Abstract
The immunological mechanisms that regulate abortion are largely unknown. Here, we found that a distinct subset of lymphocytes, Vα14 NKT cells expressing an invariant antigen receptor encoded by Vα14/Jα281 and Vβ7 segments, accumulated in the decidua during pregnancy and provoked abortion upon stimulation with α-galactosylceramide (α-GalCer), a specific ligand for Vα14 NKT cells. The α-GalCer-mediated abortion was not observed in Vα14 NKT-, IFN-γ-, tumor necrosis factor α-, or perforin-knock-out mice and appeared to be due to the degeneration of embryonic trophoblasts mediated by the activated Vα14 NKT cells whose perforin-dependent killing and production of IFN-γ and tumor necrosis factor α were essential. The possible role of the decidual Vα14 NKT cells in the pathogenesis of abortion is discussed.
Although fetal tissues possess paternal antigens, the fetus is thought to be protected from the maternal immune system. However, the precise cellular and molecular mechanisms underlying the maintenance of pregnancy and the induction of abortion remain unclear. Various immunological mechanisms have been proposed (1–6). Cytokines produced by type 2 helper T (Th2) cells, including IL-10 and tumor growth factor β (TGF-β), seem to be important for the maintenance of pregnancy through their immunosuppressive effects (2–5). On the other hand, Th1 cytokines, such as IL-2, IFN-γ, and tumor necrosis factor α (TNF-α), induce abortion (1, 6). In addition to these cytokines, a certain type of cells seems to be involved. Recent findings by Munn et al. (7, 8) suggest that indoleamine 2,3-dioxygenase (IDO), an enzyme that catabolizes tryptophan, plays a critical role for the maintenance of pregnancy by suppressing maternal allospecific T cell activation in the decidua. Moreover, the involvement of natural killer (NK) cell-mediated immune mechanisms also has been reported (6, 9, 10). Thus, it is conceivable that several types of lymphocytes with different functional activities and their cytokines contribute to pregnancy and abortion in different pathological situations. Despite the above experimental findings, immunological mechanisms involved in the fetomaternal interaction remain controversial. This might be due to the lack of an appropriate experimental model system for addressing the critical questions about the maintenance of pregnancy and the induction of abortion.
Vα14 NKT cells, recently defined as a distinct subset of lymphocytes, are characterized by the coexpression of an NK marker, NK1-1, and a single invariant antigen receptor encoded by Vα14 and Jα281 gene segments (11–13). Conventional T cells do not express the invariant Vα14Jα281 antigen receptor (14), indicating their selective usage in Vα14 NKT cells. Thus, disruption of the invariant Vα14 receptor results in the selective loss of Vα14 NKT cells, leaving other immune systems intact (15). It is thus indicated that Vα14 NKT cells are a distinct lymphoid lineage expressing several characteristics that demarcate them from T cells, NK cells, and B cells. Vα14 NKT cells produce both IFN-γ and IL-4 upon stimulation with their specific ligand, α-galactosylceramide (α-GalCer) (16). Other lymphoid populations, such as T cells or NK cells, are not activated by α-GalCer (16, 17). Moreover, the ligand-activated Vα14 NKT cells directly kill various tumor cells by an NK-like mechanism after direct contact with targets, resulting in the inhibition of tumor metastasis in vivo (18). Thus, Vα14 NKT cells appear to play a crucial role in various immune responses.
Recently, parasitic glycosyl-phosphatidylinositols derived from Plasmodium, Trypanosoma, or Leishmania, which are known to induce abortion at high frequency (4, 19–21), were shown to stimulate Vα14 NKT cells (22). Therefore, it is of interest to determine whether Vα14 NKT cells are responsible for abortion. Here, we demonstrate that Vα14 NKT cells accumulate in the decidua and provoke abortion upon stimulation with α-GalCer. The abortion appears to be due to the degeneration of embryonic trophoblasts mediated by the activation of Vα14 NKT cells in which perforin-dependent killing and the secretion of TNF-α and IFN-γ are essential.
Materials and Methods
Mice.
Specific pathogen-free C57BL/6 (B6) mice were purchased from Japan SLC (Hamamatsu, Japan). Vα14 NKT-knock-out (Vα14 NKT-KO) mice established by the specific deletion of the Jα281 gene segment (15) were backcrossed nine generations with B6. In Vα14 NKT-KO mice, only Vα14 NKT cells are missing, whereas other lymphoid populations, such as T, B, and NK cells, remain intact. The perforin-KO, TNF-α-KO, IFN-γ-KO, and ROSA βgeo 26 (ROSA 26) mice used here were of B6 background. Perforin-KO mice were kindly provided by H. Hengartner, University of Zurich (23). TNF-α-KO and IFN-γ-KO mice were provided by Y. Iwakura, Institute of Medical Science, University of Tokyo (24). ROSA 26 male mice expressing β-galactosidase were purchased from The Jackson Laboratory (25). Recombination activating gene-1 (RAG-1)-KO mice were provided by P. Mombaerts, Massachusetts Institute for Technology, Cambridge (26).
