Abstract
Kaposi's sarcoma (KS)-associated herpesvirus (KSHV) (also known as human herpesvirus 8) is a gamma-2 herpesvirus believed to be the etiologic agent responsible for KS. The pathogenesis of this potentially life-threatening neoplasm is complex and unclear, and it is currently unknown how KSHV causes KS. Id (named for inhibitor of DNA binding or inhibitor of differentiation) proteins were identified in 1990 and found to be naturally occurring dominant-negative inhibitors of basic helix-loop-helix transcription factors. Id-1, the most well-studied member of this family, has since been shown to play a key role in several biological systems including cellular differentiation, cell cycle regulation, and tumorigenesis. In this report, we demonstrate that Id-1 is expressed at high levels in KS tumor cells both in vitro and in vivo but is expressed at relatively modest levels in endothelial cells (ECs), the likely precursor of the KS tumor cell. Infection of precursor cells with KSHV may be responsible for this enhanced expression, as KSHV infection induced Id-1 27-fold in ECs under our experimental conditions. Furthermore, we demonstrate that the KSHV-encoded latency-associated nuclear antigen (LANA) protein appears to be involved. Expression of LANA in ECs resulted in Id-1 induction that was almost identical to the induction seen with KSHV-infected ECs. These results demonstrate the expression of Id-1 in KS tumor cells and indicate the KSHV LANA protein may be, at least in part, responsible. This may be an important mechanism by which KSHV allows KS tumor cells to escape normal cell cycle regulation and enhances their proliferation.
Id proteins are a family of four helix-loop-helix (HLH) proteins (Id-1 to Id-4) initially recognized as growth factor-inducible genes that inhibit cellular differentiation (5, 36). These proteins lack the basic amino acid sequence characteristic of basic HLH (bHLH) transcription factors that is necessary for DNA binding and target gene transcription (5). Instead, Id proteins bind to bHLH transcription factors and form nonfunctional heterodimers, thereby acting as naturally occurring dominant-negative inhibitors (5).
Although Id proteins have traditionally been viewed as negative regulators of cell differentiation, recent studies indicate they have wider biological roles, including cell cycle regulation, embryonic development, cell death, and tumorigenesis (36). Several of these functions may be related to recently described interactions of Id proteins with certain non-HLH proteins, including ETS transcription factors, pRb, the pocket proteins p107 and p130, MIDA1, and Pax proteins (22, 51). With respect to normal cell cycle regulation, Id genes are induced in the G1 phase and expression is down regulated in quiescent, senescent, or terminally differentiated cells. These proteins can promote cell cycle progression through several distinct mechanisms. Id proteins prevent expression of the cyclin-dependent kinase inhibitor (CDKI) p21 by blocking bHLH transcription factor activity (41). Id-2 and Id-4, but not Id-1 or Id-3, directly interact with pRb, p107, and p130 and stimulate cell cycle progression by preventing pRb/E2F interactions (22, 26). Finally, Id-1 appears to regulate proliferation by repressing p16 expression through direct interactions with the p16 promoter and/or inactivation of ETS2 transcription factors (2, 37).
Given the role of Id proteins in cellular differentiation and cell cycle regulation, it is not surprising that Id genes display an altered expression pattern in some tumor cells. Id-1 expression has been demonstrated in several primary human tumors, including squamous cell carcinoma and endometrial, breast, and cervical cancers (3, 25, 31, 32, 45, 47). Studies have also found higher levels of Id-1 in invasive or high-grade tumors, indicating a possible correlation with more-aggressive disease (31, 47). A potential role for Id proteins in tumorigenesis has been supported experimentally in several cell types forced to overexpress Id-1. Recent studies have found that Id-1 overexpression in human keratinocytes and endothelial cells (ECs) significantly delayed the onset of cellular senescence (35, 48). However, other studies indicate that sustained elevation of Id-1 expression may lead to cellular transformation. Alani et al. demonstrated that overexpression of Id-1 in human keratinocytes resulted in immortalization of the cells, which was characterized by telomerase activity and inhibition of pRb function (1).
Kaposi's sarcoma (KS) is the most common neoplasm in AIDS patients. The pathogenesis of this disease is complex and not well understood; however, in recent years, it has become clear that KS-associated herpesvirus (KSHV) (also known as human herpesvirus 8) is the likely etiologic agent of KS (14). A vast majority of KS tumor cells in vivo are latently infected with KSHV, with less than 1% undergoing lytic cycle replication (38). The latently infected cells express a limited number of viral proteins, including the latency-associated nuclear antigen (LANA, ORF73), viral cyclin (v-cyclin, ORF72), and viral FLICE inhibitory protein (v-FLIP, ORF71, or K13) (44). Both v-cyclin and v-FLIP are functionally active homologues of host cellular proteins (cyclin D and FLIP, respectively) (8, 13, 28). LANA, however, is a unique multifunctional KSHV protein that has been shown to be involved in episome maintenance, transcriptional activation of several cellular and viral genes, and inhibition of p53 (4, 11, 18, 24, 30, 39).
In an attempt to better understand the pathogenesis of KS, we evaluated the expression of Id-1 in this disease. Id-1 levels were elevated in KS tumor cells both in vitro and in vivo, and this expression appears to be directly related to KSHV infection, as ECs (the likely precursor of the KS tumor cell) infected with KSHV dramatically increase Id-1 expression. Furthermore, forced expression of the LANA protein in ECs significantly increases Id-1 levels and appears to be responsible, at least in part, for the induction seen during viral infection. These results indicate that Id-1 may be an important mechanism by which KSHV circumvents normal cell cycle control in ECs, which may have important implications in KS tumor cell proliferation and the initiation and/or progression of KS.
MATERIALS AND METHODS
Cell culture.
Human dermal microvascular ECs were purchased from Cambrex Corporation (East Rutherford, N.J.) and cultured in EGM-2MV medium (Cambrex Corp.) on plates coated with EC attachment factor (Cell Systems, Kirkland, Wash.). Human umbilical vein ECs were isolated from freshly obtained human umbilical cords by collagenase treatment as previously described (16). The cells were plated on gelatin-coated tissue culture dishes and maintained in EGM-2MV.
KS tumor cells were isolated from primary lesions and characterized as previously described (17). The cells were plated on tissue culture dishes coated with EC attachment factor (Cell Systems) and maintained in RPMI 1640 and 20% fetal bovine serum (FBS) supplemented with 10% Nutridoma HU (Roche Molecular Biochemicals), 2 mmol of l-glutamine per liter, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 50 μg of gentamicin per ml, 50 μg of EC growth supplement (ICN Biochemicals, Aurora, Ohio) per ml, and 16 U of heparin per ml. JSC-1 cells, a KSHV- and Epstein-Barr virus (EBV)-positive cell line, was kindly provided by Richard Ambinder, and cultured in RPMI 1640 supplemented with 15% FBS and 5% normal human AB serum (7, 10).
Replicative capacity and cell proliferation.
Population doublings (PD) were calculated as described previously (48). With microvascular ECs, this calculated value represents the replicative capacity in our experiments and does not include proliferation prior to passage 3 (the passage provided by the manufacturer). Therefore, the PD value is slightly lower than that reported by other investigators (50). Cell proliferation was also evaluated using 5-bromo-2′-deoxyuridine (BrdU) labeling (BrdU enzyme-linked immunosorbent assay [ELISA]; Roche Molecular Biomedicals, Indianapolis, Ind.). Briefly, ECs were plated at 104 cells/well in a 96-well plate and incubated overnight. Fresh medium (100 μl) and BrdU (10 μM) were added to the cells, and the plates were incubated for 5 h at 37°C. The labeling medium was then removed, and the cells were fixed for 1 h at room temperature with FixDenat (200 μl) prior to labeling with a peroxidase-labeled BrdU antibody. After 60 min, the wells were washed, and 100 μl of tetramethyl-benzidine (TMB) substrate was added for color development. Results were read on an ELISA plate reader at A405 to A490. Statistical differences between each group were determined by analysis of variance.
