Abstract
The Hot (homolog of theta) protein of bacteriophage P1 can substitute for the Escherichia coli DNA polymerase III θ subunit, as evidenced by its stabilizing effect on certain dnaQ mutants that carry an unstable polymerase III ɛ proofreading subunit (antimutator effect). Here, we show that Hot can also cause an increase in the mutability of various E. coli strains (mutator effect). The hot mutator effect differs from the one caused by the lack of θ. Experiments using chimeric θ/Hot proteins containing various domains of Hot and θ along with a series of point mutants show that both N- and C-terminal parts of each protein are important for stabilizing the ɛ subunit. In contrast, the N-terminal part of Hot appears uniquely responsible for its mutator activity.
The Escherichia coli chromosome is replicated with high efficiency and fidelity by DNA polymerase (Pol) III holoenzyme (HE), a large dimeric multisubunit enzyme complex that simultaneously copies the leading and lagging strands at the replication fork (for reviews, see references 15 and 24). HE contains two core polymerase subassemblies, each composed of three separate subunits, α, ɛ, and θ, which are tightly bound in the linear order α-ɛ-θ. The α subunit (dnaE gene product) (135 kDa) is the polymerase, while the ɛ subunit (dnaQ gene product) (28.5 kDa) is the 3′→5′ exonucleolytic activity that functions as a proofreader for replication errors.
The precise function of the θ subunit (holE gene product) (8 kDa) within the Pol III core is less clear. θ binds tightly to the ɛ subunit and does not seem to interact with the α subunit (2, 32). Strains lacking θ (ΔholE strains) are viable, indicating that the subunit is not essential (31). On the other hand, our laboratory has shown that such deletion strains possess a mutator phenotype in mismatch repair-defective strains, suggesting that θ may have a positive effect on the accuracy of DNA replication, likely through its effect on the ɛ proofreading activity (33). This fidelity role is consistent with the increase in the ɛ exonuclease activity observed in an in vitro exonuclease assay in the presence of θ (32). In addition, large effects of the deletion of θ were observed in strains containing an impaired or unstable ɛ subunit (33). For example, the mutability of the temperature-sensitive dnaQ49 mutator strain was increased nearly 1,000-fold upon the loss of θ (ΔholE strain). Based on these and other results, it was postulated (33) that the θ subunit fulfills a general stabilizing role for the intrinsically unstable ɛ subunit (7, 10, 12).
θ appears to be well preserved throughout the enterobacteria, suggestive of a meaningful role for the protein. Homologs of θ have also been found, surprisingly, to be encoded by two conjugative plasmids as well as by bacteriophage P1 (3). In experiments with this P1 homolog produced by the P1 hot gene (homolog of theta) (22), we showed that the resulting Hot protein can substitute for θ in certain dnaQ mutators such as dnaQ49, as it was capable of reducing the high mutability of a dnaQ49 ΔholE strain (3). In fact, for dnaQ49, which carries a V96G mutation in ɛ (33), Hot appeared to be significantly more efficient than θ in stabilizing this mutant. For other mutants, such as dnaQ920 (R56W), dnaQ923 (H66Y), and dnaQ924 (L171F), Hot proved as efficient as θ. In contrast, Hot was not able to substitute for the θ function in dnaQ928 (G17S), indicating that the precise interactions of θ and Hot with ɛ differ in certain details and that their effects may depend on the precise defect in ɛ (3).
The interaction between ɛ and θ has also been pursued by structural studies (5-7, 11, 13, 16). Nuclear magnetic resonance (NMR) solution structures of both θ and Hot have been obtained, revealing a largely superimposable three-part helical structure with unstructured N- and C-terminal segments (6, 26). Nevertheless, some small differences between the protein structures are apparent, as expected for two proteins that are approximately 50% identical (65% homologous). In addition, the biochemical behavior of Hot is slightly different from that of θ, as the purified protein appears to be more stable and better structured, allowing its solution structure to be determined in aqueous solution (6), whereas that of θ required mixed alcohol-water solvents (26).
In the present report, we describe and investigate certain instances where the substitution of θ by Hot produced, surprisingly, a mutator effect. This proved to be the case for the dnaQ930 (H89Y) mutator strain as well as the dnaQ+ (wild-type [wt]) strain. In addition, we made use of the sequence and structural homologies of the two proteins to create a number of chimeric protein molecules, which might permit a more precise definition of the subdomains of either protein responsible for the antimutator and mutator effects. The results indicate that the N-terminal domain of Hot, which appears unstructured in NMR solution spectra, is specifically responsible for the Hot mutator effect. We also show that Hot and θ compete for their incorporation into the Pol III holoenzyme.
MATERIALS AND METHODS
Strains and media.
The E. coli strains used, along with information on their source or construction, are listed in Table 1. P1 transductions were performed using P1virA. The various dnaQ alleles (Table 1) were transduced using linkage (∼40%) with transposon zae-502::Tn10 as described previously (33), followed by testing for the associated dnaQ mutator phenotype (scoring for rifampin-resistant mutants). The donor strains NR11569 (dnaQ920), NR11572 (dnaQ923), NR11573 (dnaQ924), NR11641 (dnaQ928), and NR11642 (dnaQ930) were dnaQ derivatives of NR9501 or NR9601 as described previously (33). Plasmid pKO3 (21) was obtained from G. Church (Harvard Medical School). Minimal media (MM) containing either glucose or lactose as a carbon source or LB broth were standard recipes (29). Antibiotics were added as follows: ampicillin, 100 μg/ml; chloramphenicol, 20 μg/ml; tetracycline, 15 μg/ml; rifampin (Rif) 100 μg/ml. Solid media contained 1.5% agar (Difco).
