). With this technique, the appearance of a holdfast at the cell pole was confined to the first 15 to 30 min of SW cell differentiation (Fig. 1 and 2 and data not shown). While no holdfast structures were visible at time zero, almost 75% of the SW cells exhibited a detectable holdfast after 15 min of development (Fig. 2). At this stage, all cells were still motile (Fig. 1). This is consistent with the view that during a short window of development, which coincides with optimal attachment, an active flagellum and holdfast coexist at the same pole of the differentiating cell. Cell motility could contribute to attachment by bringing cells in close contact with the surface, where pilus- and holdfast-mediated adhesion and anchoring can occur. Alternatively, it is possible that the flagellar motor is part of a signaling cascade required for optimal expression of adhesive properties upon surface contact (16, 17).
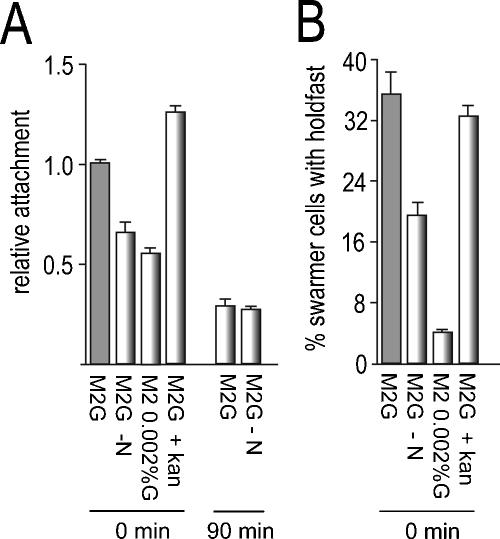
FIG. 2.
Holdfast formation and optimal surface attachment requires SW cell development but not de novo protein synthesis. (A) Purified SW cells of C. crescentus CB15 wild type (ATCC 19089) were released into glucose minimal medium (M2G), M2G lacking nitrogen (M2G −N), M2 with a 100-fold-reduced glucose concentration (M2 0.002%G), and M2G containing kanamycin (50 μg/ml; the MIC of kanamycin for C. crescentus is 1 μg/ml). Culture aliquots were immediately (0 min) transferred to microtiter plates and allowed to attach to the plastic surface for 30 min. Attachment was quantified as described in the legend to Fig. 1. As a control, purified SW cells were released into M2G and allowed to go through the SW-to-ST cell transition for 90 min (90 min) before cells were harvested, washed, and released into either M2G or M2G lacking nitrogen (M2G −N). The cells were transferred to microtiter plates and allowed to attach to the plastic surface for 30 min. (B) Purified SW cells were treated as in panel A and incubated for 30 min at 30°C, and the fraction of cells with a visible holdfast was determined by fluorescent labeling. The error bars indicate standard deviations of the mean of triplicate experiments.
The finding that holdfast formation occurs very early in development offers a plausible explanation for the sharp peak of attachment during C. crescentus SW-to-ST cell transition. For technical reasons, binding to a plastic surface had to be determined during a 15-minute time window (e.g., the value determined for the 0-min time point corresponds to the 0- to 15-min time window). Bearing in mind that most cells had already formed a holdfast structure after 15 min of development (Fig. 1 and 2), the surface binding capacity of newborn SW cells was most likely overestimated in this experiment. Thus, the actual peak of surface attachment during cell differentiation might be even more pronounced than is shown in Fig. 1. Bodenmiller et al. (4) provided evidence for the idea that in C. crescentus, attachment could be developmentally controlled. They observed a constant, low-level attachment throughout the SW-to-ST cell transition (4). Based on our observation that nonmotile mutants show constant but low-level attachment during development, this discrepancy is likely due to damage or loss of flagellar motility during the synchronization process in their experiment (4).
The C. crescentus SW-to-ST cell transition is blocked in the absence of a nitrogen or carbon source (5, 10). In agreement with this, SW cells released into M2 minimal medium (15) without nitrogen or with a 100-fold-reduced glucose concentration (0.002%) retained motility for several hours without forming stalks (data not shown). Under these conditions, attachment of SW cells was significantly reduced (Fig. 2A). When SW cells were first allowed to differentiate into ST cells for 90 min in M2G minimal medium before they were transferred to a medium lacking nitrogen, surface binding, even though reduced to the level typically observed for ST cells (Fig. 1), was no longer dependent on nitrogen (Fig. 2A). This indicated that the reduction in surface binding is not a direct consequence of limited nutrients but is caused by an indirect effect on SW cell development. The reduction in surface binding in the absence of nitrogen or at low glucose concentrations correlated with a significant drop in holdfast formation during the first 30 min of development (Fig. 2B and data not shown). In contrast, the addition of kanamycin (Fig. 2 and data not shown), chloramphenicol, or tetracycline (data not shown) at growth-inhibitory concentrations had no effect on attachment or on holdfast biogenesis. Based on this, we propose that optimal attachment is dependent on active development and that de novo protein synthesis is not required for holdfast formation and the differentiation of SW cells into an adhesion-competent form. This is consistent with the view that newborn SW cells are fully equipped with the holdfast synthesis machinery, which in turn must be activated posttranslationally at an early stage of SW cell development.
