Abstract
Gut-associated lymphoid tissue (GALT) is a significant but understudied lymphoid organ, harboring a majority of the body's total lymphocyte population. GALT is also an important portal of entry for human immunodeficiency virus (HIV), a major site of viral replication and CD4+ T-cell depletion, and a frequent site of AIDS-related opportunistic infections and neoplasms. However, little is known about HIV-specific cell-mediated immune responses in GALT. Using lymphocytes isolated from rectal biopsies, we have determined the frequency and phenotype of HIV-specific CD8+ T cells in human GALT. GALT CD8+ T cells were predominantly CD45RO+ and expressed CXCR4 and CCR5. In 10 clinically stable, chronically infected individuals, the frequency of HIV Gag (SL9)-specific CD8+ T cells was increased in GALT relative to peripheral blood mononuclear cells by up to 4.6-fold, while that of cytomegalovirus (CMV)-specific CD8+ T cells was significantly reduced (P = 0.012). Both HIV- and CMV-specific CD8+ T cells in GALT expressed CCR5, but only HIV-specific CD8+ T cells expressed αΕβ7 integrin, suggesting that mucosal priming may account for their retention in GALT. Chronically infected individuals exhibited striking depletion of GALT CD4+ T cells expressing CXCR4, CCR5, and αΕβ7 integrin, but CD4+/CD8+ T-cell ratios in blood and GALT were similar. The percentage of GALT CD8+ T cells expressing αΕβ7 was significantly decreased in infected individuals, suggesting that HIV infection may perturb lymphocyte retention in GALT. These studies demonstrate the feasibility of using tetramers to assess HIV-specific T cells in GALT and reveal that GALT is the site of an active CD8+ T-cell response during chronic infection.
Gut-associated lymphoid tissue (GALT) is the largest lymphoid organ in the body, containing the majority of the body's lymphocytes (39). GALT is also an important portal of entry for human immunodeficiency virus (HIV) and a frequent site of AIDS-associated opportunistic infections (10, 72). HIV infection of GALT is characterized by active viral replication (15, 23, 34, 55), depletion of CD4+ T cells (48, 49, 63), and expansion or infiltration of CD8+ T cells (64). Because the majority of HIV transmission occurs via mucosal surfaces, it is generally agreed that a successful vaccine will need to induce strong immune responses in these tissues. However, there are few studies describing HIV-specific T-cell responses in human GALT (50, 51), and analysis of these responses has been hampered by the difficulty of obtaining tissue from human subjects (3, 41).
Studies of murine viral infections demonstrated the cytotoxic effector functions of GALT CD8+ T cells (30, 58) and established a role for local T cells in protection from mucosal challenge (4). These studies have also been critical in determining the molecular interactions responsible for lymphocyte homing to GALT (28, 35, 36). GALT contains inductive sites, Peyer's patches and mesenteric lymph nodes, and effector sites, located within the lamina propria and epithelium of the intestinal wall (reviewed in reference 39). Antigen-specific T cells that are primed in inductive sites enter peripheral circulation but are apparently programmed for eventual return to the intestinal lining (16, 33).
Two integrins, α4β7, which interacts with mucosal vascular addressin on capillary endothelial cells (mucosal addressin cell adhesion molecule-1 [MadCAM-1]), and αEβ7, whose ligand is E-cadherin on intestinal epithelial cells, are critical for lymphocyte homing to GALT (1, 7, 52). α4β7 is found on mucosally primed T cells in blood that will eventually return to GALT, while αEβ7 (CD103) is expressed once cells reach GALT, tethering intraepithelial lymphocytes (IEL) to epithelial cells (1, 7, 52). The chemokine CCR9 is also expressed on T cells homing to the small, but not large, intestine. Its ligand, TECK, is expressed selectively in this tissue (2, 19, 25, 45, 71). In addition to GALT-specific interactions, some T-cell migration may occur in response to more general, inflammation-related chemotactic signals (14, 33, 53). Recently, intestinal epithelial cells were shown to express ligands for CXCR3 (i.e., IP-10, Mig, and I-TAC) during inflammatory responses (14, 53), and IEL and lamina propria lymphocytes (LPL) were found to express CXCR3 (1, 2). Expression of β-chemokines RANTES, MIP-1α, and MIP-1β, as well as their receptor, CCR5, was increased in GALT of patients with HIV infection and chronic inflammatory bowel disease (44). Thus, T cells expressing CXCR3 and CCR5 may be recruited to GALT during an inflammatory response (33).
