Abstract
The gene function of the locus of enterocyte effacement (LEE) is essential for full virulence of enterohemorrhagic Escherichia coli (EHEC). Strict control of LEE gene expression is mediated by the coordinated activities of several regulatory elements. We previously reported that the ClpX/ClpP protease positively controls LEE expression by down-regulating intracellular levels of GrlR, a negative regulator of LEE gene expression. We further revealed that the negative effect of GrlR on LEE expression was mediated through GrlA, a positive regulator of LEE expression. In this study, we found that the FliC protein, a major component of flagellar filament, was overproduced in clpXP mutant EHEC, as previously reported for Salmonella. We further found that FliC expression was reduced in a clpXP grlR double mutant. To determine the mediators of this phenotype, FliC protein levels in wild-type, grlR, grlA, and grlR grlA strains were compared. Steady-state levels of FliC protein were reduced only in the grlR mutant, suggesting that positive regulation of FliC expression by GrlR is mediated by GrlA. Correspondingly, cell motility was also reduced in the grlR mutant, but not in the grlA or grlR grlA mutant. Because overexpression of grlA from a multicopy plasmid strongly represses the FliC level, as well as cell motility, we conclude that GrlA acts as a negative regulator of flagellar-gene expression. The fact that an EHEC strain constitutively expressing FlhD/FlhC cannot adhere to HeLa cells leads us to hypothesize that GrlA-dependent repression of the flagellar regulon is important for efficient cell adhesion of EHEC to host cells.
Enterohemorrhagic Escherichia coli (EHEC) is an important class of diarrheagenic E. coli associated with severe diseases, such as hemorrhagic colitis and hemolytic uremic syndrome (reviewed in references 20 and 55). The majority of EHEC strains share with enteropathogenic E. coli (EPEC) and Citrobacter rodentium an island of pathogenicity determinants within their genomes termed the locus of enterocyte effacement (LEE) (52). The LEE is responsible for the production of attaching and effacing lesions, which are characterized by disruption of microvilli and formation of actin-accumulated pedestal-like structures beneath bacteria attached to intestinal epithelial cells (53).
The LEE encompasses 41 genes, which encode structural components of the specialized type III protein secretion system (T3SS), translocator (EspA, -D, and -B) and effector proteins secreted through the LEE-encoded T3SS, chaperones, adhesin (intimin), and the intimin receptor, Tir (16, 34-37). The LEE genes are organized into at least five operons and are under strict regulatory control (54). Three regulators, Ler, GrlR, and GrlA, also are encoded within the LEE: Ler is a central activator necessary for the transcription of most LEE genes (17, 54); GrlA and GrlR are positive and negative regulators, respectively, of ler transcription (3, 13, 47). The negative effect of GrlR on LEE expression depends on the GrlA function (33). Consistent with this evidence, GrlR has been considered to interact with GrlA (11). Therefore, in the absence of GrlR, free GrlA up-regulates LEE gene expression through Ler (3, 13, 33, 47). Furthermore, intracellular protein levels of GrlR are regulated in a growth phase-dependent manner; GrlR levels are minimal as the EHEC cells enter the stationary phase (33), when LEE gene expression is induced (60).
Since LEE expression has been shown to be under the control of various regulatory elements encoded outside the locus (8, 21, 23, 24, 60, 62, 65, 66, 68-70, 74, 78), its regulatory mechanism seems to be complicated and awaits clarification. However, it has been demonstrated that multiple pch genes (pchABC) encode positive regulators important for LEE expression and the adhesion phenotype of EHEC (32, 58). PchABC positively regulate ler transcription and act as key regulators in response to various environmental stimuli (33). For example, the RcsCDB phosphorelay system has been shown to control the expression of LEE both positively and negatively in EHEC (70); a multicopy plasmid carrying rcsB or rcsD activated transcription of LEE through a newly identified positive regulator, GrvA, while deletion of rcsB resulted in increased LEE expression via activation of pchA and pchC transcription (70). In addition, the ATP-dependent protease complex ClpX/ClpP positively regulates LEE expression in EHEC by regulating intracellular protein levels of GrlR and RpoS (33, 72); the latter negatively affects LEE expression by reducing the transcription of pchA by a currently unknown mechanism (33).
ClpXP similarly regulates the stability of many cellular proteins, including several transcriptional regulators (18). In Salmonella, ClpXP negatively controls flagellar synthesis by altering the stability of FlhD/FlhC master regulators (71, 73), which are essential for the expression of all other flagellar genes (28, 29, 42, 48).
The supramolecular structure of the flagellar-hook-basal-body complex resembles the needle structure of T3SS, and they are evolutionarily related (40). Several lines of evidence suggest that regulatory genes for the flagellar regulon also control the expression of T3SS and subsequent virulence phenotypes in some bacteria (7, 14, 26, 31, 49, 57). However, the biological significance and molecular mechanism of this cross-regulation remain unclear. Here, we report that a LEE-encoded positive regulator, GrlA, negatively regulates flagellar-gene expression in EHEC by reducing transcription of the flhD operon. Our results indicate that GrlA is a key regulator of inversely regulated flagellar and LEE-encoded T3SS expression in EHEC, and this regulation may be important for efficient cell adhesion of EHEC.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this study are summarized in Table 1. SKI-5142 is a Lac-negative derivative of EHEC O157:H7 strain Sakai, described previously (33), and was used as a wild-type strain in this study. Unless otherwise specified, bacteria were grown in Luria broth (LB), and ampicillin (100 μg/ml), chloramphenicol (25 μg/ml), and kanamycin (50 μg/ml) antibiotics were added as required. LB and LB-agar plates were prepared as described previously (32). Motility agar plates were prepared by adding 0.25% agar (Shoei) to 1% Bacto-Trypton (Difco) broth containing 0.5% NaCl.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant phenotype | Source or reference |
|---|---|---|
| Strains | ||
| SKI-5142 | Wild-type EHEC O157:H7 Sakai Δ(lacZYA) | 33 |
| SKI-5143 | SKI-5142 Δ(ler)::kan | 33 |
| SKI-5149 | SKI-5142 Δ(clpXP)::kan | 33 |
| SKI-5152 | SKI-5142 Δ(grlR)::cat | 33 |
| SKI-5153 | SKI-5142 Δ(grlA)::cat | 33 |
| SKI-5154 | SKI-5142 Δ(grlR-grlA)::cat | 33 |
| SKI-5156 | SKI-5149 Δ(grlR)::cat | 33 |
| SKI-5200 | SKI-5142 Δ(fliC):: kan | This study |
| Plasmids | ||
| pGEM-T-Easy | TA cloning vector, Apr | Promega |
| pGEM-self | Self-ligated pGEM-T-Easy | 33 |
| pGEMGA | pGEM-T-Easy-grlA | 33 |
| pGEMGR | pGEM-T-Easy-grlR | This study |
| pGEMFHDC | pGEM-T-Easy-flhDC | This study |
| pACYC184 | Cloning vector; Cmr Tcr | 9 |
| pACGA | pACYC184-grlA | This study |
| pACYC177 | Cloning vector; Apr Kmr | 9 |
| pAC177FHDC | pACYC177-flhDC | This study |
| pRL124 | Promoter probe vector; Apr | 51 |
| pRLFHD | pRL124-flhD promoter region | This study |
| pRLFGA | pRL124-flgA promoter region | This study |
| pRLFLC | pRL124-fliC promoter region | This study |
| pRLLER | pRL124-ler promoter region | 33 |
A one-step inactivation method was used to construct SKI-5200 as described previously (12, 32, 33). The PCR primers used to construct SKI-5200 were 5′-AAACCCAATACGTAATCAACGACTTGCAATATAGGATAACGAATCGTGTAGGCTGGAGCTGCTTC-3′ and 5′-ACGGGGTGCGGTGAAACCGCATAAAAATAAAGTTGGTTATTCTGGGTGTAGGCTGGAGCTGCTTC-3′.
