Abstract
The differentiation state of CD8+ T cells has emerged as a crucial determinant of their ability to respond to tumor and infection. Signals from T-cell receptors, co-stimulatory molecules and cytokine receptors direct the differentiation process. These signals ‘program’ sustained and heritable gene expression patterns that govern progressive differentiation and lineage commitment. The epigenetic mechanisms by which T cells are programmed are just beginning to be elucidated. Understanding the mechanisms that control CD8+ T-cell differentiation is important in the development of novel immunotherapy strategies.
Introduction
Antigen-presenting cells (APCs) and other immune and non-immune cells direct CD8+ T-cell differentiation by engaging receptors for a host of membrane-bound and soluble molecules [1••,2,3]. Signals from these receptors induce epigenetic changes that ‘program’ sustained but mutable gene expression patterns that govern progressive differentiation and lineage commitment decisions [4,5]. Emerging evidence indicates that the differentiation state crucially determines CD8+ T-cell effectiveness in responding to infection and tumor [6,7••,8,9••,10,11]. What are the differentiation states that characterize effective CD8+ T cells? How can CD8+ T cells be programmed to differentiate into optimal effector cells? Here we discuss the concepts of T-cell programming and differentiation and their implications for the development of potent new CD8+ T cell-based immunotherapy.
CD8+ T cells exist in dynamic states of progressive differentiation
Dynamic CD8+ T cell–APC interactions drive CD8+ T-cell proliferation, differentiation and lineage commitment. This process results in the generation of cells that have diverse phenotypic and functional characteristics [3,12,13]. The nomenclature, definitions, characteristics and differentiation pathways for CD8+ T cells are controversial, but two broad categories are generally acknowledged and have been named for their apparent function [12,14,15]. ‘Effector’ cells (TEFF) are highly cytolytic in vitro and express high levels of molecules required for cell killing, such as perforin, granzymes, interferon (IFN)-γ, tumor necrosis factor (TNF) and FAS ligand (FASL) [13,14]. ‘Memory’ cells are less cytolytic in in vitro assays, but exhibit increased survival, show the capacity for antigen-independent self-renewal, and respond vigorously to secondary antigen challenge [8,12,14,16].
Two subsets of memory cells, ‘effector memory’ (TEM) and ‘central memory’ (TCM), were originally identified on the basis of tissue homing molecules and effector function [17]. TCM were described as CD62L+CCR7+ cells that home to lymph nodes and have relatively low immediate effector function. TEM were defined as CD62L−CCR7− cells that preferentially home to peripheral tissues and inflammatory sites and possess relatively high immediate effector function. More recently, the distinction between TEM and TCM based on effector function has been questioned [18,19]. Generalizations about these two memory subsets have been further confused by the discovery of CCR7− antigen-experienced T cells in lymph nodes [20,21]. Another subset, ‘memory stem cells’, was recently identified in a murine model of graft-versus-host disease. These post-mitotic CD44loCD62LhiCD8+ T cells were characterized by the expression of Sca-1, CD122 and BCL-2 and were capable of self-renewal and could generate all subsets of memory and effector CD8+ T cells [22••].
Memory CD8+ T cells are intermediates in the progressive differentiation pathway
Conflicting models of CD8+ T-cell differentiation have been proposed to explain the generation of memory subsets. A model suggesting that TCM and TEM arise from distinct lineages was based on the finding that T-cell receptor (TCR) repertoires of TCM and TEM in the peripheral blood of healthy individuals are largely distinct [23]. However, subsequent studies showing that TCM and TEM clones can derive from a common naïve precursor have cast doubt on the separate lineage hypothesis [24].
A linear pathway of differentiation has instead gained acceptance; however, contention remains about whether memory cells arise from TEFF or vice versa. A progressive sequence of naïve → TEFF → TEM → TCM differentiation has been proposed based on the finding that CD62L+ cells emerged after the adoptive transfer of CD62L− enriched memory cells [6,25•]. Whether these results reflect a true TEM → TCM conversion or a selective survival and/or proliferation advantage of TCM over TEM has not been convincingly demonstrated.
Instead, mounting data, including ex vivo phenotypic analyses of virus-specific CD8+ T cells in acute and chronic viral disease, measures of telomere length, and in vitro differentiation studies, support a linear sequential progression (naïve → TCM → TEM → TEFF) model [7••, 9••,12,26]. The recent finding that the gene expression signature of TCM lies between that of naïve cells and TEM further supports the naïve → TCM → TEM → TEFF sequence [27].