KSHV infection of ECs.
ECs were infected with KSHV by the method described by Ciufo et al. (10). Briefly, JSC-1 cells (106 cells/ml) were stimulated with 20 ng of 12-O-tetradecanoylphorbol 13-acetate (TPA) per ml for 96 h, and the supernatant was harvested and filtered through a 0.45-μm-pore-size filter. The virus-containing supernatant was centrifuged at 20,000 × g for 2.5 h at 4°C to pellet the virus. The pellet was resuspended in EBM medium (Cambrex Corp) and immediately used to infect ECs. Following a 2-h adsorption step, complete EGM-2MV medium was added to the cells, and they were maintained using identical conditions to untreated ECs.
Infection of the ECs was characterized by immunostaining for the KSHV LANA protein (see below) and reverse transcriptase PCR (RT-PCR) to detect KSHV and EBV transcripts. RT-PCR was performed using standard methodologies as described previously (14). Primers used for analysis included ORF73 (LANA) (5′-AAG CGG TGG CCT TTG AGA AG-3′ and 5′-TGG AAG TCC CAC AGT GTT CAC-3′), EBNA-1 (5′-CTC CCT TTA CAA CCT AAG GC-3′ and 5′-CAA GGT CCT TAA TCG CAT CC-3′), and glyceraldehyde-3-phosphate dehydrogenase (5′-GGT AAG GAG ATG CTG CAT TCG C-3′ and 5′-GCC ATG GGT GGA ATC ATA TTG G-3′).
For experiments utilizing conditioned medium, KSHV-infected ECs were cultured for 3 days with no fresh medium added. The conditioned medium was collected and centrifuged briefly to remove contaminating cells. The supernatant was harvested and used either directly or as a 3:1 mixture with fresh medium for experiments as stated below in Results. Virus-depleted conditioned medium was prepared by centrifuging the medium at 20,000 × g for 2.5 h at 4°C. Under these conditions, KSHV was no longer detected in the conditioned medium by PCR (data not shown).
Immunostaining.
Five-micron-thick, formalin-fixed, paraffin-embedded tissue sections were dewaxed in xylene and ethanol, rehydrated, and briefly microwaved in citrate buffer (0.01 mol/liter) (pH 6.0) to optimize antigen retrieval (17). Sections were immunostained to detect Id-1 (SC-488; Santa Cruz Biotechnology, Santa Cruz, Calif.) using a highly sensitive avidin-biotin immunoperoxidase technique (Vectastain ABC kit; Vector Laboratories, Burlingame, Calif.) as described previously (17). To detect KSHV-encoded proteins in KSHV-infected ECs, cytospin cell preparations or cells grown on LabTek Slide Systems (Nalge Nunc International, Naperville, Ill.) were fixed and stained using the Vectastain system and anti-LANA, anti-ORF59, or anti-K8.1 antibodies (kindly provided by Bala Chandran). As LANA is expressed at low levels in infected cells, slides were processed both with and without a 3-min tyramide signal amplification (TSA Biotin system; Perkin-Elmer Life Sciences, Boston, Mass.) according to the manufacturer's instructions.
Western blot analysis.
Whole-cell extracts were prepared by lysing cells in 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) buffer containing a mixture of protease inhibitors (Mini-Complete; Roche Molecular Biochemicals), incubating the cells for 20 min on ice, and centrifuging to remove insoluble cellular debris (29). Protein concentration was determined using a Bradford Assay kit (Bio-Rad Laboratories, Hercules, Calif.). Protein (15 to 50 μg) was loaded on a sodium dodecyl sulfate-polyacrylamide gel (12.5% or 4 to 20% gradient polyacrylamide), transferred to an Immobilon-P (polyvinylidene difluoride) membrane, and blocked with 5% powdered milk in TBST (50 mM Tris [pH 7.5], 150 mM NaCl, 0.01% Tween 20). The membrane was then incubated with primary antibodies diluted in 2.5% powdered milk in TBST, washed extensively, and incubated with horseradish peroxidase-conjugated species-specific secondary antibodies (Amersham Biosciences, Piscataway, N.J.). Proteins were visualized with ECL reagents (Amersham Biosciences) according to the manufacturer's instructions. Even loading of proteins in the wells were confirmed by Ponceau S staining. Antibodies against Id-1 (SC-488), p21 (SC-397), p16 (SC-468), and p27 (SC-528) were purchased from Santa Cruz Biotechnology. The company certifies that the Id-1 antibody was prepared to the C terminus of the protein (a unique region) and does not cross-react with other members of the Id protein family (Id-2, Id-3, or Id-4). The Flag M2 antibody was purchased from Sigma-Aldrich Corp. (St. Louis, Mo.), and actin antibody (clone C4) was purchased from ICN Biomedicals, Inc. (Aurora, Ohio). Differences in protein expression were determined by densitometry analysis using Scion Image software (Scion Corporation, Frederick, Md.).
As our previous studies have demonstrated that Id-1 levels steadily decrease in normal ECs as the number of passages in culture increases, each Western blot experiment was performed using control ECs from the same donor at the same passage (48). A majority of the experiments, unless otherwise noted, were performed on “mid-passage” cells (passage 7 to 9) so that control cells expressed moderate levels of Id-1 for comparison purposes.
Retroviral expression vectors and transduction of ECs.
The LZRS vector was provided by Paul Khavari (35). The coding sequence for LANA was subcloned from pSG5-LANA (kindly provided by Kenneth Kaye), the sequences for v-cyclin and v-FLIP were amplified using RT-PCR as described previously, and the products were inserted into the LZRS vector using directional cloning (13, 20). Each construct contained an N-terminal Flag tag for rapid detection by Western blotting or flow cytometry. The Phoenix-Ampo retroviral packaging cells were obtained from the American Type Culture Collection with permission from Garry P. Nolan (23). The packaging cells were cultured in Dulbecco modified Eagle medium containing 10% FBS and transfected with the LZRS vectors using standard CaCl2 and 2× Hanks balanced salt solution methodologies. After overnight incubation of the cells, fresh medium was added, and the cells were incubated at 32°C for an additional 24 to 48 h. The retrovirus-containing supernatants were collected, filtered to remove contaminating cells, and used to infect ECs.
ECs were seeded into six-well plates and infected with 1 ml of viral supernatant in the presence of 8 μg of Polybrene per ml for 1 h at 32°C. After the cells were infected, fresh medium was added, and the cells were incubated overnight at 37°C in 5% CO2. The cells were then washed and propagated using standard tissue culture techniques. The LZRS vector containing green fluorescent protein (GFP) was used as a control to determine infection efficiency. Studies showed that we could reproducibly infect more than 85% of ECs (range, 87.4 to 93%) with the LZRS-GFP vector under these experimental conditions (data not shown). To confirm expression of the KSHV proteins in the transduced cells, flow cytometry was performed using saponin for cell permeabilization as described previously (17). Transduced cell cultures were utilized only if more than 50% of the cells were found to be expressing the viral protein.
Northern blot analysis.
Northern blot analysis was performed as previously described (33). Briefly, total RNA was extracted from cells using Trizol reagent (Invitrogen, Carlsbad, Calif.) following the manufacturer's instructions. Total RNA (12 μg) was fractionated on a 1% agarose formaldehyde gel and transferred to a nylon membrane (Zetabind; Cuno, Inc., Meriden, Conn.). The blot was prehybridized with Church's hybridization buffer and probed with 32P-labeled dCTP probes generated using gel-purified PCR products and a random prime label kit (Roche Molecular Biochemicals). The blots were washed with sodium phosphate buffers containing sodium dodecyl sulfate, EDTA, and bovine serum albumin and exposed to Kodak film. Differences in mRNA expression were determined by densitometry analysis using Scion Image software.