Although studies of mucosal HIV-specific T-cell responses have been limited, vaccine studies with rhesus macaques have supported a protective role for mucosal CD8+ T cells against simian immunodeficiency virus (SIVmac) challenge (20, 29, 40). In addition, CD8+ T cells specific for SIVmac Gag have been detected in GALT during chronic infection (12, 38, 47, 64). During acute infection of rhesus macaques, large numbers of SIV Gag-specific CD8+ T cells (3 to 11.5% of CD8+ T cells) were detected in intestinal mucosa (64). Concurrent with this influx of CD8+ T cells was dramatic depletion of CD4+ T cells in the jejunum, ileum, and colon (61, 63). This depletion has been found at all stages of experimental SIVmac infection (56).
In HIV-infected humans, profound CD4+ T-cell depletion also occurs in both the small and large intestines (11, 48, 49). However, the kinetics of this depletion have not been thoroughly studied, nor has the magnitude of the HIV-specific CD8+ T-cell response in GALT been determined. The development of major histocompatibility complex (MHC) class I tetrameric complexes has enabled rapid assessment of the frequency of antigen-specific T cells isolated from primate tissues (17, 57), and similar methods may be applied to human tissues. In an earlier study, we generated bulk cultures of rectal and duodenal mononuclear cells from HIV-positive individuals (50) and identified HIV- and cytomegalovirus (CMV)-specific CD8+ T cells in these cultures. However, these expanded cultures could not give an accurate assessment of CD8+ T-cell frequency and phenotype. In this report, we assess the phenotype, trafficking patterns, and relative frequencies of HIV- and CMV-specific CD8+ T cells in rectal mucosa. Our findings demonstrate that GALT is the site of an active HIV-specific CD8+ T-cell response during chronic infection.
MATERIALS AND METHODS
Biopsy and blood samples.
Eleven HIV-positive individuals and five seronegative controls were studied. Informed consent was obtained from all volunteers, and the study protocol was approved by the Committee for Human Research of the University of California, San Francisco. Viral load and CD4+ T-cell counts were obtained from clinic records.
Rectal biopsy tissue was obtained by flexible sigmoidoscopy from sites located in the rectum at 10 cm from the anal verge. A flexible sigmoidoscope with a biopsy channel (EC3831L; Pentax Precision Instrument Corporation, Orangeburg, N.Y.) was employed with single-use biopsy forceps (Radial Jaw 3; Boston Scientific, Miami, Fla.) for rectal biopsies. At each procedure, a total of 20 to 25 tissue samples were obtained and the specimens were pooled for lymphocyte extraction and analysis. As previously described by Anton et al. (3), flexible sigmoidoscopy at 10 to 30 cm from the anal verge, accompanied by up to 30 pinch biopsies, was well tolerated. Patients reported a mild pinching sensation during the procedure and mild bleeding that generally subsided within 24 h. No adverse clinical events were reported. Some patients were biopsied on multiple occasions, up to a maximum of three visits within a 2-year period.
Patient screening and inclusion criteria.
Data for the HIV-positive patients and controls are summarized in Table 1. Eleven HIV-positive individuals were selected from volunteers who were clinically stable and had CD4+ T-cell counts above 250/μl. Peripheral blood mononuclear cells (PBMC) and rectal tissue were also obtained from five healthy, HIV-negative controls. Most patients were not on antiretroviral therapy at the time of biopsy and had plasma viral loads of >10,000 copies/ml (Table 1). All HIV-positive patients also had circulating antibodies indicating previous exposure to CMV. The study was designed to include patients likely to have detectable HIV- and CMV-specific CD8+ T-cell responses in peripheral blood and tissues (42, 43). To facilitate quantification of antigen-specific CD8+ T cells by using MHC class I tetramers, potential volunteers were prescreened for the presence of the HLA-A*0201 allele. Candidates' PBMC were screened by flow cytometry with a monoclonal antibody specific for HLA-A2 (ExAlpha, Inc.) and/or by molecular genotyping (HLA-ABC SSP UniTray system; Pel-Freez Clinical Systems, Brown Deer, Wis.). PBMC from HLA-A*0201-positive subjects were then tested for binding to the following tetramers: HIV-1 Gag (SL9) (amino acids [aa] 77 to 85, SLYNTVATL) and Pol (IV9) (aa 476 to 484, ILKEPVHGV) and CMV pp65 (aa 495 to 503, NLVPMVATV). The tetramer staining protocol is described below. Subjects with detectable responses to one or more of these tetramers were recruited for GALT biopsy.