Expanding on this model, memory stem cells might represent the earliest antigen-experienced cell population to emerge in the CD8+ T-cell differentiation pathway [22••]. Paralleling B-cell differentiation, memory CD8+ T cells might represent effector cells in an arrested differentiation state [15,28]. Thus, CD8+ T-cell differentiation could be regarded in terms of a continuum from early to late effectors rather than the movement of a T cell between subsets descriptively named memory stem cell, TCM, TEM and TEFF subsets [14].
Early effectors provide the most potent immune response
Where on this differentiation continuum are CD8+ T cells most capable of eradicating infection or tumor? Late effectors, owing to their high in vitro cytotoxicity, were initially thought to be the most effective cells. However, increasing evidence indicates that progressive differentiation leads to decreased ability to eliminate infection or tumor. For example, impaired function of late effector cells has been observed in patients that have progressive human immunodeficiency virus infection. CD8+ T cells in these patients displayed a late effector (CD27−CD28−) phenotype and decreased proliferative capacity, deemed ‘replicative senescence’ [9••]. Furthermore, in the absence of early effectors, late effectors were unable to control cytomegalovirus replication in patients co-infected with human immunodeficiency virus [29]. In clinical trials of adoptive cell transfer therapy for cancer, clonal populations of CD8+ T cells that had been multiply stimulated proved ineffective [30,31]. These cells appear to represent late effectors that have poor survival capability as they have a late effector phenotype and do not engraft or persist after adoptive transfer [30,31]. In contrast, treatment with less-expanded tumor infiltrating lymphocytes caused objective responses in about 50% of treated patients in a recent clinical trial [32]. In patients treated with tumor infiltrating lymphocytes, tumor regression and T-cell persistence correlate with increased telomere length [10,33], and cells that persist express an early effector (CD27+CD28+) phenotype [34]. These data suggest that an early differentiation state is important for T-cell efficacy.
Studies in murine models have confirmed the superior function of less-differentiated CD8+ T cells and have offered insight into the mechanisms that confer a functional advantage to these cells in vivo. In models of viral and intracellular bacterial infection, early effectors provide greater protective immunity, eliminate virus more efficiently, and display greater replicative capacity than late effectors [6,11,13,35]. Similarly, when adoptively transferred, early effectors induce better tumor regression [7••,8], display higher engraftment efficiency, and mediate more severe graft-versus-host disease than more highly differentiated cells [36,22••]. The mechanisms that underlie the loss of function associated with progressive differentiation are complex and include decreased survival and proliferation capacity, reduced responsiveness to homeostatic cytokines, decreased capacity for self-renewal, inability to differentiate into diverse cell types, and impairment of lymphoid tissue homing (Figure 1) [6,7••,14,15].
Figure 1.
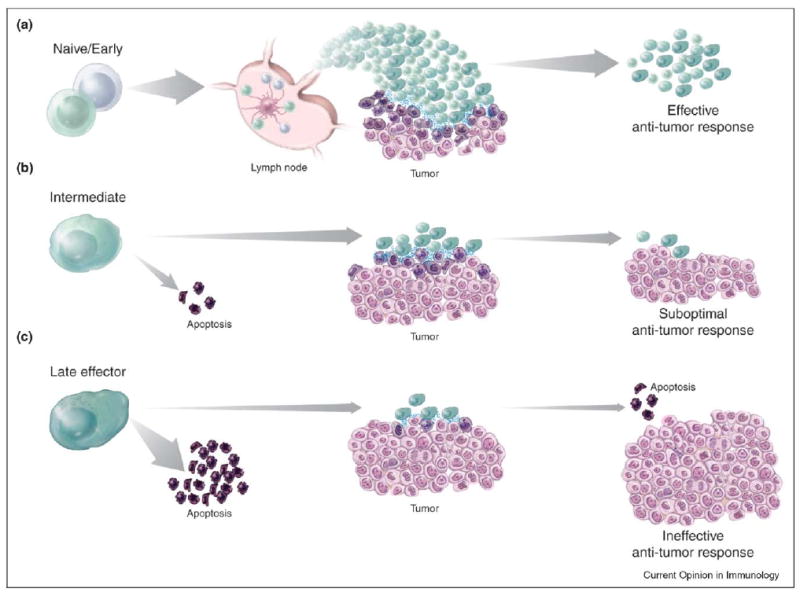
Progressive differentiation of adoptively transferred CD8+ T cells inversely correlates with in vivo anti-tumor efficacy. (a) Following adoptive transfer, naïve and early effector CD8+ T cells migrate to lymphoid tissues where they can interact with dendritic cells that are presenting cognate antigens. CD8+ T cells are programmed to proliferate, differentiate and traffic to tumor sites where they can mediate effective anti-tumor responses. After tumor clearance, T cells persist in a variety of differentiation states, providing protective immunity. (b) Intermediate effector cells are characterized by down-regulation of lymphoid homing molecules as well as having low proliferative and survival capacity. Following adoptive transfer, these cells become apoptotic or can proliferate moderately and home to tumor sites, where they can exert their cytotoxic potential. Tumor responses are sub-optimal and ultimately result in the exhaustion of T-cell responses. (c) Late effectors are characterized by poor survival and proliferative capability. After transfer, the majority of these cells undergo apoptosis. The few surviving late effectors migrate to tumor sites but are insufficient to trigger anti-tumor responses and impact on tumor growth. T cells ultimately are deleted and the tumor inexorably progresses.