RESULTS
Id-1 is expressed in KS tumor cells.
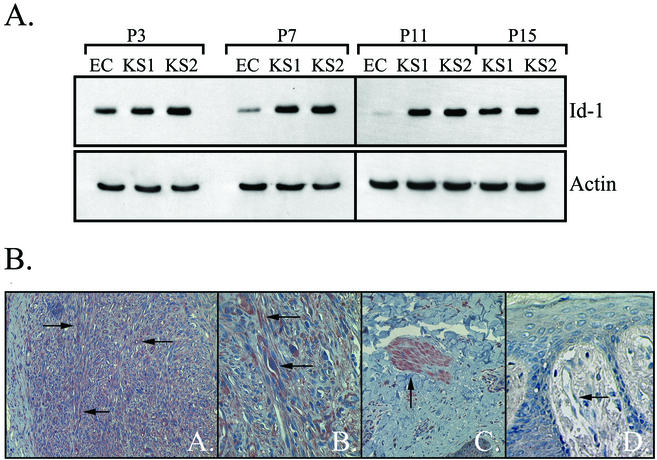
As Id-1 is expressed in normal ECs and has been shown to be upregulated in some tumors, experiments were designed to investigate expression of this protein in KS tumor cells in vitro. ECs and KS tumor cells (derived from two different patients) were grown in culture, and protein was isolated at every other passage. Although the ECs became senescent at passage 11, the KS tumor cells were actively growing in culture through passage 15 (the last passage analyzed). Western blot analysis demonstrated significantly higher levels of Id-1 in KS tumor cells than ECs at all passages tested (Fig. 1A). The ECs showed a marked decrease in Id-1 as they were passaged in culture as previously described; however, Id-1 expression persisted in both KS tumor cell lines through passage 7 after which the levels gradually began to decline (Fig. 1A) (48).
FIG. 1.
(A) Western blot analysis of Id-1 expression in ECs and KS tumor cells in culture. KS cells demonstrated higher levels of Id-1 protein than ECs. The following increases in Id-1 expression compared to that in ECs were found: at passage 3 (P3), 2.0-fold increase for KS1 cells and 3.0-fold increase for KS2 cells; at P7, 9.4-fold increase for KS1 and 10.6-fold increase for KS2; at P11, 37.0-fold increase for KS1 and 42.0-fold increase for KS2. The levels of Id-1 were 32.5- and 36.0-fold higher in KS1 and KS2 cells at P15 than in ECs at P11 (ECs were senescent at P11 in this experiment; therefore, a passage-paired control for P15 KS cells was not available). The blot was probed for expression of Id-1 and reprobed for actin to demonstrate equal loading of the proteins. A representative experiment of two independent experiments is shown. (B) Immunohistochemical staining for Id-1 in KS lesions. The spindle-shaped tumor cells of KS lesions were highly positive for Id-1 as demonstrated by the intense positive (red reaction product) staining (indicated by the arrows in micrographs A and B). The characteristic “slit-like” vascular spaces and normal ECs adjacent to the lesion were also positive for Id-1 (indicated by the arrows in micrographs A and B). Smooth muscle cells, which are known to express Id-1, served as an internal positive control (arrow in micrograph C). Quiescent ECs in normal human skin did not constitutively express Id-1 as expected (arrow in micrograph D). The results are representative of six cutaneous KS nodules from six different patients immunostained for Id-1.
As previous studies have found that in vitro, KS tumor cells lose KSHV infection, the KS cells used in this experiment were immunostained for LANA at select passages (15, 27). The results demonstrated that KS1 and KS2 cells at passage 3 were LANA positive, although the positive signal was detected only in some cells (data not shown). By passage 5, LANA positivity was not detected in either KS tumor cell culture by immunostaining (data not shown).
Id-1 is expressed in KS tumors in vivo.
To confirm and extend these results, paraffin-embedded tissue sections from KS tumor nodules were immunostained for Id-1, and the results were compared to those for normal human skin. The results demonstrated intense positive staining for Id-1 in the spindle-shaped tumor cells, the characteristic “slit-like” vascular structures, and ECs surrounding this angiogenic lesion (Fig. 1B). Smooth muscle cells were positive for Id-1, providing an internal positive control, while quiescent ECs in normal human skin did not express Id-1 (Fig. 1B).
Infection of ECs with KSHV induces Id-1 expression.
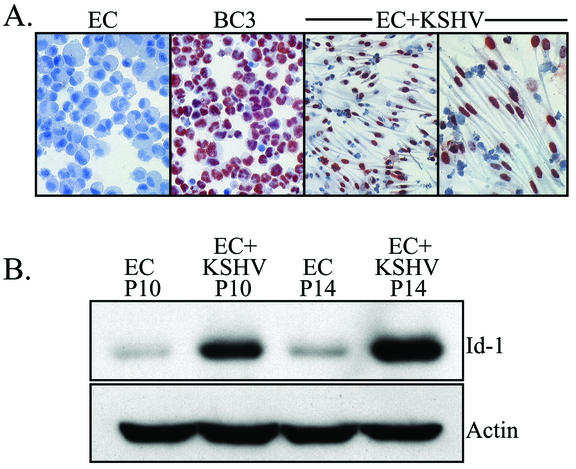
To determine whether KSHV infection was responsible for elevated Id protein expression in KS, normal human microvascular ECs were infected with KSHV using the method described by Ciufo et al. (10). Within days of infection, KSHV could be detected in a modest number of the cells as determined by immunostaining for LANA. However, as the cells were passaged in culture, the percentage of KSHV-infected cells increased as previously described (10). In our studies, 70 to 90% of the cells demonstrated KSHV positivity after approximately 30 days in culture (data not shown). The morphology of the infected ECs changed from the typical cobblestone shapes seen in EC cultures to elongated, spindle-shaped cells reminiscent of the classic appearance of KS tumor cells (Fig. 2A). A vast majority of the cells were latently infected, as indicated by positive nuclear immunostaining for LANA (Fig. 2A), and only a small percentage were undergoing spontaneous reactivation (3.0 to 6.7% ORF59 positive and 1.5 to 2.7% K8.1 positive [data not shown]). Cells were not utilized in subsequent experiments unless a majority (more than 50%) were LANA positive with immunostaining. As JSC-1 cells, the source of KSHV for infection, are coinfected with EBV, KSHV-infected ECs were also tested for the presence of this virus. RT-PCR revealed no evidence of EBV infection in the KSHV-infected EC cultures as previously described (data not shown) (10).
FIG. 2.
(A) Immunostaining of KSHV-infected ECs for LANA. Note the intense positive red reaction product in the nuclei of the KSHV-infected ECs (EC+KSHV). KSHV-infected ECs were cultured in LabTek chambers to preserve their spindle-shaped morphology during staining. Normal ECs (negative control) and BC-3 (positive control) cells are cytospin preparations and do not show cellular morphology. (B) Western blot analysis of Id-1 expression in ECs and KSHV-infected ECs (EC+KSHV). KSHV-infected cells expressed significantly higher levels of Id-1 than paired, control ECs. KSHV-infected ECs at passage 10 (P10) and P14 exhibited 11.3- and 25.3-fold increases, respectively, in Id-1 expression compared to Id-1 expression of ECs at the same passage number. The blot was probed for expression of Id-1 and reprobed for actin to demonstrate equal loading of the proteins. A representative experiment is shown. At least three independent experiments were performed and gave similar results.
Total cellular protein was isolated from KSHV-infected ECs and paired, uninfected ECs, and expression of Id-1 was evaluated by Western blotting. As shown in Fig. 2B, KSHV-infected ECs contained significantly higher levels of Id-1 than uninfected, control ECs (average fold increase of 27.3 ± 4.6). The levels of Id-1 appeared to increase with time in the KSHV-infected EC cultures, which is likely due to the increase in the number of infected cells found in later passages (Fig. 2B). This is in contrast to normal ECs, which show a steady decrease in Id-1 levels as the cells are passaged in culture (48).
