Abstract
Anabaena variabilis fixes nitrogen under aerobic growth conditions in differentiated cells called heterocysts using either a Mo nitrogenase or a V nitrogenase. The nifH1 gene, which encodes the dinitrogenase reductase of the Mo nitrogenase that is expressed only in heterocysts, is cotranscribed with nifD1 and nifK1, which together encode the Mo dinitrogenase. These genes were expressed in the presence or absence of molybdate or vanadate. The vnfH gene, which encodes the dinitrogenase reductase of the V nitrogenase, was located about 23 kb from vnfDGK, which encodes the V dinitrogenase; however, like vnfDGK, vnfH was expressed only in the absence of molybdate, with or without vanadate. Like nifH1, the vnfH gene was expressed exclusively in heterocysts under either aerobic or anaerobic growth conditions and thus is under the control of developmental factors. The vnfH mutant was able to grow diazotrophically using the V nitrogenase, because NifH1, which was also made in cells starved for molybdate, could substitute for VnfH. Under oxic conditions, the nifH1 mutant grew in the absence of molybdate but not in its presence, using VnfH, while the nifH1 vnfH double mutant did not grow diazotrophically with or without molybdate or vanadate. A nifH1 mutant that expressed nifDK and vnfH but not vnfDGK was able to grow and fix nitrogen normally, indicating that VnfH could substitute for NifH in the Mo nitrogenase and that these dinitrogenase reductases are not involved in determining the metal specificity of the Mo nitrogenase or the V nitrogenase.
Anabaena variabilis ATCC 29413 is a filamentous heterocyst-forming cyanobacterium that fixes nitrogen in the absence of fixed nitrogen, such as ammonium or nitrate, using nitrogenases. Nitrogenases are oxygen labile; therefore, nitrogen fixation must be separated from oxygen-evolving photosynthesis either spatially or temporally (reviewed in references 9 and 10). Under aerobic conditions, nitrogen fixation is sequestered in morphologically and biochemically differentiated cells called heterocysts, which comprise 5 to 10% of the cells in the filament (11, 36, 37). Heterocysts lack oxygen-evolving photosystem II (21) and have extracellular layers of glycolipid and polysaccharide that are important in maintaining microoxic conditions for nitrogen fixation (19, 20).
In A. variabilis, three distinct nitrogenase complexes fix nitrogen. Two of these nitrogenases, encoded by the nif1 and nif2 gene clusters, contain a Mo-Fe cofactor (4, 17, 25, 29, 30), whereas the third, encoded by the vnf genes, has a V-Fe cofactor (26, 28). The nif1-encoded Mo nitrogenase is expressed under diazotrophic conditions only in heterocysts (7, 30). The nif2-encoded Mo nitrogenase functions in vegetative cells and heterocysts but only under strictly anoxic conditions (29, 30). The organization of the nif1 and nif2 clusters in A. variabilis is similar and includes nifBSUHDKENXW as well as three additional open reading frames that are conserved in both clusters (29). The nif2 cluster differs from the nif1 cluster in that it lacks the fdxN gene, and the nifE1 and nifN1 homologs in the nif2 cluster are fused into a single open reading frame, nifEN2 (29, 30).
Like the V nitrogenase of Azotobacter vinelandii, a nonphotosynthetic soil bacterium, the V nitrogenase of A. variabilis includes an α subunit encoded by vnfD, a β subunit encoded by vnfK, a δ subunit encoded by vnfG, and scaffolding proteins encoded by vnfE and vnfN (13, 26, 28, 35). However, in A. variabilis the vnfD and vnfG genes are fused into a single open reading frame. In contrast to the vnf gene clusters in A. vinelandii and Rhodopseudomonas palustris (15), there is no vnfH gene near the vnfDGKEN cluster. In A. variabilis, the genes for the V nitrogenase are transcribed under diazotrophic conditions in media lacking molybdate, with or without vanadate (26). Mutations in vnfDG or vnfEN of A. variabilis abolish V nitrogenase activity (26, 28).
The nifH-encoded dinitrogenase reductase of A. vinelandii is a multifunctional protein. It is responsible for the ATP-dependent reduction of dinitrogenase (encoded by nifD and nifK) and for the biosynthesis of the FeMo cofactor of dinitrogenase. In addition, it is required for the maturation of the dinitrogenase protein to its catalytically active form (reviewed in reference 23). Since the three nitrogenase systems in A. vinelandii, nif, vnf, and anf (encoding a Fe-Fe nitrogenase), each have their own dinitrogenase reductase, it would be reasonable to infer that this protein confers metal (Mo, V, or Fe) specificity. Such a hypothesis is difficult to test in vivo in A. vinelandii because the nif genes are expressed only in the presence of Mo, the vnf genes only in the absence of Mo, and the anf genes only in the absence of Mo and V (16). However, in A. vinelandii, NifH can support weakly the V-dependent growth in a vnfH mutant (14). We report here that in A. variabilis there is a vnfH gene located 21 kb from the other V nitrogenase genes and that VnfH functions well in vivo for the biosynthesis and enzymatic activity of the Mo nitrogenase. Similarly, NifH1 functions well for the biosynthesis and activity of the V nitrogenase.
MATERIALS AND METHODS
Strains and growth conditions.