TABLE 1.
Patient characteristics
| Patienta | Genderb | Age (yr) | Ethnicityc | HLA-A2 | % of blood CD8+ T cells specific for HLA-A*0201-restricted peptidesd in:
|
CD4+ T cellse | Viral load (Copies/ml)e | Antiretroviral therapy | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV Gag
|
HIV Pol
|
CMV pp65
|
|||||||||||
| PBMC | GALT | PBMC | GALT | PBMC | GALT | ||||||||
| C01 | M | 23 | H | + | 0.00 | 0.00 | ND | ND | 0.33 | 0.20 | ND | ||
| C02 | F | 40 | C | + | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | ND | ||
| G01 | M | 54 | C | − | 0.02 | ND | 0.02 | ND | 0.01 | ND | 871 | <50 | Yes |
| G02 | M | 33 | C | + | 0.28 | 0.55 | 0.02 | ND | 1.06 | 0.09 | 394 | 72,000 | No |
| G03 | M | 41 | C | + | 0.02 | 0.02 | 0.01 | 0.02 | 0.68 | 0.30 | 136 | 169,000 | Yes |
| G04 | F | 46 | C | + | 0.69 | 1.20 | 0.85 | 1.00 | 2.02 | 0.18 | 170 | 260,000 | No |
| G05 | M | 35 | C | + | 0.00 | 0.07 | 0.00 | ND | 3.53 | 0.27 | 417 | 13,017 | No |
| G10 | M | 45 | C | + | 0.00 | 0.00 | 0.02 | ND | 1.22 | 0.07 | 235 | 8,000 | No |
| G11 | F | 44 | A | + | 0.68 | 2.60 | 0.02 | ND | 0.15 | 0.02 | 324 | 5,130 | No |
| G12 | M | 52 | C | + | 0.17 | 0.28 | ND | ND | 0.42 | 0.18 | 350 | 12,858 | No |
| G13 | M | 37 | A | + | 1.53 | 0.57 | 0.02 | ND | 2.81 | 0.41 | 329 | 148,000 | Yes (6 days) |
| G22 | M | 29 | C | + | 1.28 | 1.22 | 0.76 | ND | 0.05 | 0.03 | ND | ND | No |
| G25 | M | 39 | A, H | + | 0.18 | 0.60 | 0.00 | ND | 0.13 | 0.04 | 671 | 45,378 | Yes |
C01 and C02 are HLA-A*0201-positive, HIV-negative controls. C01 is CMV seropositive, and C02 is CMV seronegative.
M, male; F, female.
C, Caucasian; A, African American; H, Hispanic or Latino.
As determined by tetramer staining. Samples with a distinct tetramer binding population consisting of >0.03% CD8+ T cells were considered positive (see text).
CD4+ T-cell count and viral load were obtained from most recent clinic records.
ND, not determined.
Mononuclear cell preparation.
Tissue biopsies were washed once in RPMI medium containing 15% fetal calf serum (FCS), l-glutamine, and antibiotics (designated R-15 medium) and then transferred to a 50-ml conical tube (5 to 10 samples per tube). Samples were incubated for 30 min at 37°C, with shaking, in RPMI-7.5% FCS containing 0.5 mg of collagenase type II (Sigma-Aldrich, St. Louis, Mo.) per ml. After the collagenase digestion, tissue fragments were further disrupted by repeated passage through a 10-ml disposable syringe with a blunt-ended 16-gauge needle (Stem Cell Technologies, Vancouver, British Columbia, Canada). Cells liberated from the tissue matrix were then separated from the remaining fragments by passage through a sterile plastic strainer (Falcon 2350). These cells were immediately washed twice in R-15 medium to remove excess collagenase. The remaining tissue fragments were returned to a 50-ml conical tube, and the entire procedure, including 30-min incubations, was repeated two additional times.