As Id-1 is normally associated with increased replication, BrdU incorporation experiments were performed to compare proliferation rates between paired EC and KSHV-infected EC cultures. As shown in Fig. 3A, no difference in BrdU incorporation was found between ECs (average absorbance, 0.90 ± 0.02) and KSHV-infected ECs (average absorbance, 0.95 ± 0.07). To evaluate overall replicative capacity of the cells, PD were also calculated. The results demonstrated that KSHV-infected ECs and uninfected ECs grew similarly, and despite high levels of Id-1, the infected cells appeared to have little proliferative advantage (Fig. 3B). This finding was consistent with previously published results (10). Western blot analysis was then utilized to further investigate these findings and examine expression of CDKIs in infected and uninfected cells. Under our experimental conditions, KSHV infection of ECs resulted in an induction of p16 and p21 (average fold increases of 8.6 ± 4.8 and 2.9 ± 0.42, respectively) but not p27 (average fold increase of 1.0 ± 0.1) compared to control ECs (Fig. 3C).
FIG. 3.
(A) Infection of ECs with KSHV had no effect on EC proliferation as determined by BrdU incorporation (n.s., not significant). Similarly, transduction of ECs with the empty LZRS vector or with LZRS-vFLIP had no effect on proliferation. In contrast, expression of v-cyclin or LANA in ECs resulted in a small, but statistically significant, increase in cell proliferation (absorbance values of 1.14 ± 0.03 for v-cyclin-transduced ECs [EC+v-cyclin] and 1.17 ± 0.04 for LANA-transfected ECs [EC+LANA] were significantly different [P < 0.05] from the 0.96 ± 0.03 value for ECs). Four independent experi-ments were performed. (B) Infection with KSHV had little to no effect on the proliferative capacity of ECs. Population doubling (PD) was calculated in three independent experiments, and a representative experiment is shown. It should be noted, however, that in several experiments, including some in which PD was not calculated, the KSHV-infected ECs (EC+KSHV) appeared to grow slower than the uninfected ECs during the early passages immediately after infection. (C) Western blot analysis of CDKI expression in KSHV-infected ECs. A representative experiment is shown where expression of the following proteins in KSHV-infected ECs (EC+KSHV) was analyzed and compared to expression in ECs: Id-1 (24.8-fold increase), p16 (14.5-fold increase), p21 (2.7-fold increase), and p27 (1.0-fold increase). The blot was probed for expression of the proteins and reprobed for actin to demonstrate equal loading of the proteins. Three independent experiments were performed and gave similar results.
Soluble factors released from KSHV-infected cells play a minor role in Id-1 induction.
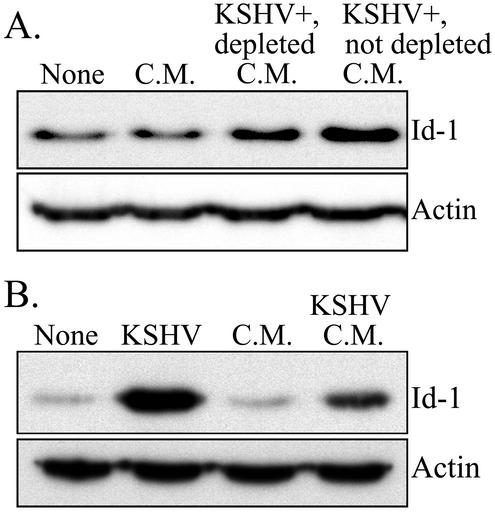
To determine whether factors released by KSHV-infected ECs, such as cytokines and/or growth factors, were responsible for the induction of Id-1, we examined Id-1 expression in ECs cultured in conditioned medium collected from either normal or KSHV-infected ECs. ECs grown in medium from uninfected cells showed no induction of Id-1 (average fold increase of 1.5 ± 0.9), while ECs cultured in conditioned medium from infected cells demonstrated a definitive increase in Id-1 expression (average fold increase of 6.0 ± 3.5-fold) (Fig. 4). As conditioned medium from the infected cells may contain infectious KSHV virions, which would complicate interpretation of the results, samples of the medium were centrifuged to pellet contaminating virus. KSHV could not be detected in this virus-depleted medium by PCR, although it was readily detected in the medium prior to centrifugation (data not shown). Virus-depleted conditioned medium was less effective (average fold increase of 3.5 ± 2.0) than untreated medium in inducing Id-1 expression (Fig. 4A), indicating that infectious virions were responsible, in part, for the Id-1 induction with conditioned medium treatment. However, the induction of Id-1 in cells cultured in conditioned medium was not nearly as dramatic as in the KSHV-infected cells from which the conditioned medium was derived (average fold increase of 27.3 ± 4.6 [Fig. 4B]). This result suggests that infection of ECs with KSHV is primarily responsible for the induction of Id-1 and that soluble factors released from the infected cells play a minor role under our experimental conditions.
FIG. 4.
Western blot analysis of Id-1 protein expression in ECs cultured in conditioned medium. (A) ECs were grown in medium (None) as a control. ECs were cultured in conditioned medium (C.M.) collected from normal ECs. ECs were also cultured in virus-depleted, conditioned medium from KSHV-infected ECs (KSHV+, depleted C.M.) and in conditioned medium from KSHV-infected ECs (KSHV+, not depleted C.M.). ECs grown in conditioned medium collected from normal ECs had a 1.1-fold increase in Id-1 expression compared to that in control ECs. ECs cultured in virus-depleted, conditioned medium from KSHV-infected ECs had a 2.8-fold increase in Id-1 compared to that in control ECs, and ECs cultured in conditioned medium from KSHV-infected ECs had a 4.3-fold increase compared to that in control ECs. (B) Id-1 expression in ECs grown in medium (None) (as a control), KSHV-infected ECs (KSHV), ECs cultured in conditioned medium collected from normal ECs (C.M.), and ECs grown in conditioned medium from KSHV-infected ECs (KSHV C.M.). The fold increases in Id-1 expression compared to that in control ECs were 37.0 for KSHV-infected ECs, 1.7 for ECs cultured in conditioned medium collected from normal ECs, and 8.0 for ECs cultured in conditioned medium from KSHV-infected ECs. In each panel, the blot was probed for expression of Id-1 and reprobed for actin to demonstrate equal loading of the proteins. Representative experiments are shown. At least three independent experiments were performed and gave similar results.
KSHV-encoded LANA and v-cyclin proteins increase Id-1 expression in ECs.
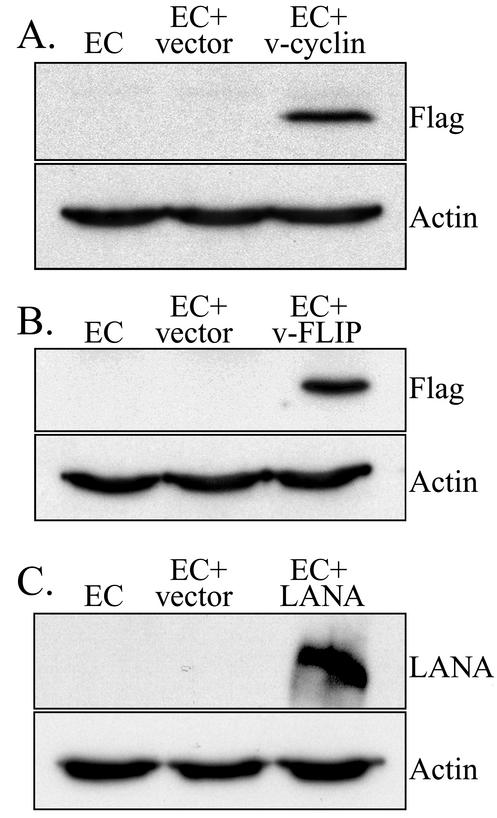
To determine whether expression of latency-associated KSHV proteins (with an emphasis on LANA, v-FLIP, and v-cyclin) was involved in Id-1 induction in KSHV-infected ECs, retroviral expression vectors coding for these three proteins were created as described in Materials and Methods. Construct integrity was evaluated using sequence analysis and/or diagnostic restriction enzyme digestion (data not shown), in addition to confirming the expression of an appropriately sized Flag-tagged product by transduced ECs by Western blot analysis (Fig. 5). Transduced cells were not selected with antibiotics, which could inadvertently select for clones that had acquired additional mutations affecting proliferative capacity and/or Id protein expression. Instead, heterogeneous mixtures of transduced cells were examined by flow cytometry to confirm expression of the Flag-tagged protein in a majority of the cells (more than 50% positive) prior to protein isolation and detection of Id-1 (data not shown).