CD8+ T-cell differentiation is epigenetically programmed
How can CD8+ T-cell differentiation be directed to generate early effectors that are programmed for optimal immune function? Stimulation of CD8+ T cells through TCRs, co-stimulatory molecules and cytokines program changes in gene expression that can be heritable owing to epigenetic modifications in gene transcription [3–5,37]. Although the precise mechanisms are largely unknown, it is likely that these changes include DNA methylation, methyl-CpG-binding proteins, and histone modifications that affect the accessibility to regulatory regions of transcription factors that serve as ‘master regulators’ [4,5].
Advances have been made in identifying the master regulator transcription factors that govern T-cell differentiation [4,5,38–40]. GATA-binding protein 3 (GATA-3) induces the uncommitted CD4+ T cells that emerge from the thymus to differentiate into IL-4-, IL-5- and IL-13-releasing T helper (Th)2 cells, whereas T-bet (encoded by Tbx21−/−) promotes differentiation into IFN-γ -releasing Th1 cells. Expression of these master regulators, and therefore lineage commitment, is stabilized by amplification loops and by repression of alternative pathway genes [37]. Nevertheless, the functionality of cells polarized to the Th1 lineage by IFN-γ or the Th2 lineage by IL-4 can be partially reversed by switching to the opposite polarizing cytokine; however, this plasticity decreases with progressive differentiation [38,41].
Less is known about lineage commitment in CD8+ T cells. Commitment to the CD8+ effector cell linage is redundantly determined by T-bet and the T-bet paralog eomesodermin (Eomes). Expression of these transcription factors is also important for maintenance of committed cells. Eomes+/−Tbx21−/− mice are deficient in IL-15-dependent lymphocyte lineages including CD8+ memory cells [42••]. Paralleling memory B-cell development, maintenance of T-cell memory might also require transcriptional repressors that arrest the differentiation process. The transcriptional repressor B-cell CLL/lymphoma 6 (Bcl-6) represses B lymphocyte-induced maturation protein-1 (Blimp-1), a transcriptional activator responsible for plasma-cell differentiation. This repression arrests the differentiation of germinal center B cells, thus enabling the generation and maintenance of B-cell memory [28]. Recent studies in Bcl6−/− and Bcl6 transgenic mice have also revealed a role for Bcl-6 in CD8+ T-cell memory formation [43,44]. Furthermore, a Bcl-6 homologue, Bcl-6b, was recently reported to be important in maintaining memory CD8+ T-cell replication potential [45]. Transcription-repressing isoforms of lymphoid enhancer-binding factor 1 (Lef1) and transcription factor 7 (Tcf7), which maintain hematopoietic stem cells in an undifferentiated pluripotent state, might also be required to arrest differentiation and to maintain memory CD8+ T cells [46]. These molecules are highly expressed in naïve and TCM CD8+ T cells, and their expression decreases with progressive differentiation [47]. Furthermore, T cells from TCF7−/− mice spontaneously differentiate more rapidly than cells from TCF7+/− littermates, supporting the hypothesis that TCF7 is important in preventing differentiation [48]. New data derived from the analysis of global gene expression are consistent with the hypothesis that hematopoietic stem cells and memory T and B cells — the only cells of the hematopoietic system able to undergo self-renewal for the lifetime of the organism — might share a common pattern of gene expression [49•]. We are just beginning to elucidate how transcription factors determine T-cell fate. Understanding how these master regulators guide T-cell differentiation is crucial to our efforts to generate optimal effector cells.