Induction of Abortion by α-GalCer.
Normal B6 and various KO mice of B6 background were mated at 8–12 weeks of age. The day a vaginal plug was found was taken as day 0 of pregnancy. The pregnant females were injected i.p. with α-GalCer at a dose of 100 μg/kg or 0.025% Polysolvate 20 (Nikko Chemical, Tokyo) in PBS as a vehicle control. Three days later, the numbers of resorbing and living embryos were counted.
PCR.
Total RNAs were isolated by using Trizol reagent (GIBCO/BRL). Reverse transcription–PCR was carried out with 10 μg of the RNA (27). The primers used for PCR amplifications were the following: IL-2, 5′-GTCAACAGCGCACCCACTTCAAGC-3′ and 5′-GCTTGTTGAGATGATGCTTTGACA-3′; IL-4, 5′-ACGGAGATGGATGTGCCAAACGTC-3′ and 5′-CGAGTAATCCATTTGCATGATGC-3′; IFN-γ, 5′-TACTGCCACGGCACAGTCATTGAA-3′ and 5′-GCAGCGACTCCTTTTCCGCTTCCT-3′; and TNF-α, 5′-ATGAGCACAGAAAGCATGATC-3′ and 5′-TACAGGCTTGTCACTCGAATT-3′.
Antibodies and Flow Cytometry.
mAbs used were as follows: antibodies against NK1.1 (PK136-PE), T cell antigen receptor β (TCRβ) (H57–597-biotin), Vβ7 (TR310-FITC), and Vβ8 (MR5–2-FITC) were purchased from PharMingen. Cychrome-streptavidin (PharMingen) was used to detect the biotinylated antibodies. Mononuclear cells in the decidua and spleen were collected by Ficoll/Hypaque (Amersham Pharmacia) gradient centrifugation and analyzed by EPICS XL (Coulter) as described (27).
Cytokine Production of Decidual NKT Cells.
The decidual mononuclear cells were stimulated with α-GalCer-pulsed dendritic cells (DCs) prepared from the spleens of RAG-1-KO mice (16). Because the number of DCs in the mononuclear cell preparations varied in different experiments, α-GalCer-pulsed DCs were used to obtain maximal responses. Cytokine concentrations in the supernatants were measured with ELISA kits (Endogen, Cambridge, MA).
Histology.
Uteri were fixed in 4% paraformaldehyde overnight at 4°C. They then were treated with ethanol and xylene, immersed in paraffin, and embedded. Sections of 4 μm in thickness were prepared and stained with hematoxylin and eosin solution.
β-Galactosidase Staining and Methacrylate-Embedded Sections.
B6 females were mated to ROSA βgeo 26 (ROSA 26) male mice expressing β-galactosidase (25). The uteri of F1 females were fixed in 2% paraformaldehyde/5 mM EGTA/2 mM magnesium chloride in 0.1 M sodium phosphate buffer, pH 7.3, for 30 min at room temperature. After washing twice in 2 mM magnesium chloride in 0.1 M sodium phosphate buffer, pH 7.3, the samples were stained in 0.1 M sodium phosphate buffer, pH 7.3, containing 1 mg/ml X-Gal (5-bromo-4-chloro-3-indolyl β-d-galactoside; Wako), 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide (Sigma), 2 mM magnesium chloride, and 0.2% Nonidet P-40. X-Gal-stained samples were embedded in methacrylate with a JB-4 embedding kit (Polysciences) (28). Sections were cut at a thickness of 3–4 μm and observed under a microscope in a dark field.
Detection of Apoptotic Cells.
Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay on the uterus section was performed by following the manufacturer's protocol (Boehringer Mannheim). After signal conversion by alkaline phosphatase reaction, specimens were counterstained with 1% methyl green solution.
Results
Vα14 NKT Cells in the Decidua.