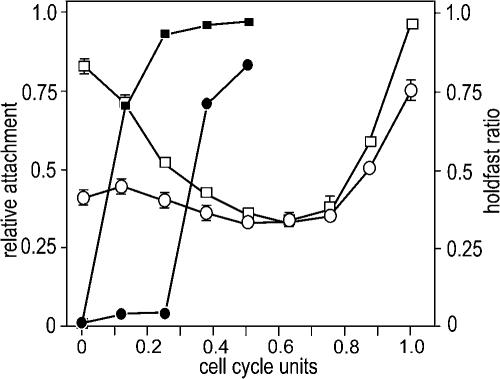
While posttranslational regulation of holdfast biosynthesis in SW cells would be consistent with the observation that known holdfast genes are transcribed in predivisional cells (13), it raised the question of the molecular mechanisms and signals involved in the initiation of holdfast synthesis during SW cell development. One of the transposon insertions isolated in the screen for surface binding mutants mapped to the pleD gene (data not shown). PleD is a diguanylate cyclase that, upon phosphorylation, sequesters to the developing pole (2, 20). Mutants lacking PleD fail to efficiently eject the flagellum and synthesize stalks during development (1, 11). An in-frame deletion in pleD in the CB15 wild-type (ATCC 19089) background also reduced surface binding by about 70% (data not shown). This was surprising, since earlier results had indicated that neither pilus nor holdfast biogenesis was affected in pleD mutants (2, 11). Surface binding of the pleD mutant strain was exclusively affected during the early stages of development, while at later stages of the cell cycle, attachment was similar to that of the wild type (Fig. 3). Reduced attachment during the SW-to-ST cell transition correlated with a considerable delay in holdfast biogenesis. While wild-type SW cells acquire a holdfast more or less immediately after entry into development, the exposure of a visible holdfast was delayed for almost one-third of the entire cell cycle in the pleD mutant (Fig. 3). Since the cell cycle lengths of the wild type and the pleD mutant are similar (1), this argued that PleD is a timing device for the formation of the adhesive organelle during C. crescentus cell differentiation. Mutants lacking PleD are sensitive to bacteriophage φCbK (2), and cell cycle-dependent fluctuations of the major pilin subunit PilA (28) were identical in wild-type and pleD mutant cells (data not shown). In cells lacking PleD, the holdfast was detectable only about 30 min after the PilA protein had disappeared (data not shown). Thus, we propose that the surface adhesion defect of a pleD mutant is due to a timing defect of holdfast synthesis during development and, as a result, the temporal uncoupling of the two adhesive organelles, pili and holdfast. In agreement with this role, PleD is activated by phosphorylation during the SW-to-ST cell transition and, as a consequence, sequesters to the differentiating pole (20). Phosphorylation of PleD results in the activation of the C-terminal diguanylate cyclase domain, which catalyzes the production of c-di-GMP from two molecules of GTP (20). The signaling molecule c-di-GMP plays a prominent role in the transition between the planktonic and surface-attached modes of bacterial growth (reviewed in references 14 and 21). While c-di-GMP effector proteins have not yet been identified, signaling by c-di-GMP seems to take place, at least in part, at the posttranslational level (12, 29, 31). It is thus conceivable that a PleD-catalyzed burst of c-di-GMP is responsible for the correct temporal control of holdfast formation during C. crescentus development.
FIG. 3.
A pleD mutant shows reduced surface binding and delayed holdfast formation. SW cells of CB15 wild type (ATCC 19089) (open squares) and an isogenic ΔpleD mutant (open circles) were purified and suspended in fresh PYE medium. Aliquots were removed from the synchronized culture throughout the cell cycle at 15-minute intervals, transferred to microtiter plates, and allowed to attach to the plastic surface for 15 min. Attachment (open symbols) and holdfast formation (closed symbols) were quantified as indicated in Fig. 2. Cell cycle progression is indicated as cell cycle units. The error bars indicate standard deviations.
Acknowledgments
This work was supported by Swiss National Science Foundation fellowships 31-59050.99 and 3100A0-108186 to U.J.
REFERENCES
- 1.Aldridge, P., and U. Jenal. 1999. Cell cycle-dependent degradation of a flagellar motor component requires a novel-type response regulator. Mol. Microbiol. 32:379-391. [DOI] [PubMed] [Google Scholar]
- 2.Aldridge, P., R. Paul, P. Goymer, P. Rainey, and U. Jenal. 2003. Role of the GGDEF regulator PleD in polar development of Caulobacter crescentus. Mol. Microbiol. 47:1695-1708. [DOI] [PubMed] [Google Scholar]
- 3.Alley, M. R., J. R. Maddock, and L. Shapiro. 1992. Polar localization of a bacterial chemoreceptor. Genes Dev. 6:825-836. [DOI] [PubMed] [Google Scholar]
- 4.Bodenmiller, D., E. Toh, and Y. V. Brun. 2004. Development of surface adhesion in Caulobacter crescentus. J. Bacteriol. 186:1438-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiaverotti, T. A., G. Parker, J. Gallant, and N. Agabian. 1981. Conditions that trigger guanosine tetraphosphate accumulation in Caulobacter crescentus. J. Bacteriol. 145:1463-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole, J. L., G. G. Hardy, D. Bodenmiller, E. Toh, A. Hinz, and Y. V. Brun. 2003. The HfaB and HfaD adhesion proteins of Caulobacter crescentus are localized in the stalk. Mol. Microbiol. 49:1671-1683. [DOI] [PubMed] [Google Scholar]
- 7.Reference deleted.