Inputs from T-cell receptors, cytokine receptors and costimulatory receptors program CD8+ T cells
Membrane-bound and soluble factors direct programmed changes in CD8+ T-cell development. Increases in duration, magnitude and frequency of the TCR stimulus drive progressive differentiation [2,3,50,51]. The TCR signal is integrated with signals from diverse costimulatory, inhibitory and cytokine receptors. The program imparted by the TCR is sustained by demethylation of the IL-2 promoter, and, depending on the strength of the antigen stimulus, one or both IL-2 alleles can be activated [52,53]. IL-2 expression is also regulated by the costimulatory molecule CD28, which induces histone acetylation and loss of cytosine methylation at the IL-2 promoter/enhancer [54]. Costimulation through CD28 also induces preferential differentiation of T cells into TCM, revealing some of the complexity of the differentiation process [36].
The activities of diverse soluble factors help to shape the complex differentiation process of T-cell differentiation. The impact of distinct cytokines on lineage commitment decisions is better established in CD4+ than CD8+ T cells. IFN-γ, acting through T-bet, induces naïve CD4+ T cells to become IFN-γ-releasing Th1 cells, whereas IL-4, acting through GATA-3, programs differentiation into IL-4-, IL-5- and IL-13-releasing Th2 cells. Evidence for additional CD4+ lineages generated under the influence of diverse cytokines is now emerging: in the absence of IFN-γ and IL-4, IL-23 can induce naïve CD4+ T cells to differentiate into IL-17-releasing Th-17 cells [55]. Alternatively, Th-17 cells might be induced by the exposure to TGF-β and IL-6. Transcriptional factors responsible for the generation of Th-17 cells have not been yet identified, but it appears that neither GATA-3 nor T-bet play a role [56]. Furthermore, TGF-β and IL-10 might influence CD4+ T cells to acquire the regulatory attributes of Th3 and Tr1 cells, respectively [57,58].
Lineage commitment by CD8+ T cells is also affected by the cytokine milieu. CD8+ T cells can be induced to differentiate into cytotoxic T cells secreting Th1-like (Tc1) or Th2-like (Tc2) cytokines by Th1-and Th2-polarizing cytokines respectively [59,60]. However, just as it is becoming clearer that CD4+ T cells differentiate along manifold lineages, it seems likely that CD8+ T-cell differentiation occurs along multiple lines (Figure 2). Many cytokines, including the common gamma-chain (γC) cytokines, are integral to CD8+ T-cell differentiation [61]. For example, IL-15 directs CD8+ T cells to preferentially differentiate into TCM whereas IL-2 promotes them to differentiate into TEM. These phenotypic and functional differences are reflected in the distinct gene expression patterns of IL-15- and IL-2-programmed cells [7••,8,62,63].
Figure 2.