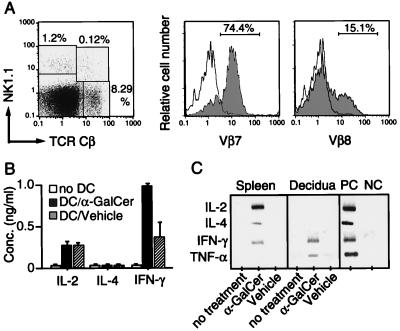
We first investigated the feature of Vα14 NKT cells accumulated in the decidua during pregnancy. The decidual mononuclear cells were prepared from three pregnant B6 mice on day 14 of gestation and stained with anti-NK1.1, anti-TCRβ, and anti-Vβ7 or anti-Vβ8. As shown in Fig. 1A, 0.12% of mononuclear cells in the decidua were NKT cells expressing both NK1.1 and TCRβ. The majority of the NKT cells were found to express Vβ7 (74.4%), whereas Vβ8+ cells accounted for only 15.1%. PCR analysis on Vβ usage in the decidual mononuclear cells at different time points in pregnancy also confirmed that Vβ7 dominated from the beginning to the end of pregnancy (data not shown). Moreover, the majority of the Vα14 sequences (ICVGGDRGS in the CDR3 region; 18 of 19 sequenced) in decidual mononuclear cells were identical to that of the canonical Vα14 NKT cell receptor (11–13). In addition, the dominant Vβ7 sequences were mostly invariant (ASSLWQGEQY in the CDR3 region; 21 of 23 sequenced), and not found in other tissues, including bone marrow, spleen, liver, and thymus (27). These results indicate that a unique subset of Vα14/Vβ7 NKT cells accumulates in the decidua during pregnancy.
Figure 1.
Characterization of decidual Vα14 NKT cells. (A) Flow cytometric analysis of decidual NKT cells. The frequencies of Vβ7- and Vβ8-bearing TCRCβ+NK1.1+ NKT cells in decidual mononuclear cells were assessed by a flow cytometer. (B) Cytokine production of decidual Vα14 NKT cells after stimulation with α-GalCer in vitro. The decidual mononuclear cells were stimulated with vehicle-pulsed (DC/vehicle, hatched bar) or α-GalCer-pulsed DCs (DC/α-GalCer, solid bar) for 48 hr. The open bars represent responder mononuclear cells alone (no DC). The concentrations of IL-2, IL-4, and IFN-γ in the culture supernatant were determined by ELISA and are shown as a mean value of three samples with SD. (C) Induction of cytokine gene expression in decidual Vα14 NKT cells after in vivo activation with α-GalCer. Spleen cells and decidual mononuclear cells were prepared 4 hr after the administration of α-GalCer or control vehicle. The amounts of mRNA of IL-2, IL-4, IFN-γ, and TNF-α were assessed by reverse transcription–PCR. For a positive control (PC), Con A-stimulated splenic blastoid cells, and a negative control (NC), freshly prepared spleen cells were used.
Cytokine Production by Decidual Vα14 NKT Cells.
Vα14 NKT cells in adult lymphoid tissues are known to produce both IFN-γ and IL-4 upon stimulation with α-GalCer (16). Cytokine production by decidual Vα14 NKT cells was examined after stimulation with α-GalCer-pulsed DCs in vitro. Interestingly, the decidual Vα14 NKT cells produced a significant amount of IFN-γ but not IL-4 or IL-2 (Fig. 1B). Similarly, IFN-γ and TNF-α transcripts but not those of IL-4 or IL-2 were induced in the decidual mononuclear cells of pregnant mice receiving α-GalCer (Fig. 1C). These cytokine profiles are clearly distinct from those of splenic Vα14 NKT cells (16).
Involvement of Decidual Vα14 NKT Cells in α-GalCer-Induced Abortion.
To investigate the function of decidual Vα14 NKT cells, α-GalCer was injected to B6 pregnant mice at different gestation times. Three days later, the numbers of resorbing and living embryos were counted. As summarized in Table 1, injection of α-GalCer on days 4–8 of pregnancy induced fetal resorption at a significantly high frequency (71–100% by α-GalCer vs. 17–31% by vehicle) in B6 mice. Interestingly, when α-GalCer was injected even before implantation, i.e., on day 4 of gestation, hemosiderin stains, generally observed as a trace of fetal implantation, were obvious in the uteri (see Fig. 2A). This finding implies that embryos implanted once, but failed to develop further.
Table 1.
The incidence of abortion induced by α-GalCer in B6 mice
| Date of injection (days of pregnancy) | Number of aborted/total embryos (%)
|
|
|---|---|---|
| Vehicle | α-GalCer | |
| 4 | 13/49 (26.5) | 58/81 (71.6)* |
| 6 | 9/46 (20.0) | 55/66 (83.3)* |
| 7 | 10/32 (31.2) | 36/36 (100)** |
| 8 | 6/34 (17.6) | 30/31 (96.8)* |
*, P < 0.005 and **, P < 0.01, by χ2 test.
Figure 2.
