Abstract
Different tissue culture cell lines infected with a number of alphaherpesviruses produce, in addition to virions, light particles (L particles). L particles are composed of the envelope and tegument components of the virion but totally lack the proteins of the capsid and the virus genome; therefore, they are noninfectious. In this electron microscopy report, we show that L particles are produced during primary replication of the alphaherpesvirus pseudorabies virus (PRV) in the nasal mucosa of experimentally infected swine, its natural host. Although PRV infected different types of cells of the respiratory and olfactory mucosae, PRV L particles were found to be produced exclusively by epithelial cells and fibroblasts. We observed that formation of noninfectious particles occurred by budding of condensed tegument at the inner nuclear membrane and at membranes of cytoplasmic vesicles, resulting in intracisternal and intravesicular L particles, respectively. Both forms of capsidless particles were clearly distinguishable by the presence of prominent surface projections on the envelope and the higher electron density of the tegument, morphological features which were only observed in intravesicular L particles. Moreover, intravesicular but not intracisternal L particles were found to be released by exocytosis and were also identified extracellularly. Comparative analysis between PRV virion and L-particle morphogenesis indicates that both types of virus particles share a common intracellular pathway of assembly and egress but that they show different production patterns during the replication cycle of PRV.
The only purpose of a virus is to perpetuate its genome, and this objective is achieved through parasitization of a host cell and subsequent production of infectious progeny. In the case of alphaherpesviruses, virions released from infected cells are the sole product of virus replication with the capacity to infect new cells and thus to maintain the virus in nature. Alphaherpesvirus virions consist of four distinct morphological components, each of them playing an important role in the infectious process: (i) an external lipid bilayer envelope that contains virus-encoded proteins essential for the attachment and fusion of the virus envelope with the plasma membrane of target cells (11, 25, 36); (ii) a characteristic layer (known as the tegument) consisting of proteins that are transferred into the cytosol immediately after virus entry and that enhance the ability of virions to initiate the process of infection (7, 8, 32); and (iii) an icosahedral capsid (consisting of 162 capsomers) that contains and transports (iv) a double-stranded linear DNA molecule up to the nuclear envelope, where the capsid interacts with the nuclear pore complexes to release the virus genome into the nucleus (15, 27, 32). After virus gene expression and DNA replication, progeny nucleocapsids assemble in the nucleus and reach the cytoplasm by envelopment followed by deenvelopment at the nuclear membranes. Recent data indicate that the definitive virus envelope is acquired by secondary envelopment of intracytoplasmic nucleocapsids at membranes of the trans-Golgi network, resulting in mature alphaherpesvirions contained in transporting vesicles that finally egress by exocytosis (1, 4, 5, 14, 15, 16, 26, 35, 40, 42). The above-described events leading to the production of progeny virions can occur immediately after virus entry into a cell or, when a latent infection is established, following an episode of reactivation (reviewed in reference 19).
Although the virion is the only infectious particle, it is not the only product of alphaherpesvirus replication. Apart from the fact that egress of immature and empty enveloped capsids has been reported previously (15, 34), cell cultures infected with alphaherpesviruses release a distinct type of noninfectious particle designated light particle (L particle). L particles lack the virus genome and the capsid but appear to contain all the envelope and tegument proteins of virions and, in addition, proteins not present in the infectious particles (23, 39). They were first shown in herpes simplex virus type 1 (HSV-1)-infected cells (39), but subsequent studies demonstrated that particles analogous to those produced by HSV-1 are identifiable in cell cultures infected with equine herpesvirus 1, pseudorabies virus (PRV) (23), bovine herpesvirus 1, varicella-zoster virus, HSV-2 (9), and gallid herpesvirus 1 (16).
Various studies carried out mainly with HSV-1 have shown a number of interesting features of these noninfectious particles (reviewed in reference 37). Of major significance among them is the fact that L particles seem to behave like virions with regard to the processes of attachment, fusion, and release of functional tegument proteins into the cytoplasm of infected cells (8, 24) and that these tegument proteins are active in enhancing the infectivity of both homologous and heterologous transfected alphaherpesvirus DNA (8). The biological competence of L particles, at least in cell cultures, has prompted several authors to propose that these virus particles boost successful initiation of the infectious process under adverse conditions for virions, i.e., during natural infection (8, 24, 37). In line with this, the present study set out to investigate whether L particles are produced concurrently with virions in vivo and, if so, to clarify the morphological events of L-particle formation and egress with the aim of gaining insights into the possible role played by them in the infectious process. To this end, we used a virulent strain of the alphaherpesvirus PRV and performed a transmission electron microscopy (TEM) study on the nasal mucosa of swine, its natural host. The nasal mucosa was chosen because this tissue usually supports primary replication of PRV during natural infection; consequently, it is the most common port of entry of this neurotropic virus into the nervous system of infected animals.
MATERIALS AND METHODS
Animals.
The study was conducted using two 2-month-old conventional pigs that were not vaccinated against PRV and were determined by enzyme-linked immunosorbent assay to be PRV seronegative before the start of the experiment. The animals were caged individually under strict isolation containment, with water and commercial food freely available.
Virus and inoculation procedure.
The E-974 strain is a high-virulence field strain of PRV which was isolated in northwestern Spain from the brain tissue of naturally infected pigs and adapted to cell culture. Information about the preparation, characterization, and use of this strain has been published previously (2, 10, 29, 30, 31). The titer of the virus suspension, determined on BHK-21 cells, was 106.5 50% tissue culture infective doses of PRV-E-974 per ml. The pigs were placed on their backs and inoculated intranasally by inserting a catheter connected to a syringe until it reached the ethmoturbinates. A total of 2 ml of virus suspension (1 ml in each nostril) was slowly administered, and the animals were kept in that position for a few minutes to ensure contact of the inoculum with the nasal epithelium. The pigs were euthanized with an intravenous injection of sodium pentobarbital 72 h after inoculation.