Strains of A. variabilis FD, a derivative of A. variabilis 29413 that can grow at 40°C and support the growth of bacteriophages better than the parent strain (6), were maintained on agar-solidified Allen and Arnon (AA) medium (1) supplemented, when appropriate, with 5.0 mM NH4Cl and 10 mM N-Tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (TES), pH 7.2, 40 μg ml−1 neomycin sulfate, or 3 μg ml−1 each of spectinomycin and streptomycin. Strains were grown photoautotrophically in liquid cultures in an eightfold dilution of AA (AA/8) or AA/8 supplemented with 5.0 mM NH4Cl and 10 mM TES, pH 7.2, at 30°C with illumination at 50 to 80 μE m−2 s−1. Mo-free medium was prepared from stocks scrubbed free of contaminating Mo using activated charcoal (24). The microelement stock was prepared without molybdate but was not treated with activated charcoal. To remove traces of Mo, glassware was treated with 1% Count-Off (New England Nuclear) and 10 mM EDTA for 24 h and then thoroughly rinsed with deionized water purified through a Millipore water purification system. Cyanobacteria were subcultured in molybdate-free medium for at least 15 generations to deplete internal molybdate reserves and then, in some instances, supplemented with 1 μM sodium orthovanadate. Growth experiments were repeated at least three times, and representative graphs are shown. Anoxic cultures were first grown in air in AA/8 with 5.0 mM fructose, 5.0 mM NH4Cl, and 10 mM TES, pH 7.2, washed with AA/8, resuspended in AA/8 containing 5.0 mM fructose and 10 μM dichlorophenyldimethylurea (to inhibit oxygen evolution from photosystem II), and flushed with dinitrogen.
Construction of nifH1, vnfH, and nifH1 vnfH mutants and vnfH:lacZ transcriptional fusion.
The vnfH gene was identified in a clone from a λ EMBL3 library of A. variabilis by hybridization with a radiolabeled internal fragment of the nifH gene of Anabaena sp. PCC 7120. The 3.8-kb vnfH fragment of the λ EMBL3 clone was inserted into pUC118. The 4.1-kb fragment containing the wild-type allele of nifH1 was subcloned into pUC118 from a cosmid clone from a library of A. variabilis. nifH1 and vnfH mutants were constructed by insertion of antibiotic resistance cassettes at restriction sites in these genes (Table 1), followed by replacement of the wild-type gene in the chromosome by the mutant allele using conjugative nonreplicative plasmids as described previously (26, 27, 32). All mutants were segregated as described previously (26, 27, 32) and tested by PCR to verify that no wild-type copies of the gene remained. The vnfH:lacZ transcriptional fusion was constructed by inserting a promoterless lacZ gene into the EcoRV site of vnfH (Table 1).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Cyanobacteria | ||
| A. variabilis FD | Anabaena variabilis strain that grows well at 40°C | 6 |
| BP227-4 | A. variabilis strain FD; nifH1::Smr Spr at AgeI | This work |
| BP227-43 | Spontaneous mutant of BP227-4 capable of growth on AA/8 | This work |
| BP227-44 | Spontaneous mutant of BP227-4 capable of growth on AA/8; probable Mo transport mutant | This work |
| MV4-167 | A. variabilis strain FD; nifH1::Smr Spr at AgeI and vnfH::Nmr at EcoRV | This work |
| TT167 | A. variabilis strain FD; vnfH::Nmr at EcoRV | This work |
| BP272 | A. variabilis strain FD; vnfH::lacZ at EcoRV | This work |
| Plasmids | ||
| Cosmid 33D12 | Cosmid hybridizing to nifH1, ∼40-kb insert of A. variabilis genomic DNA | This work |
| λ EMBL3 | λ clone with A. variabilis vnfH gene | This work |
| pBP263 | 5-kb fragment of pPE20, containing lacZ, inserted at EcoRV site of vnfH in pTT150 | This work |
| pBP272 | 4.6-kb EcoRV fragment of pRL1075 ligated into the SmaI site of pBP263 | This work |
| pEL1 | 4.1-kb BamHI fragment of cosmid 33D12 ligated to BamH1 site of pUC118; Apr | This work |
| pMV2 | 1.9-kb XmaI fragment of pRL5801, containing a Smr Spr cassette, inserted at AgeI site in nifH1 in pEL1 | This work |
| pMV4 | 4.8-kb EcoRV fragment of pRL1075 ligated into the EcoRV site of pMV2 | This work |
| pPE20 | Source of lacZ for transcriptional fusions | 30 |
| pRL277 | Cloning vector for conjugation into A. variabilis; Spr Smr | C. P. Wolk |
| pRL648 | Kmr/Nmr cassette in a polylinker (C.K.3) | 8 |
| pRL1075 | Source of mobilization site, oriT, and sacB gene, which confers sucrose sensitivity; Cmr Emr | 3 |
| pRL5801 | Source of Smr Spr cassette; Apr Smr Spr | C. P. Wolk |
| pTT150 | 3.8-kb HincII fragment of the λ EMBL3 clone inserted into at the HincII site of pUC118; Apr | This work |
| pTT165 | 1.1-kb SmaI fragment of pRL648, containing a Nmr cassette, inserted at EcoRV site of vnfH in pTT150 | This work |
| pTT167 | 4.7-kb EcoRI fragment of pTT165, containing mutated vnfH, inserted into the EcoRI site of pRL277 | This work |
Transcript analysis.
