Abstract
The mgtC gene of Salmonella enterica serovar Typhimurium encodes a membrane protein of unknown function that is important for full virulence in the mouse. Since mgtC is part of an operon with mgtB which encodes a Mg2+-transporting P-type ATPase, MgtC was hypothesized to function in ion transport, possibly in Mg2+ transport. Consequently, MgtC was expressed in Xenopus laevis oocytes, and its effect on ion transport was evaluated using ion selective electrodes. Oocytes expressing MgtC did not exhibit altered currents or membrane potentials in response to changes in extracellular H+, Mg2+, or Ca2+, thus ruling out a previously postulated function as a Mg2+/H+ antiporter. However, addition of extracellular K+ markedly hyperpolarized membrane potential instead of the expected depolarization. Addition of ouabain to block the oocyte Na+,K+-ATPase completely prevented hyperpolarization and restored the normal K+-induced depolarization response. These results suggested that the Na+,K+-ATPase was constitutively activated in the presence of MgtC resulting in a membrane potential largely dependent on Na+,K+-ATPase. Consistent with the involvement of Na+,K+-ATPase, oocytes expressing MgtC exhibited an increased rate of 86Rb+ uptake and had increased intracellular free [K+] and decreased free [Na+] and ATP. The free concentrations of Mg2+ and Ca2+ and cytosolic pH were unchanged, although the total intracellular Ca2+ content was slightly elevated. These results suggest that the serovar Typhimurium MgtC protein may be involved in regulating membrane potential but does not directly transport Mg2+ or another ion.
The mgtCB locus of Salmonella enterica serovar Typhimurium (19, 39, 43) on Salmonella pathogenicity island 3 (3) encodes the 22.5-kDa MgtC (24) and 102-kDa MgtB integral membrane proteins (19, 41). Expression of mgtCB is induced upon invasion of macrophages (38) and during serovar Typhimurium infection of mice (2). Transcription of the mgtCB operon is controlled by extracellular Mg2+ concentration through the Mg2+ binding PhoQ sensor kinase which in turn activates the PhoP transcription factor (5, 12, 13, 16, 38, 42, 44). This two-component signal transduction system controls a number of genes important for serovar Typhimurium virulence (4, 12, 16). mgtB encodes a P-type ATPase that mediates the influx of Mg2+ (43), but despite its induction via a signal transduction system controlled by its substrate, MgtB's importance during infection is unclear (2, 24, 38). Conversely, MgtC is required for optimal serovar Typhimurium virulence (2), but its function has been elusive.
The N terminus of MgtC is extremely hydrophobic, with four or five transmembrane segments, followed by a soluble probably cytosolic C-terminal tail of about 90 amino acids. Given an association in an operon with the MgtB transporter and its location in the membrane, it is reasonable to speculate that MgtC encodes some type of transporter or an accessory protein to MgtB or another transport system. However, MgtC is neither required for nor influences MgtB transport activity (24). MgtC has also been suggested to be a Mg2+/H+ antiporter (2), but this is unlikely based on expression and phenotypic data (24). Since 28Mg2+ is not readily available, a direct test of the ability of MgtC to transport Mg2+ is not possible.
Expression in Xenopus laevis oocytes, however, offers an alternative approach to determining MgtC function. Because of the size of the oocyte and its ability to express mRNA from a heterogeneous source, it has long been used to characterize transporters and channels (8, 32, 33, 48). Thus, the expression of MgtC in the oocyte offers the possibility of detecting ion movements with great sensitivity.
The results presented here demonstrate that, when expressed in oocytes, MgtC does not transport Mg2+. Instead, it can apparently activate the endogenous Na+,K+-ATPase of the oocyte plasma membrane. This ubiquitous enzyme, the “sodium” pump, is largely responsible for maintenance of membrane potential in most mammalian cells (9, 35). Thus, during serovar Typhimurium infection MgtC could have significant effects on cellular ion homeostasis through a prolonged alteration of Na+ and K+ homeostasis and membrane potential.
MATERIALS AND METHODS
Oocyte preparation and bath solutions.
Experiments were carried out using Xenopus laevis oocytes (Xenopus Express, Beverly Hills, FL). Surgery and oocyte dissociation and defolliculation were performed as previously described (31). Oocytes were kept in OR3 medium (34) at 15 to 18°C for about 12 h before injection with cRNA or water. Injected oocytes were incubated in the same medium until used for experiments, 2 to 10 days after injection.
For electrophysiological experiments, oocytes were transferred to an experimental chamber with a volume of about 500 μl that was continuously perfused with saline at room temperature (19-22°C). The standard saline contained 90 mM NaCl, 2 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 18 mM choline chloride, 3 mM HEPES, 3 mM MES (morpholineethanesulfonic acid), and 3 mM Tris (pH 7.5) adjusted with NaOH. The use of three pH buffers allowed titration over a wide range of pH values. Depending on the experimental protocol, the ionic composition of the saline could be modified without changing the osmolarity. Complete replacement of choline chloride with KCl gave 20 mM KCl, whereas 0 K+ and 0 Na+ solutions were obtained by equimolar replacement with choline chloride. A complete replacement of NaCl with KCl gave 92 mM K+ solutions, which were also nominally Na+ free. In all Na+-free solutions, pH was titrated with choline hydroxide instead of NaOH. Solutions of 0 Mg2+ or 0 Ca2+ were obtained by omitting the respective cation and increasing the choline chloride content by 1.5 and 3 mM, respectively.
Ion-selective microelectrodes.
All microelectrodes were pulled from capillaries of WPI 1B150F borosilicate glass using a P-97 puller (Sutter, Novato, CA) and silanized as described previously (36). The microelectrodes were filled with ion sensors (Fluka) for Mg2+ (sensor ETH 5214, cocktail IIa), Na+ (sensor ETH 227, cocktail Ia), K+ (sensor valinomycin), Cl− (chloride ionophore I, cocktail A, Fluka 24902), or pH (sensor ETH 1907, cocktail IIa) as described by Günzel and Schlue (18). The pH microelectrodes were back-filled with the standard saline solution (described above), Mg2+ microelectrodes with 1 mM MgCl2 plus 100 mM KCl, Cl−, and K+ microelectrodes with 100 mM KCl and Na+ microelectrodes with 100 mM NaCl. Separate conventional microelectrodes were filled with 3 M KCl and served as intracellular reference electrodes.
All potentials were measured against the potential of an extracellular reference electrode (agar bridge containing 3 M KCl and an Ag/AgCl cell), using voltmeters with an input resistance of 1015 Ω (FD223; WPI). The output signals were A/D-converted and continuously recorded by using HEKA software. Pure ionic potentials (difference potentials between the potentials of the ion-sensitive microelectrode and the intracellular reference microelectrode) were obtained using the software's subtraction mode.