TEM.
Small pieces from the respiratory and olfactory mucosae were fixed with cold 2.5% glutaraldehyde buffered in 0.1 M cacodylate (pH 7.3). They were then postfixed in 1.0% aqueous osmium tetroxide, stained in 0.5% alcoholic uranyl acetate, and dehydrated in a graded ethanol series. Thereafter, samples were cleared in propylene oxide and embedded in Epon 812. Semithin sections (0.5 to 1.0 μm) stained with toluidine blue were used to select areas for thin sectioning. Ultrathin sections were counterstained with uranyl acetate and lead citrate and examined under a JEOL SX100 TEM.
Immunohistochemistry (IHC).
Samples from the respiratory and olfactory mucosae adjacent to those taken for ultrastructural examination were fixed in 10% buffered formalin, processed by routine histological methods, embedded in paraffin, and sectioned at 4 μm. PRV antigen was detected on paraffin sections by using a rabbit polyclonal antiserum and a large-volume DAKO LSAB peroxidase kit (Dako Corp., Carpinteria, Calif.) according to the manufacturer's instructions. In each series of stained slides, positive- and negative-control sections of porcine nasal mucosa were included to assess the specificity of the assay.
RESULTS
IHC.
To confirm PRV infection, IHC tests were performed on paraffin sections of nasal mucosa from the intranasally inoculated pigs. PRV antigen was readily detected within small and large foci of infection in both the respiratory and olfactory mucosae (data not shown). In small foci of infection, immunopositive cells were located in the epithelial lining and the uppermost layer of the lamina propria. Large foci of infection extended into the lamina propria and even reached the underlying bone or cartilage. The centers of these large foci often appeared ulcerated. Positive immunoreaction was detected in the cytoplasm of lining and glandular epithelial cells, fibroblasts, endothelial cells, and infiltrated macrophages and lymphocytes. Olfactory neurons located in the epithelial lining of the olfactory mucosa were included among the cells that formed foci of infection, and many infected olfactory nerves were also observed in the lamina propria.
Identification of PRV L particles.
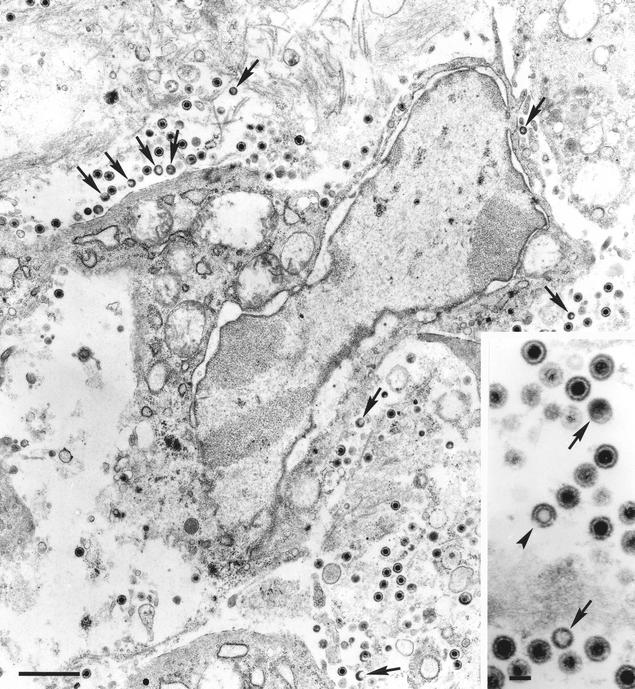
L particles were first identified in areas of the nasal mucosa in which virions were detected. They were found extracellularly located both in the lumen of the nasal cavity and in the connective matrix of the lamina propria, as well as intracellularly within membranous organelles in epithelial cells and fibroblasts. Noninfectious particles consisted of a mass of proteinaceous material of variable electron density, sometimes exhibiting an eccentric electron-lucent area, surrounded by an envelope and with diameters ranging from 120 to 200 nm (Fig. 1).
FIG. 1.
Low-magnification TEM micrograph of the lamina propria of the porcine nasal mucosa 72 h after intranasal inoculation of PRV strain E-974. Extracellular L particles (arrows) and virions can be observed in the connective matrix that surrounds the infected fibroblast. The inset shows two L particles (arrows), numerous virions, and an empty enveloped capsid (arrowhead). Bars, 1 μm and 150 nm (inset).
Morphogenesis of PRV L particles.
