Abstract
Acyl-CoA-dependent O-acyltransferases catalyze reactions in which fatty acyl-CoAs are joined to acyl acceptors containing free hydroxyl groups to produce neutral lipids. In this report, we characterize a human multifunctional O-acyltransferase (designated MFAT) that belongs to the acyl-CoA:diacylglycerol acyltransferase 2/acyl-CoA:monoacylglycerol acyltransferase (MGAT) gene family and is highly expressed in the skin. Membranes of insect cells and homogenates of mammalian cells overexpressing MFAT exhibited significantly increased MGAT, acyl-CoA:fatty acyl alcohol acyltransferase (wax synthase), and acyl-CoA:retinol acyltransferase (ARAT) activities, which catalyze the synthesis of diacylglycerols, wax monoesters, and retinyl esters, respectively. Furthermore, when provided with the appropriate substrates, intact mammalian cells overexpressing MFAT accumulated more waxes and retinyl esters than control cells. We conclude that MFAT is a multifunctional acyltransferase that likely plays an important role in lipid metabolism in human skin.
Supplementary key words: neutral lipids, diacylglycerol, fatty alcohol, esterification
Abbreviations: ARAT, acyl-coenzyme A:retinol acyltransferase; DGAT, acyl-coenzyme A:diacylglycerol acyltransferase; MFAT, multifunctional acyltransferase; MGAT, acyl-coenzyme A:monoacylglycerol acyltransferase; NCBI, National Center for Biotechnology Information
Located at the interface between higher organisms and their environment, skin serves protective, regulatory, and sensory functions. A wide variety of lipids are found in the skin and at the skin surface, where they contribute to skin functions (1). For example, ceramides, free fatty acids, and cholesterol are synthesized and secreted by keratinocytes in the epidermis. These lipids are organized into intercellular lamellae in the stratum corneum (the outermost layer of the epidermis), where they help form a permeability barrier that prevents transepidermal water loss and the entry of harmful chemical, biological, or physical agents (e.g., allergens, bacteria, or ultraviolet light) (2–4). Other skin lipids, such as the neutral lipids triacylglycerols, sterol esters, and wax monoesters and diesters, are synthesized and secreted from sebaceous glands as sebum onto the surfaces of skin and hair (5). Here, these highly nonpolar lipids serve as water repellents, lubricants, or pheromones. Sebum also delivers the antioxidant vitamin E (6) and is a major source of glycerol, which is implicated in maintaining stratum corneum hydration (7). The lipid composition of sebum varies among species. For example, triacylglycerols (58%) and wax monoesters (26%) are the most abundant lipids in human sebum (8), whereas mouse sebum has high levels of wax diesters (65%), which are believed to confer water-repulsion properties on fur (9, 10).
Recently, the identification and subsequent disruption of genes encoding lipid-synthesizing enzymes in mice have revealed functional roles for these enzymes in skin homeostasis. For example, mice lacking ACAT1, which is expressed highly in sebaceous glands and meibomian glands (11, 12), have dry skin and eyes (13). In the setting of hypercholesterolemia, these mice accumulate huge amounts of cholesterol in the skin (14), probably because there is too much cholesterol for skin histiocytes and macrophages to detoxify. Mice lacking acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1), another enzyme expressed highly in sebaceous glands, have atrophic sebaceous glands and reduced levels of type II wax diesters in their fur lipids, resulting in impaired water repulsion (15). This phenotype resembles that in mice lacking stearoyl-CoA desaturase 1, which is also expressed in sebaceous glands (16, 17). Mice lacking the fatty acid elongase ELOVL3, also expressed abundantly in sebaceous glands, exhibit impaired water repulsion and increased transepidermal water loss (18). The latter finding suggests that sebum may be important in lipid organization in the stratum corneum. Moreover, mice lacking DGAT2, a DGAT enzyme that is not homologous with DGAT1, have impaired epidermal barrier function and increased transepidermal water loss, probably because they lack essential fatty acids required for the proper organization and function of the intercellular lamellar lipids (19). Together, these studies highlight the essential role of lipid metabolism in normal skin functions.
In this study, we identified another enzyme that may play a significant role in human skin lipid metabolism. We examined the uncharacterized members [previously termed DC3, DC4, and DC7 by Cases et al. (20)] of a gene family that includes DGAT2 and three acyl-CoA:monoacylglycerol acyltransferases (MGATs) (21–24), focusing on one member (DC4) that was cloned from human skin. We studied the in vitro biochemical activities of the expressed enzyme and show that the DC4-encoded enzyme uses a variety of acyl acceptors in in vitro assays, catalyzing the synthesis of diacylglycerols, wax monoesters, and retinyl esters. Therefore, we designated this enzyme multifunctional acyltransferase (MFAT). Recently, two other groups showed that this enzyme possesses wax monoester synthase activity and is expressed in sebaceous-type glands in mice and humans (25, 26). Our findings are discussed in light of these other reports.
EXPERIMENTAL PROCEDURES
Cloning of MFAT cDNA
Human expressed sequence tags encoding MFAT were identified by Basic Local Alignment Search Tool database searches through their sequence homology to DGAT2 from Mortierella rammaniana (accession number AF391089). Based on these sequence tags, primers introducing restriction sites were designed (forward primer, 5′-GTG CCC GGG ACT CAT ATG CTC TTG CCC TCT AAG AAG GAC C-3′; reverse primer, 5′-GTG GCG GCC GCT CTT CCA GCC AGG GTG AAG GCT ACT GGG-3′) to amplify its complete coding sequence from human skin cDNA (Clontech, Palo Alto, CA). The MFAT clone was sequenced and is identical to the sequence of GenBank XM_088683.
MFAT tissue expression pattern in human
A human multiple tissue blot (SeeGene, Seoul, Korea) was hybridized with 32P-labeled probes generated by random priming (Amersham) using human MFAT cDNA as the template. Primers used for quantitative PCR (MFAT forward primer, 5′-GTG GTT CCA GAG CAT GGT ACA C-3′; MFAT reverse primer, 5′-CTT GGT GAA GCC ACG TCC A-3′; cyclophilin forward primer, 5′-TTA TTT GGG TTG CTC CCT TC-3′; cyclophilin reverse primer, 5′-AAG TGT GCC AAA TCT GCA AG-3′) were selected with Primer Express software (Applied Biosystems, Foster City, CA). Human cDNAs of various tissues were from Biochain (Hayward, CA).
Insect cell expression studies
MFAT cDNAs [with and without an N-terminal FLAG epitope: MGDYKDDDDG (epitope underlined)] were expressed in Sf9 insect cells as described (21). FLAG-tagged mouse DGAT1, mouse DGAT2, mouse MGAT1, and human MGAT2 (accession numbers AF078752, AF384160, AF384162, and AY157608, respectively) were expressed as controls. To prepare membrane fractions, cells were typically infected with virus for 3 days, washed with PBS, and homogenized by 10 passages through a 27 gauge needle in 1 mM EDTA, 200 mM sucrose, and 100 mM Tris-HCl, pH 7.4. Total membrane fractions (100,000 g pellet) were isolated, resuspended in homogenization buffer, and frozen at −80°C until use. Expression of FLAG-tagged proteins was verified by immunoblotting of samples (5 μg of membrane proteins) with anti-Flag M2 antibody (Sigma, St. Louis, MO).