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Reference, source, or construction |
|---|---|---|
| E. coli strains | ||
| CAG18486 | eda-51::Tn10 | 30 |
| CAG12068 | zeb-3190::Tn10 | 30 |
| CC101 to CC106 | F′CC101 to F′CC106 | 4 |
| KA796 | ara thi Δ(pro lac) | 29 |
| MG1655 | Wild type | 30 |
| NR9464 | mutL::Tn5 | 27 |
| NR9695 | dnaQ49 zae-502::Tn10 | 28 |
| NR11572 | dnaQ923 zae-502::Tn10 | 33 |
| NR11573 | dnaQ924 zae-502::Tn10 | 33 |
| NR11641 | dnaQ928 zae-502::Tn10 | 33 |
| NR11642 | dnaQ930 zae-502::Tn10 | 33 |
| NR13104 | ΔholE203 | 3 |
| NR16315 | ΔholE204::hot | 3 |
| NR16319 | dnaQ923 | MG1655 × P1/NR11572 |
| NR16320 | ΔholE203 dnaQ923 | NR13104 × P1/NR11572 |
| NR16321 | ΔholE204::hot dnaQ923 | NR16315 × P1/NR11572 |
| NR16322 | dnaQ924 | MG1655 × P1/NR11573 |
| NR16323 | ΔholE203 dnaQ924 | NR13104 × P1/NR11573 |
| NR16324 | ΔholE204::hot dnaQ924 | NR16315 × P1/NR11573 |
| NR16325 | dnaQ928 | MG1655 × P1/NR11641 |
| NR16326 | ΔholE203 dnaQ928 | NR13104 × P1/NR11641 |
| NR16327 | ΔholE204::hot dnaQ928 | NR16315 × P1/NR11641 |
| NR16328 | dnaQ930, zae-502::Tn10 | MG1655 × P1/NR11642 |
| NR16329 | ΔholE203 dnaQ930 | NR13104 × P1/NR11642 |
| NR16330 | ΔholE204::hot dnaQ930 | NR16315 × P1/NR11642 |
| NR16785 | ΔholE204::hot eda-51::Tn10 | NR16315 × P1/CAG18486 |
| NR16786 | ΔholE204::hot zeb-3190::Tn10 | NR16315 × P1/CAG12068 |
| NR16787 | ara, thi, Δprolac, ΔholE202::cat | KA796 × P1/RM4193 |
| NR17012 | ΔholE204::hot eda-51::Tn10 | KA796 × P1/NR16785 |
| NR17013 | ΔholE204::hot zeb-3190::Tn10 | KA796 × P1/NR16786 |
| NR17116 | dnaQ49 | MG1655 × P1/NR9695 |
| NR17117 | ΔholE203 dnaQ49 | NR13104 × P1/NR9695 |
| NR17118 | ΔholE204::hot dnaQ49 | NR16315 × P1/NR9695 |
| NR17119 | mutL::Tn5 | MG1655 × P1/NR9464 |
| NR17120 | ΔholE203 mutL::Tn5 | NR13104 × P1/NR9464 |
| NR17121 | ΔholE204::hot mutL::Tn5 | NR16315 × P1/NR9464 |
| RM4193 | ΔholE202::cat | 31 |
| Plasmids | ||
| pKO3 | 21 | |
| pKO3-ΔholE | 3 | |
| pKO3-holE | 3 | |
| pKO3-hot | 3 | |
| pKO3-θ49Hot | This work | |
| pKO3-Hot50θ | This work | |
| pKO3-θ11Hot | This work | |
| PKO3-Hot12θ | This work |
DNA isolation, PCR, and DNA sequencing.
E. coli genomic DNA was prepared using the DNeasy tissue kit (QIAGEN Sciences). Plasmid DNAs were purified from 10-ml liquid cultures by using a Qiaprep Spin Miniprep kit (QIAGEN Sciences). All preparative PCRs were performed using the Expand High Fidelity PCR system (Roche). Diagnostic PCRs were performed using Taq DNA polymerase (Invitrogen, Carlsbad, CA). Conditions for PCRs were recommended by the manufacturer. Oligo 6.8′ software (Molecular Biology Insights, Inc., Cascade, CO) was used to design oligonucleotide primers (Table 2) and to determine annealing temperatures. DNA sequencing was performed using the Big Dye Terminator v1.1 cycle sequencing kit (Applied Biosystems, Foster City, CA) and Perkin-Elmer ABI models 377 and 3100 sequencers. Both DNA strands were sequenced.
TABLE 2.
Oligonucleotide primers
| Primer | Sequencea |
|---|---|
| Pr1 | 5′-GAGAAATGCGGCCGCTGTAGTGTCCTTTCGTTTTATGCCC-3′ |
| Pr6 | 5′-CAAATCAGTCGACGCCAGCAGGTCGGGTTCTCC-3′ |
| hotN-lowb | 5′-CAGCTGCGCAGATTCTCTGGTTG-3′ |
| holEC-upb | 5′-CAGAGAATCTGCGCAGCTGGTTTCG-3′ |
| holEN-lowc | 5′-ATGAAATAGGTGCGCAAATGTTCAGGCTG-3′ |
| hotC-upc | 5′-TGAACATTTGCGCACCTATTTCATGGAAC-3′ |
| HotNLowd | 5′-CACTTTATCCATTTCTTCCTGACTTTTAGCTGC-3′ |
| HolECUpd | 5′-GTCAGGAAGAAATGGATAAAGTGAATGTCG-3′ |
| HolENLowe | 5′-CTTATCCCGTTCTGTTTGATCCAGTTTAGC-3′ |
| HotCUpe | 5′-GATCAAACAGAACGGGATAAGGTTAACG-3′ |
| SeqHotUp | 5′-GGAATATTGCAGCTAAAAGTC-3′ |
| SeqHotLow | 5′-CTATTTCTTTACGGCATCATC-3′ |
| SeqholE Up | 5′-CTGAAGAATCTGGCTAAACTG-3′ |
| SeqholE Low | 5′-GTTTTATTTAAGTTTGGGCTC-3′ |
Sequences in boldface type correspond to holE or hot coding sequences.
Primers with complementary 5′ ends used to create pKO3-Hot50θ.
Primers with complementary 5′ ends used to create pKO3-θ49Hot.
Primers with complementary 5′ ends used to create pKO3-Hot12θ.
Primers with complementary 5′ ends used in creation of pKO3-θ11Hot.
Chimeric plasmids.