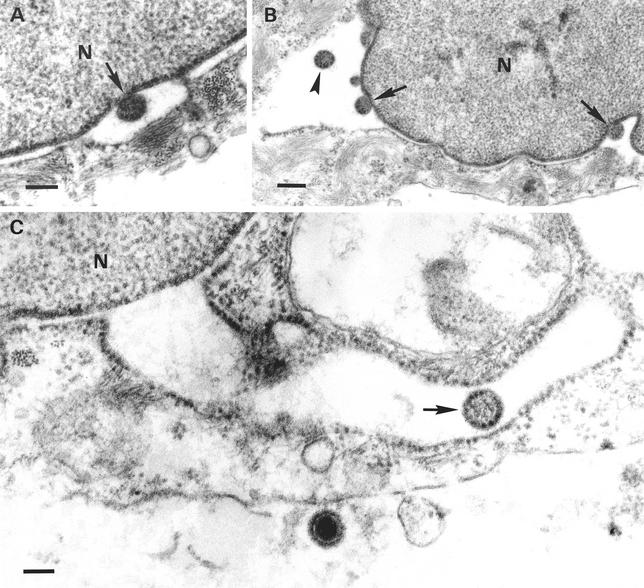
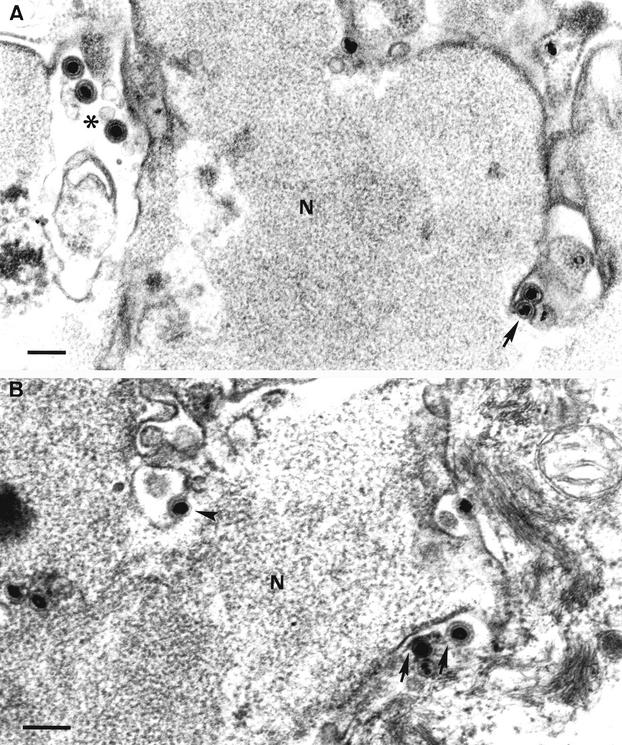
Formation of capsidless particles was found to occur by budding of condensed tegument associated with the inner nuclear membrane into the perinuclear cisterna. The resulting intracisternal L particles presented a spherical shape, ranged in size between 150 and 200 nm (average, 170 nm), and exhibited a few delicate surface projections at the envelope, and the underlying tegument appeared as a homogeneous granular material of moderate electron density. Interestingly, the inner surface of the vaulting membrane characteristically bore electron-dense granules approximately 15 nm in diameter, similar to those associated with the nuclear surface of the inner nuclear membrane (Fig. 2). L particles were frequently seen passing into distended cisternae of the rough endoplasmic reticulum (RER) that were continuous with the perinuclear space (Fig. 2C), and L particles morphologically identical to perinuclear L particles were also detected inside distended RER profiles in the cytoplasm.
FIG. 2.
Formation of intracisternal L particles and passage into the RER. Budding of condensed tegument at the inner nuclear membrane (arrows in panels A and B) resulted in perinuclear L particles (arrowhead in B). An L particle (arrow) within a distended RER profile continuous with the nuclear envelope is shown in panel C, as is an extracellular virion. Bars, 150 nm in panels A and C and 250 nm in panel B. N, nucleus.
The tegument contained within intracisternal L particles appeared to gain access to the cytosol by fusion of the particle envelope with the RER membrane (Fig. 3A to C). First, intracisternal L particles were observed in intimate contact with the organelle membrane; interestingly, dense granules were absent from the inner surface of the particle envelope at the predestined site of fusion (Fig. 3A). After completion of the fusion process, the cytoplasmic surface of the RER membrane showed electron-dense granules at the site of release, similar to those associated with the envelope of intracisternal L particles (Fig. 3B and C).
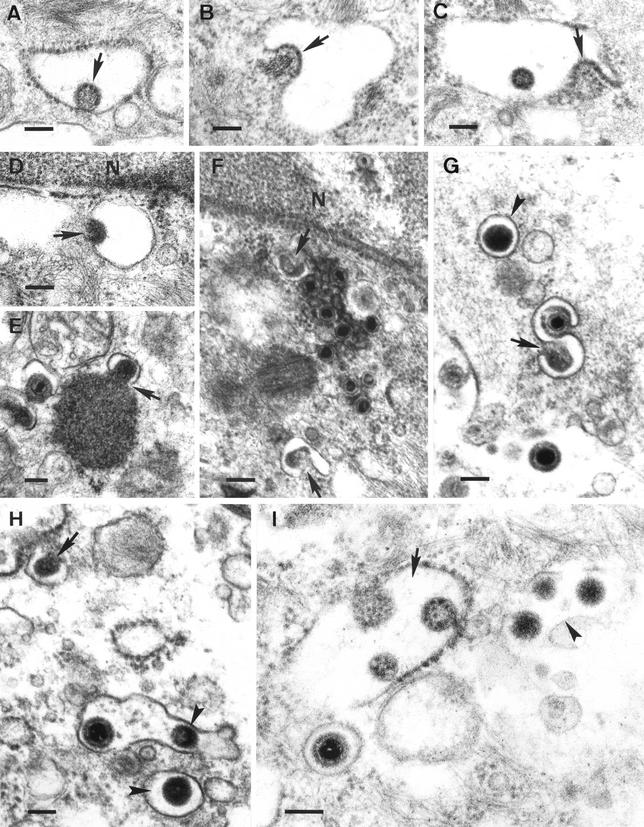
FIG. 3.
(A to C) Fusion between the envelope of intracisternal L particles (arrows) and the RER membrane. (D to H) Formation of intravesicular L particles. Budding of condensed tegument into cytoplasmic vesicles (arrows) resulted in L particles inside exocytic vesicles (arrowheads). Note the simultaneous budding of condensed tegument and a nucleocapsid into the same vesicle (G) as well as the presence of an exocytic vesicle containing both a virion and an L particle (H). (I) Intracisternal (arrow) and intravesicular (arrowhead) L particles. Note also the presence of an intravesicular virion. Bars, 150 nm. N, nucleus.
Formation of L particles was also observed to occur by budding of condensed tegument at membranes of cytoplasmic vesicles (Fig. 3D to H). This budding process was occasionally observed at the periphery of intracytoplasmic aggregations of electron-dense material (Fig. 3E). The resulting intravesicular L particles were spherical, with diameters ranging between 120 and 160 nm (average, 140 nm [though we also detected a small number with maximum diameter up to 400 nm]); the tegument showed a higher electron density than that observed within intracisternal L particles and, in addition, often exhibited an eccentric electron-lucent area. Intravesicular L particles displayed another distinctive feature, which was the presence of prominent surface projections on the envelope. Remarkably, the maximum condensation of the tegument contained within intravesicular L particles seemed to occur after completion of the process of budding of condensed tegument into cytoplasmic vesicles (Fig. 3F and G). Thus, not only the budding process but also the morphological features of the resulting intravesicular L particles were clearly distinguishable from those described above for intracisternal L particles (Fig. 3I).