Construction of plasmids.
pGEMGA was described previously (33). A 0.52-kbp DNA fragment of pGEMGA excised by BamHI-StuI digestion was inserted into the BamHI-NruI site of pACYC184 to yield pACGA. Plasmids pGEMFHDC and pGEMGR were constructed by cloning PCR-amplified flhDC and grlR genes, respectively (primers for pGEMFHDC, 5′-CGGGATCCCGGAGAAACGACGCAATCCCAA-3′ and 5′-GAAGGCCTTCAATGTTGCGCCACACCGTAT-3′; for pGEMGR, 5′-AACAAATTGAAAGGAGTGAG-3′ and 5′-ATCGACATAAAAAACATACA-3′), into pGEM-T-Easy vector (Promega). Plasmids pACFHDC and pAC177FHDC were constructed by cloning a BamHI-StuI fragment of pGEMFHDC into the BamHI-NruI site of pACYC184 (9) and the BamHI-ScaI site of pACYC177 (9), respectively. Plasmids pRLFHD, pRLFGA, and pRLFIC contain PCR-amplified DNA fragments encoding transcriptional-regulatory sequences of flhD, flgA, and fliC, respectively, as deduced from E. coli and Salmonella sequences (10, 25, 29, 64, 76). These three plasmids were cut with KpnI and EcoRI to excise the promoter fragments, which were ligated into the corresponding sites of a promoter-probe vector, pRL124 (51). For pRLFHD, a 0.74-kbp DNA fragment including −717 to +23 relative to the flhD initiation codon (the region corresponding to −527 to +212 with respect to the transcriptional start site determined in the E. coli K-12 laboratory strain) (64) was similarly generated by PCR (5′-GGGGTACCCCGAGAAACGACGCAATCCCAA-3′/5′-CGGAATTCCGTCGGAGGTATGCATTATTCC-3′). For pRLFGA, a 0.25-kbp DNA fragment including −182 to +24 relative to the flgA initiation codon was amplified (5′-CGGAATTCCGCACGCTACGTTTTATTGCCA-3′/5′-GGGGTACCCCTAAGGCGGCGTCGAGCTTAT-3′). For pRLFIC, a 0.31-kbp DNA fragment including −281 to +35 relative to the fliC initiation codon was isolated (5′-GGGGTACCCCATGAAATACTTGCCATGCGA-3′/5′-CGGAATTCCGCAGCGAGAGGCTGTTGGTAT-3′). All of these promoter fragments were similarly cloned into pRL124. pRLLER was described previously (33).
Analysis of proteins in culture supernatant and whole-cell lysates.
Bacteria were grown in LB medium with or without antibiotics at 37°C with shaking until they reached an optical density at 600 nm of 0.8. Proteins in culture supernatants and whole-cell lysates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting as described previously (30, 32, 33). Western blotting was performed with polyclonal anti-H7 (FliC) or anti-EspB antibodies (32) to detect flagellin or EspB, respectively, in whole-cell lysates. Binding of secondary anti-rabbit (or anti-mouse) immunoglobulin G antibody conjugated to horseradish peroxidase was detected using enhanced chemiluminescence Western blotting detection reagents (Amersham). All assays were performed in duplicate and were repeated at least three times.
Assay of β-galactosidase activity.
β-Galactosidase activity was assayed as described previously (30, 31, 44). Bacteria grown in LB medium with or without antibiotics at 37°C with shaking were harvested at an optical density at 600 nm of 0.8. All assays were performed in triplicate and were repeated at least three times.
Motility assay and measurement of flagellar number.
The motility of bacterial cells was measured by the spread of colonies on motility agar plates incubated for 5 to 6 h at 37°C. To count the flagella, bacterial cells grown to late logarithmic phase were fixed in formaldehyde, stained with a flagellum-staining reagent containing 0.2% Victoria blue B (Wako) as described previously (43), and examined in an Olympus BX50 microscope. For each stained sample, the number of flagella on more than 50 bacterial cells was determined.
Adhesion and fluorescent actin-staining tests.
Fluorescent actin-staining tests were performed as described previously (38) with slight modifications. Briefly, HeLa cells maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% (vol/vol) fetal bovine serum (Invitrogen) were plated onto coverslips (Fisher Scientific) in a 24-well plastic plate (Corning) at 1 × 105 cells/ml and incubated for 24 h in the presence of 5% CO2. After the HeLa cells were washed three times with DMEM without fetal bovine serum, 107 EHEC cells cultured overnight at 37°C with shaking in DMEM containing 0.5% (wt/vol) glycerol with or without antibiotics were inoculated into each well, and the plastic plate was centrifuged at 1,000 × g for 5 minutes and then incubated for 1 h at 37°C in the presence of 5% CO2. The cells were then washed three times with phosphate-buffered saline (PBS) and incubated for an additional 3 hours, after which the monolayers were washed six times with PBS, fixed in 3% paraformaldehyde for 15 min, permeabilized in 0.2% Triton X-100, and then incubated in blocking solution (Yukijirushi) for at least 1 hour. The cells were then washed twice in PBS and incubated with PBS containing 1 mg/ml bisbenzimide H33342 (Calbiochem) and rhodamine-phalloidin (Molecular Probes) for 30 min. After three washes with PBS and one with water, the coverslips were air dried and observed using an Olympus BX51. Images were captured with a digital charge-coupled-device camera (Hamamatsu). The adherence of each strain was evaluated quantitatively by plating adherent bacteria on LB agar plates with ampicillin or kanamycin after incubation with 0.2% (final concentration) Triton X-100 in PBS for 20 min at room temperature. All assays were performed in duplicate and were repeated at least four times. The statistical significance was expressed as the P value as determined by a Student t test analysis.
RESULTS
The GrlR-GrlA system controls FliC expression in EHEC.
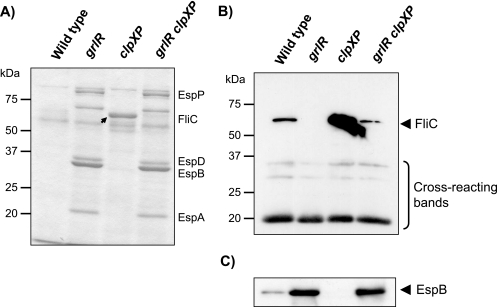
We previously reported that the ATP-dependent protease complex ClpXP positively controls LEE expression by regulating GrlR protein levels in EHEC (33). During further studies characterizing the clpXP mutant, we noticed that cells of the clpXP deletion strain secreted large amounts of protein into LB culture supernatants (Fig. 1A). The approximate molecular mass of the protein band (60 kDa), estimated by SDS-PAGE analysis was similar to that of flagellin (FliC), the component protein of the flagellar filament. Western blotting analysis using polyclonal anti-H7 (FliC) antibodies (Fig. 1B) confirmed that ClpXP in EHEC also negatively regulates FliC expression, as previously reported for Salmonella (71).