RNA was extracted from cells by using a modification of the QIAGEN RNA bacterial isolation kit, as described previously (38). Northern blot analysis was performed with 20 μg of RNA as described previously (38) with PCR-generated probes for nifH1, vnfH, or rnpB (encoding RNase P RNA) (34). Primer sets: nifH1, nifH1-L (5′-CGGCATGACCTATTGGTAGC-3′) and nifH123-R (5′-GGTGARATGATGGCGATGTAYGC-3′); vnfH, nifH3-L (5′-AGCTTCCAGTGCTTGAGCTT-3′) and nifH123-R; and rnpB, RNaseP-L (5′-AGAGTAGGCGTTGGCGGTTGC-3′) and RNaseP-R (5′-ATTGCTTTACACGAGGGCGATTAT-3′). For reverse transcription (RT)-PCR, cultures were grown initially for ∼8 generations in AA/8 containing 5.0 mM NH4Cl and 10 mM TES, pH 7.2, with 1 μM vanadate, washed free of NH4Cl, and then grown for 24 h in 50 ml AA/8 with 1 μM vanadate to optical density at 720 nm of 0.15. RNA was extracted using TriReagent, as previously described (22). The RNA was further treated to remove DNA by the Turbo DNA-free procedure (Ambion). RT-PCR was done with 25 μl single-tube reaction mixtures that contained 1× RT-PCR buffer (20 mM Tris-HCl, pH 8.33, 50 mM KCl, 2.5 mM MgCl), 5 pmol primers, 50 to 100 ng RNA, 33 U SuperScript II (Invitrogen), and 1.0 U Taq polymerase (Invitrogen). RT-PCRs used the following primer sets: nifH1, nifH1-L and nifH123-R; nifD1, nifD1-L (5′-AAAACGCGAAAAGCACCTCAAC-3′) and nifD1-R (5′-ACCAAGACCAGTAACCGCAACCTA-3′); nifK1, nifK1-L (5′-TCAAACAGCCAGAATACACC-3′) and nifK1-R (5′-AACGCAACCTTGAGAACCTT-3′); vnfH, nifH3-L and nifH123-R; vnfD, vnfD1-L (5′-AACCCAATTTCTGCCCCTATCC-3′) and vnfD1-R (5′-TTCTTGCTGTGCGCTTTTGACTAC-3′); vnfE, vnfERT3-L (5′-TTTTGGGATGCGTGCTGTTATTTA-3′) and vnfERT3-R (5′-TTTCCGGTGAGTGATTGAGCAGAT-3′); vupA, wabcA-L (5′-AGCCAACGCTCAATCTCCTA-3′) and wabcA-R (5′-CTCTTACCCCCATCAGCAAA-3′); and rnpB, RNaseP-L and RNaseP-R. The RT-PCR thermocycler program used for rnpB, nifH1, nifD1, vnfD, and vnfE primers was 55°C for 30 min, 94°C for 2 min, 94°C for 30 s, 58°C for 30 s, and 72°C for 45 s; 40 cycles of steps 3 to 5; and then 72°C for 10 min. For nifK, vnfH, and vupA, step 1 was changed to 52°C for 30 min and step 4 to 52°C for 30 s.
The transcription start site of vnfH was determined by primer extension. RNA (25 μg), extracted from cells of the strain FD grown in Mo-free AA/8 containing 1 μM sodium vanadate, was hybridized to 10 pmol of primer nifH3promo3′ (5′-TCGGGATCCGTTCAGACTGTTAAGTTACA-3′) in hybridization buffer [40 mM piperazine N,N′-bis(2-ethanesulfonic acid), 1 mM EDTA, 0.4 M NaCl, and 80% formamide] in a total volume of 200 μl. The reaction mixture was heated to 85°C for 2 min, and then hybridization was carried out at 68°C for 3 h. The RNA-primer complexes were precipitated with ethanol and combined with 10 μCi of [α-32P]dCTP, 15 μM deoxynucleoside triphosphate-dCTP mix, and 200 U of Superscript II (Invitrogen) in SuperScript buffer containing 10 μM dithiothreitol to a total volume of 20 μl. The extension reaction was incubated for 10 min at 47°C, 1 mM deoxynucleoside triphosphates was added, and the reaction mixture was incubated for another 3 h at 47°C. Unincorporated [α-32P]dCTP was removed from the reaction mixture by a phenol:chloroform extraction, and the products were separated on a 5% Long Ranger gel along with a sequencing ladder obtained using primer nifH3promo3′ end labeled with [γ-32P]ATP using the fmol DNA Cycle Sequencing system (Promega).
Acetylene reduction assays.
Aliquots (1.0 ml) of cultures at an optical density at 720 nm of about 0.15 were added to 10-ml serum bottles. The bottles were sealed with gas-tight serum stoppers, injected with 1.0 ml acetylene gas, and placed in an illuminated 30°C shaking water bath for 30 min. Samples (1.0 ml) of gas were removed via a hypodermic needle and injected into a Shimadzu gas chromatograph equipped with a 6-foot Poropak N column. The column temperature was either 60°C (to detect both ethylene and ethane) or 75°C (to detect ethylene).
Immunoblots.