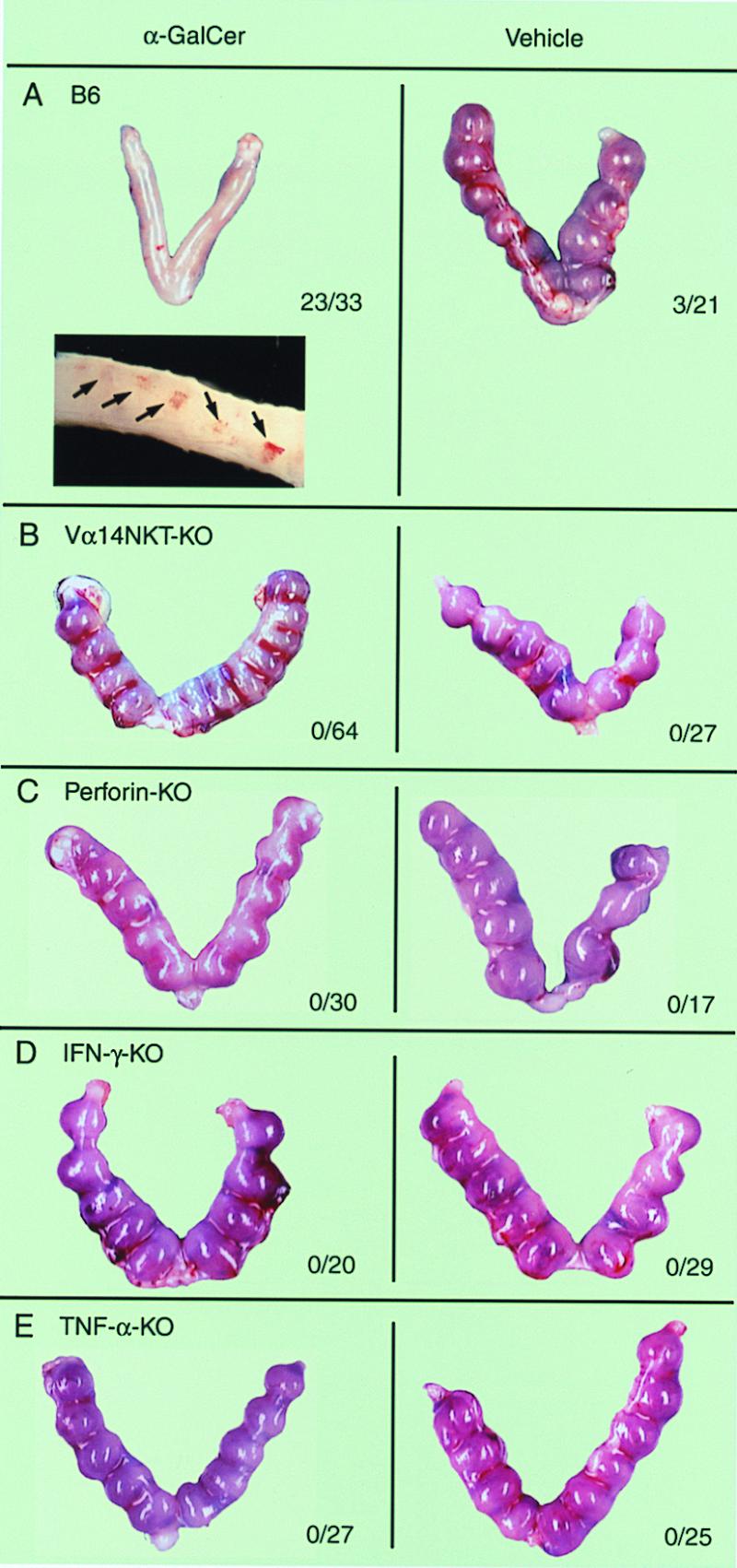
The abortion mediated by α-GalCer-activated Vα14 NKT cells. Pregnant B6 (A), Vα14 NKT-KO (B), perforin-KO (C), IFN-γ-KO (D), and TNF-α-KO (E) mice were administrated α-GalCer or control vehicle on day 6 of gestation. Three days later, pregnant mice were sacrificed and the numbers of living or resorbing embryos were counted. A representative macroscopic view of uteri in each experimental group is shown. The number of aborted fetuses per number of total fetuses is summarized and shown with each image. In A, a representative photographic view of hemosiderin deposition indicating the implantation of fertilized egg in the uterine epithelium of B6 mice is also shown (arrows, Inset).
To further investigate the requirement of Vα14 NKT cells for the abortion, B6 and Vα14 NKT-KO mice were injected with α-GalCer on day 6 of pregnancy. No fetal loss was detected in Vα14 NKT-KO mice, in which Vα14 NKT cells failed to develop, leaving other lymphoid cells, including T, B, and NK cells, intact (Fig. 2B). The results suggest that the activation of Vα14 NKT cells is crucial for the abortion.