Before each experiment, the Na+, K+, Cl−, and Mg2+ microelectrodes were calibrated with a series of four calibration solutions that contained either a constant ionic background (Na+, Cl−, and Mg2+) or, for K+ calibration solutions, a constant sum of Na+ and K+ (Table 1). The data were fitted by using the Nikolsky-Eisenman equation (1). pH-sensitive microelectrodes were calibrated in the standard saline solution (above) titrated to pH 7.5 and 5.5. The pH electrodes used showed no interference by any of the cations and exhibited a strictly Nernstian behavior within this pH range, i.e., a slope of about −57 mV/pH unit.
TABLE 1.
Microelectrode calibration media
| Electrode type (pH [medium])a | Concn (mM)
|
||||
|---|---|---|---|---|---|
| KCl | NaCl | Mg2Cl | K-gluconate | HEPES | |
| Na+ electrodes | 90 | 0 | 0.5 | 10 | |
| (7.2 [KOH]) | 2.5 | ||||
| 10 | |||||
| 50 | |||||
| K+ electrodes | 150 | 0 | 10 | ||
| (7.2 [NaOH]) | 75 | 75 | |||
| 15 | 135 | ||||
| 0 | 150 | ||||
| Mg2+ electrodes | 90 | 10 | 10 | 10 | |
| (7.2 [KOH]) | 2.5 | ||||
| 0.5 | |||||
| 0 | |||||
| Cl− electrodes | 160 | 0 | 10 | ||
| (7.2 [NaOH]) | 40 | 120 | |||
| 8 | 152 | ||||
| 0 | 160 | ||||
pH medium, the medium used to adjust the pH.
Ion microelectrodes were used if their slope in the linear range of the electrode was at least 80% of the Nernstian slope and if their detection limit was below the values recorded during the experiment. Means and standard deviations of intracellular ion concentrations are expressed as pIon values, since only pIon values are normally distributed (11). Mean ion concentrations were then calculated from the mean pIon values.
Voltage clamp experiments.
Two-electrode voltage clamp was carried out by using a OC-720C voltage clamp amplifier (Warner Instruments, Hamden, CT). Electrodes were pulled from WPI 1B150F borosilicate glass. Electrodes were filled with 3 M KCl and had resistances of 1 to 5 MΩ. Current signals were filtered with an eight-pole Bessel filter (3-dB cutoff, frequency 2 to 5 kHz) and digitized at 10 kHz. Current and voltage signals were acquired via an EPC-16 I/O interface using Pulse software, and data were analyzed by using the PulseFit program (HEKA). Oocytes were clamped at a holding potential (Vh) of −60 mV; the current was constantly monitored and recorded at 1 Hz. Current-voltage protocols consisted of 50-ms steps from Vh to potentials from −200 mV to 20 mV in 20-mV steps, returning to Vh for 100 ms between each step. Mean steady-state current was plotted against voltage.
Vector construction.
To create an oocyte expression vector for mgtC, a 891-bp HpaI-NsaI fragment of pDGK1 (24, 44) containing mgtC was gel purified and cloned into the EcoRV site of the oocyte expression vector pT7TS (courtesy P. Krieg) as pMBM60 and transformed into Escherichia coli DH5α as MM1997. To create an mgtC:EGFP fusion construct, mgtC was amplified from genomic DNA of Salmonella enterica serovar Typhimurium strain SL1344 (MM2329) using oligodeoxynucleotide primers carrying sites for NheI (5′-TTTTTGCTAGCATGGAGGAACGTATG) and BamHI (5′-TTTTTGGATCCTGACTATCAATGCTC), cut with these two enzymes and gel purified. This fragment was ligated into pEGFP-N1 (Clontech) that had been cut with NheI and BamHI and treated with alkaline phosphatase. The insert of the resulting pLMK1 plasmid was fully sequenced to ensure fidelity, and the plasmid was electroporated into E. coli DH5α (MM2426). Plasmid pOOX-mgtC:EGFP was constructed in pT7TS from pLMK1 by digestion with NheI and HpaI, followed by gel purification of the appropriate fragment. This fragment was then ligated into pLITMUS28 cut with SpeI and EcoRV and treated with calf alkaline phosphatase. pLITMUS28 was digested with SnaBI and AvrII, treated with calf alkaline phosphatase, ligated into pT7TS that had been cut with EcoRV and SpeI, and treated with calf alkaline phosphatase. The resulting vector as pOOX-mgtC:EGFP (pOOX-LMK1) was electroporated into E. coli DH5α as strain MM2494.
cRNA preparation and injection.
MgtC and MgtC:EGFP cRNA was prepared by using the mMessage mMachine Kit T7 (Ambion) according to the manufacturer's directions. The cRNA concentration was determined spectrometrically and adjusted to 1 μg/μl. Using a Nanoject injector (Drummond Scientific), 50 nl of this solution was injected per oocyte. Control oocytes were injected with 50 nl of water.
[Ca2+]i determination with Fura-2.
To determine [Ca2+]i, oocytes were pressure injected with 25 nl of a 1 mM Fura-2 penta-potassium salt solution in 100 mM KCl, resulting in cytoplasmic concentrations of approximately 50 μM. In initial experiments the oocytes were placed in the experimental chamber under an inverted microscope (Nikon Fluor, NA 1.3), taking care that the lightly colored side of the oocytes faced downward. Most experiments, however, used oocytes from albino frogs since this was more convenient. There was no difference in results using oocytes from either source. Determination of [Ca2+]i was by the method of Colegrove et al. (6). Briefly, fluorescence was excited alternately at 350 and 380 nm using a 150-W Xenon lamp as the light source, with a filter wheel rotating at 40 Hz. The emitted light was filtered at 510 nm and detected by using a photomultiplier. The ratio method of Grynkiewicz et al. (17) was used to calculate [Ca2+]i from excitation at 350 and 380 nm.
Determination of [ATP] and total [Mg2+] and [Ca2+].
ATP was determined by luminometry as previously described (44). For measurement of Mg2+ and Ca2+, oocytes were washed quickly in ice-cold 5 mM EDTA (pH 7.5) and transferred to Eppendorf microcentrifuge tubes (one per tube for Mg2+ determinations and four to eight per tube for Ca2+ determinations) containing 200 μl of a 2:1 mixture of dibutyl and dioctyl phthalate. The tubes were centrifuged for 1 min, and most of the oil was aspirated, leaving a small amount in the bottom of the tube; residual liquid on the sides of the tube was removed with a cotton swab, and 500 μl (Mg2+) or 250 μl (Ca2+) of a 10% HNO3 solution was added. The oocytes were destroyed by repetitive pipetting with a 1-ml Eppendorf pipette, followed by vortexing. Immediately before use, the samples were centrifuged for 10 min at 12,000 × g. Cation concentrations were determined by atomic absorption spectrometry as described previously (24).
86Rb+ uptake experiments.