Cultures of strains FD and TT167 grown in AA/8 with 5.0 mM NH4Cl and 10 mM TES, pH 7.2, were washed free of NH4-TES and then divided into two cultures, AA/8 alone or AA/8 with 5.0 mM NH4Cl and 10 mM TES, pH 7.2. Cultures of strains FD and TT167 grown in Mo-free AA/8 with 5.0 mM NH4Cl and 10 mM TES, pH 7.2, were washed free of NH4-TES and then split into two cultures, Mo-free AA/8 or Mo-free AA/8 containing 1 μM sodium vanadate. Cells were grown for 24 to 48 h to induce heterocysts and nitrogenase, harvested by centrifugation, and heated in sodium dodecyl sulfate lysis buffer prior to electrophoresis (2). Coomassie protein assays (Pierce) were performed on samples that had been dialyzed against 10 mM Tris-HCl, pH 7.5. Proteins (25 μg/lane) were separated on sodium dodecyl sulfate-10% polyacrylamide gels and transferred to a Hybond P polyvinylidene difluoride membrane (Amersham Pharmacia Biotech, Inc.). The membrane was blocked in 1× TBST (136 mM NaCl, 2 mM KCl, 0.05% Tween 20, 25 mM Tris-HCl, pH 8), supplemented with 10% bovine serum albumin (BSA), at room temperature for 1 h. The membrane was then incubated with a 20,000-fold dilution of a 1:1 mixture of two anti-NifH antibodies in TBST plus 10% BSA for 1 h, followed by three 5-min rinses in TBST. One antibody (kindly provided by Anneliese Ernst) was made against the NifH1 protein of A. variabilis. The other (kindly provided by Paul Ludden) was a universal anti-NifH made against a mixture of purified NifH proteins from Azotobacter vinelandii, Clostridium pasteurianum, Rhodospirillum rubrum, and Klebsiella pneumoniae. Next, the membrane was incubated in a 40,000-fold dilution of alkaline phosphatase-conjugated anti-rabbit antibodies (Sigma) in TBST plus 10% BSA at room temperature for 1 h, followed by three 5-min rinses in TBST. The membrane was briefly rinsed in AP buffer (100 mM Tris-HCl, pH 9.5, 100 mM NaCl, 50 mM MgCl2). For chemiluminescence detection, the membrane was incubated for 5 min in 0.1 mM CDP-Star substrate in 1× CDP-Star assay buffer (New England Biolabs, Inc.) and imaged using the Kodak DS Image Station 440 CF.
In situ localization of β-galactosidase activity.
Cells were fixed in 0.01% glutaraldehyde at 25°C for 15 min and washed with water. Cell pellets were resuspended in 15 μl of 100 μM C12-fluorescein-β-d-galactoside (Molecular Probes) in 25% dimethyl sulfoxide. Cells were incubated in the dark at 37°C until fluorescence was microscopically visible (15 to 60 min). Filaments were washed, resuspended in one drop of water, and photographed with a fluorescein filter set (excitation, 450 to 490 nm; dichroic, 510 nm; barrier, 520 nm) on a Zeiss epifluorescence microscope with a 560-nm shortpass filter to block the red fluorescence of the biliproteins (30). Images were acquired using a Photometrics cooled charge-coupled device camera with ScanAnalytics IPLab software. The image acquisition (exposure) time for the fluorescent photograph was about 0.5 s. The image acquisition time for the light micrograph was 0.05 s.
RESULTS AND DISCUSSION
Identification of vnfH by transcript analysis.
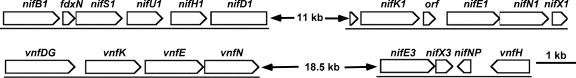
The vnfH gene (originally designated nifH3 but renamed once its function was determined) was not contiguous with vnfDGKEN in the genome of A. variabilis but was located about 21 kb downstream near a nifN-like gene, which appeared to be a truncated nifN pseudogene (indicated as nifNP) (Fig. 1). Adjacent to the nifN-like gene were complete nifX-like and nifE-like genes, in the opposite orientation to vnfH and the nifNP pseudogene. The function, if any, of these nif-like genes is unknown; however, a mutant in the nifE-like gene had no observable phenotype (Thiel, unpublished).
FIG. 1.
nifH1 and vnfH genes in the genome of A. variabilis. These gene clusters of A. variabilis were identified in the complete genome sequence (http://genome.jgi-psf.org/finished_microbes/anava/anava.home.html) using Artemis (18). The 11-kb region (which interrupts nifD1) and the 18.5-kb region of the genome that are not shown in the diagram are not drawn to scale.
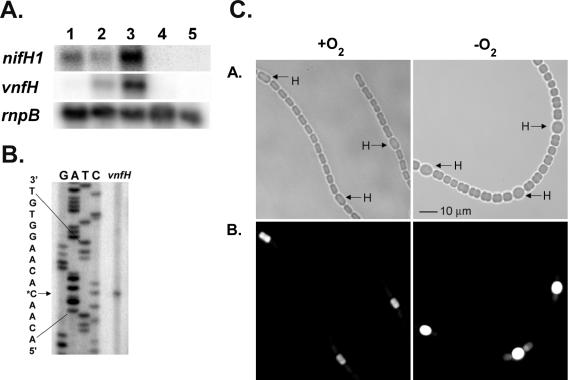
RNA extracts from cells grown under various conditions were hybridized with internal nifH1 and vnfH probes to determine the conditions under which transcripts were expressed (Fig. 2A). The nifH1 transcript was made only under conditions in which cells lacked a source of fixed nitrogen; however, molybdate was not required for transcription of nifH1. In contrast, in Azotobacter vinelandii, molybdate is required for transcription of nifH (16). The RNA from cells grown anaerobically did not contain the nifH1 transcript because RNA was extracted at 6 h after fixed nitrogen was removed from the culture, long before heterocysts developed; thus, transcription of nifH1 had not begun (7, 30). The vnfH transcript was observed only in RNA extracted from cells grown without a fixed nitrogen source and without molybdate, a pattern of expression that it shares with vnfDGK (26). Like nifH1, vnfH was not transcribed in vegetative cells grown under anaerobic conditions; thus, its expression depended on heterocyst differentiation.