FIG. 5.
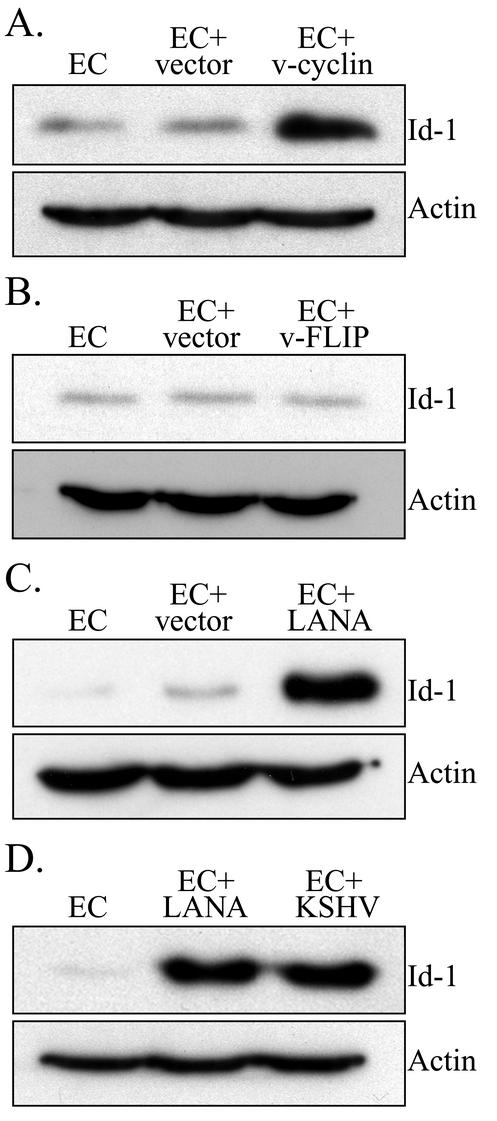
Western blot analysis for expression of Flag-tagged KSHV proteins in transduced ECs. (A) Expression of Flag-tagged KSHV proteins in ECs (as a control), ECs transduced with the empty LZRS retroviral vector (EC+vector), and ECs transduced with LZRS-v-cyclin (EC+v-cyclin). Note the band at approximately 30 kDa for ECs transduced with LZRS-v-cyclin. The Western blot was probed for expression of Flag to demonstrate expression of the viral protein and reprobed for actin (band at approximately 42 kDa) to demonstrate equal loading of proteins. (B) Expression of Flag-tagged KSHV proteins in ECs, ECs transduced with the empty LZRS retroviral vector (EC+vector), and ECs transduced with LZRS-v-FLIP. Note the band at approximately 22 kDa for ECs transduced with LZRS-v-FLIP. The Western blot was probed for expression of Flag and reprobed for actin. (C) Expression of Flag-tagged KSHV proteins in ECs, ECs transduced with the empty LZRS retroviral vector (EC+vector), and ECs transduced with LZRS-LANA. Note the band at approximately 240 kDa for ECs transduced with LZRS-LANA. The blot was probed for expression of LANA using a rabbit polyclonal anti-LANA antibody and reprobed for actin. A representative experiment is shown. At least three independent experiments were performed and gave similar results.
Western blot analysis of protein extracts from ECs transduced with v-cyclin demonstrated an increase in Id-1 expression (average fold increase of 5.1 ± 1.5), while expression of v-FLIP had no effect (average fold increase of 0.97 ± 0.09) (Fig. 6A and B). The modest Id-1 induction found in these experiments was not unexpected, as v-cyclin has been shown to be functionally active, and higher cyclin levels would promote cell cycle progression, thereby sustaining Id-1 expression. Indeed, in experiments evaluating BrdU incorporation, ECs transduced with v-cyclin showed a small, but statistically significant (P < 0.05) increase, in cell proliferation (average absorbance of 0.96 ± 0.03 for untreated ECs and 1.14 ± 0.03 for ECs transduced with v-cyclin [Fig. 3A]). In contrast, LANA dramatically increased Id-1 expression in ECs (average fold increase of 44.34 ± 12.9 [Fig. 6C and D]). LANA did not appear to cause Id-1 induction by simply increasing cellular proliferation, as BrdU incorporation studies indicate only a small increase in the proliferation of LANA-transduced ECs compared to ECs or cells transduced with empty vector (average absorbance of 0.96 ± 0.03 for ECs, 0.99 ± 0.04 for vector-transduced ECs, and 1.17 ± 0.04 for LANA-transduced ECs [Fig. 3A]). This increase in BrdU labeling, although statistically significant (P < 0.05), appears too small to be solely responsible for the dramatic increase in Id-1 expression seen under our experimental conditions (Fig. 6C and D). Interestingly, the level of Id-1 in the LANA-transduced cells was just slightly lower than that of KSHV-infected ECs analyzed on the same Western blot as control samples (Fig. 6D).
FIG. 6.
Western blot analysis for expression of Id-1 in transduced ECs. (A) Expression of Id-1 in ECs, ECs transduced with the empty LZRS retroviral vector (EC+vector), and ECs transduced with LZRS-v-cyclin. ECs transduced with the empty LZRS retroviral vector had a 0.8-fold increase in Id-1 expression compared to that in control ECs, while ECs transduced with LZRS-v-cyclin had a 7.0-fold increase compared to control ECs. (B) Expression of Id-1 in ECs, ECs transduced with the empty LZRS retroviral vector, and ECs transduced with LZRS-v-FLIP. ECs transduced with the empty LZRS retroviral vector had a 1.0-fold increase in Id-1 expression compared to that in control ECs, while ECs transduced with LZRS-v-FLIP had a 0.8-fold increase compared to control ECs. (C) Expression of Id-1 in ECs, ECs transduced with the empty LZRS retroviral vector, and ECs transducedwith LZRS-LANA. ECs transduced with the empty LZRS retroviral vector had a 2.0-fold increase in Id-1 expression compared to that of control ECs, while ECs transduced with LZRS-LANA had a 46-fold increase compared to control ECs. (D) Expression of Id-1 in ECs, ECs transduced with LZRS-LANA, and KSHV-infected ECs (EC +KSHV). ECs transduced with LZRS-LANA had a 68-fold increase in Id-1 expression compared to that in control ECs, while KSHV-infected ECs had a 70-fold increase compared to control ECs. In each panel, the blot was probed for expression of Id-1 and reprobed for actin to demonstrate equal loading of the proteins. A representative experiment is shown. At least three independent experiments were performed and gave similar results.
Transcriptional regulation appears to play a minor role in Id-1 induction by KSHV.
Interestingly, induction of Id-1 in KSHV-infected ECs has not been reported previously in studies utilizing microarray technology to identify genes induced in infected versus uninfected cells. Poole et al. analyzed the expression of more than 11,500 genes in ECs and KSHV-infected ECs in the presence or absence of TPA stimulation (40). Although Id-1 is present on both the Clontech Human Array 1.2 and the Incyte Unigem version 2.0 microarrays utilized in this study (Id-1 is listed by an alternate name, inhibitor of DNA binding 1), this gene was not identified by the researchers as significantly modulated.
