Abstract
Peptidyl-prolyl cis/trans isomerases (PPIases) play a pivotal role in catalyzing the correct folding of many prokaryotic and eukaryotic proteins that are implicated in a variety of biological functions, ranging from cell cycle regulation to bacterial infection. The nif accessory protein NifM, which is essential for the biogenesis of a functional NifH component of nitrogenase, is a PPIase. To understand the nature of the molecular signature that defines the NifM dependence of NifH, we screened a library of nifH mutants in the nitrogen-fixing bacterium Azotobacter vinelandii for mutants that acquired NifM independence. Here, we report that NifH can acquire NifM independence when the conserved Pro258 located in the C-terminal region of NifH, which wraps around the other subunit in the NifH dimer, is replaced by serine.
Peptidyl-prolyl cis/trans isomerases (PPIases) catalyze the cis/trans isomerization of the peptidyl-prolyl peptide bond in oligopeptides and proteins, a rate-limiting step in the process of protein folding that is essential for generating functional proteins (5-7). Some denatured proteins regain their native conformations within milliseconds to seconds, whereas others refold very slowly, with the time ranging from minutes to hours. The slow conformational changes arise from the well-known delocalization of electrons in the amide bond and are even more pronounced if additional steric constraints are imposed by the proline ring. PPIases enhance the rate of refolding of the slowly folding forms of denatured proteins by catalyzing the cis/trans isomerization of these peptidyl-prolyl bonds (5, 19). PPIases are regulators of a variety of biological functions, such as cell cycle progression (Pin1 and ESS1) (4, 10, 16), the inhibition of apoptosis (cyclophilins A) (17), carcinogenesis (Pin1) (2), association with hormone receptors (FKBP52/CyP40) (24), and bacterial infection (MIP) (14, 21), to name a few. The PPIase family consists of three subfamilies, the cyclophilins, the FK506 binding proteins, and the parvulins. Recently, based on homology analysis and activities, we proposed that the nif accessory protein NifM is also a PPIase and that it belongs to the family of parvulins (8, 18, 26).
Nitrogenase, the key enzyme that catalyzes biological nitrogen fixation, requires several accessory proteins for the maturation and assembly of its components, the MoFe-protein and NifH (3, 25). A major breakthrough in understanding the structural properties of nitrogenase came with the availability of the crystallographic structures of its component proteins (9, 22, 23, 27). The cloning and sequencing of most of the genes involved in the synthesis and assembly of nitrogenase, in turn, has given us an elegant picture of its genetic complexity (12). NifH, the obligate electron donor to the MoFe protein, has multiple roles in the catalytic process of biological nitrogen fixation, as well as in the maturation and assembly of nitrogenase (1, 3, 11, 20, 31). These include involvement in the initial biosynthesis of FeMo cofactor and in the insertion of preformed FeMo cofactor into an inactive FeMo cofactor-deficient MoFe protein. The synthesis of a functional NifH is dependent on the availability of a nif accessory protein, NifM (11). An examination of the consensus peptide sequence of NifH derived by comparing 60 different sequences shows the existence of seven fully conserved proline residues (Fig. 1). Therefore, it is reasonable to assume that the stability and activity of NifH largely depend upon the appropriate conformation of the peptidyl-prolyl bonds present in this protein.
FIG. 1.
Consensus sequence of NifH derived from comparison of 60 different NifH proteins showing conservation of seven proline residues. The conserved prolines are shown in boldface. Uppercase letters indicate residues that are invariant. Proline 258 is marked by an asterisk.