FIG. 2.
Expression of nifH genes. A. Northern analysis of total RNA isolated from strain FD grown aerobically in the presence of molybdate (lane 1), grown aerobically in the presence of vanadate (lane 2), grown aerobically in the absence of molybdate and vanadate (lane 3), grown anaerobically in the presence of molybdate for 6 h (lane 4), or grown aerobically in the presence of NH4Cl and molybdate (lane 5). Northern blots were hybridized with internal fragments from nifH1 or vnfH. The major 1.1-kb transcript is shown for each blot. Expression of rnpB (34) was used as a control to show approximately equal loading of RNA. B. Primer extension analysis of vnfH used RNA isolated from cultures of FD grown with vanadate in the absence of molybdate. A single primer extension product corresponded to a C in the sequence of the strand complementary to the message (indicated by the asterisk). C. In situ localization of expression of lacZ under the control of the vnfH promoter. Strain BP272 (vnfH::lacZ fusion) was grown with fructose, in the absence of molybdate, with vanadate, and with ammonium chloride to repress heterocysts. Cells were washed free of ammonium chloride and then grown for 24 h under oxic (+O2) or anoxic (−O2) conditions and incubated with C12-fluorescein-β-d-galactoside as described in Materials and Methods. A. Light micrograph. B. Fluorescein fluorescence. H = heterocysts. Bar = 10 microns.
Similarly, BP272, containing a transcriptional fusion of vnfH to a promoterless lacZ gene, resulted in high levels of β-galactosidase in cells grown in the absence of Mo and V and in cells grown with V in the absence of Mo, but essentially no enzyme activity was found in cells grown in the presence of Mo or with ammonia (data not shown). In situ localization of expression of β-galactosidase in this strain under various growth conditions demonstrated that vnfH is expressed exclusively in heterocysts under either aerobic or anaerobic growth conditions, as is nifH1 in this strain. At 6 h after ammonium withdrawal of the vnfH mutant strain under anaerobic conditions, there was no β-galactosidase activity (not shown), and at 24 h after induction, all β-galactosidase activity was confined to heterocysts, as it was in cells induced under aerobic growth conditions (Fig. 2C). These results indicate that the V-nitrogenase genes are under developmental control and require the differentiation of heterocysts for expression.
The transcriptional start site of vnfH was identified 120 bp from the translational start site (Fig. 2B). Alignment of the putative promoter regions of the nifH1 and vnfH genes with nifH1 of Anabaena sp. PCC 7120 and with the region upstream of the nifH1 gene of Nostoc punctiforme revealed near identity between the upstream regions of nifH1 in the two strains of Anabaena but limited identity between those two sequences and the upstream regions of the other two genes, thus providing few clues concerning sites that might be essential for regulation. Notably, at the position where one would expect to find an NtcA-binding site, all four genes showed very poor identity with even the minimal NtcA-binding site GTN10AC (12, 33).
Characterization of nifH1, vnfH, and nifH1 vnfH mutants.
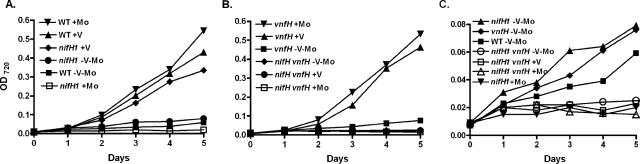
Single and double mutants of nifH1 and vnfH were constructed by inserting antibiotic resistance cassettes within each gene. The nifH1 mutant, BP227-4, could not grow using N2 in a medium containing molybdate but grew well in molybdate-free medium containing vanadate using the V nitrogenase (Fig. 3A). In contrast, the vnfH mutant, TT167, grew using N2 in medium with vanadate or with molybdate (Fig. 3B). The absence of the VnfH protein in the mutant TT167 was verified by immunoblot analysis (Fig. 4). Wild-type FD and TT167 both produced NifH1 under diazotrophic conditions with or without molybdate. Strain FD expressed VnfH in the absence of molybdate, whereas strain TT167 did not, as expected from the mutation. The double nifH1 vnfH mutant, MV4-167, lacking both the NifH1 and VnfH proteins, was unable to grow diazotrophically with or without molybdate or vanadate. Thus, NifH1 could substitute for VnfH, presumably both in the synthesis of FeV-cofactor and for the reduction of the V dinitrogenase. With A. vinelandii, a vnfH mutant grew slowly with vanadate in the absence of molybdate (14), probably because transcription of nifH requires molybdate in that strain (16), which is not the case for A. variabilis.
FIG. 3.
Growth of nifH1 and vnfH mutants of A. variabilis. The parent strain, the nifH1 mutant, the vnfH mutant, and the double nifH1 vnfH mutant were grown in AA/8 with NH4Cl in the absence of molybdate and vanadate, washed with molybdate- and vanadate-free AA/8, and transferred on day 0 to AA/8 with or without molybdate or vanadate as indicated on the graph. A. Wild-type strain compared to the nifH1 mutant. B. The vnfH mutant compared to the double mutant. C. The region of graphs A and B from 0 to 0.0.08 was expanded to show the growth of slow-growing strains.
FIG. 4.