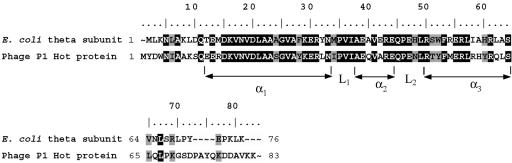
The four chimeric plasmids pKO3-θ49Hot, pKO3-Hot50θ, pKO3-θ11Hot, and pKO3-Hot12θ (Table 1) were constructed by PCR methods in two-step procedures. In the first step, two separate PCR products containing the holE or hot gene sequence of interest were created, which were then fused in a second (overlap PCR) step to yield the desired final product. The starting materials were plasmids pKO3-holE and pKO3-hot (3), which contain the holE and hot coding sequences under the control of the holE promoter and are surrounded on either side by about 500 nucleotides of chromosomal E. coli DNA (3). The various primers used are listed in Table 2. Primers Pr1 and Pr6 have been described previously (3); they are “outside” primers, complementary to the terminal 5′ and 3′ sequences of the plasmid inserts, and contain NotI and SalI recognition sequences to mediate the insertion of the final product into plasmid pKO3 (21). Chimeras were designed with aid of the amino acid alignment of θ and Hot (see Fig. 3). (In the following text, note that due to the presence of an extra residue in the N terminus of Hot compared to θ, the numberings of the corresponding θ and Hot residues differ by 1.) For example, to create pKO3-θ49Hot, we used (i) primers Pr1 and holEN-low to amplify the N-terminal part of holE (θ residues 1 to 49) from pKO3-holE and (ii) Pr6 and hotC-up to amplify the C-terminal part of hot (residues 51 to 83) from pKO3-hot. As “internal” primers, holEN-low and hotC-up contain a 24-base 5′ overlap (Table 2); a second round of PCR on the two combined PCR products using outside primers Pr1 and Pr6 could be used to create a combined (∼1,400-base) product encoding the chimeric θ49Hot protein. Using the NotI and SalI restriction sites embedded in Pr1 and Pr6, the product was then inserted into the NotI/SalI sites of plasmid pKO3, yielding pKO3-θ49Hot. The remaining chimeric plasmids (Table 1) were created in a similar fashion, using hotN-low and holEC-up for pKO3-Hot50θ, HolENLow and HotCUp for pKO3-θ11Hot, and HotNLow and HolECUp for pKO3-Hot12θ as internal primers. The resulting plasmids were analyzed by PCR and DNA sequencing. Sequencing primers were SeqHotUp and SeqHotLow (hot-specific primers) and SeqholEUp and SeqholELow (holE-specific primers). The sequencing also revealed, in addition to correct products, isolates containing one or more point mutations. Several of these mutants were included in some of the genetic experiments (see below).
FIG. 3.
Amino acid alignment of E. coli θ and P1 Hot along with secondary structure elements determined from NMR spectra (6, 26). Note that the N terminus of Hot contains one extra residue relative to that of θ and that the numbering of the corresponding residues in the two proteins differs by 1. α, α helix, L, loop.
Mutant frequency measurements.
To measure mutant frequencies, the frequency of rifampin-resistant (Rifr) mutants in cultures grown overnight was determined. In part of the experiments, we also determined the frequency of the Lac+ revertants using the series of lac alleles created previously by Cupples and Miller (4). Ten to 22 cultures for each strain started from individual colonies were grown overnight in 1 ml LB broth (containing chloramphenicol for experiments using plasmid pKO3 or its derivatives). Incubation temperatures are indicated in each of the tables or figures. For each culture, a 50-μl aliquot of a 106 dilution was spread onto an LB or minimal glucose plate to determine the total number of viable cells. Fifty microliters of the undiluted or appropriately diluted culture was spread onto LB plates with Rif to determine the number of rifampin-resistant mutants or onto minimal lactose plates to determine the number of Lac+ revertants. The mutant frequency for each culture was calculated by dividing the total number of mutants by the total number of viable cells. The mutant frequency data were analyzed using Prism statistical analysis software (GraphPad). To validate the statistical significant of observed differences, the nonparametric Mann-Whitney test was used. An exact two-tailed P value was calculated for the tested data, and differences were determined to be statistically significant when the P value was <0.05.
RESULTS
Mutator and antimutator effects of P1 Hot.
Our laboratory demonstrated previously that several dnaQ mutator strains carrying defects in the Pol III ɛ proofreading subunit are stabilized by θ (34). Their mutability, a reflection of their proofreading deficiency, was greatly enhanced in a ΔholE background. For example, the mutability of the dnaQ49 (V96G) allele was enhanced more than 1,000-fold by the lack of θ. In general, we noted a correlation between the recessive nature of dnaQ mutants, an indication of some structural impairment, and their sensitivity to the absence of θ. Subsequently, we showed that the bacteriophage P1 Hot protein, a putative θ homolog, was able to substitute for θ in several of these mutants, including dnaQ49 (3). Interestingly, for one other mutant, dnaQ928 (G17S), Hot appeared to be ineffective, although it was stabilized significantly (>100-fold) by θ (3). It was suggested that the interactions of θ and Hot with ɛ, although presumably similar, were not precisely identical in all respects.
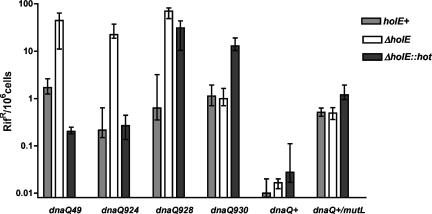
In the present study, we have extended the comparative analysis of θ and Hot to one more additional dnaQ mutator mutant, dnaQ930 (H89Y) (33, 34). The H98Y defect in dnaQ930 is genetically dominant, indicative of a mostly catalytic proofreading deficiency rather than a structural one (33). In such a case, stabilization by θ would not be expected and indeed was not observed (34). The effect of Hot on dnaQ930 is shown in Fig. 1. In this experiment, we compared the effect of several dnaQ mutator alleles in three different genetic backgrounds: wt (holE+), ΔholE, or ΔholE::hot. In the last case, the chromosomal holE gene encoding θ is replaced, precisely, by the P1 hot gene coding sequence so that Hot is expressed from the resident chromosomal E. coli holE promoter instead of θ (3).
FIG. 1.
Mutagenic and antimutagenic effects of the bacteriophage P1 Hot gene. Shown are the effects of the θ or Hot protein on several dnaQ mutator mutants and on dnaQ+ control strains. The strains are derivatives of MG1655 containing the dnaQ49, dnaQ924, dnaQ928, dnaQ930, or dnaQ+ allele in either the MG1655 (wt) (light gray), NR13104 (ΔholE) (white), or NR16315 (ΔholE::hot) (dark gray) background. All strains are mismatch repair proficient, except for the dnaQ+ mutL::Tn5 set (last entry). Cultures were grown and plated at 30°C (dnaQ49) or 37°C (others). The frequencies of rifampin-resistant mutants were calculated for 12 to 15 independent cultures for each strain, and the data were analyzed using Prism software (GraphPad). The graph shows the median values and interquartile ranges for the frequencies of rifampin-resistant mutants.
The results for dnaQ49, dnaQ924, and dnaQ928 confirm previous observations (3): dnaQ49 (V96G) and dnaQ924 (L171F) are stabilized by both θ and Hot (with dnaQ49 more effectively stabilized by Hot than by θ), while the dnaQ928 (G17S) mutator is stabilized by θ only. Interestingly, the dnaQ930 (H98Y) strain displays a strong mutator phenotype when Hot is expressed. The Hot mutator effect is about 13-fold, as observed in several experiments, compared to the corresponding strains expressing or lacking θ. Thus, in addition to the established antimutator effects, P1 Hot is also capable of producing mutator effects.