FIG. 1.
Effects of clpXP and/or grlR deletions on the expression of FliC. (A) Coomassie brilliant blue-stained SDS-PAGE profiles of secreted proteins from cells cultured in LB. An arrowhead indicates the FliC band. Strains used: wild type, SKI-5142; grlR, SKI-5152; clpXP, SKI-5149; grlR clpXP, SKI-5156. (B and C) Western blotting analyses of (B) FliC and (C) EspB in equivalent amounts of whole-cell lysates prepared from the same strains used in panel A. Cross-reacting bands recognized by the polyclonal anti-FliC (H7) serum served as loading controls.
We further observed that the amount of FliC in culture supernatants decreased significantly in strains with a deletion of grlR (Fig. 1A), which encodes a negative regulator of LEE expression (13, 47). This reduction was also observed in whole-cell samples, whereas the amounts of cross-reacting protein bands were unchanged (Fig. 1B). Since steady-state levels of FliC in the wild-type strain were abolished by a grlR deletion (Fig. 1B), we hypothesized that the expression of flagellar proteins is positively regulated by GrlR. In contrast, expression of LEE-encoded type III secreted translocator proteins (such as EspA, -B, and -D) in the grlR strain was derepressed in the same LB cultures (Fig. 1A and C), as shown in previous studies (33, 47). We also examined FliC protein levels in the same strains cultured in DMEM, conditions under which LEE expression is induced. It was found that the expression level of FliC was very low in the wild-type strain, as described previously (22, 79), and was not significantly increased even in the strain with clpXP deleted (data not shown).
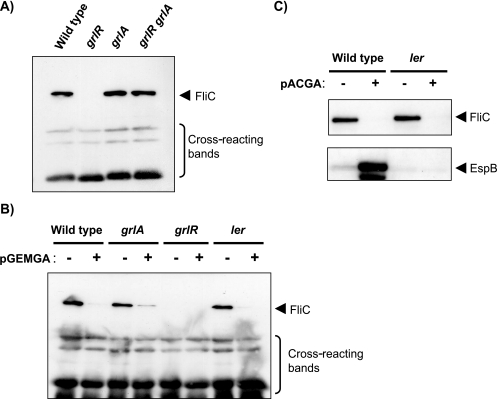
Because induction of LEE expression in the grlR mutant depends on the function of GrlA (33), a positive regulator of LEE, we compared the expression level of FliC in wild-type, grlR, grlA, and grlR grlA strains. The FliC band was absent in the grlR strain but present in the grlA and grlR grlA strains (Fig. 2A). Given that grlR deletion upregulates the expression of GrlA, as well as other LEE-encoded proteins (13, 33, 47; our unpublished data), and that GrlR is hypothesized to interact with GrlA (11), FliC expression in the grlR mutant may be inhibited by GrlA. To test this hypothesis, we examined FliC expression in a strain carrying multicopy plasmids with the grlA gene (pGEMGA and pACGA). As predicted, FliC expression in the wild type and/or a grlA mutant was significantly reduced in the presence of pGEMGA (Fig. 2B) or pACGA (Fig. 2C), indicating that GrlA participates in the repression of flagellar synthesis. A similar reduction was observed in a ler mutant strain harboring pGEMGA (Fig. 2B) or pACGA (Fig. 2C), suggesting that unlike the requirement for Ler in the GrlA-dependent activation of LEE (13) (Fig. 2C), repression of FliC by GrlA does not require Ler function.
FIG. 2.
(A and B) Repression of FliC expression by overproduced GrlA. Shown are Western blotting analyses of FliC prepared from whole-cell lysates containing an equivalent number of cells from the following strains: wild type, SKI-5142; grlR, SKI-5152; grlA, SKI-5153; grlR grlA, SKI-5154; ler, SKI-5143; and pGEM-self or pGEMGA (indicated by − or +, respectively, in panel B). (C) Western blotting analyses of FliC and EspB prepared from whole-cell lysates containing an equivalent number of cells from the same strains with plasmid: wild type, SKI-5142; ler, SKI-5143; pACYC184 or pACGA (indicated by − or +, respectively).
The GrlR-GrlA system controls the flagellar formation and function of EHEC.
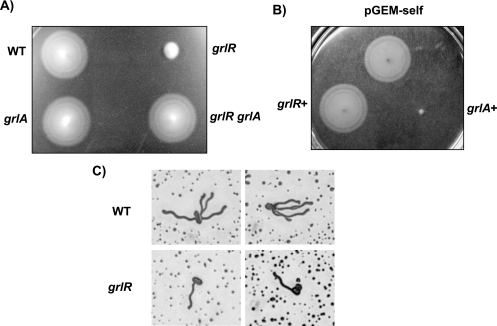
To examine whether the GrlR-GrlA regulatory system affects flagellar formation and function, we compared the motility phenotypes of wild-type, grlR, grlA, and grlR grlA EHEC strains on motility agar plates. A significant reduction in EHEC cell motility in the grlR strain, but not in the grlA or grlR grlA strain (Fig. 3A), indicated that GrlA negatively regulates EHEC motility in the absence of GrlR. Furthermore, because multicopy plasmids carrying grlA, but not grlR, strongly repressed motility (Fig. 3B), we concluded that GrlA-dependent FliC repression affects the flagellar function of EHEC. In agreement with these observations, fewer flagella were stained with Victoria blue in the grlR mutant, with the mean number of flagella per cell being 3.1 in the wild type and 0.99 in the grlR mutant strain (Fig. 3C).
FIG. 3.
Motility phenotypes of wild-type (WT), grlR, grlA, and grlR grlA strains of EHEC (A) and the wild-type strain with pGEM-self (control vector), pGEMGR (carrying grlR), or pGEMGA (carrying grlA) plasmid (B). (C) Cells grown in 1% Bacto-Trypton broth containing 0.5% NaCl were stained with Victoria blue as described in Materials and Methods. The following strains were used: WT, SKI-5142; grlR, SKI-5152; grlA, SKI-5153; and grlR grlA, SKI-5154 (A and/or C) and pGEM-self, SKI-5142 with pGEM-self; grlR+, SKI-5142 with pGEMGR; and grlA+, SKI-5142 with pGEMGA (B).
Transcription of the flagellar master operon is under the control of the GrlR-GrlA system.