Control or MgtC-injected oocytes were transferred into plastic tubes containing 5 ml of standard saline solution containing 2 mM KCl at pH 7.5 that included 2 or 35 μM 86Rb+ and incubated at room temperature up to 60 min. Eppendorf tubes (500 μl) were prepared with 200 μl of a 2:1 mixture of dibutyl and dioctyl phthalate. At each time point, the desired number of oocytes (one to four oocytes per tube, depending on the experiment) were pipetted into 0.25 ml of standard saline without isotope; the entire volume was then immediately layered over the phthalate solution, and the tube was centrifuged for 1 min at 12,000 × g. The aqueous and oil layers were carefully aspirated, leaving a small amount of the oil layer in the bottom of the tube. Residual liquid on the sides of the tube was removed with a cotton swab. The entire vial was placed into a plastic vial and counted in a Beckman LS7500 scintillation counter by Cerenkov radiation.
Immunochemistry and cytochemistry.
The antibody for MgtC has been previously described (24). For visualization of MgtC expression, oocytes were frozen in OCT compound (EM Sciences, Ft. Washington, PA) using liquid nitrogen and sliced to 10 μm with a cryotome. Slices were mounted on gelatin-coated microscope slides and stored at −20°C until use. Sections were fixed with phosphate-buffered saline (PBS) containing 4% paraformaldehyde for 10 min at room temperature, washed, and permeabilized with 0.2% Triton-X. After being blocked for 2 h in PBS containing 1% bovine serum albumin and 10% donkey serum, rabbit anti-MgtC-antibodies (24) were added at a dilution of 1:50 for 2 h at room temperature. Sections were then washed extensively in PBS and treated for 2 h with a Cy3-linked donkey anti-rabbit antibody at 1:1,000 in PBS containing 1% bovine serum albumin and 10% donkey serum, followed by three washes with PBS. Sections were mounted on microscope slides using Dako fluorescence mounting medium (Dako, Carpinteria, CA). Images were acquired with an AxioCam digital camera and AxioVision software (Carl Zeiss, Oberkochen, Germany).
RESULTS
MgtC expression.
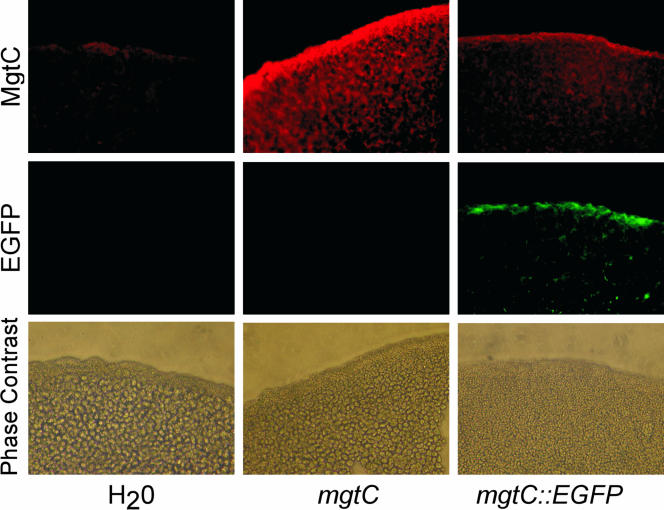
From its highly hydrophobic amino acid sequence, MgtC is predicted to be an integral membrane protein. After expression of MgtC and MgtC-enhanced green fluorescent protein (EGFP) in oocytes, Western blot analysis (data not shown) fluorescence microscopy for MgtC-EGFP (Fig. 1) shows that MgtC is associated with the plasma membrane. Oocytes did demonstrate some intracellular MgtC expression. Electrophysiological results were similar whether measured after 2 or 7 to 10 days incubation, suggesting that intracellular MgtC did not disrupt normal ion homeostasis. Further, Western blots showed full-length MgtC to be present at both shorter and longer times of expression (data not shown), suggesting that effects observed after MgtC expression were due to the membrane incorporation of full-length protein and not a proteolytic fragment.
FIG. 1.
Expression of MgtC and MgtC-EGFP in oocytes. Water or cRNA for MgtC or MgtC-EGFP was injected into oocytes (columns). The expression of the proteins was visualized using a peptide antibody against MgtC or EGFP autofluorescence (rows). The data shown are from oocytes injected 3 to 7 days previously. Similar distributions were obtained at different times after injection (data not shown).
Effect of MgtC expression on pHi and [Mg2+]i.
Since mgtC forms part of an operon with a Mg2+ transport system (40, 43) and since Mg2+ has been implicated in microbial virulence (2, 13, 15), both total and free intracellular Mg2+ and the intracellular pH were determined in MgtC-expressing oocytes and in water-injected controls. [Mg2+]i, total Mg2+, and pHi all were unchanged in MgtC-injected oocytes versus control oocytes. The pH was 7.2, the [Mg2+]i was 0.7 mM, and the total intracellular Mg2+ was 22 to 23 mM (Table 2).
TABLE 2.
Effect of MgtC expression on pHi and [Mg2+]
| Parameter | Mean ± SD (no. of expts [total no. of oocytes measured])a
|
|
|---|---|---|
| MgtC-injected oocytes | H2O-injected oocytes | |
| pHi | 7.19 ± 0.09 (7 [16]) | 7.22 ± 0.1 (8 [12]) |
| pNai | 2.13 ± 0.17 (5 [19])* | 2.03 ± 0.16 (5 [19]) |
| [Na+]i (mM) | 7.4 | 9.4 |
| pKi | 0.98 ± 0.08 (8 [31])* | 1.03 ± 0.07 (8 [22]) |
| [K+]i (mM) | 104 | 93 |
| pCli | 1.48 ± 0.21 (5 [13]) | 1.46 ± 0.11 (6 [13]) |
| [Cl−]i (mM) | 33 | 35 |
| pMgi | 3.15 ± 0.09 (4 [13]) | 3.17 ± 0.08 (4 [13]) |
| [Mg2+]i (mM) | 0.71 | 0.67 |
| [Mg]t (mM) | 23.4 ± 3.7 (6 [19]) | 22.1 ± 4.9 (6 [21]) |
| [Ca2+]i (nM) | 88.6 ± 58.6 (3 [15])* | 61.4 ± 37.8 (3 [17]) |
| [Ca]t (mM) | 2.17 ± 0.44 (6 [6])* | 1.35 ± 0.60 (5 [5]) |
| ATP (mM) | 0.71 ± 0.13 (2 [9])* | 0.90 ± 0.17 (2 [10]) |
| Vm | −42.9 ± 7.1 (22 [42]) | −46.4 ± 10.9 (22 [57]) |
The values shown are the means ± the standard deviation for the total number of oocytes examined. The number of experiments is indicated in parentheses; next to that value, the numbers in brackets indicate the total number of oocytes measured. An asterisk indicates that the indicated pIon or other value in MgtC-expressing oocytes was significantly different from the value in control oocytes (P < 0.01) calculated using analysis of variance. If, rather than combining all oocyte measurement together, values within each batch of control and MgtC-expressing oocytes are compared, the differences are still significant at P < 0.01. The measured differences for K+ and Na+ correspond to electrode potential differences of 2.6 and 6 mV, respectively, a readily detectable difference (see Fig. 2).