To further investigate this mutator activity, we analyzed the effect of Hot in the corresponding dnaQ+ (proofreading-proficient) background. A modest (about twofold) but reproducible (and statistically significant) mutator activity was also observed in this case (Fig. 1). As effects on proofreading and DNA replication errors are often best evaluated in mismatch repair-deficient strains (due to the lack of correction of the replication errors), we also investigated the activity of Hot in a dnaQ+ mutL strain. Again, a consistent two- to threefold mutator effect was observed (Fig. 1). These results with the dnaQ+ and dnaQ930 strains indicate that although Hot can substitute for θ in many dnaQ mutants and in fact is even more efficient in stabilizing the dnaQ49 mutator, it is also capable of increasing the mutant frequency in E. coli.
Competition between θ and Hot.
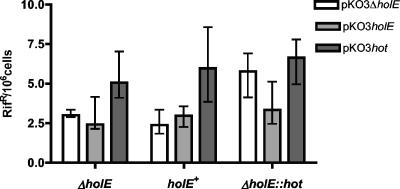
We made use of the hot mutator effect to investigate the possible competition between θ and Hot when they are present in the same cell. For this investigation, we used the mismatch repair-deficient mutL strains. High-level expression of θ or Hot is deleterious (3), but both proteins can be satisfactorily overproduced from the low-copy-number plasmid pKO3 (3). Three plasmids, pKO3-holE (containing the holE gene), pKO3-hot (containing the hot gene), and the control plasmid pKO3-ΔholE (3) (Table 1) were transformed, separately, into three E. coli strains, holE+, ΔholE, and ΔholE::hot, and the effect on the frequency of rifampin-resistant mutants was evaluated (Fig. 2). In the ΔholE strain (first set), we reproduced the hot mutator effect shown in Fig. 1. In the wild-type (holE+) strain (second set), expression of Hot from pKO3-hot also produced the mutator effect, despite the presence of θ in the cell. Finally, in the Hot-expressing (ΔholE::hot) strain (third set), overexpression of θ from pKO3-holE clearly reduced the Hot-induced mutator effect. Thus, these results indicate that θ and Hot can compete for incorporation into the Pol III holoenzyme.
FIG. 2.
Competition of θ and Hot for incorporation into the Pol III core. Shown are the effects of the overproduction of θ or Hot from low-copy-number plasmid pKO3 in a dnaQ+ strain containing the ΔholE, holE+, or ΔholE::hot genetic configuration. The mismatch repair-defective strains used were NR17119 (ΔholE), NR17120 (holE+), and NR17121 (ΔholE::hot) (Table 1) containing the indicated pKO3 plasmids (Table 1). Cultures were grown at 30°C in LB plus chloramphenicol, and the plates were likewise incubated at 30°C. The frequencies of rifampin-resistant mutants were determined for 10 to 15 independent cultures for each strain, and the data were analyzed using Prism software (GraphPad). The graph shows the median values and interquartile ranges for the frequencies of rifampin-resistant mutants. The x axis indicates, for each of the three hosts, the three pKO3 plasmids containing ΔholE (white), holE+ (light gray), or ΔholE::hot (dark gray).
Mutator effects of ΔholE.
Previously, our laboratory reported that the loss of θ (ΔholE strain) causes a modest mutator effect in dnaQ+ strains (34). This was interpreted to indicate that θ exerts a positive effect on the ɛ proofreading activity even in the proofreading-proficient (dnaQ+) background (34). This result is confirmed in the results for the dnaQ+ mutL+ strain (Fig. 1), although in this case, no obvious effect was apparent in the mutL derivative. Nevertheless, a ΔholE mutator effect in the mutL background was clearly observed in a slightly different strain, KA796 (34). Here, we made use of the KA796 series to compare the mutator effect(s) of ΔholE and ΔholE::hot. This background has the advantage that it permits the analysis of the specificities of mutations using the lac reversion system developed previously by Cupples and Miller (4). Specifically, KA796 was made ΔholE and ΔholE::hot, which was followed by an introduction of the series of F′(pro lacIZ) episomes originally present in strains CC101 through CC106. The latter permit measurement, in parallel, of each of the six possible base pair substitutions at a specific site in the lacZ gene (4). The strains were also made mutL (mutL::Tn5). The results for both Rifr mutants and lac revertants are shown in Table 3. While both ΔholE and ΔholE::hot produce a similar 1.6-fold increase in the frequency of Rifr mutants, their respective mutator effects are actually quite dissimilar, as viewed by the lac specificity data. For example, the lack of θ caused significant increases (indicated in boldface type in Table 3) for G · C→T · A, A · T→T · A, and A · T→G · C substitutions, consistent with our previous report (34). In contrast, the hot mutator did not increase the A · T→T · A transversions but instead increased the G · C→A · T transitions (1.8-fold) as well as the −1 frameshifts in strain FC40 (two- to threefold). Strain FC40 is routinely used for studying adaptive (or postplating) mutagenesis (9), but here, colonies were counted at 48 h. (We also observed a reproducible two- to threefold increase in postplating mutations in the case of the hot+ derivative but not in the ΔholE strain [data not shown].) Thus, while both Hot and the lack of θ produce a mutator effect, their specificities and, by implication, the precise mechanisms by which these effects are generated must be different.
TABLE 3.
Differential mutator effects of ΔholE and ΔholE::hot: Lac+ or Rifr mutant frequencies in mismatch repair-defective mutL strainsa
| lac allele from strain | Mutation scored | No. of mutants (per 106 cells)
|
Mutator effect (fold)
|
|||
|---|---|---|---|---|---|---|
| holE+ | ΔholE | ΔholE::hot | ΔholE/ holE+ | hot+/ holE+ | ||
| Allb | Rifr | 0.44 | 0.71 | 0.7 | 1.6 | 1.6 |
| CC101 | A · T→C · G | 0.0036 | 0.0027 | 0.0046 | 0.75 | 1.3 |
| CC102 | G · C→A · T | 0.44 | 0.45 | 0.79 | 1 | 1.8 |
| CC103 | G · C→C · G | 0 | 0 | 0 | ||
| CC104 | G · C→T · A | 0.009 | 0.038 | 0.017 | 4.2 | 1.9 |
| CC105 | A · T→T · A | 0.0037 | 0.015 | 0.004 | 4 | 1 |
| CC106 | A · T→G · C | 0.17 | 0.55 | 0.36 | 3.2 | 2.1 |
| FC40c | lac (−1) FSd | 0.096 | 0.093 | 0.27 | 0.97 | 2.8 |
Strains are mutL::Tn5 derivatives of KA796 (holE+), NR16787 (ΔholE), and NR17012 (ΔholE::hot eda-51::Tn10) or NR17013 (ΔholE::hot zeb-3190::Tn10) (Table 1) and contain the F′(pro lac) episome from strains CC101 through CC106 (Table 1), permitting measurement of the indicated, specific lac reversion frequencies. The eda or zeb transposon insertions did not affect the hot mutator effect (results not shown). For each strain with each F′(pro lac), 22 independent LB cultures were grown overnight at 37°C. The 22 cultures were derived from multiple isolates (transductants) for each strain. The frequencies of lac+ revertants and of Rifr mutants were determined as described in Materials and Methods. The mutant frequencies were analyzed by Prism software, and statistically significant differences between hot+ and ΔholE or hot+ and ΔholE::hot strains (mutator effects) were assessed using nonparametric tests. Differences with P values of <0.05 are shown in boldface type.