Requirement for Both Perforin-Dependent Killing and Production of IFN-γ and TNF-α in the Vα14 NKT Cell-Mediated Abortion.
It has been shown that Vα14 NKT cells affect target cells by a perforin-dependent mechanism after direct contact with tumor cells (18). Thus, the α-GalCer-mediated abortion also might be caused by perforin. To address this possibility, perforin-KO pregnant mice were injected with α-GalCer. As shown in Fig. 2C, no fetal resorption was observed. This result indicates that perforin-mediated cytotoxicity is requisite for the α-GalCer-induced abortion.
It has been reported that the administration of cytokines, such as IFN-γ and TNF-α, induces fetal resorption (1, 6). In addition, the decidual Vα14 NKT cells produced a considerable amount of IFN-γ and TNF-α upon stimulation with α-GalCer (Fig. 1 B and C), and, therefore, the requirement of IFN-γ and/or TNF-α for the α-GalCer-induced abortion was investigated. As shown in Fig. 2 D and E, no fetal resorption was observed after α-GalCer treatment in IFN-γ-KO or TNF-α-KO mice, indicating that both IFN-γ and TNF-α are essential for the Vα14 NKT cell-mediated abortion.
Preferential Damage in Embryonic Trophoblasts by α-GalCer Injection.
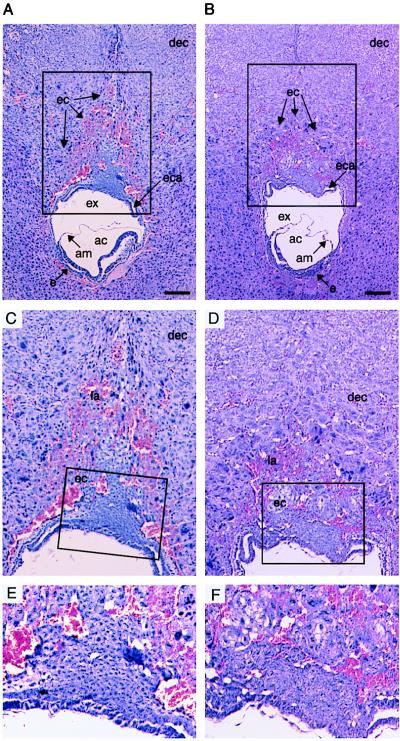
To understand the pathological process involved in the α-GalCer-induced abortion, we performed histological examination on day 8.0 embryos 12 hr after α-GalCer administration. With vehicle administration, no significant changes were observed either in embryonic or extraembryonic tissues (Fig. 3 A, C, and E). Similarly, distal structures of embryonic tissues, including chorion, amnion, and embryo, and maternal tissues surrounding the ectoplacental cone were unaffected both in the control and the α-GalCer-treated groups at this stage (Fig. 3B). However, the α-GalCer administration induced the preferential damage in the ectoplacental cone, showing that their structure was disorganized and the formation of lacunae filled with maternal blood surrounding the ectoplacental cone also was retarded (Fig. 3 B and D). In higher magnifications of the ectoplacental cone, it is easily recognized that trophoblast cells were degenerated, because their nuclei and intercellular boundaries were hardly visible (Fig. 3F).
Figure 3.
Histological analysis of embryonic and extraembryonic tissues after α-GalCer treatment. Fetomaternal junction of B6 pregnant mice on day 8 of gestation was analyzed. Twelve hours before sacrifice, mice were treated with control vehicle (A, C, and E) or α-GalCer (B, D, and F). dec, decidual stroma cells; ec, ectoplacental cone; eca, ectoplacental cavity; ex, exocoelomic cavity; am, amnion; ac, amniotic cavity; e, embryo. Higher magnifications of the ectoplacental cone (framed areas in A and B or C and D) are illustrated in C and D or E and F, respectively. dec, decidual stroma cells; la, lacunae; ec, ectoplacental cone. (Bars = 200 μm.)