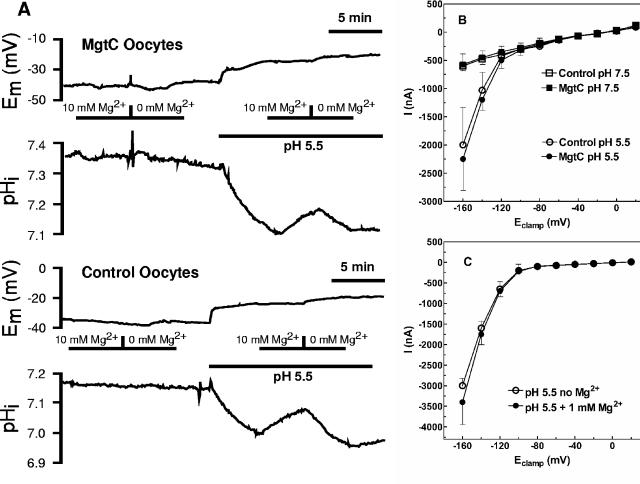
When serovar Typhimurium invades a macrophage, it resides in a vesicle that is slightly acidic and low in Mg2+ (14). We therefore varied pH and extracellular [Mg2+] to determine whether these influenced MgtC function. At an extracellular pH of 7.5, decreasing extracellular Mg2+ to a nominal 0 mM or increasing it to 10 mM had no effect on intracellular pH in MgtC-expressing or control oocytes (Fig. 2A). Decreasing the intracellular pH to 5.5 resulted in an identical, small, and slow intracellular acidification of less than 0.1 pH units/min in both MgtC-expressing and control oocytes. At this more acidic pH, the rate of acidification increased in nominally Mg2+-free solutions and decreased when 10 mM Mg2+ was present; however, these changes were similar in both MgtC-expressing and control oocytes (Fig. 2A). Further, IV-relationships obtained from voltage clamp experiments showed no differences between MgtC-expressing and control oocytes at either pH 7.5 or 5.5 (Fig. 2B) or upon alteration of extracellular Mg2+ concentration (Fig. 2C). MgtC thus does not alter Mg2+ homeostasis, indicating that it does not mediate electrogenic or electroneutral Mg2+ flux.
FIG. 2.
Lack of effect of pH or [Mg2+] in MgtC-expressing oocytes. (A) Rate of acidification in MgtC-expressing (top two panels) or control (bottom two panels) oocytes. (B) Current voltage relationships in MgtC-expressing (•, ▪) or control (○, □) oocytes were determined at extracellular pHs of 5.5 and 7.5 as described in Materials and Methods. (C) The current voltage relationship in oocytes expressing MgtC was determined at 0 and 1 mM extracellular Mg2+ at pH 5.5: without Mg2+ (○), with 1 mM Mg2+ (•). The data in all three panels are representative of at least six similar experiments.
Expression of MgtC results in activation of oocyte Na+,K+-ATPase.
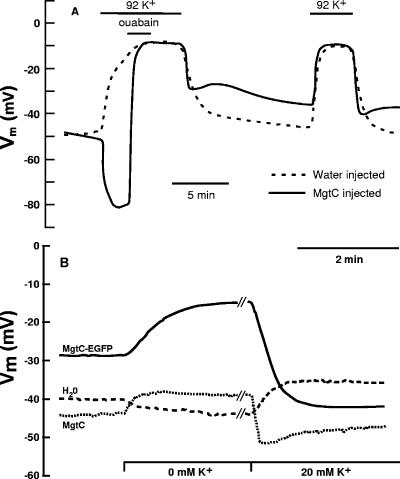
In standard buffer there was no difference in membrane potential (Vm) between water-injected control oocytes or MgtC-injected oocytes (Table 2). However, compared to control oocytes, MgtC-injected oocytes showed large changes in Vm upon alteration of extracellular [K+] (Fig. 3A) with or without concomitant changes in [Na+]. As would be expected, water-injected oocytes depolarized upon increasing extracellular [K+] (ΔVm = 29.4 ± 14.1 mV [n = 22]) and hyperpolarized upon exposure to a nominally K+-free bath solution (ΔVm = −7.8 ± 5.5 mV [n = 17]). MgtC-expressing oocytes showed the opposite response. Exposure to a nominally K+-free solution always elicited a marked depolarization (ΔVm = 8.4 ± 9.5 mV [n = 17]; Fig. 3A) rather than the hyperpolarization seen in control oocytes. Upon elevation of extracellular [K+], MgtC-injected oocytes always showed much less response than the water-injected oocytes from the same batch and usually showed a marked hyperpolarization (Fig. 3A). In a few cases, MgtC-injected oocytes responded with no change in membrane potential or a slight depolarization; in these cases, water-injected oocytes always gave a much more pronounced depolarization. This variability is presumably due to various levels of MgtC expression in different batches of oocytes. The results with the MgtC-EGFP construct were identical (Fig. 3B), indicating the functionality of the fusion protein. In addition, electrophysiological results were similar whether measured after 2 or 7 to 10 days of incubation, suggesting that intracellular MgtC did not disrupt normal ion homeostasis.
FIG. 3.
Membrane potential effects in oocytes expressing MgtC or MgtC-EGFP. In most experiments, two oocytes were measured simultaneously within the same chamber to directly compare water-injected control oocytes with MgtC-expressing oocytes. (A) The effect of ouabain and of alterations in extracellular [K+] on membrane potential was measured as described in Methods in oocytes injected with water (dashed line) or cRNA for MgtC (solid line). A representative experiment is shown. See text for replication and statistical evaluation. Ouabain is very long acting due to its affinity for Na+,K+-ATPase; therefore, there is still sufficient ouabain present during the second addition of K+ to provide continued inhibition. (B) The effect of alterations in extracellular [K+] on membrane potential was measured in oocytes injected with water (dashed line), MgtC-cRNA (dotted line), or MgtC-EGFP cRNA (solid line). The experiment shown is representative of three separate experiments that gave identical results. The average Vm for MgtC-EGFP expressing oocytes did not significantly differ from that for MgtC expressing or water-injected oocytes (data not shown, see Table 2).
These results suggested that Vm was no longer primarily dependent on the K+ electrochemical gradient across the cell membrane. We hypothesized, therefore, that some aspect of K+ homeostasis had been altered: possibly the Na+,K+-ATPase since it replenishes intracellular K+ while pumping out Na+. Therefore, we used the cardiac glycoside ouabain to selectively inhibit the oocyte Na+,K+-ATPase. Addition of 40 μM ouabain to the bath solution had virtually no effect on the response of control oocytes to a change in extracellular [K+], a finding consistent with Na+,K+-ATPase having only a minor part in maintenance of resting membrane potential. However, in MgtC-expressing oocytes (Fig. 3A) or MgtC-EGFP-expressing oocytes (data not shown), addition of ouabain in the presence of increased extracellular K+ elicited a pronounced depolarization of the oocyte to a potential equivalent to that of the control oocytes. These results are consistent with an activation of Na+,K+-ATPase in oocytes expressing MgtC.