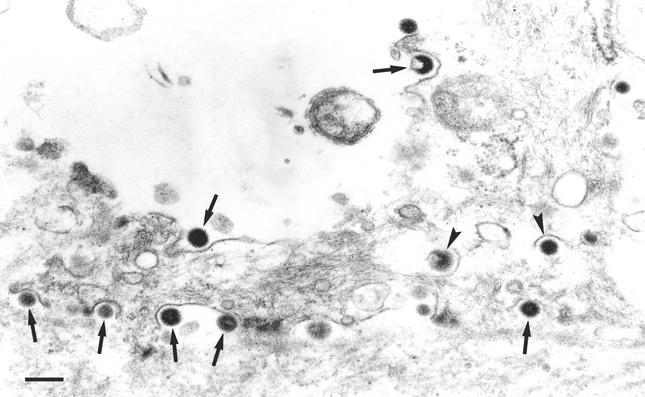
Egress of L particles occurred by exocytosis. Vesicles containing either individual L particles or groups of L particles, the latter as a consequence of the budding of multiple condensations of tegument into a single vesicle, were observed to fuse with the cell membrane, resulting in the release of L particles to the extracellular space. Morphologically, intravesicular and extracellular L particles were identical (Fig. 4).
FIG. 4.
Exocytosis of L particles. The release of L particles (arrows) similar to those present within cytoplasmic vesicles (arrowheads) can be observed at the basolateral membrane of an epithelial cell. Bar, 250 nm.
Comparative analysis of PRV virion and L-particle morphogenesis.
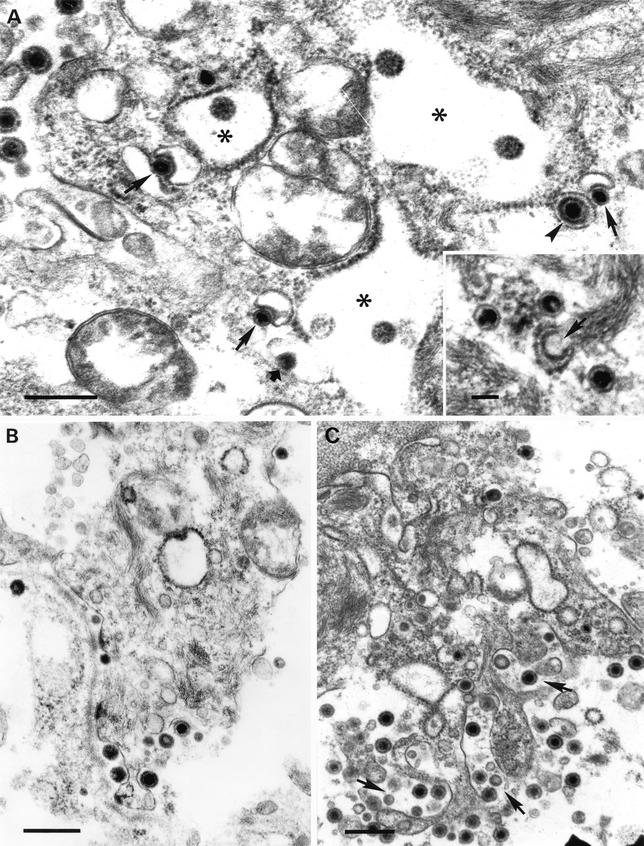
The main difference between infectious and noninfectious particle morphogenesis resided in the way of egress of virus particles from the perinuclear cisterna. Whereas L particles were found to pass into the RER lumen continuous with the perinuclear cisterna to fuse with the organelle membrane, nucleocapsids were never observed within RER profiles. Rather, nucleocapsids seemed to leave the perinuclear cisterna immediately after primary envelopment at the inner nuclear membrane (Fig. 5), mainly by direct fusion between the primary envelope and the outer nuclear membrane (Fig. 5B) but also after vesiculation at the outer nuclear membrane (data not shown). In all other respects, L particles and virions appeared to share a common pathway of assembly and egress, since both types of virus particle were detected as acquiring an envelope at the same cytoplasmic site (and even at the same vesicle) and vesicles containing simultaneously mature virions and L particles were also found in the cytoplasm of infected cells (Fig. 6A and 3E to H). Additionally, distended RER profiles containing L particles and fusion events between the envelope and the organelle membrane were often observed in cytoplasmic areas in which naked nucleocapsids accumulated and obtained a definitive envelope from cytoplasmic vesicles (Fig. 6A). Finally, exocytosis events involving virus particle-containing vesicles were found to be similar irrespective of their content: while release from the cell in epithelial cells occurred at the basal and lateral surfaces (Fig. 6B and C), egress in fibroblasts was observed at the entire surface of the cell (data not shown).
FIG. 5.
Egress of nucleocapsids from the nucleus. (A) Budding of nucleocapsids at the inner nuclear membrane (arrow) resulted in primary enveloped nucleocapsids in the perinuclear cisterna (asterisk). (B) Direct fusion of the primary envelope with the outer nuclear membrane (arrows) resulted in release of naked nucleocapsids into the cytoplasm. Note also the primary envelopment of a nucleocapsid at the inner nuclear membrane (arrowhead). Bars, 250 nm. N, nucleus.
FIG. 6.