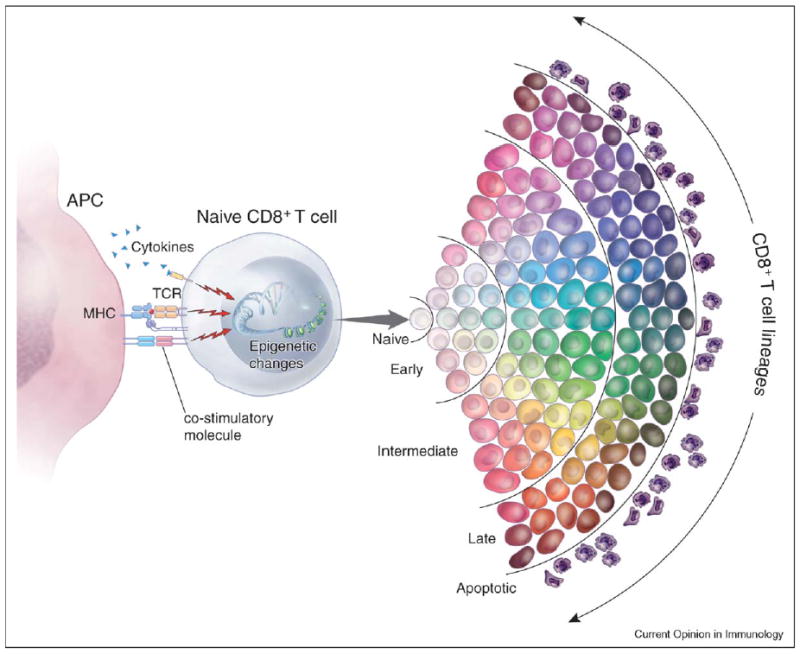
A new hypothetical multidimensional model of CD8+ T-cell programming and differentiation. CD8+ T-cell differentiation is programmed by a variety of stimulatory and inhibitory signals from T-cell receptors (TCRs), co-stimulatory molecules and cytokine receptors. These signals induce distinct patterns of gene expression that can, through epigenetic mechanisms, be sustained and heritably transmitted. The quality of the integrated signals influences T-cell lineage commitment. Phenotypic and functional attributes are symbolized by different color hues. The strength of the integrated signals drives the T cell toward progressive senescence. T-cell differentiation through early, intermediate and late stages is represented by progressive darkening shades of cell colors. In this model, de-differentiation is not possible under physiologic conditions, although lineage commitment can be mutable. Plasticity of phenotype and function is progressively reduced as the cells approach senescence.
The most recently discovered of the γC cytokines, IL-21, also confers particular features to CD8+ T cells. IL-21 generates CD8+ T cells that have a distinct CD45RO+CD28hi stable phenotype. In contrast to cells grown in IL-2, IL-21-programmed CD8+ T cells retain their capacity to produce IL-2 after antigen exposure [64•,65•]. The pattern of global gene expression by IL-21-programmed CD8+ T cells is unique compared with IL-2-, IL-7- and IL-15-programmed cells, and is characterized by simultaneously increased transcription of genes that encode lymphoid homing molecules and cytolytic effector molecules (CSH, unpublished). This surprising finding challenges the paradigm of linear differentiation for CD8+ T cells, which is characterized by an inverse relationship between the expression of genes for lymphoid homing and for effector function. Thus, differentiation of T cells appears to be multidimensional and is influenced by a host of soluble and cell-bound factors that we have only begun to explore [66–69].
Conclusions
A deeper understanding of programming and differentiation of CD8+ T cells might be valuable in the development of adoptive cell transfer-based immunotherapies for treatment of cancer and chronic infectious disease. It now seems clear that cells at early stages of differentiation have enhanced therapeutic efficacy, but attempts to generate cells that have desired programs have only just begun.
A significant problem in the translation of these ideas into human immunotherapies is that T-cell populations specific for tumor-associated antigens and antigens expressed by chronic pathogens are generally terminally differentiated (exhausted). Recent work suggests it might be possible to reprogram exhausted cells by blockade of negative regulatory receptors, such as programmed death-1 (PD-1) [70••]. Alternatively, antigen-specific cells that have optimal differentiation potential might be generated through gene transfer technology. Although this technology is still evolving, high-efficiency transfer of genetic sequences for specific TCRs into the genome of naïve or less-differentiated CD8+ T cells might soon be possible. In the future, one might be able to attenuate or even reverse progressive differentiation of T cells through manipulation of transcriptional master regulators. The reprogramming of B cells could now be a reality [71••]. As our understanding of programming and differentiation signals improves we might be able to generate a greater diversity of cells and apply them to the treatment of cancer and chronic infectious diseases.
Acknowledgments
This work was supported by the Intramural Research Program of the Center for Cancer Research, NCI, NIH. The authors thank Alan Hoofring for his excellent artistic contributions to the illustrations in this manuscript. The authors also thank Steven A Rosenberg, James C Yang, Richard M Sherry and Mark E Dudley for helpful discussions.