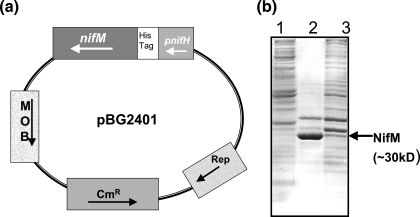
The nifM Azotobacter vinelandii strain, designated BG98, generated by inserting a kanamycin resistance (kan) gene into nifM, was used for this purpose. This strain was constructed using the same strategy that we had used for generating the nifM A. vinelandii strain that carried a lacZ-kan gene cassette (15). It showed a Nif− phenotype, since in the absence of functional NifM, NifH is nonfunctional, and it could not grow on Burke's nitrogen-free medium (BN−). To obtain enriched A. vinelandii NifM, we generated a His-tagged NifM translational fusion under the transcriptional control of the nifH promoter in the broad-host-range plasmid pBG2401 (Fig. 2a). From this construct, NifM was expressed as a His-tagged fusion protein with the His tag at the N terminus. The nifM A. vinelandii BG98 was transformed with pBG2401, and the transformants were selected on BN− Kan agar plates. These transformants could grow on BN− Kan agar plates, suggesting that the His-tagged NifM (from pBG2401) was functional and could confer a Nif+ phenotype on A. vinelandii BG98. Overexpression of the NifM protein was achieved by incubating the cells for 5 hours in BN− medium and activating the strong nifH promoter. The overexpressed NifM from A. vinelandii BG98 was partially purified using nickel columns (Fig. 2b). The PPIase activity of this NifM was measured by using the standard protease-coupled assay (5, 19, 25). The chromogenic substrate used was succinyl-Ala-Phe-Pro-Phe-4-nitroanilide. The substrate was dissolved in 460 mM LiCl in trifluoroethanol as described previously (19) to increase the concentration of the cis isomer. The assay was performed at 10°C as described previously (5, 19, 25) using different amounts of enriched NifM and substrate for a range of time periods. Addition of NifM to the oligopeptide substrate enhanced the conversion of the cis isomers to trans isomers. The appearance of nitroanilide (resulting from the cleavage of the trans isomer of succinyl-Ala-Phe-Pro-Phe-4-nitroanilide by chymotrypsin) was measured by monitoring the absorbance at 390 nm spectrophotometrically. The control sample contained crude extracts of A. vinelandii BG98, which is devoid of functional NifM. It was found that A. vinelandii NifM demonstrated PPIase activity with a specificity constant of 1.09 × 107/M/s. This value is comparable to the reported specificity constant of the Escherichia coli PPIase, PPiC (parvulin; EC 5.2.1.8), which is 1.69 × 107/M/s when succinyl-Ala-Leu-Pro-Phe-4-nitroanilide is used as a substrate (19). These results suggested that the extent of PPIase activity that NifM exhibited was comparable to that of E. coli PpiC, which shares homology with NifM.
FIG. 2.
Plasmid for overexpression of NifM and sodium dodecyl sulfate (SDS)-polyacrylamide gel showing partially purified NifM. (a) Structure of the plasmid pBG2401 that carries a His-tagged NifM translational fusion. This plasmid is a derivative of the broad-host-range plasmid pBHR1 (MoBiTec, Germany). The Kanr gene of pBHR1was replaced by a pnifH-His tag-nifM fusion gene in pBG2401. (b) Coomassie blue-stained SDS-polyacrylamide gel showing crude extracts of A. vinelandii BG98 (lane 1), A. vinelandii BG98 carrying pBG2401 (lane 3), and partially purified NifM after purification of His-tagged NifM by nickel column (lane 2).
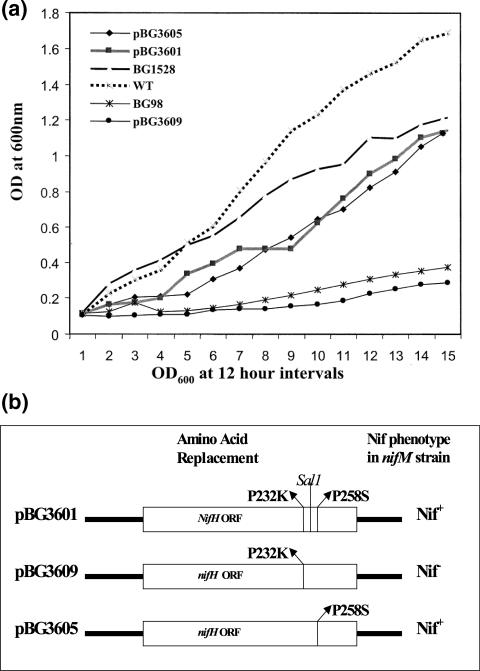
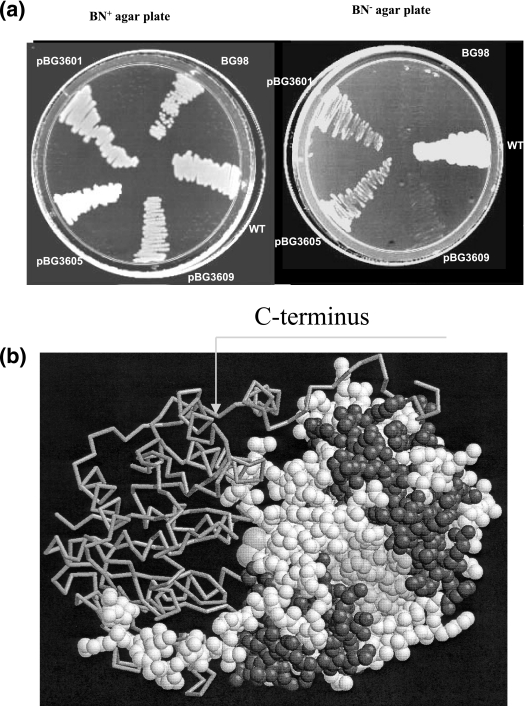