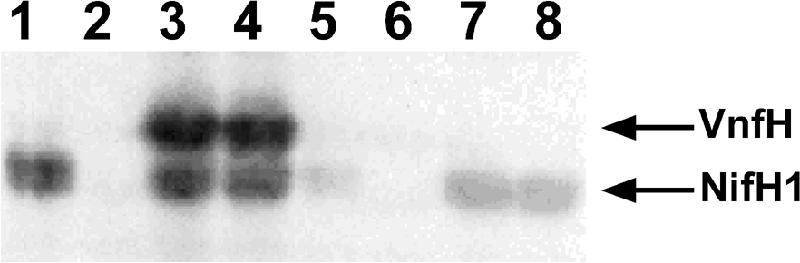
Expression of NifH1 and VnfH in strain TT167. Western blot analysis was done on total protein isolated from FD (wild type) (lanes 1 to 4) and TT167 (vnfH) cells (lanes 5 to 8) grown diazotrophically with molybdate (lanes 1 and 5), with ammonium (lanes 2 and 6), without molybdate and vanadate (lanes 3 and 7), or with vanadate (lanes 4 and 8). Bands corresponding to VnfH and NifH1 are marked with arrows.
While the wild-type strain, the nifH1 mutant, and the vnfH mutant showed very slow growth in the apparent absence of molybdate and vanadate, the nifH1 vnfH double mutant did not grow at all (Fig. 3C). Thus, trace amounts of molybdate and vanadate in a medium carefully prepared to be free of these metals were sufficient to support very slow growth of the wild-type strain and the nifH1 and vnfH mutants. A. variabilis has a high-affinity transport system for molybdate, with a Km value for transport that is less than 10−9 M (31) and a different system for transport of vanadate, encoded by vupABC, with a similar Km value (22). This suggests that the molybdate- and vanadate-free medium probably contained these metals at a concentration of about 10−9 M.
The nifH1 mutant grew slowly in molybdate- and vanadate-free medium, presumably using the V nitrogenase with the very low levels of vanadate in the medium. The nifH1 mutant, however, did not grow at all in a medium containing molybdate. The explanation for this is that the strain could not use the Mo nitrogenase because NifH1 was not made and it could not use the V nitrogenase because transcription of vnf genes was repressed by molybdate. In a medium lacking both molybdate and vanadate, the nifH1 mutant grew better than in a medium with molybdate, because in the former medium, the V nitrogenase was made, allowing the cells to grow poorly using trace amounts of vanadate. The absence of any evidence for a Fe-Fe cofactor, based on analysis of the whole genome sequence and the inability of a Mo-transport mutant to grow in the absence of vanadate (31), suggests that a Fe-Fe cofactor is not responsible for growth. However, it is possible that in the nifH1 mutant strain, VnfH substituted for NifH1, producing some functional Mo nitrogenase with the trace amounts of molybdate in the medium.
VnfH can replace NifH1 in Nif1 Mo nitrogenase.
The nifH1 mutant, BP227-4, in which the gene was interrupted by a Spr Smr gene under the control of the strong rbcL promoter, did not grow at all on a solid medium lacking a source of fixed nitrogen with molybdate, thus providing positive selection for mutations that restored growth under those conditions. With low frequency (about 1 colony per 109 cells), mutants appeared that were capable of growth and nitrogen fixation. Some of these spontaneous mutants, including BP227-44, produced ethane from acetylene (characteristic of the V nitrogenase), and growth was stimulated by the addition of vanadate, suggesting that these mutations were in modA or modBC, preventing the transport of molybdate, thus allowing full expression of the vnf genes even in the presence of molybdate in the medium. BP227-44 could not use nitrate, also characteristic of Mo transport mutants, which lack sufficient molybdate to make the molybdopterin cofactor of nitrate reductase. We confirmed by RT-PCR that these mutant strains expressed vnfH, vnfD, vnfE, and vupA (all these genes are repressed by molybdate), and they were not studied further (Fig. 5). However, another nitrogen-fixing spontaneous mutant, BP227-43, did not produce ethane, and growth was not stimulated by vanadate. This strain did not produce a transcript for vnfD, vnfE, vupA, or nifH1 but did make transcripts for vnfH, nifD1, and nifK1 (Fig. 5). The latter two transcripts were presumably made under the control of the strong rbcL promoter driving the Spr Smr gene inserted in nifH1. Thus, in BP227-43 the vnfH gene was expressed in the presence of molybdate (Fig. 5), allowing VnfH to function with the Nif1 Mo nitrogenase, which lacked only the NifH1 component. The mutant grew at about the same rate as the wild-type strain (generation time of about 18 to 19 h) under nitrogen-fixing conditions in a medium with molybdate, and the specific activity of nitrogenase as measured by acetylene reduction to ethylene was also very similar to that of the wild-type strain (300 to 400 nmol ethylene (mg chlorophyll a)−1 min−1). In contrast, the nifH1 mutant, BP227-4, did not grow or reduce acetylene under these conditions. The nature of the spontaneous mutation in BP227-43 is not yet known; however, the sequence of the promoter region of vnfH in this strain was unaltered, suggesting that the mutation might affect a protein factor required for the Mo-dependent regulation of vnfH but not vnfDGK or vnfEN. Thus, VnfH could substitute fully for NifH1 in the synthesis of the FeMo-cofactor, presumably in both the maturation of the Mo dinitrogenase and the reduction of the Mo nitrogenase. This has not been demonstrated in vivo for A. vinelandii but has been shown in an in vitro system, where VnfH replaced NifH for the synthesis of FeMo-cofactor and for the maturation of the Mo dinitrogenase (5). The results presented here for A. variabilis and the previous studies with A. vinelandii indicate that the dinitrogenase reductase proteins, VnfH and NifH, do not specify the incorporation of Mo or V into their respective nitrogenase enzyme cofactors.