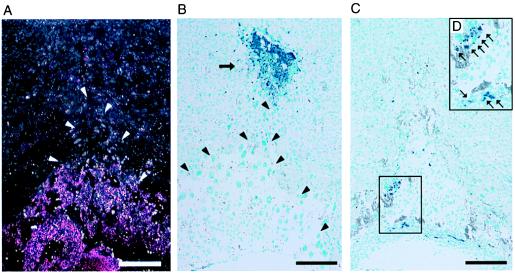
The terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling staining revealed the drastic changes in both the embryonic trophoblasts and the maternal tissues in the vicinity of the proximal end of the ectoplacental cone. By visualizing the embryonic cells as pink grains by X-Gal staining, indicating the β-galactosidase expression derived from ROSA 26 transgene of fetal origin (25), the precise boundary of embryonic and maternal tissues was determined (Fig. 4A, white arrowheads). Interestingly, a cluster of maternal cells undergoing apoptosis was seen clearly in the normal and vehicle-treated embryos (Fig. 4B, black arrow) and the structure of the ectoplacental cone was identified easily (Fig. 4B, black arrowheads). However, the maternal apoptotic cells at the proximal end of the ectoplacental cone disappeared after α-GalCer administration, whereas a number of apoptotic cells were detected in the ectoplacental cone (Fig. 4 C and D, blue grains). Therefore, the ectoplacental cone was primarily affected by the α-GalCer injection, implying that embryonic trophoblasts lose their invasive activity and undergo apoptosis after activation of Vα14 NKT cells.
Figure 4.
Apoptotic cell death of embryonic trophoblasts after α-GalCer treatment. (A) LacZ staining of the fetomaternal junction of (B6 × Rosa 26)F1 embryos at day 8.5 of gestation. The trophoblast cells were visualized by pink grains (X-Gal staining). White arrowheads show the edge of the ectoplacental cone. (B and C) Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling assay on normal B6 uteri at the fetomaternal junction on day 8.5 of gestation. Arrow indicates massive apoptosis (blue staining) at around the proximal end of the ectoplacental cone in the control specimen (B). Black arrowheads show the edge of the ectoplacental cone (B). (C) Apoptosis of embryonic trophoblasts in the α-GalCer-treated uteri is shown (blue grains). (D) Framed area in C at a higher magnification. Arrows (blue grains) indicate cells undergoing apoptosis. (Bars = 200 μm.)
Discussion
Here, we demonstrate that a distinct lymphocyte subset, Vα14 NKT cells that predominantly express Vβ7, accumulates in the decidua during pregnancy. Because Vα14 NKT cells in most peripheral tissues, including bone marrow, spleen, and liver, express Vβ8.2 (27), the preferential association with a particular Vβ7 suggests a specific selection and function of the decidual Vα14 NKT cells. In fact, we found a unique cytokine profile of the decidual Vα14 NKT cells producing IFN-γ and TNF-α but not IL-2 or IL-4 upon α-GalCer stimulation (Fig. 1 B and C). Similar to the Vβ8-bearing NKT cells, those with Vβ7 are known to react with α-GalCer, indicating that the epitope specificity of Vα14 NKT cell receptor is not altered by the Vβ7 usage (29, 30). Thus, it is most likely that the α-GalCer-induced abortion is mediated by the decidual Vα14 NKT cells bearing Vβ7, although the difference in function between Vα14 NKT cells bearing Vβ8 and Vβ7 is not clarified at this moment.
So far, the (CBA × DBA/2)F1 mouse is the only known animal model to show a high abortion rate (31). However, α-GalCer-induced Vα14 NKT cell-mediated abortion can be a universal model for a high abortion rate and can be applied to all mouse strains regardless of genetic background, because α-GalCer activates Vα14 NKT cells in various mouse strains in association with CD1d class Ib molecules that are monomorphic in nature (32).
The α-GalCer-induced abortion requires Vα14 NKT cells because no embryo loss is found in Vα14 NKT-KO mice (Fig. 2). Moreover, both IFN-γ and TNF-α are found to be essential for the Vα14 NKT cell-mediated abortion. These results are in good agreement with those by others (1–3, 33). The injection of large amounts of IFN-γ and TNF-α induces apoptosis in trophoblasts (34) and leads to abortion (1, 6). Although the mode of action of these cytokines in the decidua remains unclear, the present results obtained from various KO mice clearly indicate that both IFN-γ and TNF-α are required, but neither one is sufficient.
In addition to these cytokines, direct cell–cell contact and perforin-dependent killing appear to be indispensable for the induction of the abortion, because no fetal demise is observed in perforin-KO mice that still produced large amounts of IFN-γ and TNF-α in their sera after α-GalCer injection (data not shown). Although the molecules involved in the Vα14 NKT cell-mediated contact killing have not been elucidated precisely yet, they are likely to be similar to those defined in NK cells, whose killing system is mediated by two-step molecular events: the direct contact by adhesion molecules or killer activation receptors and the discrimination of target cells from normal cells by killer inhibitory receptors delivering negative signals through interaction with MHC-like self-molecules on target cells (35). Because embryonic trophoblasts do not express classical class I MHC molecules (36), Vα14 NKT cells after activation with α-GalCer-presented DCs are likely to directly contact and kill embryonic trophoblasts but not normal cells. In fact, histological findings in the decidua of pregnant mice undergoing the α-GalCer-mediated abortion indicate that embryonic trophoblasts are affected selectively, leading to apoptosis.