Effect of MgtC on oocyte ion concentrations.
The activation of Na+,K+-ATPase by MgtC could be direct, or it could be indirect, the consequence of an MgtC-mediated alteration of the concentration of other ions. For example, the injection of Na+ into naive oocytes significantly activates Na+,K+-ATPase (data not shown). We therefore determined total intracellular Ca2+, [Ca2+]i, [H+]i (as pHi), [Na+]i, [K+]i, and [Cl−]i using atomic absorption spectrometry and ion-selective electrodes. Intracellular [Na+]i in control oocytes was about 9.4 mM but was decreased to 7.4 mM in MgtC-injected oocytes (P < 0.01; Table 2), an indication that the change in [Na+]i was a consequence rather than a cause of Na+,K+-ATPase activation. Consistent with the decrease in [Na+]i as a result of Na+,K+-ATPase activation, [K+]i was significantly elevated in MgtC-injected oocytes compared to control oocytes (P < 0.01, Table 2). In contrast, [Cl−]i was unchanged. Interestingly, while [Ca2+]i showed no change in MgtC-injected versus control oocytes, total intracellular Ca2+ concentration was significantly elevated in MgtC-injected oocytes (P < 0.01, Table 2). Since MgtC elicits the same response in the oocyte in the presence or absence of extracellular Ca2+ (data not shown), this change in total intracellular Ca2+ is most likely a consequence rather than a cause of the activation of Na+,K+-ATPase. Finally, as noted above, neither [Mg2+]i nor total intracellular [Mg2+] were altered by expression of MgtC.
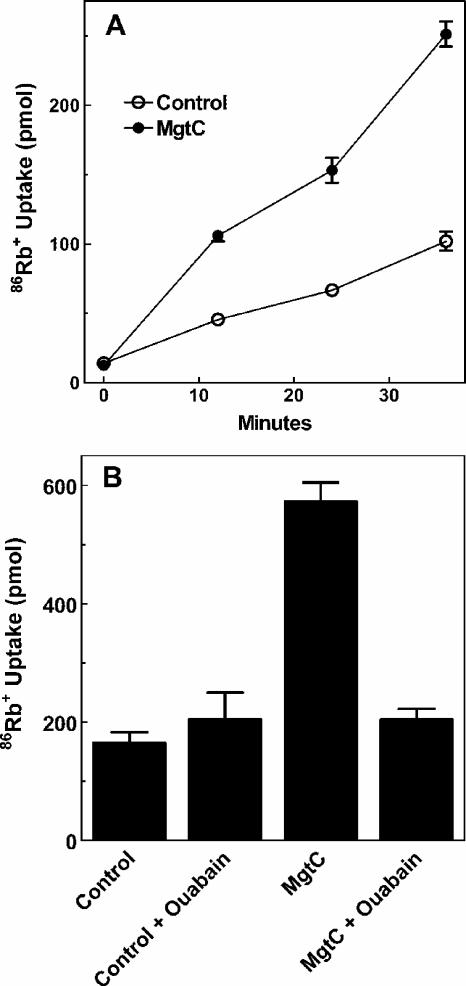
MgtC-expressing oocytes exhibit increased 86Rb+ uptake.
If oocyte Na+,K+-ATPase is activated in the presence of MgtC, an increased K+ influx should be evident. Therefore, uptake of K+ was measured by using 86Rb+ as a surrogate K+ cation. Expression of MgtC caused a marked elevation in the rate of 86Rb+ uptake (Fig. 4A) that was inhibitable by ouabain (Fig. 4B). In contrast, the basal rate of 86Rb+ uptake in control oocytes was unaffected by ouabain. These results indicated that basal K+ uptake was largely independent of the Na+,K+-ATPase, which implies that basal Na+,K+-ATPase activity in the naive oocyte is very low. In addition to increased K+ flux, an increase in Na+,K+-ATPase activity would be expected to use a large proportion of the cell's ATP to drive and maintain the altered ionic conditions. Consistent with this interpretation, ATP concentration in MgtC-expressing oocytes was decreased ca. 25% compared to that of the control oocytes (Table 2).
FIG. 4.
86Rb+ uptake in oocyte expressing MgtC. (A) Time course of 86Rb+ uptake in water-injected (○)and MgtC-injected (•) oocytes. Uptake of 2 μM 86Rb+ was measured as described in Materials and Methods using two oocytes per point determined in duplicate. (B) Uptake of 35 μM 86Rb+ was measured after 20 min incubation as described in Materials and Methods with three oocytes per point determined in duplicate. For each panel, the error bars show the range of the duplicate determinations. Each experiment is representative of three similar experiments.
DISCUSSION
The mgtC locus was discovered in serovar Typhimurium during study of Mg2+ transport systems (19, 40). CorA is the primary Mg2+ influx system of most Eubacteria and many Archaea (21). MgtA-like transporters are much less widely distributed. CorA mediated Mg2+ influx is apparently dependent on membrane potential (19, 37, 40). In contrast, the MgtA Mg2+ transporter of serovar Typhimurium belongs to the P-type ATPase superfamily; however, unlike other P-type ATPases it mediates the influx of Mg2+ with rather than against its electrochemical gradient (21, 40). Serovar Typhimurium also contains a second Mg2+-transporting P-type ATPase, MgtB, carried within Salmonella pathogenicity island 3. In serovar Typhimurium, mgtB forms the second gene of a two- gene operon with mgtC. This association naturally led to the hypothesis that MgtC was a β-subunit of MgtB (and possibly MgtA). We previously showed that MgtB expression (24) and transport kinetics (44, 45) were unaffected by the absence of mgtC. Both mgtB and mgtC have numerous homologs over a wide range of bacterial species but only very rarely form an operon. Many species possess an mgtC homolog but not an mgtB homolog. Thus, the association of mgtC and mgtB in an operon in serovar Typhimurium is likely fortuitous, perhaps as a consequence of the regulation of both genes by the PhoPQ two-component system (3, 44, 45).
MgtC function.
If MgtC is not functionally associated with MgtB, what is its function? A serovar Typhimurium strain lacking mgtC exhibits significant attenuation in a mouse model of infection after intraperitoneal injection (2). The sequence of MgtC indicates that it is an integral membrane protein. The first 130 amino acids are predicted by hydropathy analysis to form at least four and most likely five transmembrane segments, while the 100-amino-acid C-terminal residues are predicted to be cytosolic. Thus, one function of MgtC might be transport, possibly of an ion. MgtC has previously been suggested to be a fourth Mg2+ transport system in serovar Typhimurium, specifically a Mg2+/H+ antiporter (2). However, this is unlikely based on phenotypic data (19, 24, 40). Since a possible ionic substrate was unknown, we chose to express MgtC in Xenopus oocytes where ions can be readily manipulated, measuring both current and membrane potential over a wide range of conditions. The electrophysiological data presented herein provide no support for the idea that MgtC can mediate movement of Mg2+ across the membrane whether as a channel or as a Mg2+/H+ antiporter. The current-voltage relationships in Fig. 2 indicate that electrogenic Mg2+/H+ exchange does not occur, while the pH and [Mg2+]i measurements indicate that electroneutral Mg2+/H+ exchange does not occur.