In vitro acyltransferase assays
Initial acyltransferase activity assays were performed with 100 μg of membrane proteins, 5 mM MgCl2, 1.25 mg/ml BSA, 200 mM sucrose, 100 mM Tris-HCl, pH 7.4, 25 μM acyl donor, and 100 μM acyl acceptor as described (21). Nonpolar acyl acceptors (1-hexadecanol, 1,2-hexadecandiol, retinol, monoacylglycerol, diacylglycerol, and cholesterol) were dissolved in acetone, which accounted for 5% of 200 μl reactions, and the more polar glycerol-3-phosphate was dissolved in water. Reactions were started by adding membranes (from insect cells) or total protein homogenates (from mammalian cells) to the assay mix and were stopped after 10 min (increases in reaction products were linear for up to 30 min) by adding 4 ml of chloroform-methanol (2:1, v/v). The extracted lipids were dried, separated by TLC with hexane-ethyl ether-acetic acid (80:20:1, v/v/v), and visualized with iodine vapor, and products were identified by comparison with the migration of lipid standards. For experiments in which the incorporation of radioactive substrates into lipid products was measured, TLC plates were either scanned with an imaging scanner (Bioscan AR-2000, Washington, DC) or exposed to X-ray film followed by band scraping and counting in a scintillation counter.
MGAT, DGAT, wax synthase, and acyl-CoA:retinol acyltransferase (ARAT) activities were detected by assaying the incorporation of [14C]palmitoyl-CoA (25 μM; specific activity, ~20,000 cpm/nmol; Amersham Biosciences, Piscataway, NJ) into diacylglycerols, triacylglycerols, waxes (monoesters or diesters), and retinyl esters in the presence of added sn-2-monooleoylglycerol, sn-1,2-dioleoylglycerol, 1-hexadecanol, 1,2-hexadecandiol, or all-trans-retinol, respectively. The dependence of each activity on its specific acyl acceptor was determined by assaying with or without palmitoyl-CoA as the acyl donor and 200 μM [3H]sn-2-monooleoylglycerol (~3,000 cpm/nmol resuspended in acetone; American Radiolabeled Chemicals, St. Louis, MO), 100 μM [14C]1-hexadecanol (~8,000 cpm/nmol in ethanol; American Radiolabeled Chemicals), or 50 μM [3H]all-trans-retinol (~320,000 cpm/nmol in ethanol; American Radiolabeled Chemicals) as the acyl acceptor. The dependence of acyltransferase activities on the concentration of acyl acceptor substrates was assessed by assaying with 25 μM [14C]palmitoyl-CoA (~20,000 cpm/nmol) and various concentrations of monooleoylglycerol, 1-hexadecanol, or retinol. The acyl acceptors were serially diluted beforehand to maintain the percentage of acetone in each reaction at 5%. All assays were performed in duplicate, and experiments were repeated with independent protein preparations at least twice.
Mammalian cell expression studies
For mammalian cell expression, FLAG-tagged MFAT was sub-cloned into pIRESneo vector (Clontech) and transfected into COS-7 cells with Fugene 6 (Roche Diagnostics, Chicago, IL). β-Galactosidase and FLAG-tagged DGAT1, DGAT2, MGAT1, and MGAT2 were expressed as controls. The expression of FLAG-tagged proteins was verified by immunoblotting of lysate proteins (20 μg) with an anti-FLAG (M2) antibody. Acyltransferase activities in lysates of transfected cells were assayed as described above.
Esterification of monoacylglycerol, fatty acyl alcohol, and retinol in cultured cells
COS-7 cells were plated in six-well dishes (105/well) for 24 h and transfected with vectors containing cDNAs for MFAT or control enzymes. After 24 h, cells were incubated in medium containing 25 μM [3H]sn-2-monooleoylglycerol (~15,000 cpm/nmol) for 18 h, 10 μM [14C]1-hexadecanol (~47,000 cpm/nmol) for 6 h, or 2.5μM [3H]retinol (~200,000 cpm/nmol) for 18 h. After incubations, lipids were extracted, separated by TLC, visualized with a Bioscan imaging scanner, and scraped to assess the incorporation of radioactivity into diacylglycerols and triacylglycerols, wax esters, or retinyl esters.
RESULTS
Identification of the MFAT gene
The human MFAT cDNA was originally identified through its sequence similarity to DGAT2 [human DC4 in Cases et al. (20)]. Its open reading frame encodes a 333 amino acid protein with a predicted molecular mass of 38.1 kDa. MFAT is a member of the DGAT2 gene family [National Center for Biotechnology Information (NCBI) Conserved Domain Database: pfam 03982 DAGAT; Fig. 1A], which also includes MGAT1, MGAT2, MGAT3, and two additional members, DC3 and DC7. The gene encoding MFAT resides near those encoding DC3 and DC7 on the X chromosome (Fig. 1A), suggesting that one of these genes is the ancestral form and the two others arose from gene duplication. Consistent with this conjecture, MFAT is more similar to DC3 and DC7 than to other family members. MFAT shares 52% amino acid identity with DC3 and 50.7% with DC7, compared with 42.3% identity with DGAT2 and ~40% with the MGAT proteins. MFAT has an ortholog in the mouse genome (accession number NM_177746) located near the DC3 and DC7 orthologs on the X chromosome.
Fig. 1.
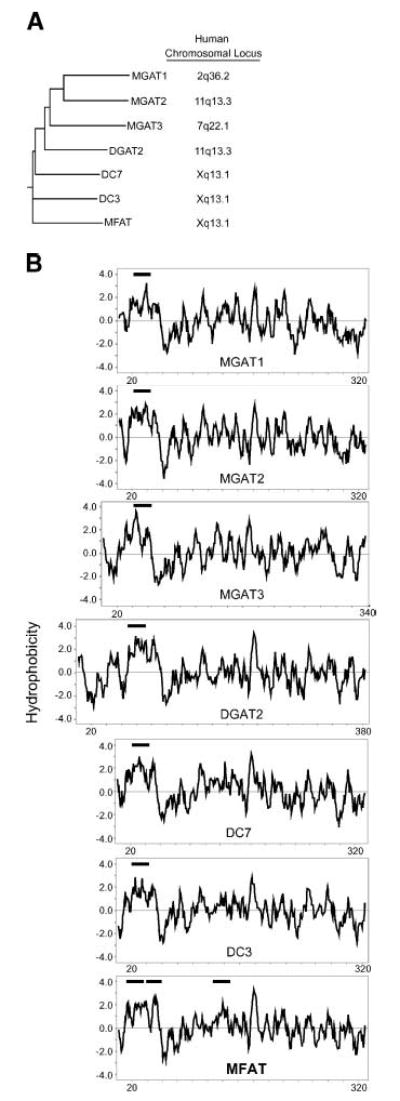
Human multifunctional acyltransferase (MFAT), a member of the acyl-CoA:diacylglycerol acyltransferase 2/acyl-CoA: monoacylglycerol acyltransferase (DGAT2/MGAT) gene family. A: Dendrogram of the human DGAT2/MGAT gene family. Chromosome locations were from the National Center for Biotechnology Information LocusLink database (http://www.ncbi.nlm.nih.gov/Locus-Link/list.cgi). MGAT1 was designated as DC2, MGAT2 as DC5, and MFAT as DC4 by Cases et al. (20). B: Hydrophobicity plots of DGAT2/MGAT gene family members as assessed by Kyte-Doolittle analysis (35). Thick horizontal lines indicate predicted transmembrane domains (http://www.cbs.dtu.dk/services/TMHMM/). The X axis represents the positions of amino acids, numbering from the N terminus to the C terminus.