- 8.Entcheva-Dimitrov, P., and A. M. Spormann. 2004. Dynamics and control of biofilms of the oligotrophic bacterium Caulobacter crescentus. J. Bacteriol. 186:8254-8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reference deleted.
- 10.Gorbatyuk, B., and G. T. Marczynski. 2005. Regulated degradation of chromosome replication proteins DnaA and CtrA in Caulobacter crescentus. Mol. Microbiol. 55:1233-1245. [DOI] [PubMed] [Google Scholar]
- 11.Hecht, G. B., and A. Newton. 1995. Identification of a novel response regulator required for the swarmer-to-stalked cell transition in Caulobacter crescentus. J. Bacteriol. 177:6223-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, B., C. B. Whitchurch, and J. S. Mattick. 2003. FimX, a multidomain protein connecting environmental signals to twitching motility in Pseudomonas aeruginosa. J. Bacteriol. 185:7068-7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janakiraman, R. S., and Y. V. Brun. 1999. Cell cycle control of a holdfast attachment gene in Caulobacter crescentus. J. Bacteriol. 181:1118-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenal, U. 2004. Cyclic di-guanosine-monophosphate comes of age: a novel secondary messenger involved in modulating cell surface structures in bacteria? Curr. Opin. Microbiol. 7:185-191. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, R. C., and B. Ely. 1977. Isolation of spontaneously derived mutants of Caulobacter crescentus. Genetics 86:25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawagishi, I., M. Imagawa, Y. Imae, L. McCarter, and M. Homma. 1996. The sodium-driven polar flagellar motor of marine Vibrio as the mechanosensor that regulates lateral flagellar expression. Mol. Microbiol. 20:693-699. [DOI] [PubMed] [Google Scholar]
- 17.Lauriano, C. M., C. Ghosh, N. E. Correa, and K. E. Klose. 2004. The sodium-driven flagellar motor controls exopolysaccharide expression in Vibrio cholerae. J. Bacteriol. 186:4864-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong, C. J., M. L. Wong, and J. Smit. 1990. Attachment of the adhesive holdfast organelle to the cellular stalk of Caulobacter crescentus. J. Bacteriol. 172:1448-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 20.Paul, R., S. Weiser, N. C. Amiot, C. Chan, T. Schirmer, B. Giese, and U. Jenal. 2004. Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain. Genes Dev. 18:715-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romling, U., M. Gomelsky, and M. Y. Galperin. 2005. C-di-GMP: the dawning of a novel bacterial signalling system. Mol. Microbiol. 57:629-639. [DOI] [PubMed] [Google Scholar]
- 22.Reference deleted.
- 23.Reference deleted.
- 24.Skerker, J. M., and L. Shapiro. 2000. Identification and cell cycle control of a novel pilus system in Caulobacter crescentus. EMBO J. 19:3223-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith, C. S., A. Hinz, D. Bodenmiller, D. E. Larson, and Y. V. Brun. 2003. Identification of genes required for synthesis of the adhesive holdfast in Caulobacter crescentus. J. Bacteriol. 185:1432-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommer, J. M., and A. Newton. 1988. Sequential regulation of developmental events during polar morphogenesis in Caulobacter crescentus: assembly of pili on swarmer cells requires cell separation. J. Bacteriol. 170:409-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens, C. M., and L. Shapiro. 1993. An unusual promoter controls cell-cycle regulation and dependence on DNA replication of the Caulobacter fliLM early flagellar operon. Mol. Microbiol. 9:1169-1179. [DOI] [PubMed] [Google Scholar]
- 28.Viollier, P. H., N. Sternheim, and L. Shapiro. 2002. A dynamically localized histidine kinase controls the asymmetric distribution of polar pili proteins. EMBO J. 21:4420-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinhouse, H., S. Sapir, D. Amikam, Y. Shilo, G. Volman, P. Ohana, and M. Benziman. 1997. c-di-GMP-binding protein, a new factor regulating cellulose synthesis in Acetobacter xylinum. FEBS Lett. 416:207-211. [DOI] [PubMed] [Google Scholar]
- 30.Wu, J., and A. Newton. 1997. Regulation of the Caulobacter flagellar gene hierarchy; not just for motility. Mol. Microbiol. 24:233-239. [DOI] [PubMed] [Google Scholar]
- 31.Zogaj, X., M. Nimtz, M. Rohde, W. Bokranz, and U. Romling. 2001. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol. Microbiol. 39:1452-1463. [DOI] [PubMed] [Google Scholar]