The Rifr data reflect the median value of eight independent experiments, each comprised of 22 cultures.
This strain was tested in the mismatch repair-proficient background.
FS, frameshift mutation.
θ-Hot chimeric proteins.
The mutator effect exerted by the P1 Hot protein presumably reflects certain differences in the ɛ-Hot interaction compared to the ɛ-θ interaction. In the simplest model, Hot might bind more strongly to ɛ than θ, a possibility suggested by its stronger stabilization of dnaQ49 (Fig. 1) (3). Such stronger binding, while greatly stabilizing dnaQ49, might lead to some structural or functional impairment of the exonuclease, observable as a mutator effect in some other dnaQ alleles. In an expanded version of this model, the mutator/antimutator effects might be ascribable to separate (sub)interactions within the ɛ-Hot and ɛ-θ complexes. We have investigated the latter possibility by creating several θ-Hot chimeric proteins and assaying their effect on some of the dnaQ mutator alleles.
Figure 3, displays the amino acid alignment of θ and Hot, including the secondary structural elements, as defined by structural studies (6, 26). Overall, θ and Hot are approximately 48 to 53% identical (60 to 66% similar). These numbers increase to approximately 70% and 80%, respectively, when considering the structured α1-L1-α2-L2-α3 core. The N- and C-terminal regions appear unstructured in the NMR spectra (6, 26), although it has been assumed that they will gain structure in the complex with ɛ and may play an important role in the interactions with ɛ (16, 20).
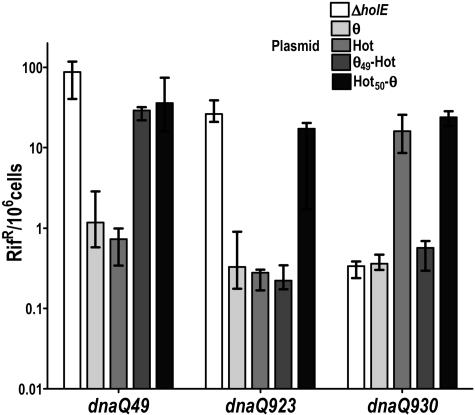
The first pair of chimeric plasmids that we constructed were pKO3-θ49Hot and pKO3-Hot50θ (Table 1) (see Materials and Methods). pKO3-θ49Hot encodes a chimeric protein consisting of the first 49 residues of θ fused to the corresponding C terminus of Hot (residues 51 to 83). (Note that due to the extra N-terminal residue of Hot [Fig. 3], the numberings for the corresponding θ and Hot residues differ by 1.) The reciprocal plasmid pKO3-Hot50θ produces the corresponding N terminus of Hot (residues 1 to 50) fused to the C terminus of θ (residues 50 to 76). In the simplest view, these two constructs involve a swap of the α3 helix plus the remaining C terminus, which appear to be relatively most dissimilar between the two proteins.
In Fig. 4, we show the effects of these plasmids, along with those of the control plasmids pKO3, pKO3-holE, and pKO3-hot, in a series of dnaQ mutator mutants. E. coli contained the chromosomal ΔholE, so that all θ/Hot activities emanate from the pKO3 plasmids. The results indicate that neither chimeric plasmid was able to stabilize the dnaQ49 mutant, suggesting that perhaps neither protein was sufficiently structured to stabilize the DnaQ49 protein. On the other hand, the θ49Hot protein was able to fully complement the dnaQ923 defect, while the reciprocal Hot50θ protein clearly reproduced the Hot mutator effect on dnaQ930, suggesting that the proteins were at least modestly structured. Importantly, the results suggest that the Hot mutator effect is specifically associated with the action of its N-terminal half.
FIG. 4.
Effects of θ-Hot and Hot-θ chimeric proteins. The strains used were NR17117 (dnaQ49 ΔholE), NR16320 (dnaQ923 ΔholE), and NR16329 (dnaQ930 ΔholE) (Table 1) containing one of five indicated plasmids (Table 1). Cultures were grown overnight at 30°C in LB plus chloramphenicol; plates were incubated at the same temperature as the cultures. Frequencies of rifampin-resistant colonies were determined for 10 independent cultures for each strain, and the data were analyzed using Prism software (GraphPad). The graph shows the median values and interquartile ranges for the frequencies of rifampin-resistant mutants. The x axis indicates, for each dnaQ allele, the five strains containing the following plasmids: pKO3ΔholE, pKO3holE, pKO3ΔholE::hot, pKO3-θ49Hot, and pKO3-Hot50θ. See the text for details.
Chimeric θ/Hot proteins containing exchanges of the N-terminal “unstructured” residues.
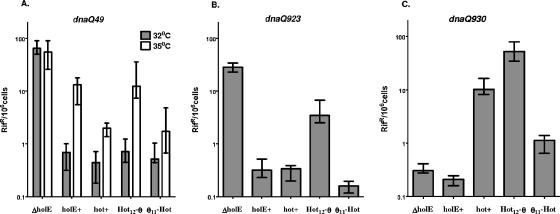
To further explore the role of the N-terminal half of Hot in its mutator activity, we focused on the region preceding the first α helix (residues 1 to 11 or 12) (Fig. 3). This stretch of residues differs substantially for θ and Hot (7 or 8 different residues out of 11 to 12). This region has been found to be nonstructured in NMR solution spectra, but it may be of importance for the interaction with ɛ (16, 20, 26). We created recombinant plasmids pKO3-θ11Hot and pKO3-Hot12θ containing the first 11 residues of θ attached to Hot residues 13 to 83 or the first 12 residues of Hot attached to θ residues 12 to 76, respectively. These new constructs were tested as described above (Fig. 5).
FIG. 5.