Since NifM is a PPIase and it interacts with NifH, the possible substrate for NifM can be any of the conserved proline residues present in NifH. Therefore, it is conceivable that NifM-independent nifH genetic mutants can be isolated. We utilized a DNA-shuffling technique (13, 29) to generate a large number of nifH mutants and analyzed whether any of the mutants attained NifM independence. This library of nifH mutants was initially screened for the Nif+ phenotype by testing their abilities to grow on agar plates with BN− (30). Then, the abilities of the NifH proteins encoded by these mutants to function independently of NifM were tested. To do this, the nifH genes of these mutants were PCR amplified using the specific nifH primers, and the resulting PCR products were individually ligated into pCR2.1TOPO vector (Invitrogen Corp., Carlsbad, CA). The cloned mutant nifH genes were subjected to nucleotide sequence analysis. Details of the mutations in these nifH mutants are described elsewhere (unpublished data). The plasmids containing mutant nifH genes with amino acid changes were then transformed into the nifM A. vinelandii BG98. The transformants were tested for the ability to grow on BN− Kan agar plates. Since the pCR2.1TOPO vector has a pUC ori, it cannot replicate in A. vinelandii. However, if the mutant nifH could confer a Nif+ phenotype in the absence of NifM, it would be rescued onto the chromosome via homologous recombination. Therefore, the nifM A. vinelandii BG98 transformants that could grow on BN− Kan plates carry a mutant nifH that is capable of participating in nitrogen fixation without the help of NifM. We found that one of the nifH mutants had two amino acid changes, P232K (CCG→AAG) and P258S (CCG→TCG), and was able to impart to the nifM A. vinelandii BG98 strain the ability to grow on BN− Kan plates. This nifH mutant was present in the original clone, designated pBG1528. The nifM A. vinelandii BG98 that rescued this nifH mutant onto the chromosome was designated A. vinelandii BG1528. Comparison of the growth of this strain in BN− liquid medium to that of the wild-type A. vinelandii is shown in Fig. 3a. Since both amino acid replacements in this mutant were conserved proline residues and the NifH encoded by this mutant could function independently of NifM, we hypothesized that these proline residues might serve as the substrates for the PPIase activity of NifM. We analyzed which of these replacements was absolutely essential for achieving NifM independence of NifH. One of the DNA-shuffling mutants carried only the P232K amino acid replacement. To generate a nifH mutant that carried only the P258S amino acid replacement, we utilized the conveniently located site for the restriction enzyme SalI in the nifH coding sequence (Fig. 3b). We separated the DNA fragments carrying the mutations by digesting them with SalI and joining the wild-type nifH fragment with the mutant nifH fragment carrying a CCG→TCG mutation at position C775. Figure 3b shows the resulting nifH plasmids and the Nif phenotypes they imparted to the nifM A. vinelandii BG98. These plasmids are derivatives of pBHR1 (a broad-host-range vector from MobBiTec, Germany) and replicate in A. vinelandii. nifM A. vinelandii BG98 was transformed using these plasmids, and the Nif phenotypes of the resulting transformants were analyzed by observing their growth on BN− plates (Fig. 4a) and also in BN− liquid medium (Fig. 3a). It was found that the P258S amino acid change (pBG3605) imparted a Nif+ phenotype to the nifM A. vinelandii BG98. The P232K amino acid change (pBG3609) was unable to do so. This result implied that the region of NifH spanning P258 serves as the substrate for the NifM protein and that the region spanning P232 is not involved in this function. According to the structure of NifH (Protein Data Bank code 2NIP) (9, 28), P258 is located in the C-terminal region of NifH, which wraps around the other subunit of the NifH dimer (Fig. 4b). It is conceivable that the trans conformation of the peptidyl-prolyl bond of P258 contributes significantly to the structure of this region and that NifM-mediated modification is essential to attain this structure.