As predicted from the similar protein sequences, the hydrophobicity plot of MFAT resembles those of other DGAT2/MGAT family members, especially its closest relatives, DC3 and DC7 (Fig. 1B). Although most members of the family have one transmembrane domain in the N-terminus, MFAT has two additional predicted transmembrane domains. Like other family members, human MFAT contains a low degree of similarity (in the region of amino acids 99–233) to a conserved domain of glycerol phosphate acyltransferases (NCBI Conserved Domain Database: COG0204 PlsC). MFAT also possesses several putative posttranslational modification sites, including one N-linked glycosylation site, one protein kinase A phosphorylation site, two protein kinase C phosphorylation sites, and three myristoylation sites (Prosite database).
Tissue distribution of human MFAT expression
As determined by Northern blot analysis, human MFAT mRNA has two major transcripts (~1.5 and ~2.2 kb), which are expressed predominantly in the skin (Fig. 2A). A smeared band was present below the ~1.5 kb band; whether this was attributable to partial RNA degradation or additional transcripts remains to be determined. Quantitative PCR revealed that MFAT was also expressed in testis and lung, although the mRNA levels were <1% of that in skin (Fig. 2B). Examination of human DC3 and DC7 expression using full-length cDNA probes cloned from human skin and the Northern blot in Fig. 2A did not show expression of either gene in the tissues represented on this blot (data not shown).
Fig. 2.
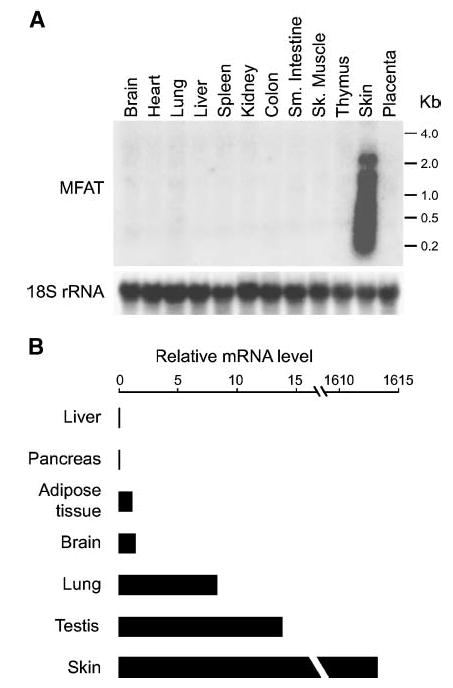
Tissue expression pattern of human MFAT. A: Northern blot analysis. Human MFAT mRNA expression was examined with 20 μg of total RNA from the indicated human tissues. Sk., skeletal; Sm., small. B: Quantitative PCR. Relative mRNA levels in the indicated human tissues (compared with white adipose tissue) were assessed with real-time PCR. Cyclophilin mRNA levels served as internal controls.
Expression of human MFAT in insect cells
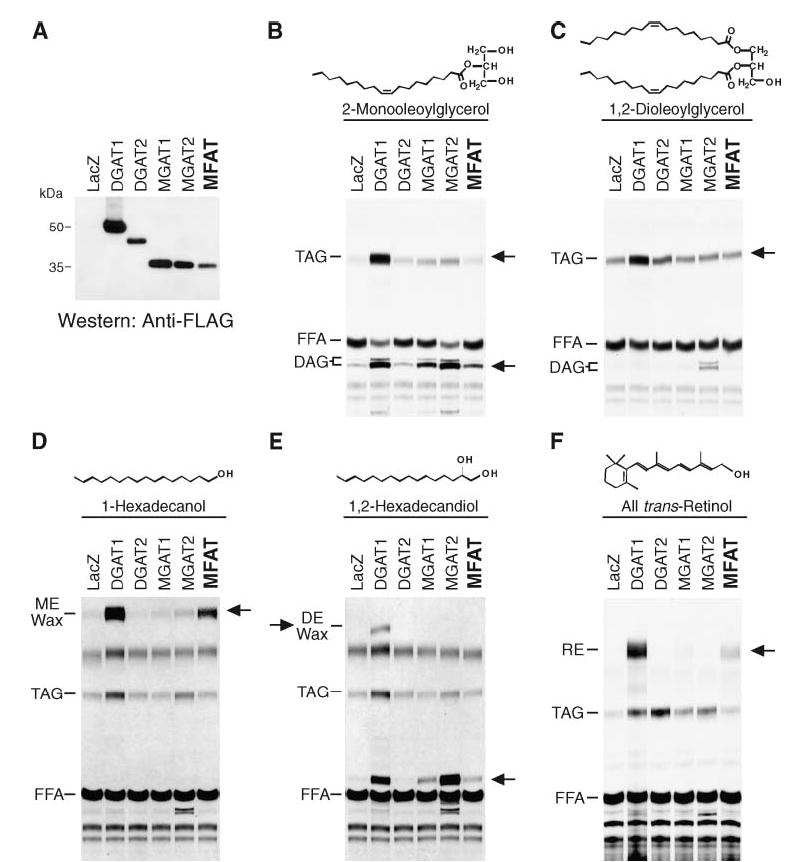
To examine the biochemical activities of MFAT protein, we expressed FLAG-tagged and nontagged versions of the cDNA in insect cells. The FLAG-tagged MFAT migrated on SDS-PAGE with an apparent molecular mass of ~35 kDa (Fig. 3A). The level of MFAT expression was ~2-fold higher than that of DGAT1 and DGAT2 and was similar to that of MGAT1 and MGAT2 (Fig. 3A).
Fig. 3.