Secondary envelopment of nucleocapsids and exocytosis of virions. (A) Budding of intracytoplasmic nucleocapsids into cytoplasmic vesicles (arrows) resulted in mature virions inside exocytic vesicles (arrowhead). Note the presence of L particles within distended RER profiles (asterisks) and the formation of an intravesicular L particle (short arrow). The inset shows naked intracytoplasmic nucleocapsids adjacent to a RER profile marked with electron-dense granules (arrow) similar to those found after completion of the fusion event between the envelope of intracisternal L particles and the organelle membrane. (B and C) Simultaneous egress of virions and L particles at the basolateral membranes of epithelial cells. (C) Exocytosis of vesicles containing both virions and L particles (arrows). Bars, 500 nm and 100 nm (inset in panel A).
Also of interest is the fact that intravesicular infectious and noninfectious particles were clearly distinguishable from the respective intracisternal forms. In the case of virions, primary enveloped nucleocapsids inside the perinuclear cisterna were 125 nm in diameter, the capsid contained a visible electron-dense nucleoprotein core, and a tegument of moderate electron density separated the nucleocapsid from the envelope, which showed scarce surface projections. Notably, the primary envelope did not show associated electron-dense granules (Fig. 5A). After secondary envelopment at cytoplasmic vesicles, mature virions were (on average) 160 nm in diameter, prominent spikes projected from the envelope surface, the nucleocapsid was scarcely distinguishable (as it appeared in association with a highly electron-dense tegument), and a characteristic low-electron-density halo of tegument was present between the nucleocapsid core and the envelope (Fig. 2C and 3I). It is noteworthy that the capsid shell and the inner nucleoprotein core were readily identified as distinct structures during the process of budding of naked nucleocapsids into cytoplasmic vesicles; only after completion of the envelopment event did they show modified morphology (Fig. 3G and 6A). As expected, mature virions inside cytoplasmic vesicles and extracellular virions were morphologically indistinguishable. In conclusion, then, intravesicular L particles and virions typically showed an increase in both number of surface projections and tegument electron density with respect to intracisternal forms.
Cells producing PRV L particles.
All of the above-described stages of L-particle morphogenesis were detected in epithelial cells and fibroblasts of the respiratory and olfactory mucosae of the nasal cavity. We were not able to identify any ultrastructural feature indicative of L-particle production in olfactory neurons or in other cell types infected by PRV.
Regarding production of PRV L particles, we observed in epithelial cells and fibroblasts three distinct categories of cells apparently corresponding to stages of the virus replication cycle: (i) cells that seemed to produce only L particles (Fig. 4); (ii) cells that produced infectious and noninfectious virus particles in variable proportions; and (iii) cells that were found to produce large numbers of virions but few or no L particles. The cells that were observed to produce only L particles appeared to be recently infected cells, since they were located at the periphery of the foci of infection and displayed either no morphological signs of virion morphogenesis or only initial stages such as nucleocapsid assembly in the nucleus. These cells also exhibited morphological changes indicative of cell injury, such as high-amplitude swelling of mitochondria, extreme dilatation of the RER and, in epithelial cells, loss of cilia and microvilli. The ultrastructural changes displayed by infected cells correlated directly with the course of infection: the larger the number of virions inside intracytoplasmic vesicles and released by exocytosis, the greater the extent of cell injury. Among these changes, we observed a progressive disintegration of the Golgi apparatus and RER into small, distended cytoplasmic vesicles. The cells that exhibited the most prominent morphological changes, including in many instances breakdown of the nuclear envelope and plasma membrane, were consistently located at the center of the foci of infection. In these cells, formation and egress of intravesicular L particles were rarely observed, although a few L particles were identified at the cell surface or within the perinuclear cisterna (Fig. 1).
DISCUSSION
The results of the present TEM study confirm the cooccurrence of L particles and virions during primary replication of PRV in the nasal mucosa of swine, its natural host. This is the first demonstration of noninfectious virus particle production during in vivo infection by an alphaherpesvirus, but this possibility has long been suspected, since L particles are generated in cell cultures infected by all alphaherpesviruses so far examined (9, 16, 23, 39). Consequently, it seems reasonable to assume that most, if not all, members of the subfamily Alphaherpesvirinae produce L particles not only in culture but also during natural infection.
We observed that formation of PRV L particles occurred by budding of condensed tegument at the inner nuclear membrane as well as at membranes of cytoplasmic vesicles, resulting in intracisternal and intravesicular L particles, respectively. Regarding the subcellular compartments in which intravesicular L particles are formed, we assume that they originated from the budding of condensed tegument into Golgi-derived vesicles. This contention is supported by our observations of the simultaneous budding of nucleocapsids and condensed tegument into the same vesicle and of vesicles containing both mature virions and L particles, since a previous study has demonstrated that nucleocapsids and L particles are enveloped at membranes of the trans-Golgi network in cell cultures infected with PRV (16).