It is also speculated that activated-Vα14 NKT cells in tissues other than decidua produce various cytokines, including IFN-γ, which, in turn, activates other cell types, such as NK cells in the decidua, because NK cells are reported to be activated by IFN-γ and kill targets lacking class I MHC expression in a perforin-dependent fashion (37). In any event, the direct cell–cell contact followed by perforin-dependent killing and certain cytokines produced by Vα14 NKT cells appears to be essential for the induction of the abortion. Thus, the process of embryonic cell apoptosis during abortion seems to involve multifactorial events.
A high incidence of maternal complications, such as abortion (9.7%), premature labor (59.6%), and still-births (5.7%), in malaria (Plasmodium falciparum)-infected pregnant women has been reported (19). Trypanosoma brucei and Leishmania mexicana infections in pregnant women also result in abortion at a high frequency (4, 20, 21). Recently, glycosyl-phosphatidylinositols (GPIs) from P. falciparum, Leishmania, and T. brucei have been found to be ligands for Vα14 NKT cells (22). These findings suggest the possible involvement of Vα14 NKT cells in the fetomaternal immune responses, particularly against pathogens, such as parasites.
Th2 cells are reported to be involved in the maintenance of the embryo, whereas Th1 cells are responsible for abortion (1–6). It thus is speculated that Vα14 NKT cells in the decidua have a role in preventing rejection of the fetus under physiological conditions, because Vα14 NKT cells have been shown to down-regulate Th1-type immune responses under certain conditions (38, 39), regardless of their ability to produce both Th1 and Th2 cytokines (16). However, there is no direct evidence to support the above notion in normal pregnancy at present, because differences in the production rate between Vα14 NKT-KO and wild-type mice are found to be insignificant (data not shown).
Contrary to the above speculation, our findings clearly indicate that the decidual Vα14 NKT cells reject embryos upon stimulation with their ligands. Thus, the most conceivable function of the decidual Vα14 NKT cells seems to be the protection of the maternal body from serious infections. Our recent experiments also suggest that bacteria-derived material can activate Vα14 NKT cells to produce cytokines such as IFN-γ (data not shown). The decidual Vα14 NKT cells might play a unique role in the defense system against pathogens in the pregnant uterus.
Acknowledgments
We thank Dr. Y. Koezuka, Kirin Brewery (Tokyo), for providing α-GalCer (KRN7000), Ms. T. Kagami for technical assistance, and Ms. H. Tanabe for secretarial assistance. This work is supported by a Grant-in-Aid for Priority Areas for Scientific Research (06282103) from the Ministry of Education, Culture, Sports, and Science, Japan.
Abbreviations
- α-GalCer
α-galactosylceramide
- NK
natural killer
- KO
knock-out, DC, dendritic cell
- Th2
type 2 helper T
- TGF-β
tumor growth factor β
- TNF-α
tumor necrosis factor α
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
References
- 1.Chaouat G, Menu E, Clark D A, Dy M, Minkowski M, Wegmann T G. J Reprod Fertil. 1990;89:447–458. doi: 10.1530/jrf.0.0890447. [DOI] [PubMed] [Google Scholar]
- 2.Wegmann T G, Lin H, Guilbert L, Mosmann T R. Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 3.Chaouat G, Assal Meliani A, Martal J, Raghupathy R, Elliot J, Mosmann T, Wegmann T G. J Immunol. 1995;154:4261–4268. [PubMed] [Google Scholar]
- 4.Krishnan L, Guilbert L J, Russell A S, Wegmann T G, Mosmann T R, Belosevic M. J Immunol. 1996;156:644–652. [PubMed] [Google Scholar]
- 5.Arck P C, Ferrick D A, Steele-Norwood D, Croitoru K, Clark D A. Am J Reprod Immunol. 1997;37:492–502. doi: 10.1111/j.1600-0897.1997.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 6.Clark D A, Chaouat G, Arck P C, Mittruecker H W, Levy G A. J Immunol. 1998;160:545–549. [PubMed] [Google Scholar]
- 7.Munn D H, Zhou M, Attwood J T, Bondarev I, Conway S J, Marshall B, Brown C, Mellor A L. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 8.Munn D H, Shafizadeh E, Attwood J T, Bondarev I, Pashine A, Mellor A L. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King A, Burrows T, Loke Y W. Nat Immun. 1996;15:41–52. [PubMed] [Google Scholar]