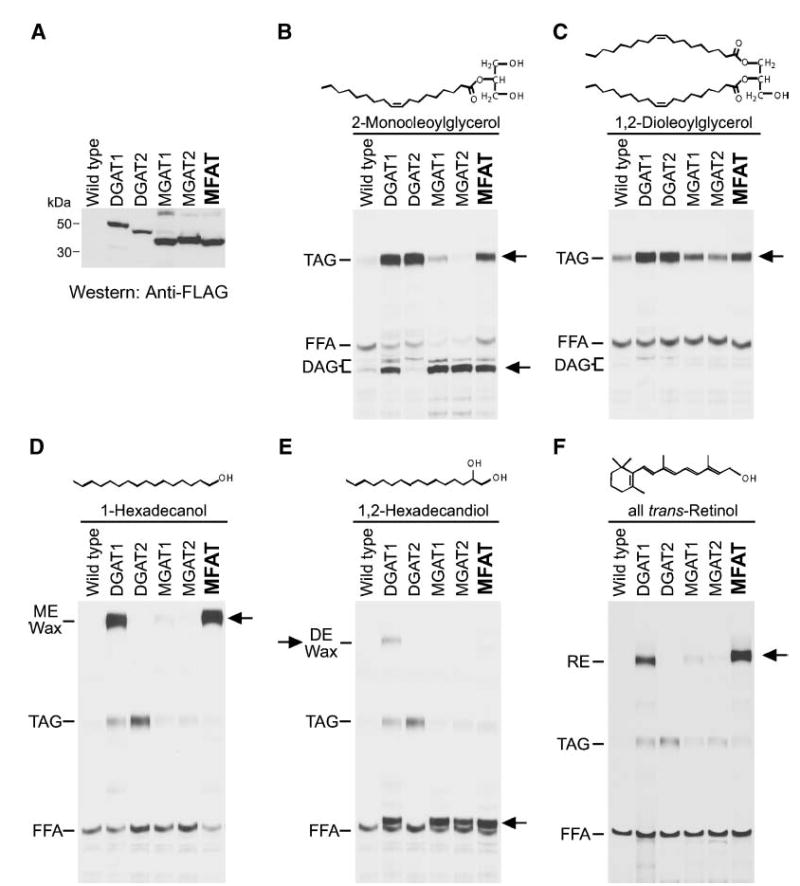
Expression of MFAT in Sf9 insect cells. A: Immunoblots of insect cell membranes. Expression of MFAT and control proteins was verified by immunoblotting with an anti-FLAG antibody. Membrane proteins (5 μg) were analyzed from Sf9 cells infected with wild-type virus or FLAG-tagged versions of mouse DGAT1, mouse DGAT2, mouse MGAT1, human MGAT2, or human MFAT recombinant baculoviruses. B–F: Expression of MFAT confers in vitro acyltransferase activities. MGAT (B), DGAT (C), wax monoester synthase (D), wax diester synthase (E), and acyl-CoA:retinol acyltransferase (ARAT; F) activities were detected by incorporation of [14C]palmitoyl-CoA (25 μM) into diacylglycerol (DAG), triacylglycerol (TAG), wax monoester (ME), wax diester (DE), or retinyl ester (RE) in the presence of 100 μM added sn-2-monooleoylglycerol, sn-1,2-dioleoylglycerol, 1-hexadecanol, 1,2-hexadecandiol, or all-trans-retinol, respectively. Neutral lipids were extracted, separated by TLC, and exposed to X-ray film. Arrows indicate the putative products of each acyltransferase activity. In E, the prominent acylation product when 1,2-hexadecandiol was provided is most likely the wax monoester 2-hydroxylhecadecyl hexadecanoate (lower arrow). Results are representative of three independent experiments.
Because MFAT shares sequence homology with MGATs and DGAT2, we first examined whether membranes expressing MFAT have MGAT and DGAT activity. With [14C]palmitoyl-CoA as a tracer, MFAT-expressing membranes incorporated more radioactivity into diacylglycerol in the presence of added monoacylglycerol than membranes expressing wild-type viral proteins (Fig. 3B), indicating that MFAT possesses MGAT activity. Because more radioactivity was also incorporated into triacylglycerol, MFAT may also possess DGAT activity. To directly examine this possibility, MFAT-expressing membranes were assayed by measuring triacylglycerol synthesis in the presence of [14C]palmitoyl-CoA and diacylglycerol substrates. MFAT appeared to have some DGAT activity (e.g., greater than that found for MGAT enzymes), but it was considerably less than that of DGAT enzymes (even though MFAT was expressed at a higher level than DGAT1 or DGAT2) (Fig. 3C).
Because MFAT is highly expressed in skin, we examined whether MFAT uses fatty acyl alcohol as an acyl-CoA acceptor to generate wax monoesters, a major type of lipid (second only to triacylglycerol) found on the surface of human skin. DGAT1, which catalyzes the synthesis of both wax monoesters and diesters (27), was included as a positive control. In the presence of 1-hexadecanol, membranes expressing MFAT, like those expressing DGAT1, catalyzed wax monoester synthesis using [14C]palmitoyl-CoA as the acyl donor (Fig. 3D). However, in the presence of 1,2-hexadecandiol, MFAT, unlike DGAT1, did not catalyze the synthesis of wax diesters (Fig. 3E). With this substrate, MFAT appeared to catalyze the esterification of only one hydroxyl group of 1,2-hexadecandiol (but not both), producing a wax monoester (most likely 2-hydroxylhecadecyl hexadecanoate). Interestingly, DGAT1, MGAT1, and MGAT2 also exhibited this wax monoester synthase activity with this substrate (Fig. 3E, lower arrow).
Because retinol has roles in skin biology and retinol esterification has been reported for skin (28, 29), we next tested whether MFAT can catalyze the synthesis of retinyl esters in assays with retinol and palmitoyl-CoA substrates. Like DGAT1, MFAT possessed robust ARAT activity (Fig. 3F).
We tested membranes expressing non-FLAG-tagged MFAT and found similar activities (data not shown), indicating that the FLAG epitope did not alter the substrate specificity of MFAT. We also tested other substrates. Consistent with the requirement for a free hydroxyl group, the retinol derivatives, including retinal and retinoic acid, did not serve as acyl acceptors in reactions catalyzed by MFAT, nor did other potential acyl acceptors, including cholesterol, glycerol, glycerol-3-phosphate, and 1-acylglycerol-3-phosphate (data not shown).
We next examined whether the MGAT, wax synthase, and ARAT activities of MFAT could be demonstrated with labeled acyl acceptors that were specific for the respective activities, and we determined whether these acyltransferase activities were dependent on the presence of acyl-CoA. When [3H]sn-2-monooleoylglycerol was used as the tracer, MFAT catalyzed the production of radiolabeled diacylglycerol and triacylglycerol, confirming its acylglycerol acyltransferase activity (Fig. 4A). Likewise, MFAT catalyzed the incorporation of [14C]1-hexadecanol and [3H]all-trans-retinol into wax ester and retinyl esters, respectively, confirming its wax synthase and ARAT activities (Fig. 4B, C). Each of these activities was dependent on the presence of palmitoyl-CoA.
Fig. 4.