FIG. 3.
The P258S mutation is sufficient to confer NifM independence on NifH. (a) Growth curves of the nifM A. vinelandii BG98 and its derivatives carrying different plasmids harboring different nifH mutants in BN− medium compared with the growth curve of A. vinelandii BG1528 and wild-type A. vinelandii (WT). BG1528 is the A. vinelandii strain that was transformed with pBG1528, the pCR2.1TOPO vector derivative carrying the nifH mutant with two amino acid changes, P232K and P258S. Since this plasmid is not a broad-host-range plasmid, it cannot replicate in A. vinelandii. Therefore A. vinelandii BG1528 was generated via homologous recombination and subsequent rescuing of the mutant nifH gene that can confer NifM independence on the chromosome. OD600, optical density at 600 nm. (b) Amino acid changes in the nifH mutants present in plasmids pBG3601, -3605, and -3609. The SalI site (at position 754) present between the mutations (CCG→AAG at positions C697 and C698, resulting in a P232K amino acid change, and CCG→TCG at position C775, resulting in a P258S amino acid change) is marked. Since these plasmids are broad-host-range plasmids, they replicated in nifM A. vinelandii BG98. The growth curves show the Nif phenotypes conferred by these plasmids on nifM A. vinelandii BG98, and they are marked in panel b.
FIG. 4.
(a) Comparison of the growth of wild-type A. vinelandii (WT) with that of nifM A. vinelandii BG98 and its derivatives carrying pBG3605 (p258S mutant), pBG3601 (P232K P258S double mutant), or pBG3609 (P232K mutation alone) on a BN− agar plate. All strains were plated on a BN+ agar plate and a BN−agar plate and incubated at 30°C for 72 h. On the BN− agar plate, the growth of wild-type A. vinelandii was comparable to the growth of nifM A. vinelandii BG98 carrying the plasmid pBG3601 or pBG3605. Neither nifM A. vinelandii BG98 nor its derivative carrying pBG3609 could grow on BN− agar plates. (b) Ribbon diagram of the Av2 dimer (Protein Data Bank code 2NIP) (27). The location of P258 (marked by the arrow) is in the C-terminal region of the NifH subunit, which wraps around the other subunit of the NifH dimer. It is conceivable that the structure of this region, caused by the specific conformation of the rigid peptidyl-prolyl bond (presumably converted to trans conformation by NifM) of P258, plays a significant role in the folding of NifH to generate a functional molecule.
Our results suggest that the NifM-mediated conversion to the trans conformation of the peptidyl-prolyl bond of Pro258 is crucial for the proper folding, maturation, and assembly of the functional NifH dimer. We also show that altering this molecular signature of NifM dependence by replacing P258 with serine is sufficient to generate a NifM-independent NifH. Thus, our results delineate the specific site on NifH where NifM exerts its effect as a molecular chaperon of NifH and demonstrate an alternative, chaperon-independent mechanism by which the functional conformation can be achieved. More importantly, the P258S mutant nifH is the first example of a PPIase-independent functional mutant. Our approach has allowed us to differentiate a proline-peptide bond (P258) in NifH that serves as a substrate for the PPIase activity of NifM from one that does not (P232). The PPIases are ubiquitous and influence several fundamental biological functions, including cell division and oncogenesis. Therefore, a method to identify their site of action in a given substrate, similar to the approach used here, can be utilized to delineate PPIase-mediated influences.
Acknowledgments
This research was supported by the NSF (N.G. and L.P.).
We thank members of the Gavini-Pulakat laboratory for helpful discussions and technical help.
Footnotes
Dedicated to the memory of the late Barbara K. Burgess, of the University of California at Irvine, who strove to improve the lives of foreign and female students.