Although Mg2+/H+ exchange can be excluded, expression of MgtC in oocytes nonetheless elicits a marked change in the cell's response to manipulations that affect membrane potential, Vm. In most animal cells, membrane potential is almost completely determined by the K+ gradient and lies close to the K+ equilibrium potential. We hypothesized, therefore, that some aspect of K+ homeostasis had been altered. Vm is maintained by a variety of ion channels and to a smaller extent by the Na+,K+-ATPase which is largely responsible for keeping intracellular [Na+] low. Substitution of extracellular Na+ by K+ would collapse the K+ electrochemical gradient and membrane potential. Our data, however, indicate that addition of extracellular K+ to MgtC-expressing oocytes hyperpolarizes rather than depolarizes the oocyte. This suggests that Vm is no longer entirely dependent on the K+ electrochemical gradient but is being actively maintained by the Na+,K+-ATPase. In addition to the hyperpolarization upon addition of K+, MgtC-expressing oocytes exhibit a higher [K+]i, a lower [Na+]i and lower [ATP], all consistent with activation of the Na+,K+-ATPase in the normal direction, i.e., three Na+ out and two K+ in. Addition of ouabain, a selective inhibitor of the Na-K+-ATPase, further confirms this interpretation. In the presence of ouabain, MgtC-expressing oocytes have the same response as water-injected oocytes, a finding consistent with the conclusion that the Na+,K+-ATPase has little to do with maintenance of resting membrane potential.
The 86Rb+ uptake data (Fig. 4) support an activation of the Na+,K+-ATPase by MgtC. In normal oocytes, the Na+,K+-ATPase appears to be minimally active, contributing little to basal K+ uptake as demonstrated by the absence of ouabain-inhibitable K+ influx in control oocytes. In MgtC-expressing oocytes in contrast, there is a large component of K+ influx mediated by the Na+,K+-ATPase, since ouabain inhibits K+ uptake, but only back to basal levels. The change in steady-state intracellular [K+] and [Na+] is about a 20% decrease for Na+ and a 10% increase for K+. If we assume an intracellular volume of 1 mm3 for the oocyte, this would correspond to ca. 1 × 1015 fewer Na+ ions and 5 × 1015 to 6 × 1015 more K+ ions. The measured 86Rb+ uptake rate is 1 nmol oocyte−1 h−1, which translates to 1.7 × 1011 ions s−1 taken up in the MgtC-injected oocytes. Thus, ignoring all other Na+ and K+ transporters, it would take about 1.6 h of Na+,K+-ATPase activity to change [Na+] by 2 mM and about 10 h to change K+ by 10 mM. Thus, the apparent level of Na+,K+-ATPase activity in MgtC-expressing oocytes is sufficient to achieve this degree of change in intracellular ion concentrations in the oocyte.
In contrast, the change in Vm is not a direct result of steady-state changes in intracellular Na+ and K+ concentration but rather a rapid change in ion flux across the cell membrane. This requires only about 8,000 ions for an oocyte (calculated assuming a cell diameter of 1 mm3 [and thus a surface area of 3.14 × 10−8 cm2], a voltage change of 40 mV, and a specific capacitance of 1 μF/cm2). Inhibition of the Na+,K+-ATPase by ouabain should, within a few seconds, abolish the contribution of the Na+,K+-ATPase to Vm. Vm therefore quickly returns to a value close to the K+ equilibrium potential since equilibration of intracellular ion concentrations is not necessary. This conclusion is consistent with the fact that the addition of ouabain to inhibit the Na+,K+-ATPase appears to revert the oocyte to normal behavior in less than a minute. The oocytes expressing MgtC and thus having an activated Na+,K+-ATPase have presumably developed substantial steady-state compensation for MgtC as reflected in identical resting membrane potentials in water-injected and MgtC-injected oocytes; however, they exhibit far less compensation on the time scale of depolarization.
Physiological role of MgtC.
During serovar Typhimurium invasion of a mammalian host cell, many bacterial proteins are injected into the host cell via a type III secretion system (7, 51). Thus, activation of Na+,K+-ATPase by MgtC suggests the obvious hypothesis that MgtC interacts with the Na+,K+-ATPase in a host cell membrane. Alternatively, since it is unclear whether MgtC is a substrate for a type III secretion system, MgtC could interact with an unknown protein within the bacterium.
We favor the simplest hypothesis, that MgtC would act as a subunit of the host cell's Na+,K+-ATPase, either by replacing the endogenous β-subunit or by binding to the existing αβ heterodimer. Such interactions have been well documented in Xenopus oocytes for the Slc7 neutral amino acid transporters (47) and the non-pore-forming members of the Slc3 gene family (29). Alternatively, the effects on Vm and on intracellular Na+ and Ca2+ content could be due to a general ability of MgtC to activate P-type ATPases, the Ca2+- and Na+,K+-ATPases being the most prominent in the cell and thus giving the most pronounced phenotypic results. Third, and least likely, MgtC might not interact with a P-type ATPase at all but with another, unknown, host cell system that would indirectly cause ion homeostatic changes that would indirectly activate the Na+,K+-ATPase.
Since MgtC is required for full virulence of serovar Typhimurium, an ability to constitutively activate host cell Na+,K+-ATPase (or induce other ion homeostatic changes) presumably has a role in the infectious process. One of the hallmarks of invasion of many enteric bacteria into mammalian cells is the induction of apoptosis (10, 22, 23, 25). Apoptotic cell death induces a relatively mild inflammatory reaction in the host, thus minimizing mobilization of host cell defenses against the pathogen. Inhibition of Na+,K+-ATPase is known to initiate or accelerate apoptosis in numerous cell types (20, 26-28, 30, 49, 50). Perhaps activation of macrophage Na+,K+-ATPase by MgtC serves the purpose of slowing apoptosis rather than initiating or promoting it. It is of interest that Yersinia pestis, Listeria monocytogenes, and serovar Typhimurium persist for a significant period of time in the host cell before final apoptotic events, and all have mgtC. In contrast, neither Shigella nor pathogenic E. coli strains have an mgtC, and neither persist for lengthy periods of time in host cells, instead causing rapid cell death (46, 51).