Effects of the θ11Hot and Hot12θ chimeric proteins. The three panels show the effect of the θ11Hot and Hot12θ chimeric proteins along with the control proteins on the mutability of dnaQ49 (A), dnaQ923 (B), and dnaQ930 (C). The strains used were NR17117 (dnaQ49 ΔholE), NR16320 (dnaQ923 ΔholE), and NR16329 (dnaQ930 ΔholE) containing various plasmids as indicated along the x axis: pKO3-ΔholE, pKO3-holE, pKO3-hot, pKO3-Hot12θ, or pKO3-θ11Hot. The cultures were grown in LB with chloramphenicol at 30°C and 35°C for the dnaQ49 strains (A) and at 30°C for the dnaQ923 and dnaQ930 strains (B and C). Plates were incubated at the same temperature as the cultures. The frequencies of rifampin-resistant colonies were determined for 8 to 12 independent cultures for each strain, and the data were analyzed using Prism software (GraphPad). The graph shows the median values and interquartile ranges for the frequencies of rifampin-resistant mutants.
The results for dnaQ49 at two temperatures, 30° and 35°C, are presented in Fig. 5A. The mutator activity of dnaQ49 is temperature dependent (14, 34), and previous studies have shown that while both Hot and θ can stabilize this allele, Hot is more effective than θ, a difference that becomes more pronounced at higher temperatures (3). The present data reproduce these findings. The results further indicate that the Hot12θ protein behaves essentially like θ, while the θ11Hot protein behaves like Hot. These results suggest that while the N-terminal 11 to 12 residues of θ or Hot may have a role in stabilizing the DnaQ49 protein, they are not the primary determinants for the greater efficiency of Hot in this respect. Apparently, these determinants lie in the remainder of the respective proteins.
A most informative result was obtained for the dnaQ930 allele (Fig. 5C). Here, the mutator effect of Hot was strongly reproduced by the Hot12θ protein, indicating that the N-terminal 12 residues of Hot are primarily responsible for the Hot mutator activity. A weaker mutator effect was also seen for the θ11Hot protein, suggesting that some other structures within Hot might also be relevant for the Hot mutator effect for dnaQ930.
Further corroborative insights were obtained from the experiment with the dnaQ923 mutant (Fig. 5B). While, ostensibly, θ and Hot are similarly active in stabilizing this dnaQ allele, the two chimeric proteins clearly reveal a split phenotype. The Hot12θ chimera increased the dnaQ923 mutant frequency up to threefold in comparison to either θ or Hot, while the θ11Hot protein showed a three- to fivefold decrease. Although other explanations are possible, it appears that the N-terminal 11 to 12 residues of Hot are responsible for a mutator effect in both dnaQ923 and dnaQ930, but in the dnaQ923 mutant, this mutator effect is compensated for by a simultaneous antimutator effect of the remaining C-terminal part of Hot. As dnaQ930 does not require any stabilization by either θ or Hot, this second effect is not observed for this mutant.
Effects of point mutants in chimeric proteins.
During the creation of pKO3-θ11Hot and pKO3-Hot12θ plasmids, we also obtained (presumably through PCR amplification errors) several mutants carrying amino acid substitutions. Several of these mutants were tested in parallel with the plasmids described above. The results are listed in Table 4.
TABLE 4.
Effect of point mutants in Hot12θ and θ11Hot chimeric proteins on the mutability of dnaQ49, dnaQ923, and dnaQ930 mutantsa
| Proteinb | No. of Rifr mutants per 106 cells
|
|||
|---|---|---|---|---|
|
dnaQ49
|
dnaQ923 | dnaQ930 | ||
| 32°C | 35°C | |||
| None (ΔholE) | 65 | 55 | 27 | 0.33 |
| θ | 0.69 | 13 | 0.33 | 0.29 |
| Hot | 0.4 | 2.0 | 0.30 | 13 |
| Hot12θ | 0.72 | 12 | 2.4 | 44 |
| Hot12θ (Y2Η) | 0.5 | 34 | 37 | 50 |
| Hot12θ (Α7Τ) | 12 | 55 | 0.42 | 0.59 |
| Hot12θ (N17H) | 0.66 | 20 | 38 | 40 |
| Hot12θ (W51C) | 71 | 27 | 28 | 3.2 |
| θ11Hot | 0.52 | 1.7 | 0.11 | 1.4 |
| θ11Hot (D9Y) | 0.78 | 8.8 | 0.17 | 0.96 |
| θ11Hot (V17I) | 0.39 | 0.12 | 0.64 | |
| θ11Hot (F53S) | 63 | 92 | 7.5 | 0.87 |
Results are the averages of two or three determinations. The dnaQ49 experiments were performed at 32° and 35°C; the dnaQ923 and dnaQ930 experiments were performed at 30°C.
Amino acid numbers (in parentheses) refer to the original residue numbers in θ or Hot.
For the Hot12θ protein, two substitution mutants were obtained in the N-terminal Hot region. The Y2H and A7T mutations significantly increased the mutability of the dnaQ49 strain, indicating that this N-terminal portion of Hot is important for the stabilization of the DnaQ49 protein. Y2H also increased the mutability of dnaQ923, consistent with the requirement of this allele for stabilization by Hot or θ. Y2H did not affect the Hot12θ mutator effect on dnaQ930, consistent with the notion that dnaQ930 does not require stabilization by Hot or θ. Interestingly, the A7T mutation lowered the mutability of both dnaQ923 and dnaQ930 and in fact nearly completely abolished the mutator effect of Hot12θ on both alleles. These results are fully consistent with our proposal that the N-terminal 12 residues of Hot are responsible for the Hot mutator effect.
Two mutations, N17H and W51C, were obtained in the θ-specific part of Hot12θ. The N17H defect was generally deleterious for dnaQ49 and, especially, dnaQ923, increasing the mutation frequency while not affecting the mutability of dnaQ930. These results are consistent with a stabilizing role of the C-terminal part. In contrast, W51C likely impairs the overall integrity of the protein, as the resulting hybrid lacked the ability to stabilize (dnaQ49 and dnaQ923) and greatly reduced the mutator effect on dnaQ930. W51 in θ and the corresponding Y52 in Hot likely occupy a critical position within helix α3, as discussed previously (26).
The amino acid substitutions in the reciprocal θ11Hot protein are less informative. The D9Y and V17I mutations are largely neutral, whereas F53S resembles the case of W51C discussed above, reducing both the stabilizing effect on dnaQ49 and dnaQ923 and the mutator effect on dnaQ930. Thus, F53S likely represents a structurally impaired protein that has lost most of its capacity to interact with ɛ.