REFERENCES
- 1.Allen, R. M., H. M. R. Chatterjee, P. W. Ludden, G. P. Roberts, and V. K. Shah. 1993. Dinitrogenase reductase- and MgATP-dependent maturation of apodinitrogenase from Azotobacter vinelandii. J. Biol. Chem. 268:23670-23674. [PubMed] [Google Scholar]
- 2.Bao, L., A. Kimzey, G. Sauter, J. M. Sowadski, K. P. Lu, and D. G. Wang. 2004. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am. J. Pathol. 164:1727-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgess, B. K., and D. J. Lowe. 1996. Mechanism of molybdenum nitrogenase. Chem. Rev. 96:2983-3011. [DOI] [PubMed] [Google Scholar]
- 4.Crenshaw, D. G., J. Yang, A. R. Means, and S. Kornbluth. 1998. The mitotic peptidyl-prolyl isomerase, Pin1, interacts with Cdc25 and Plx1. EMBO J. 17:1315-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer, G. 1994. Peptidyl-prolyl cis/trans isomerases and their effectors. Angew. Chem. Int. 33:1415-1436. [Google Scholar]
- 6.Fischer, G., T. Tradler, and T. Zarnt. 1998. The mode of action of peptidyl prolyl cis/trans isomerases in vivo: binding vs. catalysis. FEBS Lett. 426:17-20. [DOI] [PubMed] [Google Scholar]
- 7.Galat, A. 2003. Peptidylprolyl cis/trans isomerases (immunophilins): biological diversity targets—functions. Curr. Top. Med. Chem. 3:1315-1347. [DOI] [PubMed] [Google Scholar]
- 8.Gavini, N., and L. Pulakat. 2002. Role of NifM in maturation of the Fe-protein of nitrogenase, p. 228-232. In T. M. Finan, M. R. O'Brian, D. B. Layzell, J. K. Vessey, and W. Newton (ed.), Nitrogen fixation—global perspectives. CABI Publishing, New York, N.Y.
- 9.Georgiadis, M. M., H. Komiya, P. Chakrabarti, D. Woo, J. J. Kornuc, and D. C. Rees. 1992. Crystallographic structure of the nitrogenase iron protein from Azotobacter vinelandii. Science 257:1653-1659. [DOI] [PubMed] [Google Scholar]
- 10.Hanes, S. D., P. R. Shank, and K. A. Bostian. 1989. Sequence and mutational analysis of Ess1, a gene essential for growth in Saccharomyces cerevisiae. Yeast 5:55-72. [DOI] [PubMed] [Google Scholar]
- 11.Howard, K. S., P. A. McLean, F. B. Hansen, P. V. Lemley, K. S. Koblan, and W. H. Ormejohnson. 1986. Klebsiella pneumoniae nifM gene product is required for stabilization and activation of nitrogenase iron protein in Escherichia coli. J. Biol. Chem. 261:772-778. [PubMed] [Google Scholar]
- 12.Jacobson, M. R., K. E. Brigle, L. T. Bennett, R. A. Setterquist, M. S. Wilson, V. L. Cash, J. Beynon, W. E. Newton, and D. R. Dean. 1989. Physical and genetic map of the major nif gene cluster from Azotobacter vinelandii. J. Bacteriol. 171:1017-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kikuchi, M. H. S. 2002. DNA shuffling and family shuffling for in vitro gene evolution. Methods Mol. Biol. 182:243-257. [DOI] [PubMed] [Google Scholar]
- 14.Kohler, R., J. Fanghanel, B. Konig, E. Luneberg, M. Frosch, J. U. Rahfeld, R. Hilgenfeld, G. Fischer, J. Hacker, and M. Steinert. 2003. Biochemical and functional analyses of the Mip protein: influence of the N-terminal half and of peptidylprolyl isomerase activity on the virulence of Legionella pneumophila. Infect. Immun. 71:4389-4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei, S., L. Pulakat, and N. Gavini. 1999. Regulated Expression of the nifM of Azotobacter vinelandii in response to molybdenum and vanadium supplements in Burk's nitrogen free growth medium. Biochem. Biophys. Res. Commun. 264:186-190. [DOI] [PubMed] [Google Scholar]