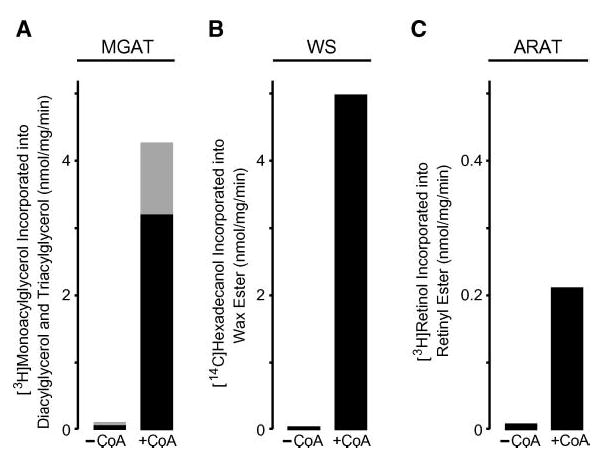
Demonstration of multiple acyl-CoA acyltransferase activities for MFAT by labeled activity-specific acyl acceptors and by dependence on acyl-CoA as an acyl donor. A: MGAT activity assessed by the amount of [3H]sn-2-monooleoylglycerol (~3,000 cpm/nmol) incorporated into both diacylglycerol (black bar) and triacylglycerol (gray bar). B: Wax monoester synthase (WS) activity assessed by the amount of [14C]1-hexadecanol (~8,000 cpm/nmol) incorporated into wax monoester. C: ARAT activity assessed by the amount of [3H]all-trans-retinol (~320,000 cpm/nmol) incorporated into retinol ester. For all panels, each acyl acceptor was tested without (−CoA) and with (+CoA) added palmitoyl-CoA. Values shown are means of duplicate measurements. The experiment was repeated twice with similar results.
We also examined the acyltransferase activities of MFAT as a function of varying the acyl acceptor concentrations in assays containing 25 μM [14C]palmitoyl-CoA. For each acyl acceptor, enzymatic activity increased with the concentration of the acceptor (Fig. 5A–D). The highest enzymatic activity was found with monoacylglycerol as the acceptor, a condition in which the results reflect both MGAT and DGAT activities (Fig. 5A). Most of the label accumulated in diacylglycerol (Fig. 5A, inset; reflecting MGAT activity), with only a minor portion accumulating in triacylglycerol, suggesting that the acylglycerol acyltransferase activity of MFAT is mainly MGAT activity. Consistent with this finding, the levels of palmitoyl-CoA incorporated into triacylglycerol when diacylglycerol was provided (reflecting DGAT activity) were much lower (Fig. 5B). Membranes expressing MFAT also exhibited wax monoester synthase activity (Fig. 5C) and ARAT activity (Fig. 5D). The results shown are from one of three experiments; we noted variability in the maximal levels of each activity in different experiments (1.8–6.7 nmol/min/mg for MGAT, 1.5–5.0 nmol/min/mg for wax synthase, and 0.2–0.8 nmol/min/mg for ARAT). In general, the maximal levels of MGAT and wax monoester synthase activities were similar, and the maximal levels of ARAT activity were 50–95% lower.
Fig. 5.
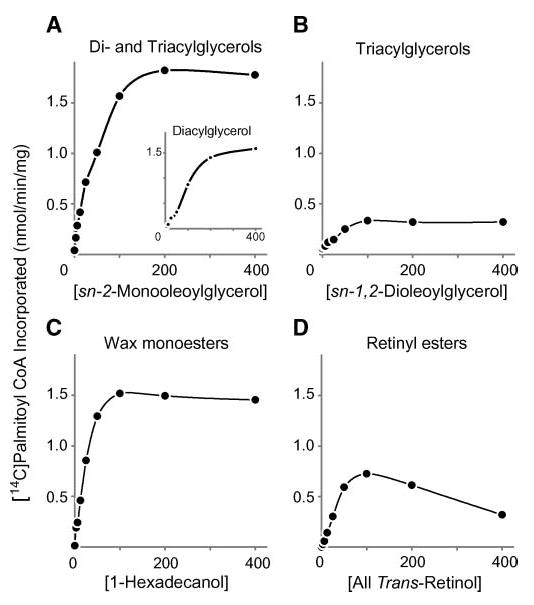
Dependence of acyltransferase activities in membranes expressing MFAT on acyl acceptor concentrations. A: Acylglycerol acyltransferase (both DGAT and MGAT) activities assessed by the amount of [14C]palmitoyl-CoA incorporated into both diacylglycerol and triacylglycerol in the presence of different concentrations of sn-2-monooleoylglycerol. The inset shows the amount of [14C]palmitoyl-CoA incorporated into diacylglycerol only. B: DGAT activity assessed by the amount of [14C]palmitoyl-CoA incorporated into triacylglycerol in the presence of different concentrations of sn-1,2-dioleoylglycerol. C: Wax monoester synthase activity assessed by the amount of [14C]palmitoyl-CoA incorporated into wax monoester in the presence of different concentrations of 1-hexadecanol. D: ARAT activity assessed by the amount of [14C]palmitoyl-CoA incorporated into retinyl ester in the presence of different concentrations of all-trans-retinol. Values are means of duplicate measurements. The experiment was repeated twice with similar results.
Expression of human MFAT in mammalian cells
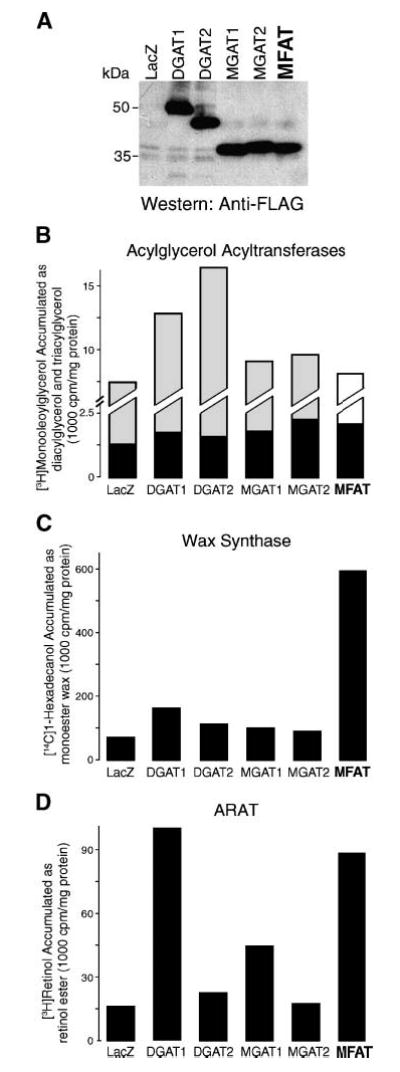
To determine whether MFAT also possesses acylglycerol acyltransferase, wax synthase, and ARAT activities in mammalian cells, we transiently overexpressed human MFAT and control enzymes in COS-7 cells and determined enzyme activities in homogenates. The expression level of FLAG-tagged MFAT was lower than those of control proteins, as demonstrated by immunoblotting (Fig. 6A). Despite the relatively low expression level, homogenates of COS-7 cells overexpressing MFAT exhibited activities similar to those of insect cell membranes expressing MFAT. Like those overexpressing DGAT1 and MGATs, these homogenates incorporated more radioactivity into diacylglycerols than controls overexpressing β-galactosidase (LacZ) or DGAT2 in assays using monoacylglycerol and [14C]palmitoyl-CoA as substrates (Fig. 6B); however, unlike those overexpressing DGAT1 and DGAT2, MFAT-overexpressing homogenates did not incorporate more radioactivity into triacylglycerols when diacylglycerol served as the acyl-CoA acceptor (Fig. 6C). Similarly, with 1-hexadecanol as the acyl-CoA acceptor, homogenates from cells expressing MFAT and DGAT1 exhibited wax monoester synthase activity (Fig. 6D), whereas with 1,2-hexadecandiol, only homogenate of DGAT1-expressing cells generated wax diester (Fig. 6E). When all-trans-retinol was the acyl-CoA acceptor, homogenates from cells expressing MFAT and DGAT1 exhibited significant ARAT activity (Fig. 6F). In some experiments, homogenates from cells expressing MGAT1 also exhibited very low levels of activity (Fig. 6F).