Footnotes
This review comes from a themed issue on Lymphocyte effector functions Edited by Stephen Schoenberger
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1••.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. This study elucidates a novel mechanism by which CD4+ T cells program CD8+ T-cell memory formation. CD8+ T cells primed in the absence of CD4+ T cells undergo death via TNF-related apoptosis-inducing ligand (TRAIL)-mediated signaling after re-challenge with antigen. [DOI] [PubMed] [Google Scholar]
- 2.van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 3.Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Curr Opin Immunol. 2004;16:217–225. doi: 10.1016/j.coi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Wilson CB, Makar KW, Shnyreva M, Fitzpatrick DR. DNA methylation and the expanding epigenetics of T cell lineage commitment. Semin Immunol. 2005;17:105–119. doi: 10.1016/j.smim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Wilson CB, Merkenschlager M. Chromatin structure and gene regulation in T cell development and function. Curr Opin Immunol. 2006;18:143–151. doi: 10.1016/j.coi.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 7••.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. The authors describe a comprehensive panel of phenotypic and functional characteristics that define the progressive differentiation process in vitro. By adoptively transferring progressively differentiated cells, they demonstrate the detrimental effect of progressive differentiation on CD8+ T-cell function in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, Dong T, Chesney G, Waters A, Easterbrook P, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2:E20. doi: 10.1371/journal.pbio.0020020. This study elegantly demonstrates progressive differentiation of human CD8+ T cells in vitro and in vivo based on expression of the molecules CD27 and CD28. Furthermore the loss of proliferative capacity associated with this process is firmly established in this work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 13.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 14.Appay V, Rowland-Jones SL. Lessons from the study of T-cell differentiation in persistent human virus infection. Semin Immunol. 2004;16:205–212. doi: 10.1016/j.smim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Rocha B, Tanchot C. Towards a cellular definition of CD8+ T-cell memory: the role of CD4+ T-cell help in CD8+ T-cell responses. Curr Opin Immunol. 2004;16:259–263. doi: 10.1016/j.coi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 18.Ravkov EV, Myrick CM, Altman JD. Immediate early effector functions of virus-specific CD8+CCR7+ memory cells in humans defined by HLA and CC chemokine ligand 19 tetramers. J Immunol. 2003;170:2461–2468. doi: 10.4049/jimmunol.170.5.2461. [DOI] [PubMed] [Google Scholar]
- 19.Unsoeld H, Krautwald S, Voehringer D, Kunzendorf U, Pircher H. Cutting edge: CCR7+ and CCR7− memory T cells do not differ in immediate effector cell function. J Immunol. 2002;169:638–641. doi: 10.4049/jimmunol.169.2.638. [DOI] [PubMed] [Google Scholar]
- 20.Chen G, Shankar P, Lange C, Valdez H, Skolnik PR, Wu L, Manjunath N, Lieberman J. CD8 T cells specific for human immunodeficiency virus, Epstein-Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood. 2001;98:156–164. doi: 10.1182/blood.v98.1.156. [DOI] [PubMed] [Google Scholar]
- 21.Ellefsen K, Harari A, Champagne P, Bart PA, Sekaly RP, Pantaleo G. Distribution and functional analysis of memory antiviral CD8 T cell responses in HIV-1 and cytomegalovirus infections. Eur J Immunol. 2002;32:3756–3764. doi: 10.1002/1521-4141(200212)32:12<3756::AID-IMMU3756>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 22••.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:299–1305. doi: 10.1038/nm1326. The authors describe a novel subset of postmitotic CD44loCD62LhiCD8+ cells that emerges early in the CD8+ T-cell differentiation pathway. These cells are capable of self-renewal as well as the generation of TEM, TCM and TEFF. [DOI] [PubMed] [Google Scholar]
- 23.Baron V, Bouneaud C, Cumano A, Lim A, Arstila TP, Kourilsky P, Ferradini L, Pannetier C. The repertoires of circulating human CD8+ central and effector memory T cell subsets are largely distinct. Immunity. 2003;18:193–204. doi: 10.1016/s1074-7613(03)00020-7. [DOI] [PubMed] [Google Scholar]