- 10.Verma S, King A, Loke Y W. Eur J Immunol. 1997;27:979–983. doi: 10.1002/eji.1830270426. [DOI] [PubMed] [Google Scholar]
- 11.Imai K, Kanno M, Kimoto H, Shigemoto K, Yamamoto S, Taniguchi M. Proc Natl Acad Sci USA. 1986;83:8708–8712. doi: 10.1073/pnas.83.22.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koseki H, Imai K, Nakayama F, Sado T, Moriwaki K, Taniguchi M. Proc Natl Acad Sci USA. 1990;87:5248–5252. doi: 10.1073/pnas.87.14.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makino Y, Kanno R, Ito T, Higashino K, Taniguchi M. Int Immunol. 1995;7:1157–1161. doi: 10.1093/intimm/7.7.1157. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi M, Koseki H, Tokuhisa T, Masuda K, Sato H, Kondo E, Kawano T, Cui J, Perkes A, Koyasu S, et al. Proc Natl Acad Sci USA. 1996;93:11025–11028. doi: 10.1073/pnas.93.20.11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 16.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Van Kaer L, et al. J Exp Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T, et al. Proc Natl Acad Sci USA. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair L S, Nair A S. Indian J Malariol. 1993;30:207–214. [PubMed] [Google Scholar]
- 20.Hernandez-Matheson I M, Frankowski R F, Held B. Trans R Soc Trop Med Hyg. 1983;77:405–411. doi: 10.1016/0035-9203(83)90174-8. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan L, Guilbert L J, Wegmann T G, Belosevic M, Mosmann T R. J Immunol. 1996;156:653–662. [PubMed] [Google Scholar]
- 22.Schofield L, McConville M J, Hansen D, Campbell A S, Fraser-Reid B, Grusby M J, Tachado S D. Science. 1999;283:225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 23.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Nature (London) 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 24.Tagawa Y, Sekikawa K, Iwakura Y. J Immunol. 1997;159:1418–1428. [PubMed] [Google Scholar]
- 25.Zambrowicz B P, Imamoto A, Fiering S, Herzenberg L A, Kerr W G, Soriano P. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mombaerts P, Iacomini J, Johnson R S, Herrup K, Tonegawa S, Papaioannou V E. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 27.Masuda K, Makino Y, Cui J, Ito T, Tokuhisa T, Takahama Y, Koseki H, Tsuchida K, Koike T, Moriya H, et al. J Immunol. 1997;158:2076–2082. [PubMed] [Google Scholar]
- 28.Lazik A, Liu Y, Bringas P, Sangiorgi F, Maxson R. Trends Genet. 1996;12:445–447. doi: 10.1016/0168-9525(96)99999-0. [DOI] [PubMed] [Google Scholar]
- 29.Burdin N, Brossay L, Koezuka Y, Smiley S T, Grusby M J, Gui M, Taniguchi M, Hayakawa K, Kronenberg M. J Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- 30.Brossay L, Naidenko O, Burdin N, Matsuda J, Sakai T, Kronenberg M. J Immunol. 1998;161:5124–5128. [PubMed] [Google Scholar]
- 31.Clark D A, Chaput A, Tutton D. J Immunol. 1986;136:1668–1675. [PubMed] [Google Scholar]
- 32.Porcelli S A. Adv Immunol. 1995;59:1–98. doi: 10.1016/s0065-2776(08)60629-x. [DOI] [PubMed] [Google Scholar]
- 33.Arck P C, Troutt A B, Clark D A. Am J Reprod Immunol. 1997;37:262–266. doi: 10.1111/j.1600-0897.1997.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 34.Yui J, Garcia-Lloret M, Wegmann T G, Guilbert L J. Placenta. 1994;15:819–835. doi: 10.1016/s0143-4004(05)80184-5. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Botet M, Bellon T. Curr Opin Immunol. 1999;11:301–307. doi: 10.1016/s0952-7915(99)80048-x. [DOI] [PubMed] [Google Scholar]
- 36.Loke Y W, King A. Mol Med Today. 1997;3:153–159. doi: 10.1016/s1357-4310(97)01011-3. [DOI] [PubMed] [Google Scholar]
- 37.Yu Y Y, George T, Dorfman J R, Roland J, Kumar V, Bennett M. Immunity. 1996;4:67–76. doi: 10.1016/s1074-7613(00)80299-x. [DOI] [PubMed] [Google Scholar]
- 38.Lehuen A, Lantz O, Beaudoin L, Laloux V, Carnaud C, Bendelac A, Bach J F, Monteiro R C. J Exp Med. 1998;188:1831–1839. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson S B, Kent S C, Patton K T, Orban T, Jackson R A, Exley M, Porcelli S, Schatz D A, Atkinson M A, Balk S P, et al. Nature (London) 1998;391:177–181. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]