Fig. 6.
In vitro MGAT, wax synthase, and ARAT activities in mammalian cells overexpressing MFAT. A: Immunoblotting of FLAG-tagged proteins demonstrating the expression of FLAG-tagged MFAT and control proteins. Cells were transfected with expression vectors containing cDNAs for β-galactosidase (LacZ) or FLAG-tagged versions of DGAT1, DGAT2, MGAT1, MGAT2, or MFAT. B–F: In vitro acyltransferase activities conferred by MFAT expression. MGAT (B), DGAT (C), wax monoester synthase (D), wax diester synthase (E), and ARAT (F) activities were detected by the incorporation of [14C]palmitoyl-CoA (25 μM) into diacylglycerol (DAG), triacylglycerol (TAG), wax monoester (ME), wax diester (DE), or retinyl ester (RE) in the presence of 100 μM added sn-2-monooleoylglycerol, sn-1,2-dioleoylglycerol, 1-hexadecanol, 1,2-hexadecandiol, or all-trans-retinol, respectively. Neutral lipids were extracted, separated by TLC, and exposed to X-ray film. Arrows indicate the putative products of each acyltransferase activity. In E, the prominent acylation product when 1,2-hexadecandiol was provided is most likely the wax monoester 2-hydroxylhecadecyl hexadecanoate (lower arrow). Results are representative of two experiments.
Finally, to determine whether human MFAT possesses acylglycerol acyltransferase, wax synthase, and ARAT activities in intact cells, we measured the accumulation of radiolabeled substrates ([3H]sn-2-monooleoylglycerol, [14C] 1-hexadecanol, and [3H]all-trans-retinol) into esterification products in COS-7 cells overexpressing MFAT and control enzymes (Fig. 7A). Compared with LacZ-expressing controls, cells overexpressing MFAT had only a moderate increase in cellular diacylglycerol accumulation (150% of controls), similar to that in cells overexpressing MGAT enzymes (Fig. 7B). This most likely reflects hydrolysis of the labeled monoacylglycerol substrate or metabolism of the diacylglcyerol product in COS-7 cells, which would preclude accumulation of the label in the diacylglycerol pool. On the other hand, like cells expressing DGAT1, cells overexpressing MFAT accumulated significantly higher levels of labeled substrates in wax esters (Fig. 7C) and retinyl esters (Fig. 7D) than cells expressing other control enzymes.
Fig. 7.
Esterification of monoacylglycerol, fatty acyl alcohol, and retinol in intact cells overexpressing MFAT. A: Immunoblotting of FLAG-tagged proteins demonstrating the expression of FLAG-tagged DGAT1 and control proteins (LacZ). B–D: Esterification of monoacylglycerol, fatty acyl alcohol, and retinol in COS-7 cells overexpressing MFAT as assessed by the accumulation of prospective products. Cells were plated in six-well dishes (105/well) for 24 h and transfected with vectors containing cDNAs for MFAT or control enzymes. Twenty-four hours later, cells were incubated in medium containing 25 μM [3H]sn-2-monooleoylglycerol (specific activity, ~15,000 cpm/nmol) for 18 h (B), 10 μM [14C]1-hexadecanol (~47,000 cpm/nmol) for 6 h (C), or 2.5 μM [3H]retinol (~200,000 cpm/nmol) for 18 h (D). Neutral lipids were separated by TLC, visualized with a Bioscan imaging scanner, and scraped to assess the incorporation of radioactivity into diacylglycerol (black bar) and triacylglycerol (gray bar) (B), wax esters (C), and retinol esters (D). Values represent results from pooled six-well dishes for each group. Similar results were found in two experiments.
DISCUSSION
In this study, we show that a member of the DGAT2/MGAT gene family encodes an MFAT that is highly expressed in human skin. In in vitro assays in the membranes of insect cells and homogenates of mammalian cells overexpressing the enzyme, MFAT catalyzed esterification reactions that use monoacylglycerol, long-chain alcohol, and retinol as acyl acceptor substrates to synthesize diacylglycerols, wax monoesters, and retinyl esters, respectively. The wax synthase and ARAT activities of this enzyme were also demonstrated in intact cells. Some or all of the enzymatic activities of MFAT may be important in human skin function.
The wax synthase activity for this enzyme was recently described by two other groups, one designating the enzyme as wax synthase and one as acyl-CoA wax alcohol acyltransferase (AWAT) (25, 26). In our in vitro assays, we tested a variety of acyl-CoA acceptors and found that the enzyme had additional acylglycerol acyltransferase and retinol acyltransferase activities; therefore, we designated the enzyme as MFAT. Similarly, we recently reported that DGAT1 possesses multiple acyltransferase activities in in vitro assays, including those of monoacylglycerol acyltransferase, wax monoester and diester synthases, and retinol acyltransferase (27). Thus, DGAT1 appears also to function as an MFAT. This and other studies (25, 27) suggest that several of the enzymes in the DGAT1 and DGAT2 gene families may have broader substrate specificity than was originally recognized.
In humans, skin surface lipids are mainly composed of triacylglycerol and wax monoesters (8), and large amounts are found primarily where sebaceous glands are dense, such as scalp and forehead (10). Indeed, we found that human MFAT was expressed most highly in skin, and, using in situ hybridization, Turkish et al. (26) showed that the enzyme (AWAT2 in their report) was expressed in sebaceous glands of the scalp. Similarly, Cheng and Russell (25) found that the murine enzyme was expressed highly in the preputial glands and the meibomian glands of the eyelid, which are specialized sebaceous-like glands that produce large amounts of wax monoesters. Thus, the data collectively suggest that human MFAT expression is restricted primarily to sebaceous glands, where it synthesizes skin surface lipids.