DISCUSSION
The current results provide new evidence for the importance of the ɛ-θ interaction within the Pol III core in determining the fidelity of replication in E. coli. Previously, we demonstrated that the lack of the θ subunit caused a mutator effect in wild-type cells and in several proofreading-impaired dnaQ mutants (34). This strongly suggested a stabilizing role for the θ subunit, presumably keeping ɛ in a structural conformation favorable for proofreading. Currently, we show that Hot, the P1 homolog of θ that is capable of substituting for θ in this stabilizing role, can also generate a mutator effect, thus providing a further indication of the importance of the ɛ-θ (or ɛ-Hot) interaction in the optimal functioning of the proofreading activity. Specifically, the present results indicate that the extreme N-terminal residues (residues 1 to 12) of Hot are responsible for this mutator activity. In the published structure of Hot (or θ), this segment is not observed, presumably because it is poorly structured or conformationally active (6, 26). However, it is very likely that this segment plays an important role in the interaction with the ɛ subunit. The isolation of the A7T mutant in the Hot12θ chimera, showing abolishment of the mutator effect, further supports the importance of this segment for the ɛ-Hot interaction. Further structural studies on ɛ-Hot or ɛ-θ complexes may shed light on this important aspect.
Different mutational specificities for ΔholE and ΔholE::hot.
It is interesting that both the lack of θ and the substitution of θ by Hot lead to a very similar mutator phenotype when assayed by the frequency of rifampin-resistant mutants. However, more careful analysis using a series of defined lac reversions shows that the mutator effects are clearly distinct. If both effects result simply from the impairment of ɛ proofreading, one must assume that different base-base mismatches are differentially sensitive to the two modes of proofreading disturbance. As exonucleolytic proofreading involves multiple steps, including the conformational changes associated with the transfer of the terminal mismatch from the polymerase active site to the exonuclease site, such differential effects should be considered. Alternatively, the differential specificities could reflect a more complicated mode of mutation production in ΔholE versus ΔholE::hot strains. For example, accessory DNA polymerases, such as Pol II or Pol IV, have been shown to be involved in the production of mutations, particularly when Pol III has difficulty extending certain terminal mispairs (1, 17). Possibly, HE without θ and HE containing Hot behave differently with respect to this phenomenon of polymerase trafficking. This will be an interesting area for further studies. Finally, a phylogenetic tree relating P1 Hot to other sequenced θ homologs clearly suggests that Hot is more closely related to homologs from more distant species such as Klebsiella than to θ itself (3). Thus, Hot is likely optimized for interaction with the ɛ subunit from other enterobacterial species, possibly explaining the observed mutator effect.
The role of hot for P1.
A noteworthy aspect of the present work is the demonstration of direct competition between θ and Hot (Fig. 2). While we have not demonstrated a direct incorporation of Hot into the Pol III HE, the straightforward interpretation is that Pol III HE, when replicating the E. coli chromosome, contains Hot at least part of the time in the mixed holE/hot experiments and completely in the case of the ΔholE strain. This is, to our knowledge, the first demonstration of such a heterologous substitution in the Pol III HE. Such substitutions may also occur in P1-infected cells or in lysogens carrying the P1 prophage. These conclusions are relevant for the question as to why P1 carries, specifically, a gene for a θ homolog but no homolog for any other Pol III accessory subunit.
One possibility is that the increased mutation rate resulting from the incorporation of Hot in the HE is beneficial to the phage. As optimal mutation rates are proportional to genome size in DNA-based microbes (8), P1, based on its smaller genome, might tolerate and benefit from an increased mutation rate. Alternatively, the beneficial effect of Hot may be derived from an increased efficiency of replication. As the number of Pol III HE molecules per cell is very limited (23, 25), phage replication would probably benefit from an increase in their number. As ɛ is an intrinsically unstable protein (7, 10, 12) and θ and Hot stabilize ɛ, increased amounts of θ or Hot may result in increased amounts of the Pol III core and, ultimately, HE. The precise order of assembly of HE and its rate-limiting steps are unknown, and it might be worthwhile to study this under conditions of increased θ or Hot protein. Another interesting question is in which stage of the P1 life cycle the hot gene product is expressed or active. The gene has been classified as a “late” gene based on its predicted promoter structure (18, 19, 22), suggesting that it might be produced primarily late in the lytic cycle, when DNA replication has ceased and packaging ensues. It is hard to envision a role for a replication protein at this late stage, and this issue has to be investigated further, including expression of hot during lysogeny. Preliminary results from our laboratory (our unpublished data) have indicated that hot is expressed at least from the P1 prophage (lysogenic state).
In future studies, we will attempt to address the issue of the role of Hot in the P1 life cycle by investigating the properties of a phage deleted for Hot as well as the important question of the timing of Hot expression as either a later or early gene.
Acknowledgments
We thank Gregory Stuart and Giang Nguyen of the NIEHS for their helpful comments on the manuscript and Emily Gifford and Andrew DeBrecht for technical assistance.
This research was supported by the Intramural Research Program of the NIH and NIEHS.