Although an argument regarding activation of host cell Na+,K+-ATPase and apoptosis is attractive, a major puzzle remains regarding both the timing and location of mgtC expression. The presence or absence of mgtC has no effect on the invasion or survival of serovar Typhimurium within macrophage or epithelial cell lines for at least several hours after infection (38). Transcription of the mgtCB operon is greatly increased upon invasion, but it is not known whether both MgtC and MgtB proteins are actually expressed. Studies of expression in free-living bacteria indicate that the expression of the two proteins is not tightly coupled. After activation of mgtCB transcription in serovar Typhimurium via a decrease in extracellular [Mg2+], MgtC, encoded by the first gene of the operon, cannot be detected for several hours although abundant MgtB protein is expressed (24, 44, 45). This is not due to differential degradation of the mRNA (L. Shi and M. E. Maguire, unpublished observations). Nonetheless, demonstration that mgtC is required for long-term survival of the pathogen within the macrophage (2) implies that the role of the MgtC protein occurs late in the infection pathway. While this could be due to activation of a host cell protein such as the Na+,K+-ATPase, MgtC might be expressed late in infection but be inserted only into the bacterial membrane. Regardless of the identity of any protein(s) with which MgtC interacts, we suggest that MgtC mediates a constitutive alteration of ion homeostasis most likely in the host cell, but possibly in the bacterium, and that this affects host-pathogen interactions, either by slowing the apoptotic process or protecting the bacterium from host cell defenses.
Acknowledgments
D.G. gratefully acknowledges support from the Deutsche Forschungsgemeinschaft (Gu 447/5-1). This research was supported by National Institutes of Health grants GM39447 (M.E.M.) and DK56218 (M.F.R.).
We thank Christopher M. Sciortino for assistance with electrophysiological experiments and Mary Beth Moncrief for construction of pMBM60. We thank David Friel for help with Ca2+ measurements and Andrea Romani for use of the atomic absorption spectrometer.
REFERENCES
- 1.Ammann, D. 1986. Ion-selective microelectrodes: principles, design and application. Springer-Verlag, Berlin, Germany.
- 2.Blanc-Potard, A. B., and E. A. Groisman. 1997. The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 16:5376-5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanc-Potard, A. B., F. Solomon, J. Kayser, and E. A. Groisman. 1999. The SPI-3 pathogenicity island of Salmonella enterica. J. Bacteriol. 181:998-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowe, F., C. J. Lipps, R. M. Tsolis, E. A. Groisman, F. Heffron, and J. G. Kusters. 1998. At least four percentage of the Salmonella typhimurium genome is required for fatal infection of mice. Infect. Immun. 66:3372-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cano, D. A., M. Martinez-Moya, M. G. Pucciarelli, E. A. Groisman, J. Casadesus, and F. Garcia-del Portillo. 2001. Salmonella enterica serovar Typhimurium response involved in attenuation of pathogen intracellular proliferation. Infect. Immun. 69:6463-6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colegrove, S. L., M. A. Albrecht, and D. D. Friel. 2000. Dissection of mitochondrial Ca2+ uptake and release fluxes. in situ after depolarization-evoked [Ca2+]i elevation in sympathetic neurons. J. Gen. Physiol. 115:351-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornelis, G. R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54:735-774. [DOI] [PubMed] [Google Scholar]
- 8.Dascal, N. 1987. The use of. Xenopus oocytes for the study of ion channels. CRC Crit. Rev. Biochem. 22:317-387. [DOI] [PubMed] [Google Scholar]
- 9.Dostanic-Larson, I., J. N. Lorenz, J. W. Van Huysse, J. C. Neumann, A. E. Moseley, and J. B. Lingrel. 2006. Physiological role of the a1- and a2-isoforms of the Na+-K+-ATPase and biological significance of their cardiac glycoside binding site. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290:R524-R528. [DOI] [PubMed] [Google Scholar]
- 10.Finlay, B. B., and P. Cossart. 1997. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276:718-725. [DOI] [PubMed] [Google Scholar]
- 11.Fry, C. H., S. K. Hall, L. A. Blatter, and J. A. McGuigan. 1990. Analysis and presentation of intracellular measurements obtained with ion-selective microelectrodes. Exp. Physiol. 75:187-198. [DOI] [PubMed] [Google Scholar]
- 12.Garcia, V. E., F. C. Soncini, and E. A. Groisman. 1994. The role of the PhoP/PhoQ regulon in Salmonella virulence. Res. Microbiol. 145:473-480. [DOI] [PubMed] [Google Scholar]
- 13.Garcia, V. E., F. C. Soncini, and E. A. Groisman. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165-174. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-del Portillo, F., J. W. Foster, M. E. Maguire, and B. B. Finlay. 1992. Characterization of the micro-environment of. Salmonella typhimurium-containing vacuoles within MDCK epithelial cells. Mol. Microbiol. 6:3289-3297. [DOI] [PubMed] [Google Scholar]
- 15.Groisman, E. A. 1998. The ins and outs of virulence gene expression: Mg2+ as a regulatory signal. Bioessays 20:96-101. [DOI] [PubMed] [Google Scholar]
- 16.Groisman, E. A. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 183:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grynkiewicz, G., M. Poenie, and R. Y. Tsien. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440-3450. [PubMed] [Google Scholar]
- 18.Günzel, D., and W. R. Schlue. 1996. Sodium-magnesium antiport in Retzius neurones of the leech Hirudo medicinalis. J. Physiol. 491:595-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hmiel, S. P., M. D. Snavely, J. B. Florer, M. E. Maguire, and C. G. Miller. 1989. Magnesium transport in Salmonella typhimurium: genetic characterization and cloning of three magnesium transport loci. J. Bacteriol. 171:4742-4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaev, N. K., E. V. Stelmashook, A. Halle, C. Harms, M. Lautenschlager, M. Weih, U. Dirnagl, I. V. Victorov, and D. B. Zorov. 2000. Inhibition of Na+,K+-ATPase activity in cultured rat cerebellar granule cells prevents the onset of apoptosis induced by low potassium. Neurosci. Lett. 283:41-44. [DOI] [PubMed] [Google Scholar]
- 21.Kehres, D. G., C. H. Lawyer, and M. E. Maguire. 1998. The CorA magnesium transporter gene family. Microb. Comparative Genomics 43:151-169. [DOI] [PubMed] [Google Scholar]