FIG. 5.
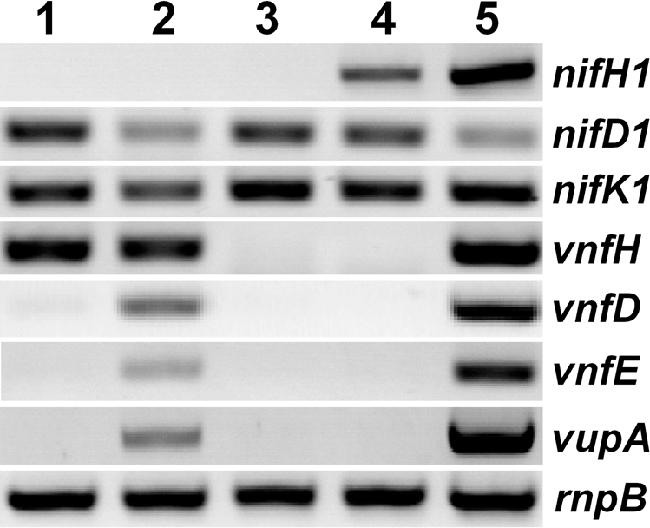
RT-PCR analysis of mutant and wild-type strains. RNA was extracted from cells grown under nitrogen-fixing conditions in AA/8 with 1 μm molybdate and 1 μm vanadate. Specific transcripts were detected with primers designed to amplify part of the genes shown: nif1 genes for the Mo nitrogenase, vnf genes for the V nitrogenase, a vup gene for the V transport system (which is repressed by Mo), and rnpB for a constitutive gene. Lane 1, BP227-43 (point mutation restoring growth of nifH1 mutant); lane 2, BP227-44 (point mutation restoring growth of nifH1 mutant; probable Mo transport mutant); lane 3, BP227-4 (nifH1 insertion mutation); lane 4, FD (wild-type); lane 5, chromosomal DNA from FD. (Not shown, negative controls verifying that no DNA contaminated the RNA extracts).
Acknowledgments
This work was supported by National Science Foundation grant MCB-0416663 and USDA grant 99-35100-7582.
REFERENCES
- 1.Allen, M. B., and D. I. Arnon. 1955. Studies on nitrogen-fixing blue-green algae. I. Growth and nitrogen fixation by Anabaena cylindrica. Lemm. Plant Physiol. 30:366-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1992. Current protocols in molecular biology. Greene Publishing and Wiley-Interscience, New York, N.Y.
- 3.Black, T. A., Y. Cai, and C. P. Wolk. 1993. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol. Microbiol. 9:77-84. [DOI] [PubMed] [Google Scholar]
- 4.Brusca, J. S., M. A. Hale, C. D. Carrasco, and J. W. Golden. 1989. Excision of an 11-kilobase-pair DNA element from within the nifD gene in Anabaena variabilis heterocysts. J. Bacteriol. 171:4138-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee, R., R. M. Allen, P. W. Ludden, and V. K. Shah. 1997. In vitro synthesis of the iron-molybdenum cofactor and maturation of the nif-encoded apodinitrogenase. Effect of substitution of VNFH for NIFH. J. Biol. Chem. 272:21604-21608. [DOI] [PubMed] [Google Scholar]
- 6.Currier, T. C., and C. P. Wolk. 1979. Characteristics of Anabaena variabilis influencing plaque formation by cyanophage N-1. J. Bacteriol. 139:88-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elhai, J., and C. P. Wolk. 1990. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 9:3379-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elhai, J., and C. P. Wolk. 1988. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene 68:119-138. [DOI] [PubMed] [Google Scholar]
- 9.Fay, P. 1992. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol. Rev. 56:340-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallon, J. R. 1992. Reconciling the incompatible: N2 fixation and O2. New Phytol. 122:571-609. [Google Scholar]
- 11.Golden, J. W., and H. S. Yoon. 2003. Heterocyst development in Anabaena. Curr. Opin. Microbiol. 6:557-563. [DOI] [PubMed] [Google Scholar]
- 12.Jiang, F., S. Wisen, M. Widersten, B. Bergman, and B. Mannervik. 2000. Examination of the transcription factor NtcA-binding motif by in vitro selection of DNA sequences from a random library. J. Mol. Biol. 301:783-793. [DOI] [PubMed] [Google Scholar]
- 13.Joerger, R. D., T. M. Loveless, R. N. Pau, L. A. Mitchenall, B. H. Simon, and P. E. Bishop. 1990. Nucleotide sequences and mutational analysis of the structural genes for nitrogenase 2 of Azotobacter vinelandii. J. Bacteriol. 172:3400-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joerger, R. D., E. D. Wolfinger, and P. E. Bishop. 1991. The gene encoding dinitrogenase reductase 2 is required for expression of the second alternative nitrogenase from Azotobacter vinelandii. J. Bacteriol. 173:4440-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larimer, F. W., P. Chain, L. Hauser, J. Lamerdin, S. Malfatti, L. Do, M. L. Land, D. A. Pelletier, J. T. Beatty, A. S. Lang, F. R. Tabita, J. L. Gibson, T. E. Hanson, C. Bobst, J. L. Torres, C. Peres, F. H. Harrison, J. Gibson, and C. S. Harwood. 2004. Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nat. Biotechnol. 22:55-61. [DOI] [PubMed] [Google Scholar]
- 16.Luque, F., and R. N. Pau. 1991. Transcriptional regulation by metals of structural genes for Azotobacter vinelandii nitrogenases. Mol. Gen. Genet. 227:481-487. [DOI] [PubMed] [Google Scholar]
- 17.Lyons, E. M., and T. Thiel. 1995. Characterization of nifB, nifS, and nifU genes in the cyanobacterium Anabaena variabilis: NifB is required for the vanadium-dependent nitrogenase. J. Bacteriol. 177:1570-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mural, R. J. 2000. ARTEMIS: a tool for displaying and annotating DNA sequence. Brief Bioinform. 1:199-200. [DOI] [PubMed] [Google Scholar]