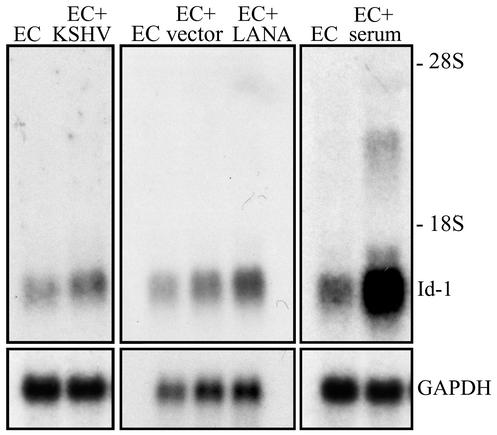
Therefore, Northern blot analysis was performed to examine Id-1 mRNA expression under our experimental conditions. There was only a modest increase in Id-1 mRNA following KSHV infection of ECs (average fold increase of 3.3 ± 2.0) compared to paired, uninfected ECs (Fig. 7). Similarly, Id-1 was not significantly induced in ECs transduced with empty vector (average fold increase of 1.75 ± 0.1) or ECs transduced with LANA (average fold increase of 3.2 ± 0.2) (Fig. 7). As a control, induction of Id-1 mRNA expression in serum-stimulated ECs is shown. ECs were serum starved overnight, and serum-containing medium was added to the cultures 1 h prior to RNA isolation. Addition of complete medium resulted in a rapid increase in Id-1 mRNA (11.3-fold increase) as previously described (49). The relatively modest induction of Id-1 mRNA expression in KSHV-infected and LANA-expressing ECs (compared to Id-1 mRNA expression after serum starvation as well as the dramatic increase in protein levels seen in these cells) may indicate that posttranscriptional regulation plays an important role in Id-1 expression in this experimental system. Experiments are in progress to clearly define the mechanism(s) by which LANA induces Id-1 expression.
FIG. 7.
Northern blot analysis for Id-1 mRNA expression. (Left) Id-1 mRNA expression in ECs and KSHV-infected ECs (EC+KSHV). The results demonstrated an average fold induction of Id-1 mRNA after KSHV infection of 3.3 ± 2. (Middle) Id-1 mRNA expression in ECs, ECs transduced with the empty LZRS vector (EC+vector), and ECs transduced with LZRS-LANA (EC+LANA). The results demonstrated an average fold increase in Id-1 mRNA after transduction with the empty vector of 1.75 ± 0.1, while LANA expression resulted in a fold increase of 3.2 ± 0.2. (Right) Id-1 mRNA expression in ECs and in ECs serum starved for 18 h prior to the addition of serum-containing medium. The cells were then cultured in complete medium for 1 h, and total RNA was extracted. The results demonstrated an 11.3-fold increase in Id-1 mRNA in serum-stimulated cells compared to untreated ECs. In each panel, the blot was probed for expression of Id-1 mRNA and reprobed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to demonstrate equal loading of RNA. A representative experiment is shown, and at least two independent experiments were performed and gave similar results.
DISCUSSION
Since the discovery of KSHV by Chang et al. (9) in 1994, many investigators have been interested in understanding what role this virus plays in the development of KS, primary effusion lymphoma, and a subset of multicentric Castleman's disease. In particular, a variety of studies have investigated whether KSHV is a transforming virus and have attempted to determine how this virus alters normal cell cycle regulation, resulting in neoplasia. We became interested in studying Id-1 as part of our ongoing investigations to better understand the pathogenesis of KS. As Id-1 is a critical factor involved in regulating the expression of key cell cycle regulatory proteins, such as p16 and p21, modulation of Id-1 expression may be an important, upstream event facilitating uncontrolled cellular proliferation (36). In addition, previous studies from our laboratory have shown that overexpression of Id-1 in human ECs significantly extends their replication capacity and alters their morphology, resulting in elongated spindle-shaped cells (48).
In the present study, we demonstrate that Id-1 is expressed at high levels in KS tumor cells both in vitro and in vivo, while this protein is expressed at more modest levels in ECs grown in culture and is not expressed by quiescent ECs in normal human skin. This finding is consistent with studies reporting elevated Id-1 levels in several primary human tumors; however, it is the first report, to our knowledge, demonstrating overexpression in an EC-derived tumor (3, 25, 31, 32, 45, 47). Our results have also demonstrated that induction of Id-1 in KS may be related to infection of the cells with KSHV. KSHV-infected ECs express, on average, 27.3-fold more Id-1 than paired, control ECs. Furthermore, these studies demonstrate that expression of the LANA protein dramatically induced Id-1. KSHV v-cyclin also induced Id-1, but the induction was modest compared to that of LANA-transduced ECs, and v-FLIP had no effect on Id-1 expression. Although quantitative analysis cannot be performed (it is unclear if LANA or v-cyclin expression using retroviral vectors is similar to that in KSHV-infected ECs or KS cells in vivo), it is interesting that LANA-transduced ECs and KSHV-infected ECs induced Id-1 expression similarly (Fig. 6). Further investigation is needed to determine whether LANA is primarily responsible for Id-1 induction by KSHV or if other KSHV proteins (including v-cyclin) also play a significant role.
Although KS tumor cells in vitro clearly overexpress Id-1 compared to ECs, it is unknown why the KS cells expressed constant levels of Id-1 for at least four passages after the loss of detectable LANA expression (Fig. 1A). Even though immunostaining is a relatively insensitive method to detect low levels of protein expression, the loss of detectable LANA after passage 3 is consistent with previous studies utilizing PCR to detect KSHV infection in cultured KS cells (15, 27). It is, however, possible that the alterations responsible for Id-1 induction by LANA are not immediately reversed following loss of the virus, allowing for sustained Id-1 expression for a period of time. The gradual loss of Id-1 seen in cells at later passages in this study was not unexpected, as KS tumor cells in culture are not immortalized and eventually undergo senescence. Clearly, additional KS tumor cell cultures (only two cultures were studied in Fig. 1A) need to be examined to determine the reproducibility of this finding and to examine the mechanism involved in persistent Id-1 expression.
As Id-1 is expressed in most replicating cells, it could be argued that KSHV or KSHV LANA increases Id-1 by providing a persistent mitogenic signal, resulting in a larger number of replicating cells and thus an increase in Id-1 as a result of increased proliferation. We do not believe this is the case for several reasons. First, increased cell proliferation would result in higher Id-1 levels based on transcriptional regulation, which has been shown to be crucial to Id-1 induction in normal replicating cells (49). Indeed, data presented here (Fig. 7) show that release of ECs from a block in the G1 phase of the cell cycle (by serum starvation, followed by serum stimulation) results in an 11.3-fold increase in Id-1 mRNA expression. In contrast, there is only a modest increase in Id-1 transcription in KSHV-infected and LANA-transduced ECs (3.3- and 3.2-fold increases, respectively). These results suggest posttranscriptional regulation may be the predominant mechanism by which Id-1 is regulated by KSHV. If correct, this conclusion would be consistent with the results of Poole et al. (40), as posttranscriptional regulation of Id-1 expression would be missed by microarray analysis despite the dramatic induction of Id-1 protein demonstrated in the present study. Studies are in progress to further define the transcriptional and posttranscriptional mechanisms involved in Id-1 induction by KSHV. Second, the BrdU incorporation and PD data (Fig. 3A and B) indicate that KSHV-infected ECs are not replicating faster than uninfected cells. In fact, in several experiments, ECs grew more slowly after infection than normal ECs. It is unclear whether the slight increase in cell proliferation noted in the BrdU studies with LANA- and v-cyclin-transduced ECs is physiologically important. It is, however, interesting that there was no difference in the proliferation of the v-cyclin- and LANA-transduced ECs despite the dramatic differences in Id-1 protein expression found in these cells. Finally, studies by Hollnagel et al. (21) found that stimulation of ECs with bone morphogenetic proteins induced Id-1 expression, which did not correlate with DNA synthesis as indicated in [3H]thymidine incorporation assays. This result indicates Id-1 levels can be regulated posttranscriptionally and supports our conclusion.