More than 50 genes are involved in the formation and function of flagella in E. coli and Salmonella (50). They are organized within more than a dozen operons and can be classified into three hierarchical classes (classes 1, 2, and 3), which together form the flagellar regulon (39, 42, 43). Class 1 is a single operon composed of the flhD and flhC genes (4, 39, 41), which are essential for the expression of all other flagellar genes (29, 48). Class 2 operons contain genes essential for the formation of the hook-basal body and the FliA-sigma factor, all of which are responsible for the transcription of class 3 genes (42, 56). The components necessary for the formation of the flagellar filament and functions (motility and chemotaxis) are encoded by the genes belonging to class 3 operons (42). To locate a possible GrlA target site on the flagellar regulon, we measured the transcriptional activities of several flagellar promoters by fusing the transcriptional-regulatory regions of flhD (for measuring flagellar class 1 transcription), flgA (for class 2), and fliC (for class 3) to lacZ in pRL124 as described in Materials and Methods. β-Galactosidase activity mediated by each of these three promoters (on plasmids pRLFHD, pRLFGA, and pRLFIC), as well as the ler promoter (on pRLLER) (33), was higher than that of the promoterless vector (pRL124) in the wild-type strain (Table 2), indicating that all plasmids contained functional promoters. The activities of all the flagellar promoters were reduced in the grlR strain and slightly increased in the grlA and grlR grlA strains relative to those in the wild type, while the activity of the ler promoter was inversely derepressed in the grlR strain but reduced in the grlA strain (Table 2). These results indicate that these promoters are under the control of the GrlR-GrlA system. The transcriptional activity of flhD in the grlR mutant strain was decreased only twofold compared to that in the wild type. Hence, we further examined the effects of a multicopy of grlA on the transcriptional activities of flagellar promoters. Although the flgA and fliC promoters were significantly reduced in the presence of multicopy grlA (pACGA), the flhD promoter in the presence of pACGA still exhibited approximately twofold-lower activity than that in the strain with pACYC184 (Table 3). Consistent with these observations, several other mutations that affect flagellar expression reduced the transcription of the flhD operon by 2- to 3-fold but decreased FliC expression more than 10-fold in E. coli K-12 strains or an EHEC O157:H7 strain (46, 61, 64, 67). Together, this evidence suggests that the reduction of the flhD operon observed in the grlR strain is due to the effect of GrlA, and the twofold reduction alone may be enough to reduce the entire activity of the flagellar regulon.
TABLE 2.
GrlR-GrlA system controls promoter activity of flhD, flgA, fliC, and ler genes
| Strain (no.) | β-Galactosidase activity (Miller units) (SD)
|
||||
|---|---|---|---|---|---|
| pRL124 | pRLFHD | pRLFGA | pRLFIC | pRLLER | |
| Wild type (SKI-5142) | 15.8 (5.07) | 2,670 (284) | 717 (76.5) | 1,720 (98.7) | 7,600 (410) |
| grlR (SKI-5152) | 18.8 (7.04) | 1,240 (199) | 87.9 (3.18) | 147 (4.76) | 23,800 (1,600) |
| grlA (SKI-5153) | 19.3 (3.27) | 3,400 (250) | 869 (210) | 2,130 (118) | 4,390 (515) |
| grlR grlA (SKI-5154) | 23.1 (5.11) | 3,130 (138) | 848 (87.2) | 2,000 (313) | NDa |
ND, not done.
TABLE 3.
Promoter activities of flhD, flgA, and fliC genes with or without multicopy grlA
| Strain/plasmid (no.) | β-Galactosidase activity (Miller units) (SD)
|
|||
|---|---|---|---|---|
| pRL124 | pRLFHD | pRLFGA | pRLFIC | |
| SKI-5142/pACYC184 | 14.6 (5.87) | 5,460 (296) | 608 (10.5) | 1,330 (567) |
| SKI-5142/pACGA | 13.8 (4.24) | 2,620 (182) | 126 (4.29) | 10.7 (2.05) |
Constitutive expression of FlhD/FlhC inhibits efficient adhesion of EHEC to HeLa cells.
The results presented above led us to hypothesize that repression of flhD transcription by GrlA may be necessary for EHEC adherence to host cells, because reduction of the flagellar regulon was observed in the grlR strain, a condition under which LEE expression is significantly induced (33, 47). To test this hypothesis, flhD and flhC genes cloned from the wild-type EHEC into pGEM-T-Easy or pACYC177 (designated pGEMFHDC and pAC177FHDC, respectively) were introduced into wild-type and grlR strains, and the effect of constitutive FlhDC expression on EHEC adhesion to HeLa cells was examined. The strains transformed with pGEMFHDC or pAC177FHDC were highly motile on motility agar and produced high levels of flagellin even when they were grown in DMEM (data not shown).
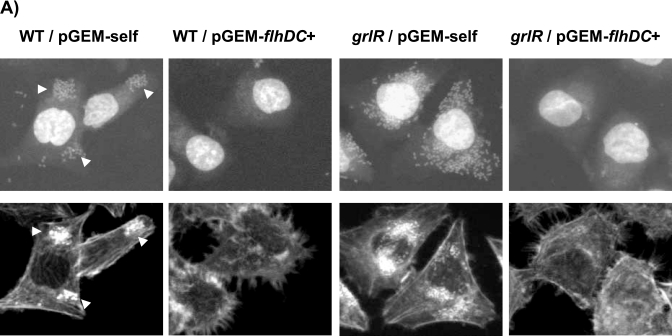
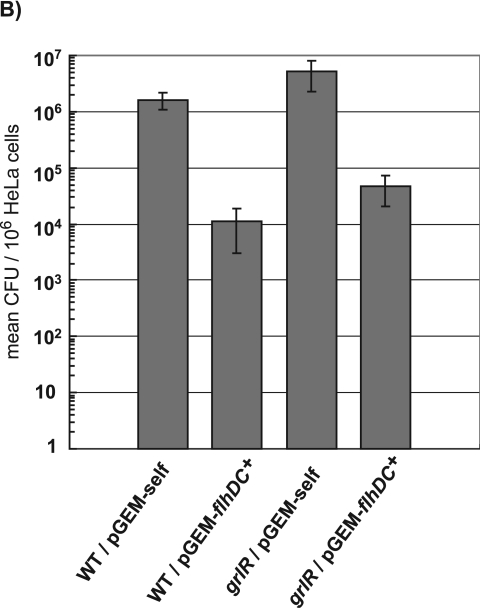
Figure 4A depicts wild-type Sakai microcolonies on cultured HeLa cells, with intense accumulations of stained actin beneath bacterial attachment sites. The grlR mutant cells transformed with the control vector (pGEM-self) adhered to HeLa cells more efficiently than wild-type cells with pGEM-self. Neither strain with pGEMFHDC adhered to HeLa cells or showed fluorescent actin staining, suggesting that constitutively expressed FlhD/FlhC inhibit efficient adhesion of EHEC to HeLa cells. The in vitro adherence levels of these strains were then compared by quantitative assay (Fig. 4B). The introduction of plasmid pGEMFHDC produced significantly reduced adherence in wild-type (1.9%; P < 0.001) and grlR mutant (0.93%; P < 0.001) strains relative to those strains with vector control (pGEM-self). We obtained the same results when the wild-type strain was transformed with pACYC177 or pAC177FHDC and used for adhesion assays (data not shown); the strain harboring pAC177FHDC did not efficiently adhere to HeLa cells. Therefore, we hypothesize that GrlA-dependent repression of the flagellar regulon is important for efficient adhesion of EHEC to host cells.
FIG.4.