Significantly, intracisternal and intravesicular PRV L particles were clearly distinguishable not only by their distinct intracellular location within membranous organelles but also by their ultrastructural features and intracellular routes. L particles inside the perinuclear cisterna were 170 nm in diameter on average, showed scarce surface projections, and contained a homogeneous tegument of moderate electron density and granules associated with the inner surface of the envelope. Although we are well aware of the fact that electron microscopy is ill suited for deducing the directionality of the observed processes, our data indicate that intracisternal L particles passed into the RER lumen to fuse then with the organelle membrane and deliver their proteinaceous content directly into the cytosol. In contrast, intravesicular L particles were significantly smaller (average diameter, 140 nm), exhibited prominent surface projections on the envelope, and contained highly electron-dense tegument, often showing an eccentric electron-lucent area. Only intravesicular L particles were observed to be released by exocytosis, and these particles were also observed to be extracellular. These observations are in agreement with the results recently reported by Granzow and colleagues (16), who demonstrated for the first time the existence in different cell lines infected with various alphaherpesviruses (including PRV) of two types of L particle, one inside the perinuclear cisterna and the other within Golgi-derived vesicles or at the cell surface. The existence of two forms of L particles prompted these authors to conclude that viral tegument proteins can interact with membrane proteins to acquire both a primary and a definitive envelope independently of the presence of a capsid. It is significant that our findings, in accordance with the data obtained by Granzow and colleagues (16), likewise indicate that the resulting L particles contain signals involved in their intracellular targeting and egress similar to those present in particles endowed with a capsid. In particular, this conclusion is suggested by our observation that in polarized cells, such as epithelial cells, exocytosis of virus particle-containing vesicles occurred specifically at the basolateral membranes, regardless of whether the vesicles contained L particles and/or virions. As for loss of the envelope of intracisternal L particles and nucleocapsids and targeting of their content to the cytoplasmic site where a Golgi-derived envelope is obtained, these events require further consideration. Perinuclear nucleocapsids were found to be released into the cytoplasm mainly by fusion of the primary envelope with the outer nuclear membrane. Irrespective of the site of egress from the perinuclear cisterna, naked nucleocapsids translocated to and accumulated in a cytoplasmic area where they acquired a definitive envelope. Sporadically, egress of primary enveloped nucleocapsids from the perinuclear cisterna was also observed to occur by vesiculation at the outer lamella of the nuclear envelope. It has been reported that these nucleocapsids (which are surrounded by a double membrane) are likewise translocated to the Golgi area, where naked nucleocapsids are released after fusion of the two membranes (15, 16). Viral tegument proteins contained within intracisternal L particles also appeared to reach the Golgi area, but in contrast to nucleocapsids, they traversed the RER to gain access to this cytoplasmic compartment. It is not presently known whether deenvelopment of nucleocapsids at the outer nuclear membrane results in complete loss of the primary tegument or, more likely, physical interaction between at least some tegument and capsid proteins is conserved (7, 26); if the latter, tegument proteins would be the only component present in each of the three forms of virus particle (“naked” nucleocapsids, nucleocapsids surrounded by a bimembranous envelope, and intracisternal L particles). It is therefore tempting to speculate that nuclear viral tegument proteins contain the signals necessary for targeting of virus particles to the Golgi area.
Even though the morphological features shared by intravesicular L particles and virions suggest that the processes involved in assembly of tegument proteins and envelopment at Golgi-derived vesicles are similar for both, it is striking that two tegument proteins (the UL36 and UL37 gene products) are not detectable in L particles released from PRV-infected cells (13) and that L particles released from HSV-1- and PRV-infected cells contain phosphoproteins (up to five [175, 134, 92, 60, and 55 kDa] in HSV-1 L particles) not detected in virions (23, 39). Lack of detection of the UL36 and UL37 gene products is to a certain extent expected, since these proteins apparently form the innermost layer of the tegument by direct interactions between the capsid and the UL36 protein and between the UL36 and UL37 proteins (21, 22, 41). It has been proposed that such semitegumented nucleocapsids are then targeted to the future budding site, which is in turn formed by interaction of UL49 (and probably other tegument proteins) with the viral glycoproteins gE/I and gM present in membranes of Golgi-derived vesicles (13, 26). Therefore, it appears likely that in the absence of semitegumented nucleocapsids, as in L-particle formation, viral envelope glycoproteins and/or tegument proteins at the budding site contact other proteins, resulting in the incorporation of components that would otherwise not be included in the tegument of virions. In this connection, some or all of the proteins unique to HSV-1 L particles have been suggested to be associated with membrane-enclosed inclusion vesicles which have been identified in the tegument of a large proportion of HSV-1 L particles but not in virions (38). On the other hand, the presence or absence of a capsid possibly determines the composition of the primary tegument as well, since perinuclear L particles and nucleocapsids were found to differ clearly in tegument appearance: whereas the former exhibited electron-dense granules associated with the inner surface of the envelope, these granules were absent from primary enveloped nucleocapsids. Lastly, it has to be pointed out that intracisternal L particles were morphologically distinct from intravesicular L particles, a fact that leads us to speculate that they are biochemically different, as demonstrated in vitro for PRV perinuclear and mature virions (20).
An interesting finding of the present study relates to the observation of three different categories of cell as regards production of PRV L particles and virions. We found that epithelial cells and fibroblasts that released high numbers of virions but few, if any, noninfectious particles characteristically showed prominent morphological changes and were located at the center of the foci of infection in the nasal mucosa. In contrast, cells that were observed to produce only L particles were located at the periphery of the foci of infection and showed evidence of mild injury and, notably, nucleocapsids at various stages of assembly were only occasionally observed in their nuclei. Finally, production of both types of virus particle was observed in cells exhibiting ultrastructural changes whose magnitude correlated directly with the number of virions assembled and released. Although TEM analysis is not an ideal approach for characterizing dynamic processes inherent in virus infection, the observation of these three different categories of cell supports the contention that virions and L particles have a distinct pattern of production during the replication cycle of PRV. We thus propose that noninfectious particles are the first products formed and released from PRV-infected cells and that as the viral replication cycle progresses and an increasing number of nucleocapsids are assembled in the nucleus, tegument proteins are coupled to virion morphogenesis to produce primarily infectious particles.
However, when we bear in mind that tegument proteins can self-assemble and acquire an envelope without any need for the presence of a capsid (16, 23, 33), the above hypothesis does not satisfactorily explain why cells at advanced stages in the course of infection were found to release few or no L particles. Since perinuclear L particles seemed to gain access to the Golgi area by traversing the RER and since during the course of infection this organelle disintegrated into vesicles with no continuity with the perinuclear cisterna, one possible explanation for the lack of L-particle production in these cells is that when the envelope of L particles fuses with the outer nuclear membrane at the perinuclear cisterna, tegument proteins disperse into the cytoplasm due to the absence of interactions with capsid proteins, thus affecting or precluding formation of intravesicular L particles. This hypothesis implies that the primary tegument of perinuclear L particles also contains proteins involved in the establishment of interactions that are necessary for assembly and/or envelopment of cytoplasmic tegument proteins and, consequently, that morphogenesis of noninfectious particles occurs, as for infectious particles, through a two-step process in which formation of immature intracisternal L particles precedes formation of mature intravesicular L particles. Another possibility that does not conflict with the proposal of a two-step process for L-particle maturation is that a viral gene(s) expressed predominately during the late phase of the replication cycle represses the budding into Golgi-derived vesicles of condensed tegument without associated nucleocapsids. In that respect, there is recent evidence that the tegument protein encoded by PRV gene UL48 and expressed during the late phase of the replication cycle is required to prevent the excessive formation of L particles, since cells infected with a UL48-negative PRV mutant released great amounts of capsidless particles late after infection (13).