- 24.Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J Exp Med. 2005;201:579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. This study describes that the frequency of antigen-specific T cells during priming phase is an important determinant of the CD8+ T-cell memory commitment. The authors find that high frequency of precursors generates central memory CD8+ T cells whereas low precursor frequency leads to an effector memory phenotype. Because competition for antigen by T cells might limit antigenic stimulation, the author’s findings might instead stem from differences in the strength and duration of TCR signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 27.Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J Immunol. 2005;175:5895–5903. doi: 10.4049/jimmunol.175.9.5895. [DOI] [PubMed] [Google Scholar]
- 28.Fearon DT, Manders P, Wagner SD. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science. 2001;293:248–250. doi: 10.1126/science.1062589. [DOI] [PubMed] [Google Scholar]
- 29.Sacre K, Carcelain G, Cassoux N, Fillet AM, Costagliola D, Vittecoq D, Salmon D, Amoura Z, Katlama C, Autran B. Repertoire, diversity, and differentiation of specific CD8 T cells are associated with immune protection against human cytomegalovirus disease. J Exp Med. 2005;201:1999–2010. doi: 10.1084/jem.20042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry RM, Marincola FM, Leitman SF, Seipp CA, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Jr, Rosenberg SA. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powell DJ, Jr, Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wherry EJ, Blattman JN, Ahmed R. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J Virol. 2005;79:8960–8968. doi: 10.1128/JVI.79.14.8960-8968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bondanza A, Valtolina V, Magnani Z, Ponzoni M, Fleischhauer K, Bonyhadi M, Traversari C, Sanvito F, Toma S, Radrizzani M, et al. Suicide gene therapy of graft-versus-host disease induced by central memory human T lymphocytes. Blood. 2006;107:1828–1836. doi: 10.1182/blood-2005-09-3716. [DOI] [PubMed] [Google Scholar]
- 37.Hatton RD, Weaver CT. Immunology. T-bet or not T-bet. Science. 2003;302:993–994. doi: 10.1126/science.1092040. [DOI] [PubMed] [Google Scholar]
- 38.Messi M, Giacchetto I, Nagata K, Lanzavecchia A, Natoli G, Sallusto F. Memory and flexibility of cytokine gene expression as separable properties of human T(H)1 and T(H)2 lymphocytes. Nat Immunol. 2003;4:78–86. doi: 10.1038/ni872. [DOI] [PubMed] [Google Scholar]
- 39.Zhou W, Chang S, Aune TM. Long-range histone acetylation of the Ifng gene is an essential feature of T cell differentiation. Proc Natl Acad Sci USA. 2004;101:2440–2445. doi: 10.1073/pnas.0306002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avni O, Lee D, Macian F, Szabo SJ, Glimcher LH, Rao A. T(H) cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat Immunol. 2002;3:643–651. doi: 10.1038/ni808. [DOI] [PubMed] [Google Scholar]
- 41.Sundrud MS, Grill SM, Ni D, Nagata K, Alkan SS, Subramaniam A, Unutmaz D. Genetic reprogramming of primary human T cells reveals functional plasticity in Th cell differentiation. J Immunol. 2003;171:3542–3549. doi: 10.4049/jimmunol.171.7.3542. [DOI] [PubMed] [Google Scholar]
- 42••.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. This publication offers new insight into how the transcriptional master regulators T-bet and Eomes redundantly determine not only effector lineage commitment but also survival of committed lineages. Mice that have compound mutations in the genes that encode these transcription factors were nearly devoid of several IL-15-dependent lineages. T-bet and Eomes were found to be responsible for enhanced expression of the IL-2/IL-15 receptor β-chain. [DOI] [PubMed] [Google Scholar]
- 43.Ichii H, Sakamoto A, Hatano M, Okada S, Toyama H, Taki S, Arima M, Kuroda Y, Tokuhisa T. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat Immunol. 2002;3:558–563. doi: 10.1038/ni802. [DOI] [PubMed] [Google Scholar]
- 44.Ichii H, Sakamoto A, Kuroda Y, Tokuhisa T. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J Immunol. 2004;173:883–891. doi: 10.4049/jimmunol.173.2.883. [DOI] [PubMed] [Google Scholar]
- 45.Manders PM, Hunter PJ, Telaranta AI, Carr JM, Marshall JL, Carrasco M, Murakami Y, Palmowski MJ, Cerundolo V, Kaech SM, et al. BCL6b mediates the enhanced magnitude of the secondary response of memory CD8+ T lymphocytes. Proc Natl Acad Sci USA. 2005;102:7418–7425. doi: 10.1073/pnas.0501585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 47.Willinger T, Freeman T, Herbert M, Hasegawa H, McMichael AJ, Callan MF. Human naïve CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T Cell Factor-1) following antigen encounter in vitro and in vivo. J Immunol. 2006;176:1439–1446. doi: 10.4049/jimmunol.176.3.1439. [DOI] [PubMed] [Google Scholar]