Effect of constitutively expressed FlhD/FlhC on adhesion of EHEC to HeLa cells. (A) The top row was stained with bisbenzimide to see the formation of microcolonies. The bottom row was stained with rhodamine-phalloidin to see accumulated actin under individual attached bacteria. Microcolonies or localized actin accumulations are indicated by arrowheads. The following strains were used: WT/pGEM-self, SKI-5142 with pGEM-self; WT/pGEM-flhDC+, SKI-5142 with pGEMFHDC; grlR/pGEM-self, SKI-5152 with pGEM-self; grlR/pGEM-flhDC+, SKI-5152 with pGEMFHDC. (B) Quantitative analysis of adhesion phenotypes of the strains used in panel A. The bars indicate the numbers of mean bacterial CFU per well. The error bars indicate standard deviations.
These results also suggest that constitutively expressed flagella may have a deleterious effect on efficient adhesion of EHEC to HeLa cells. To test this hypothesis, we constructed a fliC mutant of EHEC (designated SKI-5200) transformed with pGEM-self or pGEMFHDC and performed the same adhesion assay. The fliC mutant with pGEM-self showed an adhesion phenotype comparable to that of the wild-type strain carrying pGEM-self: constitutive expression of FlhDC reduced adhesion of EHEC to HeLa cells even in the fliC mutant (data not shown). These results suggest that overproduced flagellar filament is not the sole reason for the reduction of cell adhesion. Other genes under the control of FlhDC may have deleterious effects on the adhesion of EHEC to host cells.
DISCUSSION
In the present study, we demonstrate that the GrlR-GrlA regulatory system coordinately controls the expression of not only LEE genes but also the flagellar regulon in EHEC. When we compared the expression levels of FliC in several strains of EHEC, reduction of FliC expression was observed in the grlR mutant but not in the grlR grlA mutant. Additionally, overproduced GrlA from a multicopy plasmid strongly repressed FliC expression and the motility of EHEC, indicating that GrlA is responsible for reducing the expression of the flagellar regulon. Conversely, LEE expression was strongly derepressed in the grlR mutant and induced by the introduction of a plasmid carrying grlA, as reported previously (3, 13, 33, 47). Intracellular GrlR levels in wild-type EHEC have been shown to decrease gradually as culture times increase and to achieve minimum levels in stationary-phase cultures (33). Therefore, this reduction of GrlR levels, which leads to the derepression of GrlA (our unpublished data), is a key event in the inverse regulation of flagellar and LEE gene expression, as summarized in Fig. 5. Consistent with this model, maximum expression of a LEE gene transcription was observed as bacterial cells entered the stationary phase (60). As described above, flagellin expression is highly induced in clpXP-mutant EHEC. This may be due to the increased stability of FlhD/FlhC, as hypothesized for Salmonella (73). Since the protein levels of intracellular GrlR have also been regulated by ClpXP in EHEC (33), ClpXP directly and indirectly controls flagellar expression in EHEC by regulating the FlhD/FlhC and GrlR levels, respectively (Fig. 5). Other than these regulatory systems, LEE expression has been shown to be under the control of various regulatory elements: bacterial histone-like proteins, such as H-NS (8, 74), Hha (60), IHF (21), and Fis (23); the quorum-sensing system (65, 66, 68); and other factors, such as GadX (62), YhiE (GadE)/YhiF (69), BipA (24), GrvA (70), and EtrA/EivF (78). Therefore, these regulators should also be depicted properly in the regulation model in Fig. 5. Further studies integrating these regulatory networks must be done. Our results indicate that GrlA-dependent repression of flagellar synthesis does not require Ler, although this protein is a central regulator and is indispensable for the GrlA-dependent activation of LEE expression (13). To our knowledge, this is the first report that GrlA controls the expression of non-LEE genes without Ler function.
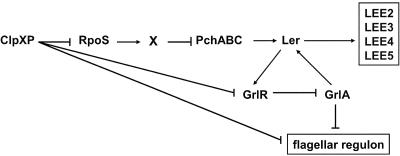
FIG. 5.
Model depicting the coordinate regulation of LEE and flagellar gene expressions by the GrlR-GrlA system in EHEC. GrlA activates LEE expression via activation of ler transcription, whereas it inhibits transcription of the flhD operon in response to GrlR levels in the cell. ClpXP positively regulates LEE expression by controlling changes in GrlR and RpoS levels and directly or indirectly represses the flagellar regulon by altering the stabilities of FlhD/FlhC and GrlR. As described in the text, additional regulatory elements, such as Fis, H-NS, IHF, Hha, GadX, YhiE, YhiF, BipA, EtrA, EivF, GrvA, and quorum sensing, have also been implicated in the regulation of LEE.
We also show that GrlA represses transcription of flhD, the master operon of the flagellar regulon. Although GrlA has a putative helix-turn-helix DNA-binding motif (13) and activates ler transcription even in an E. coli K-12 background (3), attempts to verify the DNA-binding activity of GrlA have been not successful (3). Therefore, the molecular mechanisms by which GrlA activates and represses ler and flhD transcription remain unknown. Since the transcription of the flhD operon has been shown to be under the control of multiple regulatory elements, such as cyclic AMP-CRP (42, 63, 64), OmpR (61), H-NS (6, 64, 76), CsrA (75), IHF (77), RcsB (19), RtsB (15), LrhA (46), and QseB (10, 67), in several bacteria, GrlA may alternatively affect the expression or activity of these regulators and thereby control the expression of the flagellar regulon in EHEC.
In a mouse model, GrlA is essential for full virulence of C. rodentium (13), indicating that the activation of LEE expression by GrlA is necessary for virulence. This finding raises the possibility that repression of the flagellar regulon, as well as LEE activation by GrlA, is also important for the establishment of EHEC infection. To test the effect of constitutive expression of the flagellar regulon on the LEE-mediated adhesion phenotype, we constructed EHEC strains transformed with a multicopy plasmid carrying flhDC and examined their adhesion phenotypes. The fact that the strain constitutively expressing FlhDC did not efficiently adhere to HeLa cells suggests that appropriate reduction of flagellar-gene expression is necessary for the adhesion phenotype of EHEC. Consistent with these observations, a mutant of E. coli strain K-12, MG1655, with flhDC deleted can colonize the mouse intestine more efficiently than the parent strain (45). In Bordetella bronchiseptica, a causative agent of human respiratory diseases, flagellar synthesis and virulence gene expression are coordinately regulated by a two-component regulatory system, BvgA/BvgS, in response to environmental signals. Activated BvgA, which positively regulates the transcription of several virulence genes, including adhesin and toxin, inversely represses transcription of frlAB, an analogue of flhDC (2). Constitutive expression of FrlAB in BvgAS-activated cells resulted in defective tracheal colonization by B. bronchiseptica (1), demonstrating that B. bronchiseptica down-regulates FrlAB expression to circumvent the inappropriate expression of the gene under the control of FrlAB.