- 16.Lu, K. P., S. D. Hanes, and T. Hunter. 1996. A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature 380:544-547. [DOI] [PubMed] [Google Scholar]
- 17.Nathan, M., I. Friehs, Y. H. Choi, D. B. Cowan, H. Cao-Danh, F. X. McGowan, and P. J. del Nido. 2005. Cyclosporin A but not FK-506 protects against dopamine-induced apoptosis in the stunned heart. Ann. Thoracic Surg. 79:1620-1626. [DOI] [PubMed] [Google Scholar]
- 18.Rahfeld, J. U., K. P. Rucknagel, B. Schelbert, B. Ludwig, J. Hacker, K. Mann, and G. Fischer. 1994. Confirmation of the existence of a third family among peptidyl-prolyl cis/trans isomerases—amino-acid sequence and recombinant production of parvulin. FEBS Lett. 352:180-184. [DOI] [PubMed] [Google Scholar]
- 19.Rahfeld, J. U., A. Schierhorn, K. Mann, and G. Fischer. 1994. A novel peptidyl-prolyl cis/trans isomerase from Escherichia coli. FEBS Lett. 343:65-69. [DOI] [PubMed] [Google Scholar]
- 20.Rangaraj, P., V. K. Shah, and P. W. Ludden. 1997. Apo-NifH functions in iron-molybdenum cofactor synthesis and apodinitrogenase maturation. Proc. Natl. Acad. Sci. USA 94:11250-11255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratcliff, R. M., M. A. Slavin, N. Sangster, R. M. Doyle, J. F. Seymour, and J. A. Lanser. 2003. Legionella pneumophila mip gene sequencing to investigate a cluster of pneumonia cases. Pathology 35:65-69. [PubMed] [Google Scholar]
- 22.Rees, D. C. 2002. Great metalloclusters in enzymology. Annu. Rev. Biochem. 71:221-246. [DOI] [PubMed] [Google Scholar]
- 23.Rees, D. C., F. A. Tezcan, C. A. Haynes, M. Y. Walton, S. Andrade, O. Einsle, and J. B. Howard. 2005. Structural basis of biological nitrogen fixation. Phil. Trans. R. Soc. A 363:971-984. [DOI] [PubMed] [Google Scholar]
- 24.Riggs, D. L., P. J. Roberts, S. C. Chirillo, J. Cheung-Flynn, V. Prapapanich, T. Ratajczak, R. Gaber, D. Picard, and D. F. Smith. 2003. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 22:1158-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubio, L. M., and P. W. Ludden. 2005. Maturation of nitrogenase: a biochemical puzzle. J. Bacteriol. 187:405-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudd, K. E., H. J. Sofia, E. V. Koonin, G. Plunkett, S. Lazar, and P. E. Rouviere. 1995. A new family of peptidyl-prolyl isomerases. Trends Biochem. Sci. 20:12-14. [DOI] [PubMed] [Google Scholar]
- 27.Schindelin, N., C. Kisker, J. L. Sehlessman, J. B. Howard, and D. C. Rees. 1997. Structure of ADP center dot AIF(4)(−)-stabilized nitrogenase complex and its implications for signal transduction. Nature 387:370-376. [DOI] [PubMed] [Google Scholar]
- 28.Schlessman, J. L., D. Woo, L. Joshua-Tor, J. B. Howard, and D. C. Rees. 1998. Conformational variability in structures of the nitrogenase iron proteins from Azotobacter vinelandii and Clostridium pasteurianum. J. Mol. Biol. 280:669-685. [DOI] [PubMed] [Google Scholar]
- 29.Stemmer, W. Rapid evolution of a protein in vitro by DNA shuffling. Nature 370:389-391. [DOI] [PubMed]
- 30.Strandberg, G. W., and P. W. Wilson. 1968. Formation of the nitrogen-fixing enzyme system in Azotobacter vinelandii. Can. J. Microbiol. 14:25-31. [DOI] [PubMed] [Google Scholar]
- 31.Throneley, R. N. F., H. Angrove, G. A. Ashby, M. C. Durrant, S. A. Fairhurst, S. J. George, P. C. Hallenbeck, A. Sinclair, and J. D. Tolland. 2002. Nitrogenase mechanism: can old lags teach us new tricks? p. 25-29. In T. M. Finan, M. R. O’Brian, D. B. Layzell, J. K. Vessey, and W. Newton (ed.), Nitrogen fixation—global perspectives. CABI Publishing, New York, N.Y.