- 22.Monack, D. M., J. Mecsas, N. Ghori, and S. Falkow. 1997. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA 94:10385-10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monack, D. M., B. Raupach, A. E. Hromockyj, and S. Falkow. 1996. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. USA 93:9833-9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moncrief, M. B. C., and M. E. Maguire. 1998. Magnesium and the role of mgtC in Salmonella typhimurium. Infect. Immun. 66:3802-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarre, W. W., and A. Zychlinsky. 2000. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell Microbiol. 2:265-273. [DOI] [PubMed] [Google Scholar]
- 26.Nobel, C. S., J. K. Aronson, D. J. van den Dobbelsteen, and A. F. Slater. 2000. Inhibition of Na+/K+-ATPase may be one mechanism contributing to potassium efflux and cell shrinkage in CD95-induced apoptosis. Apoptosis 5:153-163. [DOI] [PubMed] [Google Scholar]
- 27.Orlov, S. N., S. Taurin, N. Thorin-Trescases, N. O. Dulin, J. Tremblay, and P. Hamet. 2000. Inversion of the intracellular Na+/K+ ratio blocks apoptosis in vascular smooth muscle cells by induction of RNA synthesis. Hypertension 35:1062-1068. [DOI] [PubMed] [Google Scholar]
- 28.Orlov, S. N., N. Thorin-Trescases, S. V. Kotelevtsev, J. Tremblay, and P. Hamet. 1999. Inversion of the intracellular Na+/K+ ratio blocks apoptosis in vascular smooth muscle at a site upstream of caspase-3. J. Biol. Chem. 274:16545-16552. [DOI] [PubMed] [Google Scholar]
- 29.Palacin, M., and Y. Kanai. 2004. The ancillary proteins of HATs: SLC3 family of amino acid transporters. Pflugers Arch. 447:490-494. [DOI] [PubMed] [Google Scholar]
- 30.Pchejetski, D., S. Taurin, S. S. Der, O. D. Lopina, A. V. Pshezhetsky, J. Tremblay, D. deBlois, P. Hamet, and S. N. Orlov. 2003. Inhibition of Na+,K+-ATPase by ouabain trigger epithelial cell death independently of inversion of the [Na+]i/[K+]i ratio. Biochem. Biophys. Res. Commun. 301:735-744. [DOI] [PubMed] [Google Scholar]
- 31.Romero, M. F., P. Fong, U. V. Berger, M. A. Hediger, and W. F. Boron. 1998. Cloning and functional expression of rNBC, an electrogenic Na+-HC03− cotransporter from rat kidney. Am. J. Physiol. 274:F425-F432. [DOI] [PubMed] [Google Scholar]
- 32.Romero, M. F., M. A. Hediger, E. L. Boulpaep, and W. F. Boron. 1997. Expression cloning and characterization of a renal electrogenic HC03− cotransporter. Nature 387:409-413. [DOI] [PubMed] [Google Scholar]
- 33.Romero, M. F., D. Henry, S. Nelson, P. J. Harte, A. K. Dillon, and C. M. Sciortino. 2000. Cloning and characterization of a Na+-driven anion exchanger (NDAE1): a new bicarbonate transporter. J. Biol. Chem. 275:24552-24559. [DOI] [PubMed] [Google Scholar]
- 34.Romero, M. F., Y. Kanai, H. Gunshin, and M. A. Hediger. 1998. Expression cloning using Xenopus laevis oocytes. Methods Enzymol. 296:17-51. [DOI] [PubMed] [Google Scholar]
- 35.Schoner, W., and G. Scheiner-Bobis. 2005. Endogenous cardiac glycosides: hormones using the sodium pump as signal transducer. Semin. Nephrol. 25:343-351. [DOI] [PubMed] [Google Scholar]
- 36.Sciortino, C. M., and M. F. Romero. 1999. Cation and voltage dependence of rat kidney electrogenic Na+-HCO−3 cotransporter, rkNBC, expressed in oocytes. Am. J. Physiol. 277:F611-F623. [DOI] [PubMed] [Google Scholar]
- 37.Smith, R. L., J. L. Banks, M. D. Snavely, and M. E. Maguire. 1993. Sequence and topology of the CorA magnesium transport systems of Salmonella typhimurium and Escherichia coli:identification of a new class of transport protein. J. Biol. Chem. 268:14071-14080. [PubMed] [Google Scholar]
- 38.Smith, R. L., M. L. Kaczmarek, L. M. Kucharski, and M. E. Maguire. 1998. Magnesium transport in. Salmonella typhimurium: induction of MgtA and MgtCB expression during invasion of epithelial and macrophage cells. Microbiology 144:1835-1843. [DOI] [PubMed] [Google Scholar]
- 39.Smith, R. L., and M. E. Maguire. 1998. Microbial magnesium transport: unusual transporters searching for identity. Mol. Microbiol. 28:217-226. [DOI] [PubMed] [Google Scholar]
- 40.Snavely, M. D., J. B. Florer, C. G. Miller, and M. E. Maguire. 1989. Magnesium transport in. Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J. Bacteriol. 171:4761-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snavely, M. D., J. B. Florer, C. G. Miller, and M. E. Maguire. 1989. Magnesium transport in. Salmonella typhimurium: expression of cloned genes for three distinct Mg2+ transport systems. J. Bacteriol. 171:4752-4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snavely, M. D., S. A. Gravina, T. T. Cheung, C. G. Miller, and M. E. Maguire. 1991. Magnesium transport in Salmonella typhimurium: regulation of mgtA and. mgtB expression. J. Biol. Chem. 266:824-829. [PubMed] [Google Scholar]
- 43.Snavely, M. D., C. G. Miller, and M. E. Maguire. 1991. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J. Biol. Chem. 266:815-823. [PubMed] [Google Scholar]
- 44.Tao, T., P. F. Grulich, L. M. Kucharski, R. L. Smith, and M. E. Maguire. 1998. Magnesium transport in Salmonella typhimurium: biphasic time and magnesium dependence of the transcription of the mgtA and mgtCB loci. Microbiology 144:655-664. [DOI] [PubMed] [Google Scholar]
- 45.Tao, T., M. D. Snavely, S. G. Farr, and M. E. Maguire. 1995. Magnesium transport in Salmonella typhimurium: mgtA encodes a P-type ATPase and is regulated by Mg2+ in a manner similar to that of the mgtB P-type ATPase. J. Bacteriol. 177:2654-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vazquez-Torres, A., and F. C. Fang. 2000. Cellular routes of invasion by enteropathogens. Curr. Opin. Microbiol. 3:54-59. [DOI] [PubMed] [Google Scholar]
- 47.Verrey, F., E. I. Closs, C. A. Wagner, M. Palacin, H. Endou, and Y. Kanai. 2004. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 447:532-542. [DOI] [PubMed] [Google Scholar]
- 48.Wagner, C. A., B. Friedrich, I. Setiawan, F. Lang, and S. Broer. 2000. The use of. Xenopus laevis oocytes for the functional characterization of heterologously expressed membrane proteins. Cell Physiol. Biochem. 10:1-12. [DOI] [PubMed] [Google Scholar]
- 49.Xiao, A. Y., X. Q. Wang, A. Yang, and S. P. Yu. 2002. Slight impairment of Na+,K+-ATPase synergistically aggravates ceramide- and beta-amyloid-induced apoptosis in cortical neurons. Brain Res. 955:253-259. [DOI] [PubMed] [Google Scholar]
- 50.Xiao, A. Y., L. Wei, S. Xia, S. Rothman, and S. P. Yu. 2002. Ionic mechanism of ouabain-induced concurrent apoptosis and necrosis in individual cultured cortical neurons. J. Neurosci. 22:1350-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaharik, M. L., S. Gruenheid, A. J. Perrin, and B. B. Finlay. 2002. Delivery of dangerous goods: type III secretion in enteric pathogens. Int. J. Med. Microbiol. 291:593-603. [DOI] [PubMed] [Google Scholar]