In the EPEC O127:H6 strain E2348/69, most of the bacterial cells attached to the actin pedestals were flagellated at an early stage of infection of HeLa cells, while many cells associated with actin pedestals at a later stage of infection did not form flagella (77). Therefore, EPEC may repress flagellar synthesis at later stages of attaching and effacing lesion formation if strain E2348/69 has the same GrlA-dependent regulatory system for reducing flagellar expression. Because purified H6 (but not H7) flagella can bind to HeLa cells (22) and nonflagellated fliC mutant cells of E2348/69 were less adherent and were not able to produce typical large microcolonies on HeLa cells compared with the wild-type strain (22), H6 but not H7 flagella seem to be important for initial attachment of the E2348/69 strain to HeLa cells. However, both H7 and H6 flagellins from EHEC O157:H7 strains 86-24 and E2348/69, respectively, have been shown to be the main triggers inducing the expression of a neutrophil chemoattractant, interleukin-8, in human intestinal epithelial cells (5, 79). Therefore, not only the EHEC but also the EPEC strain may need to down-regulate flagellar synthesis to circumvent the immune responses of host cells at a specific stage of their infection process. Consistent with this, inversely regulated expression of flagellar and LEE-encoded T3S systems in EPEC has been reported: BipA, a positive regulator for LEE gene expression, negatively regulates bacterial-cell motility in EPEC (24).
Given that flagellar filaments achieve lengths of more than 5 μm and that each reversibly rotating flagellum consists of approximately 20,000 subunits of flagellin (50), strict control of flagellar synthesis may be necessary to minimize the energy expense of survival in changing environments. Alternatively, repressing flagellar formation may be important to avoid steric hindrance for polymerizing the EspA filament, which is essential for translocating effector proteins into host cells (38).
When a plasmid overexpressing FlhDC was introduced into the fliC mutant of EHEC, the resulting transformants still did not adhere to HeLa cells, suggesting that overproduced flagellin was not the sole reason for the reduction of EHEC cell adhesion to HeLa cells when FlhDC was constitutively expressed. This result further suggests that the function of another gene(s) under the control of FlhDC may have a deleterious effect on the adhesion of EHEC to HeLa cells. Because the flagellar regulator FlhDC and/or FliA controls the expression of several genes other than those required for flagellar formation and function in E. coli K-12 strains (27, 59), we can hypothesize that these nonflagellar genes under the control of FlhDC and/or FliA may inhibit EHEC cell adhesion when FlhDC is overproduced. Alternatively, these flagellar regulators may affect the expression of LEE genes. Supporting this idea, deletion of the flhD operon or fliA up-regulates the expression of several T3SS-related virulence genes, such as yop or ysc in Yersinia enterocolitica (7, 26).
Superstructures of the flagellar hook-basal body and needle complexes of T3SS are similar, and the genes encoding their components are phylogenetically related (40). Regulatory interactions between flagellar and other T3S systems have been reported in several bacteria (57). For example, a positive regulatory gene in the flagellar regulon, fliZ, also activates the expression of Salmonella pathogenicity island 1 genes and the virulence phenotype of Salmonella (31, 49). In contrast, as shown here, the positive regulator GrlA that is encoded within a LEE pathogenicity island negatively regulates flagellar expression. Additionally, putative regulators, EtrA and EivF, encoded in E. coli T3SS-2 (ETT2) negatively regulate LEE expression, even though ETT2 seems to be defective in the EHEC O157 Sakai strain (78). Links between T3S regulatory systems may be important for survival under various environmental conditions, including those within host cells. Future studies of coupled or mutually exclusive expression of T3S systems in various environments will further extend our understanding of the roles of these control systems in infection by each pathogenic bacterium.
Acknowledgments
We thank Kazuhiro Kutsukake and Shouji Yamamoto (Okayama University) for helpful discussions and suggestions.
This work was supported by grants-in-aid for scientific research from the Ministry of Education, Culture, Science and Technology of Japan; the Ministry of Welfare and Labor of Japan (H17-Sinkou-ippan-019); and the Japan Health Science Foundation. Yan Lu was supported by a fellowship from the Japan Health Science Foundation.
REFERENCES
- 1.Akerley, B. J., P. A. Cotter, and J. F. Miller. 1995. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell 80:611-620. [DOI] [PubMed] [Google Scholar]
- 2.Akerley, B. J., D. M. Monack, S. Falkow, and J. F. Miller. 1992. The bvgAS locus negatively controls motility and synthesis of flagella in Bordetella bronchiseptica. J. Bacteriol. 174:980-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barba, J., V. H. Bustamante, M. A. Flores-Valdez, W. Deng, B. B. Finlay, and J. L. Puente. 2005. A positive regulatory loop controls expression of the locus of enterocyte effacement-encoded regulators Ler and GrlA. J. Bacteriol. 187:7918-7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett, D. H., B. B. Frantz, and P. Matsumura. 1988. Flagellar transcriptional activators FlbB and FlaI: gene sequences and 5′ consensus sequences of operons under FlbB and FlaI control. J. Bacteriol. 170:1575-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berin, M. C., A. Darfeuille-Michaud, L. J. Egan, Y. Miyamoto, and M. F. Kagnoff. 2002. Role of EHEC O157: H7 virulence factors in the activation of intestinal epithelial cell NF-κB and MAP kinase pathways and the up-regulated expression of interleukin-8. Cell. Microbiol. 4:635-647. [DOI] [PubMed] [Google Scholar]
- 6.Bertin, P., E. Terao, E. H. Lee, P. Lejeune, C. Colson, A. Danchin, and E. Collatz. 1994. The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. J. Bacteriol. 43:5537-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleves, S., M. N. Marenne, G. Detry, and G. R. Cornelis. 2002. Up-regulation of the Yersinia enterocolitica Yop regulon by deletion of the flagellum master operon. flhDC. J. Bacteriol. 184:3214-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bustamante, V. H., F. J. Santana, E. Calva, and J. L. Puente. 2001. Transcriptional regulation of type III secretion genes in enteropathogenic Escherichia coli: Ler antagonizes H-NS-dependent repression. Mol. Microbiol. 39:664-678. [DOI] [PubMed] [Google Scholar]
- 9.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke, M. B., and V. Sperandio. 2005. Transcriptional regulation of flhDC by QseBC and σ28 (FliA) in enterohaemorrhagic Escherichia coli. Mol. Microbiol. 57:1734-1749. [DOI] [PubMed] [Google Scholar]
- 11.Creasey, E. A., R. M. Delahay, S. J. Daniell, and G. Frankel. 2003. Yeast two-hybrid system survey of interactions between LEE-encoded proteins of enteropathogenic Escherichia coli. Microbiology 149:2093-2106. [DOI] [PubMed] [Google Scholar]