The results reported here apparently suggest that noninfectious particles are a byproduct of PRV replication derived from an initial uncoupling of virion morphogenesis. When we take into account that the morphological events of virion assembly and egress are probably similar for all herpesviruses (reviewed in reference 26), this interpretation might explain why other members of this family generate enveloped tegument structures related to alphaherpesvirus L particles to greater or lesser degrees (6, 12, 17, 18, 28). However, in the nasal mucosa of experimentally infected pigs, PRV L particles were found to be produced exclusively by cells that support the first rounds of virus replication and thus allow amplification of the virus load at the inoculation site; this strongly suggests that L particles play a significant role in the initial stages of PRV infection in the natural host. In this connection, several functions have been proposed for L particles during natural infection, such as acting as decoys for the immune system, enhancing reactivation from latency, promoting infection of semipermissive cells, and complementing partially defective coinfecting virions (8, 37). In a recent study, it has been demonstrated that the polyserine tract of HSV-1 ICP4 is critical for productive growth of HSV-1 in the trigeminal ganglia whereas it is not required for viral growth in culture or at the inoculation site in experimentally infected mice (3). Strikingly, HSV-1 L particles but not virions contain the phosphoprotein ICP4 (175 kDa) and PRV L particles but not virions contain the homolog of HSV-1 ICP4 (IE180) (23, 39). Therefore, another possibility is that when entering peripheral nerve endings or neurons together with virions, alphaherpesvirus L particles released by epithelial cells and fibroblasts facilitate successful infection of neuronal cells by providing a supply of this phosphoprotein in the absence of de novo viral gene expression. This scenario implies a relationship between L-particle production and alphaherpesvirus neurotropism. Nevertheless, more detailed analyses are clearly necessary for the assessment of the biological role, if any, of alphaherpesvirus L particles.
Acknowledgments
We thank E. Puentes (CZ Veterinaria, S. L., Pontevedra, Spain) for providing the PRV strain E-974 and M. B. Pensaert (University of Ghent, Ghent, Belgium) for the generous gift of the anti-PRV antibody.
This study was supported by a research grant from the Xunta de Galicia (XUGA26105B98).
REFERENCES
- 1.Alconada, A., U. Bauer, B. Sodeik, and B. Hoflack. 1999. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J. Virol. 73:377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alemañ, N., M. I. Quiroga, M. López-Peña, S. Vázquez, F. H. Guerrero, and J. M. Nieto. 2001. Induction and inhibition of apoptosis by pseudorabies virus in the trigeminal ganglion during acute infection of swine. J. Virol. 75:469-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates, P. A., and N. A. DeLuca. 1998. The polyserine tract of herpes simplex virus ICP4 is required for normal viral gene expression and growth in murine trigeminal ganglia. J. Virol. 72:7115-7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browne, H., S. Bell, T. Minson, and D. W. Wilson. 1996. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J. Virol. 70:4311-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Card, J. P., L. Rinaman, R. B. Lynn, B.-H. Lee, R. P. Meade, R. R. Miselis, and L. W. Enquist. 1993. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J. Neurosci. 13:2515-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craighead, J. E., R. E. Kanich, and J. D. Almeida. 1972. Nonviral microbodies with viral antigenicity produced in cytomegalovirus-infected cells. J. Virol. 10:766-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham, C., A. J. Davison, A. R. MacLean, N. S. Taus, and J. D. Baines. 2000. Herpes simplex virus type 1 gene UL14: phenotype of a null mutant and identification of the encoded protein. J. Virol. 74:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dargan, D. J., and J. H. Subak-Sharpe. 1997. The effect of herpes simplex virus type 1 L-particles on virus entry, replication, and the infectivity of naked herpesvirus DNA. Virology 239:378-388. [DOI] [PubMed] [Google Scholar]
- 9.Dargan, D. J., A. H. Patel, and J. H. Dubak-Sharpe. 1995. PREPs: herpes simplex virus type 1-specific particles produced by infected cells when viral DNA replication is blocked. J. Virol. 69:4924-4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eiras, A., E. Puentes, R. Seoane, E. Cancio, M. V. Nores, and B. J. Regueiro. 1992. Antigens involved in vaccination of swine against Aujeszky's (pseudorabies) virus. J. Vet. Med. Ser. B 39:526-536. [DOI] [PubMed] [Google Scholar]
- 11.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1999. Infection and spread of alphaherpesvirus in the nervous system. Adv. Virus Res. 51:237-347. [DOI] [PubMed] [Google Scholar]
- 12.Fiala, M., R. W. Honess, D. C. Heiner, J. W. Heine, J. Murnane, R. Wallace, and L. B. Guze. 1976. Cytomegalovirus proteins. I. Polypeptides of virions and dense bodies. J. Virol. 19:243-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs, W., H. Granzow, B. G. Klupp, M. Kopp, and T. C. Mettenleiter. 2002. The UL48 tegument protein of pseudorabies virus is critical for intracytoplasmic assembly of infectious virions. J. Virol. 76:6729-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gershon, A. A., D. L. Sherman, Z. Zhu, C. A. Gabel, R. T. Ambron, and M. D. Gershon. 1994. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J. Virol. 68:6372-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granzow, H., F. Weiland, A. Jöns, B. G. Klupp, A. Karger, and T. C. Mettenleiter. 1997. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J. Virol. 71:2072-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granzow, H., B. G. Klupp, W. Fuchs, J. Veits, N. Osterrieder, and T. C. Mettenleiter. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75:3675-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenspan, J. S., J. P. Rabanus, V. Petersen, and D. Greenspan. 1989. Fine structure of EBV-infected keratinocytes in oral hairy leukoplakia. J. Oral Pathol. Med. 18:565-572. [DOI] [PubMed] [Google Scholar]