As in other recent reports (25, 26), we found that MFAT possesses robust wax synthase activity. In humans, the predominant skin waxes are wax monoesters, containing a long-chain alcohol with a single hydroxyl group linked to a fatty acyl moiety by an ester bond. MFAT possessed wax monoester synthase activity, suggesting that it plays an important role in synthesizing these waxes found on human skin. In mice, the predominant surface lipids found on the skin are wax diesters (9, 10), containing a long-chain alcohol with two hydroxyl groups (diol) that are both linked to fatty acyl moieties by ester bonds. In our assays, MFAT did not catalyze the synthesis of wax diesters. Instead, wax diesters in mice appear to be mainly synthesized by DGAT1, because DGAT1-deficient mice have markedly reduced amounts of wax diesters in their fur lipids (15). Consistent with this conclusion, MFAT mRNA levels are not induced in the skin of DGAT1-deficient mice (M. Shih, unpublished observations).
Because MFAT belongs to a gene family that includes three MGAT enzymes, as expected, MFAT possesses in vitro MGAT activity. Whether MFAT functions as an MGAT in intact cells remains unclear because of the difficulties of assessing monoacylglycerol esterification in intact cells (monoacylglycerol, the tracer specific for the MGAT reaction, can be hydrolyzed before uptake by cells, and diacylglycerol, the MGAT reaction product, can be converted to other lipids and does not accumulate in significant amounts). Our results also suggest that MFAT possesses minor DGAT activity, which is not surprising given its homology with DGAT2. However, it is also possible that MFAT acts primarily as an MGAT and that the diacylglycerol product is subsequently used as a substrate for triacylglycerol synthesis by the endogenous DGAT activity in insect cells. Studies with purified MFAT protein will be needed to address this question. Because human skin surface lipids have a high proportion of triacylglycerols, the acylglcyerol acyltransferase activities of MFAT may contribute to triacylglycerol production in human skin. We note that our findings differ from those of Cheng and Russell (25), who did not observe MGAT activity for this enzyme. However, they examined MGAT activity only by measuring labeled fatty acid incorporation in intact Sf9 cells and not by in vitro assays, which may account for the discrepancy.
We also show that MFAT exhibits ARAT activity in in vitro assays and in intact cells. A similar finding was cited as unpublished data in the characterization of the murine enzyme by Cheng and Russell (25). Retinol esterification has been reported in human skin (30), where esters of retinol account for up to 90% of total vitamin A [~1 nmol/g in both epidermis and dermis (31)]. These retinyl esters may serve as reservoirs of retinol that is subsequently metabolized to retinoids, such as retinoic acids. The latter molecules interact with nuclear hormone receptors and participate in a variety of functions in the skin, such as the development of sebaceous glands and keratinocytes. Moreover, retinyl esters may function as a sunscreen by absorbing ultraviolet radiation (32).
It is unclear whether the formation of retinyl esters in the skin is catalyzed predominantly by ARAT or by lecithin: retinol acyltransferase, which uses phosphatidylcholine as the acyl donor (30). ARAT activity is higher in differentiated keratinocytes, whereas lecithin:retinol acyltransferase activity is higher in undifferentiated keratinocytes and is the major contributor to retinyl ester synthesis in cultured human keratinocytes (30). Our data suggest that MFAT contributes to the ARAT activity in human skin, although the biological relevance of this finding remains to be determined. DGAT1 also possesses ARAT activity and may contribute to ARAT activity in this tissue (27). Human MFAT expression was also detected at low levels in testis and lung, tissues that are reported to possess ARAT activity (33, 34).
The broad biochemical activities and high expression levels of MFAT in human skin suggest that this enzyme plays a significant role in lipid metabolism in the skin. A form of X-linked anhidrotic ectodermal dysplasia has been mapped to the region of the X chromosome where the human MFAT gene is located, although current evidence indicates that mutations in ED1, the gene located next to MFAT, are responsible for most cases of this disease (Online Mendelian Inheritance in Man™ number 305100). Further studies in humans or model organisms will be required to determine the relevance of MFAT to skin biology.
Acknowledgments
The authors thank Drs. Rosalind Coleman, Sylvaine Cases, and Robert Mahley for helpful comments on the manuscript, Bryan Tow for technical assistance, Stephen Ordway and Gary Howard for editorial assistance, and Bethany Taylor for manuscript preparation. This work was supported by the American Heart Association (a fellowship grant to C-L.E.Y.), the Sarnoff Endowment (a fellowship grant to C.H.B.), the American Diabetes Association (a mentor-based fellowship grant to M.M.), the National Institutes of Health (Grant DK-56084 to R.V.F.), and the J. David Gladstone Institutes.
References
- 1.Downing DT, Stewart ME, Wertz PW, Colton SW, Abraham W, Strauss JS. Skin lipids: an update. J Invest Dermatol. 1987;88(Suppl 3):2–6. doi: 10.1111/1523-1747.ep12468850. [DOI] [PubMed] [Google Scholar]
- 2.Elias PM, Feingold KR. Lipids and the epidermal water barrier: metabolism, regulation, and pathophysiology. Semin Dermatol. 1992;11:176–182. [PubMed] [Google Scholar]
- 3.Kao JS, Fluhr JW, Man MQ, Fowler AJ, Hachem JP, Crumrine D, Ahn SK, Brown BE, Elias PM, Feingold KR. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456–464. doi: 10.1046/j.1523-1747.2003.12053.x. [DOI] [PubMed] [Google Scholar]
- 4.Elias PM. The epidermal permeability barrier: from the early days at Harvard to emerging concepts. J Invest Dermatol. 2004;122:xxxvi–xxxix. doi: 10.1046/j.0022-202X.2004.22233.x. [DOI] [PubMed] [Google Scholar]
- 5.Stewart ME. Sebaceous gland lipids. Semin Dermatol. 1992;11:100–105. [PubMed] [Google Scholar]
- 6.Thiele JJ, Weber SU, Packer L. Sebaceous gland secretion is a major physiologic route of vitamin E delivery to skin. J Invest Dermatol. 1999;113:1006–1010. doi: 10.1046/j.1523-1747.1999.00794.x. [DOI] [PubMed] [Google Scholar]
- 7.Fluhr JW, Mao-Qiang M, Brown BE, Wertz PW, Crumrine D, Sundberg JP, Feingold KR, Elias PM. Glycerol regulates stratum corneum hydration in sebaceous gland deficient (asebia) mice. J Invest Dermatol. 2003;120:728–737. doi: 10.1046/j.1523-1747.2003.12134.x. [DOI] [PubMed] [Google Scholar]
- 8.Gunstone, F. D., J. L. Harwood, and F. B. Padley. 1994. The Lipid Handbook. 2nd edition. Chapman & Hall, London.
- 9.Wilkinson DI, Karasek MA. Skin lipids of a normal and mutant (asebic) mouse strain. J Invest Dermatol. 1966;47:449–455. doi: 10.1038/jid.1966.168. [DOI] [PubMed] [Google Scholar]
- 10.Grigor, M. R. 1977. Skin. In Lipid Metabolism in Mammals. F. Snyder, editor. Plenum Press, New York. 209–235.