- 19.Murry, M. A., A. J. Horne, and J. R. Benemann. 1984. Physiological studies of oxygen protection mechanisms in the heterocysts of Anabaena cylindrica. Appl. Environ. Microbiol. 47:449-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murry, M. A., and C. P. Wolk. 1989. Evidence that the barrier to the penetration of oxygen into heterocysts depends upon two layers of the cell envelope. Arch. Microbiol. 151:469-474. [Google Scholar]
- 21.Peterson, R. B., E. R. Shaw, E. Dolan, and B. Ke. 1981. A photochemically active heterocyst preparation from Anabaena variabilis. Photobiochem. Photobiophys. 2:79-84. [Google Scholar]
- 22.Pratte, B. S., and T. Thiel. 2006. High-affinity vanadate transport system in the cyanobacterium Anabaena variabilis ATCC 29413. J. Bacteriol. 188:464-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubio, L. M., and P. W. Ludden. 2005. Maturation of nitrogenase: a biochemical puzzle. J. Bacteriol. 187:405-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider, K., A. Muller, U. Schramm, and W. Klipp. 1991. Demonstration of a molybdenum- and vanadium-independent nitrogenase in a nifHDK-deletion mutant of Rhodobacter capsulatus. Eur. J. Biochem. 195:653-661. [DOI] [PubMed] [Google Scholar]
- 25.Schrautemeier, B., U. Neveling, and S. Schmitz. 1995. Distinct and differently regulated Mo-dependent nitrogen-fixing systems evolved for heterocysts and vegetative cells of Anabaena variabilis ATCC 29413: characterization of the fdxH1/2 gene regions as part of the nif1/2 gene clusters. Mol. Microbiol. 18:357-369. [DOI] [PubMed] [Google Scholar]
- 26.Thiel, T. 1993. Characterization of genes for an alternative nitrogenase in the cyanobacterium Anabaena variabilis. J. Bacteriol. 175:6276-6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiel, T. 1994. Genetic analysis of cyanobacteria, p. 581-611. In D. A. Bryant (ed.), The molecular biology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 28.Thiel, T. 1996. Isolation and characterization of the vnfEN genes of the cyanobacterium Anabaena variabilis. J. Bacteriol. 178:4493-4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiel, T., E. M. Lyons, and J. C. Erker. 1997. Characterization of genes for a second Mo-dependent nitrogenase in the cyanobacterium Anabaena variabilis. J. Bacteriol. 179:5222-5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thiel, T., E. M. Lyons, J. C. Erker, and A. Ernst. 1995. A second nitrogenase in vegetative cells of a heterocyst-forming cyanobacterium. Proc. Natl. Acad. Sci. USA 92:9358-9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiel, T., B. Pratte, and M. Zahalak. 2002. Transport of molybdate in the cyanobacterium Anabaena variabilis ATCC 29413. Arch. Microbiol. 179:50-56. [DOI] [PubMed] [Google Scholar]
- 32.Thiel, T., and C. P. Wolk. 1987. Conjugal transfer of plasmids to cyanobacteria. Methods Enzymol. 153:232-243. [DOI] [PubMed] [Google Scholar]
- 33.Vazquez-Bermudez, M. F., E. Flores, and A. Herrero. 2002. Analysis of binding sites for the nitrogen-control transcription factor NtcA in the promoters of Synechococcus nitrogen-regulated genes. Biochim. Biophys. Acta 1578:95-98. [DOI] [PubMed] [Google Scholar]
- 34.Vioque, A. 1997. The RNase P RNA from cyanobacteria: short tandemly repeated repetitive (STRR) sequences are present within the RNase P RNA gene in heterocyst-forming cyanobacteria. Nucleic Acids Res. 25:3471-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfinger, E. D., and P. E. Bishop. 1991. Nucleotide sequence and mutational analysis of the vnfENX region of Azotobacter vinelandii. J. Bacteriol. 173:7565-7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolk, C. P. 2000. Heterocyst formation in Anabaena, p. 83-104. In Y. V. Brun and L. J. Shimkets (ed.), Prokaryotic development. ASM Press, Washington, D.C.
- 37.Wolk, C. P., A. Ernst, and J. Elhai. 1994. Heterocyst metabolism and development, p. 769-823. In D. A. Bryant (ed.), Molecular biology of the cyanobacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 38.Zahalak, M., B. Pratte, K. J. Werth, and T. Thiel. 2004. Molybdate transport and its effect on nitrogen utilization in the cyanobacterium Anabaena variabilis ATCC 29413. Mol. Microbiol. 51:539-549. [DOI] [PubMed] [Google Scholar]