It is currently unknown how LANA induces Id-1 expression. On the basis of the Northern blot analysis shown in Fig. 7, we hypothesize that Id-1 expression under our experimental conditions may be regulated primarily through a posttranscriptional mechanism. Previous studies have demonstrated posttranscriptional regulation of Id proteins via expression of splice variants and stabilization of Id proteins through heterodimerization with bHLH transcription factors (12, 34, 46). Our Northern blot data do not indicate expression of the Id-1 splice variant (termed Id-1′) in this system. However, it is possible that Id-1 binds to proteins (potentially LANA or HLH proteins), preventing its degradation by the ubiquitin-proteasome pathway (6). Previous studies have shown that LANA can interact with several cellular proteins, including pRb and p53 (18, 42). Furthermore, Id proteins are known to interact with various HLH and non-HLH proteins, including pRb. Taken together, these findings indicate that stabilization of Id-1 expression through binding of Id-1 to LANA or other proteins is a plausible explanation. Alternatively, it is possible that the modest mRNA induction seen in our Northern blot analysis directs substantial protein expression. If this were the case, then LANA may increase Id-1 by inducing expression of transcription factors, such as Egr-1, that have been shown to activate the Id-1 promoter (34, 49). However, studies have also demonstrated that LANA can directly bind DNA and can induce transcription of its own promoter (19, 43). Therefore, LANA may bind to the Id-1 promoter and directly induce protein expression.
Despite high Id-1 levels, KSHV-infected ECs appear to have little to no advantage in overall replicative capacity; a finding that is consistent with results published by Ciufo et al. (10). This may be due to the upregulation of p16 (and to a lesser extent p21), which was also detected in the infected cells. Studies from our laboratory showing that expression of high p16 levels in ECs can overcome Id-1 overexpression, resulting in cellular senescence, support this hypothesis (48). However, the induction of p16 in infected cells in vitro may be related to culture conditions, which can only partially mimic the microenvironment of KS tumor cells in vivo. Preliminary studies indicate that the tumor cells in KS nodules in vivo are weakly positive for p16, as shown by immunostaining (unpublished observation); therefore, overexpression of Id-1 in these cells may be biologically important in extending replicative capacity. Alternatively, another mechanism may be responsible for senescence of KSHV-infected ECs in vitro. Factors, such as pRb, that act downstream of CDKI activity may be modulated under KSHV-infected EC culture conditions, resulting in senescence.
In conclusion, the overexpression of Id-1 in KS tumor cells may be an important mechanism in enhancing cellular replicative capacity. As Id proteins have been associated with tumorigenesis either as an oncogene or a cooperative oncogene, induction of Id-1 by the KSHV LANA protein may be an important early event in the pathogenesis of KS. Further studies are necessary to determine the biological relevance of this novel finding.
Acknowledgments
This study was supported in part by Public Health Service grant CA76951 (K.E.F.) from the National Cancer Institute.
We thank Kenneth Kaye, Garry Nolan, Paul Khavari, Richard Ambinder, and Bala Chandran for providing reagents and Thomas Gallagher (Loyola University Medical Center) and members of the Skin Cancer Research Program for helpful discussions.
REFERENCES
- 1.Alani, R. M., J. Hasskarl, M. Grace, M. C. Hernandez, M. A. Israel, and K. Munger. 1999. Immortalization of primary human keratinocytes by the helix-loop-helix protein, Id-1. Proc. Natl. Acad. Sci. USA 96:9637-9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alani, R. M., A. Z. Young, and C. B. Shifflett. 2001. Id1 regulation of cellular senescence through transcriptional repression of p16/Ink4a. Proc. Natl. Acad. Sci. USA 98:7812-7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andres-Barquin, P. J., M. C. Hernandez, T. E. Hayes, R. D. McKay, and M. A. Israel. 1997. Id genes encoding inhibitors of transcription are expressed during in vitro astrocyte differentiation and in cell lines derived from astrocytic tumors. Cancer Res. 57:215-220. [PubMed] [Google Scholar]
- 4.Ballestas, M. E., and K. M. Kaye. 2001. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 75:3250-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benezra, R., R. L. Davis, D. Lockshon, D. L. Turner, and H. Weintraub. 1990. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell 61:49-59. [DOI] [PubMed] [Google Scholar]
- 6.Bounpheng, M. A., J. J. Dimas, S. G. Dodds, and B. A. Christy. 1999. Degradation of Id proteins by the ubiquitin-proteasome pathway. FASEB J. 13:2257-2264. [PubMed] [Google Scholar]
- 7.Cannon, J. S., D. Ciufo, A. L. Hawkins, C. A. Griffin, M. J. Borowitz, G. S. Hayward, and R. F. Ambinder. 2000. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi's sarcoma herpesvirus-containing supernatant. J. Virol. 74:10187-10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cesarman, E., R. G. Nador, F. Bai, R. A. Bohenzky, J. J. Russo, P. S. Moore, Y. Chang, and D. M. Knowles. 1996. Kaposi's sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J. Virol. 70:8218-8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 10.Ciufo, D. M., J. S. Cannon, L. J. Poole, F. Y. Wu, P. Murray, R. F. Ambinder, and G. S. Hayward. 2001. Spindle cell conversion by Kaposi's sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J. Virol. 75:5614-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotter, M. A., and E. S. Robertson. 1999. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264:254-264. [DOI] [PubMed] [Google Scholar]
- 12.Deed, R. W., S. Armitage, and J. D. Norton. 1996. Nuclear localization and regulation of Id protein through an E protein-mediated chaperone mechanism. J. Biol. Chem. 271:23603-23606. [DOI] [PubMed] [Google Scholar]
- 13.Djerbi, M., V. Screpanti, A. I. Catrina, B. Bogen, P. Biberfeld, and A. Grandien. 1999. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J. Exp. Med. 190:1025-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foreman, K. E., J. Friborg, B. Chandran, H. Katano, T. Sata, M. Mercader, G. J. Nabel, and B. J. Nickoloff. 2001. Injection of human herpesvirus-8 in human skin engrafted on SCID mice induces Kaposi's sarcoma-like lesions. J. Dermatol. Sci. 26:182-193. [DOI] [PubMed] [Google Scholar]
- 15.Foreman, K. E., J. Friborg, W. Kong, C. Woffendin, P. J. Polverini, B. J. Nickoloff, and G. J. Nabel. 1997. Propagation of a human herpesvirus from AIDS-associated Kaposi's sarcoma. N. Engl. J. Med. 336:163-171. [DOI] [PubMed] [Google Scholar]
- 16.Foreman, K. E., A. A. Vaporciyan, B. K. Bonish, M. L. Jones, K. J. Johnson, M. M. Glovsky, S. M. Eddy, and P. A. Ward. 1994. C5a-induced expression of P-selectin in endothelial cells. J. Clin. Investig. 94:1147-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foreman, K. E., T. Wrone-Smith, L. H. Boise, C. B. Thompson, P. J. Polverini, P. L. Simonian, G. Nunez, and B. J. Nickoloff. 1996. Kaposi's sarcoma tumor cells preferentially express Bcl-xL. Am. J. Pathol. 149:795-803. [PMC free article] [PubMed] [Google Scholar]