- 11.Uelmen PJ, Oka K, Sullivan M, Chang CCY, Chang TY, Chan L. Tissue-specific expression and cholesterol regulation of acylcoenzyme A:cholesterol acyltransferase (ACAT) in mice. Molecular cloning of mouse ACAT cDNA, chromosomal localization, and regulation of ACAT in vivo and in vitro. J Biol Chem. 1995;270:26192–26201. doi: 10.1074/jbc.270.44.26192. [DOI] [PubMed] [Google Scholar]
- 12.Meiner V, Tam C, Gunn MD, Dong LM, Weisgraber KH, Novak S, Myers HM, Erickson SK, Farese RV., Jr Tissue expression studies of mouse acyl CoA:cholesterol acyltransferase gene (Acact): findings supporting the existence of multiple cholesterol esterification enzymes in mice. J Lipid Res. 1997;38:1928–1933. [PubMed] [Google Scholar]
- 13.Yagyu H, Kitamine T, Osuga JI, Tozawa RI, Chen Z, Kaji Y, Oka T, Perrey S, Tamura Y, Ohashi K, et al. Absence of ACAT-1 attenuates atherosclerosis but causes dry eye and cutaneous xanthomatosis in mice with congenital hyperlipidemia. J Biol Chem. 2000;275:21324–21330. doi: 10.1074/jbc.M002541200. [DOI] [PubMed] [Google Scholar]
- 14.Accad M, Smith SJ, Newland DL, Sanan DA, King LE, Jr, Linton MF, Fazio S, Farese RV., Jr Massive xanthomatosis and altered composition of atherosclerotic lesions in hyperlipidemic mice lacking acyl CoA:cholesterol acyltransferase 1. J Clin Invest. 2000;105:711–719. doi: 10.1172/JCI9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen HC, Smith SJ, Tow B, Elias PM, Farese RV., Jr Leptin modulates the effects of acyl CoA:diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J Clin Invest. 2002;109:175–181. doi: 10.1172/JCI13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Y, Eilertsen KJ, Ge L, Zhang L, Sundberg JP, Prouty SM, Stenn KS, Parimoo S. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268–270. doi: 10.1038/15446. [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131:2260–2268. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- 18.Westerberg R, Tvrdik P, Unden AB, Mansson JE, Norlen L, Jakobsson A, Holleran WH, Elias PM, Asadi A, Flodby P, et al. Role for ELOVL3 and fatty acid chain length in development of hair and skin function. J Biol Chem. 2004;279:5621–5629. doi: 10.1074/jbc.M310529200. [DOI] [PubMed] [Google Scholar]
- 19.Stone SJ, Myers H, Brown BE, Watkins SM, Feingold KR, Elias PM, Farese RV., Jr Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem. 2004;279:11767–11776. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 20.Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, Voelker T, Farese RV., Jr Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J Biol Chem. 2001;276:38870–38876. doi: 10.1074/jbc.M106219200. [DOI] [PubMed] [Google Scholar]
- 21.Yen CLE, Stone SJ, Cases S, Zhou P, Farese RV., Jr Identification of a gene encoding MGAT1, a monoacylglylcerol acyltransferase. Proc Natl Acad Sci USA. 2002;99:8512–8517. doi: 10.1073/pnas.132274899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yen CLE, Farese RV., Jr MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J Biol Chem. 2003;278:18532–18537. doi: 10.1074/jbc.M301633200. [DOI] [PubMed] [Google Scholar]
- 23.Cao J, Lockwood J, Burn P, Shi Y. Cloning and functional characterization of a mouse intestinal acyl-CoA:monoacylglycerol acyltransferase, MGAT2. J Biol Chem. 2003;278:13860–13866. doi: 10.1074/jbc.M300139200. [DOI] [PubMed] [Google Scholar]
- 24.Cheng D, Nelson TC, Chen J, Walker SG, Wardwell-Swanson J, Meegalla R, Taub R, Billheimer JT, Ramaker M, Feder JN. Identification of acyl coenzyme A:monoacylglycerol acyltransferase 3, an intestinal specific enzyme implicated in dietary fat absorption. J Biol Chem. 2003;278:13611–13614. doi: 10.1074/jbc.C300042200. [DOI] [PubMed] [Google Scholar]
- 25.Cheng JB, Russell DW. Mammalian wax biosynthesis. II. Expression cloning of wax synthase cDNAs encoding a member of the acyltransferase enzyme family. J Biol Chem. 2004;279:37798–37807. doi: 10.1074/jbc.M406226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turkish AR, Henneberry AL, Cromley D, Padamsee M, Oelkers P, Bazzi H, Christiano AM, Billheimer JT, Sturley SL. Identification of two novel human acyl-CoA wax alcohol acyltransferases: members of the diacylglycerol acyltransferase 2 (DGAT2) gene superfamily. J Biol Chem. 2005;280:14755–14764. doi: 10.1074/jbc.M500025200. [DOI] [PubMed] [Google Scholar]
- 27.Yen CLE, Monetti M, Burri BJ, Farese RV., Jr The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J Lipid Res. 2005;46:1502–1511. doi: 10.1194/jlr.M500036-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Torma H, Vahlquist A. Retinol esterification by mouse epidermal microsomes: evidence for acyl-CoA:retinol acyltransferase activity. J Invest Dermatol. 1987;88:398–402. doi: 10.1111/1523-1747.ep12469726. [DOI] [PubMed] [Google Scholar]
- 29.Roos TC, Jugert FK, Merk HF, Bickers DR. Retinoid metabolism in the skin. Pharmacol Rev. 1998;50:315–333. [PubMed] [Google Scholar]
- 30.Torma H, Vahlquist A. Vitamin A esterification in human epidermis: a relation to keratinocyte differentiation. J Invest Dermatol. 1990;94:132–138. doi: 10.1111/1523-1747.ep12873990. [DOI] [PubMed] [Google Scholar]
- 31.Vahlquist A. Vitamin A in human skin. I. Detection and identification of retinoids in normal epidermis. J Invest Dermatol. 1982;79:89–93. doi: 10.1111/1523-1747.ep12500032. [DOI] [PubMed] [Google Scholar]
- 32.Antille C, Tran C, Sorg O, Carraux P, Didierjean L, Saurat JH. Vitamin A exerts a photoprotective action in skin by absorbing ultraviolet B radiation. J Invest Dermatol. 2003;121:1163–1167. doi: 10.1046/j.1523-1747.2003.12519.x. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhary LR, Nelson EC. Some properties and subcellular distribution of acyl-coenzyme A:retinol acyltransferase activity in rat testes. Biochim Biophys Acta. 1987;917:24–32. doi: 10.1016/0005-2760(87)90279-7. [DOI] [PubMed] [Google Scholar]
- 34.Takase S, Matsumoto Y, Goda T. Lack of lecithin:retinol acyltransferase activity in chick lungs. J Nutr Sci Vitaminol (Tokyo) 1996;42:267–275. doi: 10.3177/jnsv.42.267. [DOI] [PubMed] [Google Scholar]
- 35.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]