- 12.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng, W., J. L. Puente, S. Gruenheid, Y. Li, B. A. Vallance, A. Vazquez, J. Barba, J. A. Ibarra, P. O'Donnell, P. Metalnikov, K. Ashman, S. Lee, D. Goode, T. Pawson, and B. B. Finlay. 2004. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci. USA 101:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichelberg, K., and J. E. Galan. 2000. The flagellar sigma factor FliA (σ28) regulates the expression of Salmonella genes associated with the centisome 63 type III secretion system. Infect. Immun. 68:2735-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellermeier, C. D., and J. M. Slauch. 2003. RtsA and RtsB coordinately regulate expression of the invasion and flagellar genes in Salmonella enterica serovar Typhimurium. J. Bacteriol. 185:5096-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. K. Deng, L. C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 17.Elliott, S. J., V. Sperandio, J. A. Giron, S. Shin, J. L. Mellies, L. Wainwright, S. W. Hutcheson, T. K. McDaniel, and J. B. Kaper. 2000. The locus of enterocyte effacement (LEE)-encoded regulator controls expression of both LEE- and non-LEE-encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 68:6115-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn, J. M., S. B. Neher, Y. I. Kim, R. T. Sauer, and T. A. Baker. 2003. Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol. Cell 11:671-683. [DOI] [PubMed] [Google Scholar]
- 19.Francez-Charlot, A., B. Laugel, A. Van Gemert, N. Dubarry, F. Wiorowski, M. P. Castanie-Cornet, C. Gutierrez, and K. Cam. 2003. RcsCDB His-Asp phosphorelay system negatively regulates the flhDC operon in Escherichia coli. Mol. Microbiol. 49:823-832. [DOI] [PubMed] [Google Scholar]
- 20.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 21.Friedberg, D., T. Umanski, Y. Fang, and I. Rosenshine. 1999. Hierarchy in the expression of the locus of enterocyte effacement genes of enteropathogenic Escherichia coli. Mol. Microbiol. 34:941-952. [DOI] [PubMed] [Google Scholar]
- 22.Giron, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44:361-379. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg, M. D., M. Johnson, J. C. Hinton, and P. H. Williams. 2001. Role of the nucleoid-associated protein Fis in the regulation of virulence properties of enteropathogenic Escherichia coli. Mol. Microbiol. 41:549-559. [DOI] [PubMed] [Google Scholar]
- 24.Grant, A. J., M. Farris, P. Alefounder, P. H. Williams, M. J. Woodward, and C. D. O'Connor. 2003. Co-ordination of pathogenicity island expression by the BipA GTPase in enteropathogenic Escherichia coli (EPEC). Mol. Microbiol. 48:507-521. [DOI] [PubMed] [Google Scholar]
- 25.Hanafusa, T., A. Sakai, A. Tominaga, and M. Enomoto. 1989. Isolation and characterization of Escherichia coli hag operator mutants whose hag48 expression has become repressible by a Salmonella H1 repressor. Mol. Gen. Genet. 216:44-50. [DOI] [PubMed] [Google Scholar]
- 26.Horne, S. M., and B. M. Prüß. 2006. Global gene regulation in Yersinia enterocolitica: effect of FliA on the expression levels of flagellar and plasmid-encoded virulence genes. Arch. Microbiol. 185:115-126. [DOI] [PubMed] [Google Scholar]
- 27.Ide, N., and K. Kutsukake. 1997. Identification of a novel Escherichia coli gene whose expression is dependent on the flagellum-specific sigma factor, FliA, but dispensable for motility development. Gene 199:19-23. [DOI] [PubMed] [Google Scholar]
- 28.Ikebe, T., S. Iyoda, and K. Kutsukake. 1999. Structure and expression of the fliA operon of Salmonella typhimurium. Microbiology 145:1389-1396. [DOI] [PubMed] [Google Scholar]
- 29.Ikebe, T., S. Iyoda, and K. Kutsukake. 1999. Promoter analysis of the class 2 flagellar operons of Salmonella. Genes Genet. Syst. 74:179-183. [DOI] [PubMed] [Google Scholar]
- 30.Iyoda, S., and K. Kutsukake. 1995. Molecular dissection of the flagellum-specific anti-sigma factor, FlgM, of Salmonella typhimurium. Mol. Gen. Genet. 249:417-424. [DOI] [PubMed] [Google Scholar]
- 31.Iyoda, S., T. Kamidoi, K. Hirose, K. Kutsukake, and H. Watanabe. 2001. A flagellar gene fliZ regulates the expression of invasion genes and virulence phenotype in Salmonella enterica serovar Typhimurium. Microb. Pathog. 30:81-90. [DOI] [PubMed] [Google Scholar]
- 32.Iyoda, S., and H. Watanabe. 2004. Positive effects of multiple pch genes on expression of the locus of enterocyte effacement genes and adherence of enterohaemorrhagic Escherichia coli O157:H7 to Hep-2 cells. Microbiology 150:2357-2371. [DOI] [PubMed] [Google Scholar]
- 33.Iyoda, S., and H. Watanabe. 2005. ClpXP protease controls expression of the type III protein secretion system through regulation of RpoS and GrlR levels in enterohemorrhagic Escherichia coli. J. Bacteriol. 187:4086-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarvis, K. G., J. A. Girón, A. E. Jerse, T. K. McDaniel, M. S. Donnenberg, and J. B. Kaper. 1995. Enteropathogenic Escherichia coli contains a specialized secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 92:7996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jerse, A. E., J. Yu., B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jerse, A. E., and J. B. Kaper. 1991. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect. Immun. 59:4302-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 38.Knutton, S., T. Baldwin, P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 28:1290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komeda, Y. 1986. Transcriptional control of flagellar genes in Escherichia coli K-12. J. Bacteriol. 168:1315-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galan, and S. I. Aizawa. 1998. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280:602-605. [DOI] [PubMed] [Google Scholar]
- 41.Kutsukake, K., T. Iino, Y. Komeda, and S. Yamaguchi. 1980. Functional homology of fla genes between Salmonella typhimurium and Escherichia coli. Mol. Gen. Genet. 178:59-67. [DOI] [PubMed] [Google Scholar]
- 42.Kutsukake, K., Y. Ohya, and T. Iino. 1990. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J. Bacteriol. 172:741-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kutsukake, K., and T. Iino. 1994. Role of FliA-FlgM regulatory system on the transcriptional control of the flagellar regulon and flagella formation in Salmonella typhimurium. J. Bacteriol. 176:3598-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kutsukake, K., S. Iyoda, K. Ohnishi, and T. Iino. 1994. Genetic and molecular analyses of the interaction between the flagellum-specific sigma and anti-sigma factors in Salmonella typhimurium. EMBO J. 13:4568-4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leatham, M. P., S. J. Stevenson, E. J. Gauger, K. A. Krogfelt, J. J. Lins, T. L. Haddock, S. M. Autieri, T. Conway, and P. S. Cohen. 2005. Mouse intestine selects nonmotile flhDC mutants of Escherichia coli MG1655 with increased colonizing ability and better utilization of carbon sources. Infect. Immun. 73:8039-8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lehnen, D., C. Blumer, T. Polen, B. Wackwitz, V. F. Wendisch, and G. Umden. 2002. LrhA as a new transcriptional key regulator of flagella, motility and chemotaxis genes in Escherichia coli. Mol. Microbiol. 45:521-532. [DOI] [PubMed] [Google Scholar]
- 47.Lio, C.-W. J., and W.-J. Syu. 2004. Identification of a negative regulator for the pathogenicity island of enterohemorrhagic Escherichia coli O157:H7. J. Biomed. Sci. 11:855-863. [DOI] [PubMed] [Google Scholar]