- 18.Friborg, J., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 19.Garber, A. C., M. A. Shu, J. Hu, and R. Renne. 2001. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:7882-7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godden-Kent, D., S. J. Talbot, C. Boshoff, Y. Chang, P. S. Moore, R. A. Weiss, and S. Mittnacht. 1997. The cyclin encoded by Kaposi's sarcoma-associated herpesvirus stimulated cdk6 to phosphorylate the retinoblastoma protein and histone H1. J. Virol. 71:4193-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollnagel, A., V. Oehlmann, J. Heymer, U. Ruther, and A. Nordheim. 1999. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J. Biol. Chem. 274:19838-19845. [DOI] [PubMed] [Google Scholar]
- 22.Iavarone, A., P. Garg, A. Lasorella, J. Hsu, and M. A. Israel. 1994. The helix-loop-helix protein Id-2 enhances cell proliferation and binds to the retinoblastoma protein. Genes Dev. 8:1270-1284. [DOI] [PubMed] [Google Scholar]
- 23.Kinsella, T. M., and G. P. Nolan. 1996. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 7:1405-1413. [DOI] [PubMed] [Google Scholar]
- 24.Krithivas, A., D. B. Young, G. Liao, D. Greene, and S. D. Hayward. 2000. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J. Virol. 74:9637-9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langlands, K., G. A. Down, and T. Kealey. 2000. Id proteins are dynamically expressed in normal epidermis and dysregulated in squamous cell carcinoma. Cancer Res. 60:5929-5933. [PubMed] [Google Scholar]
- 26.Lasorella, A., A. Iavarone, and M. A. Israel. 1996. Id2 specifically alters regulation of the cell cycle by tumor suppressor proteins. Mol. Cell. Biol. 16:2570-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebbe, C., P. de Cremoux, M. Rybojad, C. Costa da Cunha, P. Morel, and F. Calvo. 1995. Kaposi's sarcoma and new herpesvirus. Lancet 345:1180. [DOI] [PubMed] [Google Scholar]
- 28.Li, M., H. Lee, D. W. Yoon, J. C. Albrecht, B. Fleckenstein, F. Neipel, and J. U. Jung. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional cyclin. J. Virol. 71:1984-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liles, W. C., J. A. Ledbetter, A. W. Waltersdorph, and S. J. Klebanoff. 1995. Cross-linking of CD45 enhances activation of the respiratory burst in response to specific stimuli in human phagocytes. J. Immunol. 155:2175-2184. [PubMed] [Google Scholar]
- 30.Lim, C., H. Sohn, Y. Gwack, and J. Choe. 2000. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J. Gen. Virol. 81:2645-2652. [DOI] [PubMed] [Google Scholar]
- 31.Lin, C. Q., J. Singh, K. Murata, Y. Itahana, S. Parrinello, S. H. Liang, C. E. Gillett, J. Campisi, and P. Y. Desprez. 2000. A role for Id-1 in the aggressive phenotype and steroid hormone response of human breast cancer cells. Cancer Res. 60:1332-1340. [PubMed] [Google Scholar]
- 32.Maruyama, H., J. Kleeff, S. Wildi, H. Friess, M. W. Buchler, M. A. Israel, and M. Korc. 1999. Id-1 and Id-2 are overexpressed in pancreatic cancer and in dysplastic lesions in chronic pancreatitis. Am. J. Pathol. 155:815-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercader, M., B. Taddeo, J. R. Panella, B. Chandran, B. J. Nickoloff, and K. E. Foreman. 2000. Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. Am. J. Pathol. 156:1961-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nehlin, J. O., E. Hara, W. L. Kuo, C. Collins, and J. Campisi. 1997. Genomic organization, sequence, and chromosomal localization of the human helix-loop-helix Id1 gene. Biochem. Biophys. Res. Commun. 231:628-634. [DOI] [PubMed] [Google Scholar]
- 35.Nickoloff, B. J., V. Chaturvedi, P. Bacon, J. Z. Qin, M. F. Denning, and M. O. Diaz. 2000. Id-1 delays senescence but does not immortalize keratinocytes. J. Biol. Chem. 275:27501-27504. [DOI] [PubMed] [Google Scholar]
- 36.Norton, J. D. 2000. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J. Cell Sci. 113:3897-3905. [DOI] [PubMed] [Google Scholar]
- 37.Ohtani, N., Z. Zebedee, T. J. Huot, J. A. Stinson, M. Sugimoto, Y. Ohashi, A. D. Sharrocks, G. Peters, and E. Hara. 2001. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature 409:1067-1070. [DOI] [PubMed] [Google Scholar]
- 38.Orenstein, J. M., S. Alkan, A. Blauvelt, K. T. Jeang, M. D. Weinstein, D. Ganem, and B. Herndier. 1997. Visualization of human herpesvirus type 8 in Kaposi's sarcoma by light and transmission electron microscopy. AIDS 11:F35-F45. [DOI] [PubMed] [Google Scholar]
- 39.Paulose-Murphy, M., N. K. Ha, C. Xiang, Y. Chen, L. Gillim, R. Yarchoan, P. Meltzer, M. Bittner, J. Trent, and S. Zeichner. 2001. Transcription program of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus). J. Virol. 75:4843-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poole, L. J., Y. Yu, P. S. Kim, Q. Z. Zheng, J. Pevsner, and G. S. Hayward. 2002. Altered patterns of cellular gene expression in dermal microvascular endothelial cells infected with Kaposi's sarcoma-associated herpesvirus. J. Virol. 76:3395-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prabhu, S., A. Ignatova, S. T. Park, and X. H. Sun. 1997. Regulation of the expression of cyclin-dependent kinase inhibitor p21 by E2A and Id proteins. Mol. Cell. Biol. 17:5888-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radkov, S. A., P. Kellam, and C. Boshoff. 2000. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 6:1121-1127. [DOI] [PubMed] [Google Scholar]
- 43.Renne, R., C. Barry, D. Dittmer, N. Compitello, P. O. Brown, and D. Ganem. 2001. Modulation of cellular and viral gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarid, R., O. Flore, R. A. Bohenzky, Y. Chang, and P. S. Moore. 1998. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1). J. Virol. 72:1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schindl, M., G. Oberhuber, A. Obermair, S. F. Schoppmann, B. Karner, and P. Birner. 2001. Overexpression of Id-1 protein is a marker for unfavorable prognosis in early-stage cervical cancer. Cancer Res. 61:5703-5706. [PubMed] [Google Scholar]
- 46.Springhorn, J. P., K. Singh, R. A. Kelly, and T. W. Smith. 1994. Posttranscriptional regulation of Id1 activity in cardiac muscle. Alternative splicing of novel Id1 transcript permits homodimerization. J. Biol. Chem. 269:5132-5136. [PubMed] [Google Scholar]
- 47.Takai, N., T. Miyazaki, K. Fujisawa, K. Nasu, and I. Miyakawa. 2001. Id1 expression is associated with histological grade and invasive behavior in endometrial carcinoma. Cancer Lett. 165:185-193. [DOI] [PubMed] [Google Scholar]
- 48.Tang, J., G. M. Gordon, B. J. Nickoloff, and K. E. Foreman. 2002. The helix-loop-helix protein id-1 delays onset of replicative senescence in human endothelial cells. Lab. Investig. 82:1073-1079. [DOI] [PubMed] [Google Scholar]
- 49.Tournay, O., and R. Benezra. 1996. Transcription of the dominant-negative helix-loop-helix protein Id1 is regulated by a protein complex containing the immediate-early response gene Egr-1. Mol. Cell. Biol. 16:2418-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, J., E. Chang, A. M. Cherry, C. D. Bangs, Y. Oei, A. Bodnar, A. Bronstein, C. P. Chiu, and G. S. Herron. 1999. Human endothelial cell life extension by telomerase expression. J. Biol. Chem. 274:26141-26148. [DOI] [PubMed] [Google Scholar]
- 51.Yates, P. R., G. T. Atherton, R. W. Deed, J. D. Norton, and A. D. Sharrocks. 1999. Id helix-loop-helix proteins inhibit nucleoprotein complex formation by the TCF ETS-domain transcription factors. EMBO J. 18:968-976. [DOI] [PMC free article] [PubMed] [Google Scholar]