- 18.Irmiere, A., and W. Gibson. 1983. Isolation and characterization of a non-infectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology 130:118-133. [DOI] [PubMed] [Google Scholar]
- 19.Jones, C. 1999. Alphaherpesvirus latency: its role in disease and survival of the virus in nature. Adv. Virus Res. 51:81-133. [DOI] [PubMed] [Google Scholar]
- 20.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2000. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J. Virol. 74:10063-10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klupp, B. G., H. Granzow, E. Mundt, and T. C. Mettenleiter. 2001. Pseudorabies virus UL37 gene product is involved in secondary envelopment. J. Virol. 75:8927-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klupp, B. G., W. Fuchs, H. Granzow, R. Nixdorf, and T. C. Mettenleiter. 2002. Pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J. Virol. 76:3065-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLauchlan, J., and F. J. Rixon. 1992. Characterization of enveloped tegument structures (L particles) produced by alphaherpesviruses: integrity of the tegument does not depend on the presence of capsid or envelope. J. Gen. Virol. 73:269-276. [DOI] [PubMed] [Google Scholar]
- 24.McLauchlan, J., C. Addison, M. C. Cragie, and F. J. Rixon. 1992. Noninfectious L-particles supply functions which can facilitate infection by HSV-1. Virology 190:682-688. [DOI] [PubMed] [Google Scholar]
- 25.Mettenleiter, T. C. 2000. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis—state of the art, June 1999. Vet. Res. 31:99-115. [DOI] [PubMed] [Google Scholar]
- 26.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ojala, P. M., B. Sodeik, M. W. Ebersold, U. Kutay, and A. Helenius. 2000. Herpes simplex virus type 1 entry into host cells: reconstitution of capsid binding and uncoating at the nuclear pore complex in vitro. Mol. Cell. Biol. 20:4922-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orenstein, J. M., D. M. Ciufo, J. P. Zoeteweij, A. Blauvelt, and G. S. Hayward. 2000. Morphogenesis of HHV8 in primary human dermal microvascular endothelium and primary effusion lymphomas. Ultrastruct. Pathol. 24:291-300. [DOI] [PubMed] [Google Scholar]
- 29.Puentes, E., E. Cancio, A. Eiras, M. V. Nores, A. Aguilera, B. J. Regueiro, and R. Seoane. 1993. Efficacy of various non-oily adjuvants in immunization against the Aujeszky's disease (pseudorabies) virus. J. Vet. Med. Ser. B 40:353-365. [DOI] [PubMed] [Google Scholar]
- 30.Quiroga, M. I., S. Vázquez, M. López-Peña, F. Guerrero, and J. M. Nieto. 1995. Experimental Aujeszky's disease in blue foxes (Alopex lagopus). J. Vet. Med. Ser. A 42:649-657. [DOI] [PubMed] [Google Scholar]
- 31.Quiroga, M. I., S. Vázquez, M. López-Peña, and J. M. Nieto. 1997. Distribution of Aujeszky's disease virus in experimentally infected mink (Mustela vison). Dtsch. Tieraerztl. Wochenschr. 104:147-150. [PubMed] [Google Scholar]
- 32.Rixon, F. J. 1993. Structure and assembly of herpesviruses. Semin. Virol. 4:135-144. [Google Scholar]
- 33.Rixon, F. J., C. Addison, and J. McLauchlan. 1992. Assembly of enveloped tegument structures (L particles) can occur independently of virion maturation in herpes simplex virus type 1-infected cells. J. Gen. Virol. 73:277-284. [DOI] [PubMed] [Google Scholar]
- 34.Schrag, J. D., B. V. Venkataram-Prasad, F. J. Rixon, and W. Chiu. 1989. Three-dimensional structure of the HSV-1 nucleocapsid. Cell 56:651-660. [DOI] [PubMed] [Google Scholar]
- 35.Skepper, J. N., A. Whiteley, H. Browne, and A. Minson. 2001. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment-deenvelopment-reenvelopment pathway. J. Virol. 75:5697-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spear, P. G. 1993. Entry of alphaherpesviruses into cells. Semin. Virol. 4:167-180. [Google Scholar]
- 37.Subak-Sharpe, J. H., and D. J. Dargan. 1998. HSV molecular biology: general aspects of herpes simplex virus molecular biology. Virus Genes 16:239-251. [DOI] [PubMed] [Google Scholar]
- 38.Szilágyi, J. F., and J. Berriman. 1994. Herpes simplex virus L particles contain spherical membrane-enclosed inclusion vesicles. J. Gen. Virol. 75:1749-1753. [DOI] [PubMed] [Google Scholar]
- 39.Szilágyi, J. F., and C. Cunningham. 1991. Identification and characterization of a novel non-infectious herpes simplex virus-related particle. J. Gen. Virol. 72:661-668. [DOI] [PubMed] [Google Scholar]
- 40.Whiteley, A., B. Bruun, T. Minson, and H. Browne. 1999. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J. Virol. 73:9515-9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou, Z. H., D. H. Chen, J. Jakana, F. J. Rixon, and W. Chiu. 1999. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J. Virol. 73:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu, Z., M. D. Gershon, Y. Hao, R. T. Ambron, C. A. Gabel, and A. A. Gershon. 1995. Envelopment of varicella-zoster virus: targeting of viral proteins to the trans-Golgi network. J. Virol. 69:7951-7959. [DOI] [PMC free article] [PubMed] [Google Scholar]