- 48.Liu, X., and P. Matsumura. 1994. The FlhD/FlhC complex, a transcriptional activator of the Escherichia coli flagellar class II operons. J. Bacteriol. 176:7345-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lucas, R. L., C. P. Lostroh, C. C. DiRusso, M. P. Spector, B. L. Wanner, and C. A. Lee. 2000. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:1872-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macnab, R. M. 1996. Flagella and motility, p. 123-146. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 51.Malo, M. S., and R. E. Loughlin. 1988. Promoter-detection vectors for Escherichia coli with multiple useful features. Gene 64:207-215. [DOI] [PubMed] [Google Scholar]
- 52.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDaniel, T. K., and J. B. Kaper. 1997. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol. Microbiol. 23:399-407. [DOI] [PubMed] [Google Scholar]
- 54.Mellies, J. L., S. J. Elliott, V. Sperandio, M. S. Donnenberg, and J. B. Kaper. 1999. The Per regulon of enteropathogenic Escherichia coli: identification of a regulatory cascade and a novel transcriptional activator, the locus of enterocyte effacement (LEE)-encoded regulator (Ler). Mol. Microbiol. 33:296-306. [DOI] [PubMed] [Google Scholar]
- 55.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohnishi, K., K. Kutsukake, H. Suzuki, and T. Iino. 1990. Gene fliA encodes an alternative sigma factor specific for flagellar operons in Salmonella typhimurium. Mol. Gen. Genet. 221:139-147. [DOI] [PubMed] [Google Scholar]
- 57.Ottemann, K. M., and J. F. Miller. 1997. Roles for motility in bacterial-host interactions. Mol. Microbiol. 24:1109-1117. [DOI] [PubMed] [Google Scholar]
- 58.Porter, M. E., P. Mitchell, A. Free, D. G. Smith, and D. L. Gally. 2005. The LEE1 promoters from both enteropathogenic and enterohemorrhagic Escherichia coli can be activated by PerC-like proteins from either organism. J. Bacteriol. 187:458-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prüß, B. M., X. Liu, H. Hendrickson, and P. Matsumura. 2001. FlhD/FlhC regulated promoters analyzed by gene array and lacZ gene fusions. FEMS Microbiol. Lett. 197:91-97. [DOI] [PubMed] [Google Scholar]
- 60.Sharma, V. K., and R. L. Zuerner. 2004. Role of hha and ler in transcriptional regulation of the esp operon of enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 186:7290-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shin, S., and C. Park. 1995. Modulation of flagellar expression in Escherichia coli by acetyl phosphate and the osmoregulator OmpR. J. Bacteriol. 177:4696-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin, S., M. P. Castanie-Cornet, J. W. Foster, J. A. Crawford, C. Brinkley, and J. B. Kaper. 2001. An activator of glutamate decarboxylase genes regulates the expression of enteropathogenic Escherichia coli virulence genes through control of the plasmid-encoded regulator Per. Mol. Microbiol. 41:1133-1150. [DOI] [PubMed] [Google Scholar]
- 63.Silverman, M., and M. Simon. 1974. Characterization of Escherichia coli flagellar mutants that are insensitive to catabolite repression. J. Bacteriol. 120:1196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soutourina, O., A. Kolb, E. Krin, C. Laurent-Winter, S. Rimsky, A. Danchin, and P. Bertin. 1999. Multiple control of flagellum biosynthesis in Escherichia coli: role of H-NS protein and the cyclic AMP-catabolite activator protein complex in transcription of the flhDC master operon. J. Bacteriol. 181:7500-7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sperandio, V., J. L. Mellies, W. Nguyen, S. Shin, and J. B. Kaper. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 96:15196-15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sperandio, V., C. C. Li, and J. B. Kaper. 2002. Quorum-sensing Escherichia coli regulator A: a regulator of the LysR family involved in the regulation of the locus of enterocyte effacement pathogenicity island in enterohemorrhagic E. coli. Infect. Immun. 70:3085-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sperandio, V., A. G. Torres, and J. B. Kaper. 2002. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol. Microbiol. 43:809-821. [DOI] [PubMed] [Google Scholar]
- 68.Sperandio, V., A. G. Torres, B. Jarvis, J. P. Nataro, and J. B. Kaper. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. USA 100:8951-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tatsuno, I., K. Nagano, K. Taguchi, L. Rong, H. Mori, and C. Sasakawa. 2003. Increased adherence to Caco-2 cells caused by disruption of the yhiE and yhiF genes in enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 71:2598-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tobe, T., H. Ando, H. Ishikawa, H. Abe, K. Tashiro, T. Hayashi, S. Kuhara, and N. Sugimoto. 2005. Dual regulatory pathways integrating the RcsC-RcsD-RcsB signalling system control enterohaemorrhagic Escherichia coli pathogenicity. Mol. Microbiol. 58:320-333. [DOI] [PubMed] [Google Scholar]
- 71.Tomoyasu, T., T. Ohkishi, Y. Ukyo, A. Tokumitsu, A. Takaya, M. Suzuki, K. Sekiya, H. Matsui, K. Kutsukake, and T. Yamamoto. 2002. The ClpXP ATP-dependent protease regulates flagellum synthesis in Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tomoyasu, T., A. Takaya, Y. Handa, K. Karata, and T. Yamamoto. 2005. ClpXP controls the expression of LEE genes in enterohaemorrhagic Escherichia coli. FEMS Microbiol. Lett. 253:59-66. [DOI] [PubMed] [Google Scholar]
- 73.Tomoyasu, T., A. Takaya, E. Isogai, and T. Yamamoto. 2003. Turnover of FlhD and FlhC, master regulator proteins for Salmonella flagellum biogenesis, by the ATP-dependent ClpXP protease. Mol. Microbiol. 48:443-452. [DOI] [PubMed] [Google Scholar]
- 74.Umanski, T., I. Rosenshine, and D. Friedberg. 2002. Thermoregulated expression of virulence genes in enteropathogenic Escherichia coli. Microbiology 148:2735-2744. [DOI] [PubMed] [Google Scholar]
- 75.Wei, B. L., A.-M. Brun-Zinkernagel, J. W. Simecka, B. M. Prüß, P. Babitzke, and T. Romeo. 2001. Positive regulation of motility and flhDC expression by the RNA-binding protein CsrA of Escherichia coli. Mol. Microbiol. 40:245-256. [DOI] [PubMed] [Google Scholar]
- 76.Yanagihara, S., S. Iyoda, K. Ohnishi, T. Iino, and K. Kutsukake. 1999. Structure and transcriptional control of the flagellar master operon of Salmonella typhimurium. Genes Genet. Syst. 74:105-111. [DOI] [PubMed] [Google Scholar]
- 77.Yona-Nadler, C., T. Umanski, S. Aizawa, D. Friedberg, and I. Rosenshine. 2003. Integration host factor (IHF) mediates repression of flagella in enteropathogenic and enterohaemorrhagic Escherichia coli. Microbiology 149:877-884. [DOI] [PubMed] [Google Scholar]
- 78.Zhang, L., R. R. Chaudhuri, C. Constantinidou, J. L. Hobman, M. D. Patel, A. C. Jones, D. Sarti, A. J. Roe, I. Vlisidou, R. K. Shaw, F. Falciani, M. P. Stevens, D. L. Gally, S. Knutton, G. Frankel, C. W. Penn, and M. J. Pallen. 2004. Regulators encoded in the Escherichia coli type III secretion system 2 gene cluster influence expression of genes within the locus for enterocyte effacement in enterohemorrhagic E. coli O157:H7. Infect. Immun. 72:7282-7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou, X., J. A. Giron, A. G. Torres, J. A. Crawford, E. Negrete, S. N. Vogel, and J. B. Kaper. 2003. Flagellin of enteropathogenic Escherichia coli stimulates interleukin-8 production in T84 cells. Infect. Immun. 71:2120-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]