REFERENCES
- 1.Banach-Orlowska, M., I. J. Fijalkowska, R. M. Schaaper, and P. Jonczyk. 2005. DNA polymerase II as a fidelity factor in chromosomal DNA synthesis in Escherichia coli. Mol. Microbiol. 58:61-70. [DOI] [PubMed] [Google Scholar]
- 2.Carter, J. R., M. A. Franden, R. Aebersold, D. R. Kim, and C. S. McHenry. 1993. Isolation, sequencing and overexpression of the gene encoding the θ subunit of DNA polymerase III holoenzyme. Nucleic Acids Res. 21:3281-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chikova, A. K., and R. M. Schaaper. 2005. The bacteriophage P1 hot gene product can substitute for the Escherichia coli DNA polymerase III θ subunit. J. Bacteriol. 187:5528-5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cupples, C. G., and J. H. Miller. 1989. A set of lacZ mutations in Escherichia coli that allow rapid detection of each of the six base substitutions. Proc. Natl. Acad. Sci. USA 86:5345-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeRose, E. F., T. Darden, S. Harvey, S. Gabel, F. W. Perrino, R. M. Schaaper, and R. E. London. 2003. Elucidation of the ɛ-θ interface of Escherichia coli DNA polymerase III by NMR spectroscopy. Biochemistry 42:3635-3644. [DOI] [PubMed] [Google Scholar]
- 6.DeRose, E. F., T. W. Kirby, G. A. Mueller, A. K. Chikova, R. M. Schaaper, and R. E. London. 2004. Phage like it HOT: solution structure of the bacteriophage P1-encoded HOT protein, a homolog of the θ subunit of E. coli DNA polymerase III. Structure 12:2221-2231. [DOI] [PubMed] [Google Scholar]
- 7.DeRose, E. F., D. Li, T. Darden, S. Harvey, F. W. Perrino, R. M. Schaaper, and R. E. London. 2002. Model for the catalytic domain of the proofreading epsilon subunit of Escherichia coli DNA polymerase III based on NMR structural data. Biochemistry 41:94-110. [DOI] [PubMed] [Google Scholar]
- 8.Drake, J. W. 1991. A constant rate of spontaneous mutation in DNA-based microbes. Proc. Natl. Acad. Sci. USA 88:7160-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster, P. L. 1999. Mechanisms of stationary phase mutation: a decade of adaptive mutation. Annu. Rev. Genet. 33:57-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster, P. L., and M. G. Marinus. 1992. Levels of epsilon, an essential replication subunit of Escherichia coli DNA polymerase III, are controlled by heat shock proteins. J. Bacteriol. 174:7509-7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta, R., S. M. Hamdan, N. E. Dixon, M.M. Sheil, and J. L. Beck. 2004. Application of electrospray ionization mass spectrometry to study the hydrophobic interaction between the ɛ and θ subunits of DNA polymerase III. Protein Sci. 13:2878-2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamdan, S., E. M. Bulloch, P. R. Thompson, J. L. Beck, J. Y. Yuang, J. A. Crowther, P. E. Lilley, P. D. Carr, D. L. Ollis, S. E. Brown, and N. E. Dixon. 2002. Hydrolysis of the 5′-p-nitrophenyl ester of TMP by the proofreading exonuclease (ɛ) subunit of Escherichia coli DNA polymerase III. Biochemistry 41:5266-5275. [DOI] [PubMed] [Google Scholar]
- 13.Hamdan, S., P. D. Carr, S. E. Brown, D. L. Ollis, and N. E. Dixon. 2002. Structural basis of proofreading during replication of the Escherichia coli chromosome. Structure 10:535-546. [DOI] [PubMed] [Google Scholar]
- 14.Horiuchi, T., H. Maki, and M. Sekiguchi. 1978. A new conditional lethal mutator (dnaQ49) in Escherichia coli K12. Mol. Gen. Genet. 163:277-283. [DOI] [PubMed] [Google Scholar]
- 15.Kelman, Z., and M. O'Donnell. 1995. DNA polymerase III holoenzyme: structure and function of a chromosomal replicating machine. Annu. Rev. Biochem. 64:171-200. [DOI] [PubMed] [Google Scholar]
- 16.Keniry, M. A., H. A. Berthon, J. Y. Yang, C. S. Miles, and N. E. Dixon. 2000. NMR solution structure of the θ subunit of DNA polymerase III from Escherichia coli. Protein Sci. 9:721-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuban, W., M. Banach-Orlowska, M. Bialoskorska, A. Lipowska, R. M. Schaaper, P. Jonczyk, and I. J. Fijalkowska. 2005. Mutator phenotype resulting from DNA polymerase IV overproduction in Escherichia coli: preferential mutagenesis on the lagging strand. J. Bacteriol. 187:6862-6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehnherr, H., A. Guidolin, and W. Arber. 1991. Bacteriophage P1 gene 10 encodes a trans-activating factor required for late expression. J. Bacteriol. 173:6438-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehnherr, H., A. Guidolin, and W. Arber. 1992. Mutational analysis of the bacteriophage P1 late promoter sequence Ps. J. Mol. Biol. 228:101-107. [DOI] [PubMed] [Google Scholar]
- 20.Li, D., D. L. Allen, S. Harvey, F. W. Perrino, R. M. Schaaper, and R. E. London. 1999. A preliminary CD and NMR study of the Escherichia coli DNA polymerase III θ subunit. Proteins Struct. Funct. Genet. 36:111-116. [DOI] [PubMed] [Google Scholar]
- 21.Link, A. J., D. Phillips, and G. M. Church. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobocka, M. B., D. J. Rose, G. Plunkett III, M. Rusin, A. Samojedny, H. Lehnherr, M. B. Yarmolinsky, and F. R. Blattner. 2004. Genome of bacteriophage P1. J. Bacteriol. 186:7032-7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maki, H., S. Maki, and A. Kornberg. 1988. DNA polymerase III holoenzyme of Escherichia coli. IV. The holoenzyme is an asymmetric dimer with twin active sites. J. Biol. Chem. 263:6570-6578. [PubMed] [Google Scholar]
- 24.McHenry, C. S. 2003. Chromosomal replicases as asymmetric dimers: studies of subunit arrangement and functional consequences. Mol. Microbiol. 29:1157-1165. [DOI] [PubMed] [Google Scholar]
- 25.McHenry, C. S., and A. Kornberg. 1977. DNA polymerase III holoenzyme of Escherichia coli. J. Biol. Chem. 252:6478-6484. [PubMed] [Google Scholar]
- 26.Mueller, G. A., T. W. Kirby, E. F. DeRose, D. Li, R. M. Schaaper, and R. E. London. 2005. Nuclear magnetic resonance solution structure of the Escherichia coli DNA polymerase III θ subunit. J. Bacteriol. 187:7081-7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaaper, R. M. 1993. The mutational specificity of two Escherichia coli dnaE antimutator alleles as determined from lacI mutation spectra. Genetics 134:1031-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaaper, R. M., and R. Cornacchio. 1992. An Escherichia coli dnaE mutation with suppressor activity toward mutD5. J. Bacteriol. 174:1974-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaaper, R. M., B. N. Danforth, and B. W. Glickman. 1985. Rapid repeated cloning of mutant lac repressor genes. Gene 39:181-189. [DOI] [PubMed] [Google Scholar]
- 30.Singer, M., T. A. Baker, G. Schnitzler, S. M. Deischel, M. Goel, W. Dove, K. J. Jaacks, A. D. Grossman, J. W. Erickson, and C. A. Gross. 1989. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol. Rev. 53:1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slater, S. C., M. R. Lifsics, M. O'Donnell, and R. Maurer. 1994. holE, the gene coding for the θ subunit of DNA polymerase III of Escherichia coli: characterization of a holE mutant and comparison with a dnaQ (ɛ-subunit) mutant. J. Bacteriol. 176:815-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Studwell-Vaughan, P. S., and M. O'Donnell. 1993. DNA polymerase III accessory proteins: θ encoded by holE. J. Biol. Chem. 268:11785-11791. [PubMed] [Google Scholar]
- 33.Taft-Benz, S. A., and R. M. Schaaper. 1998. Mutational analysis of the 3′→5′ proofreading exonuclease of Escherichia coli DNA polymerase III. Nucleic Acids Res. 26:4005-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taft-Benz, S. A., and R. M. Schaaper. 2004. The θ subunit of Escherichia coli DNA polymerase III: a role in stabilizing the ɛ proofreading subunit. J. Bacteriol. 186:2774-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]