- 48.Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol. 1998;161:3984–3991. [PubMed] [Google Scholar]
- 49•.Luckey CJ, Bhattachararya D, Goldrath AW, Weissman IL, Benoist C, Mathis D. Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc Natl Acad Sci. 2006;103:3304–3309. doi: 10.1073/pnas.0511137103. By using global gene array analyses, the authors show that memory T cells and B cells share a transcriptional program of self-renewal with hematopoietic stem cells. These findings might be helpful for shedding light on the transcriptional factors governing CD8+ T-cell memory formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naïve CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 52.Hollander GA, Zuklys S, Morel C, Mizoguchi E, Mobisson K, Simpson S, Terhorst C, Wishart W, Golan DE, Bhan AK, et al. Monoallelic expression of the interleukin-2 locus. Science. 1998;279:2118–2121. doi: 10.1126/science.279.5359.2118. [DOI] [PubMed] [Google Scholar]
- 53.Naramura M, Hu RJ, Gu H. Mice with a fluorescent marker for interleukin 2 gene activation. Immunity. 1998;9:209–216. doi: 10.1016/s1074-7613(00)80603-2. [DOI] [PubMed] [Google Scholar]
- 54.Thomas RM, Gao L, Wells AD. Signals from CD28 induce stable epigenetic modification of the IL-2 promoter. J Immunol. 2005;174:4639–4646. doi: 10.4049/jimmunol.174.8.4639. [DOI] [PubMed] [Google Scholar]
- 55.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 56.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Weiner HL. Induction and mechanism of action of transforming growth factor-β -secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 58.Battaglia M, Gregori S, Bacchetta R, Roncarolo M-G. Tr1 cells: from discovery to their clinical application. Semin Immunol. 2006;18:120–127. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 59.Cerwenka A, Carter LL, Reome JB, Swain SL, Dutton RW. In vivo persistence of CD8 polarized T cell subsets producing type 1 or type 2 cytokines. J Immunol. 1998;161:97–105. [PubMed] [Google Scholar]
- 60.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 61.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 62.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manjunath N, Shankar P, Wan J, Weninger W, Crowley MA, Hieshima K, Springer TA, Fan X, Shen H, Lieberman J, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. These studies [64•, 65• ] describe how IL-21 influences the phenotypic and functional characteristic CD8+ T cells in mouse and human. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol. 2005;175:2261–2269. doi: 10.4049/jimmunol.175.4.2261. See annotation to [64•]. [DOI] [PubMed] [Google Scholar]
- 66.Ahmadzadeh M, Rosenberg SA. TGF-β 1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J Immunol. 2005;174:5215–5223. doi: 10.4049/jimmunol.174.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang SS, Allen PM. Priming in the presence of IL-10 results in direct enhancement of CD8+ T cell primary responses and inhibition of secondary responses. J Immunol. 2005;174:5382–5389. doi: 10.4049/jimmunol.174.9.5382. [DOI] [PubMed] [Google Scholar]
- 68.Owaki T, Asakawa M, Morishima N, Hata K, Fukai F, Matsui M, Mizuguchi J, Yoshimoto T. A role for IL-27 in early regulation of Th1 differentiation. J Immunol. 2005;175:2191–2200. doi: 10.4049/jimmunol.175.4.2191. [DOI] [PubMed] [Google Scholar]
- 69.Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol. 2005;175:1686–1693. doi: 10.4049/jimmunol.175.3.1686. [DOI] [PubMed] [Google Scholar]
- 70••.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. This work shows that it might be possible in one model (lymphocytic choriomeningitis virus) to reverse T-cell ‘exhaustion’ by blocking negative regulatory signals sent by way of PD-1. These findings vividly illustrate the kind of maneuver that might enable vaccines and immunotherapies for cancer and chronic infectious disease to overcome effector exhaustion associated with terminal differentiation. [DOI] [PubMed] [Google Scholar]
- 71••.Fujita N, Jaye DL, Geigerman C, Akyildiz A, Mooney MR, Boss JM, Wade PA. MTA3 and the Mi-2/NuRD complex regulate cell fate during B lymphocyte differentiation. Cell. 2004;119:75–86. doi: 10.1016/j.cell.2004.09.014. This study describes how the manipulation of key transcriptional factors might be used to reprogram the lymphocyte fate. The authors were able to de-differentiate plasma cells into less differentiated B cells by forcing the expression of BCL-6 and MTA-3. [DOI] [PubMed] [Google Scholar]