Cells liberated from all three collagenase incubations were combined, overlaid onto a discontinuous 35 to 60% Percoll gradient (Pharmacia, Uppsala, Sweden), and then centrifuged at 1,800 rpm in a Sorvall Legend RT centrifuge for 20 min at 4°C. Epithelial cells, located at the interface between the medium and 35% Percoll, were discarded. Mononuclear cells, located at the interface between 35 and 60% Percoll, were harvested, transferred to a 50-ml conical tube, and washed twice in 40 ml of phosphate-buffered saline. Yield and viability were assessed by manual cell counts with trypan blue staining. Typical yields ranged from 3 × 106 to 8 × 106 viable cells for 20 to 25 pinch biopsy samples.
Tetramer staining.
To assess the percentage of antigen-specific CD8+ T cells in blood and GALT, cells were stained with a mixture of surface antibodies and MHC class I tetramers conjugated to phycoerythrin or allophycocyanin for 30 min at 4°C. Cells were then washed twice in phosphate-buffered saline-2% FCS, fixed in 1% paraformaldehyde, and assessed by four-color flow cytometry, collecting 100,000 events in the live lymphocyte gate whenever possible. MHC class I tetramers were provided by Graham S. Ogg (Oxford University, Oxford, United Kingdom), and by Beckman-Coulter Immunomics (Hialeah, Fla.). Specificity of the MHC class I tetramers was assessed by using blood and GALT from control individuals who were either HIV positive and HLA-A*0201 negative or HIV negative and HLA-A*0201 positive. Results for three such individuals (patients C01, C02, and G01) are presented in Table 1. Based upon these results, responses were considered positive if a distinct population representing ≥0.03% of CD8+ T cells bound tetramer. This cutoff was similar to that used in previous studies (42, 43). To increase the level of confidence in low-frequency populations, apparent tetramer binding populations representing fewer than 0.1% of CD8+ T cells were considered equivocal unless observed in at least two independently stained samples. Because of the limited number of cells available, GALT from patients whose PBMC did not recognize the HIV Pol (IV9) tetramer were not tested with this tetramer.
Surface antigen staining and phenotypic analysis.
Fresh mucosal lymphocytes were assessed for expression of phenotypic markers by using fluorescent antibodies specific for CD3, CD4, CD8, T-cell receptor α and γ chains, activation markers CD25 and CD69, maturation markers CD45RA and CD45RO, chemokine receptors CXCR4 and CCR5 (BD-PharMingen), and integrins CD103 (αEβ7integrin; Caltag Laboratories, Burlingame, Calif.) and α4β7 (Act-1; Millenium Pharmaceuticals, Cambridge, Mass.). Because the yield of viable mucosal lymphocytes varied from approximately 3 × 106 to 10 × 106, it was not possible to perform all staining combinations with each sample.
Stained mononuclear cell populations were analyzed for expression of cell surface markers by flow cytometry on a FACSCalibur (Becton Dickinson). Lymphocyte populations were gated based on forward and side scatter and in some cases based on expression of the CD3 surface antigen. Appropriate isotype controls were used to set quadrant markers. For multicolor analysis, electronic compensation for spectral overlap was set by using PBMC stained with single-color reagents. Preliminary studies demonstrated that collagenase treatment did not significantly affect monoclonal antibody recognition of most surface and intracellular proteins. All statistical analyses, including linear regression analysis, Student's t tests, and Mann-Whitney rank sum tests, were performed with the Sigma Plot and Sigma Stat software packages (SPSS Software, Chicago, Ill.).
RESULTS
Chronically infected individuals show significant CD4+ T-cell depletion in rectal tissue.
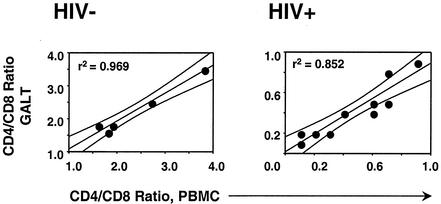
We used four-color flow cytometry to assess the relative percentages of CD4+ and CD8+ lymphocytes in peripheral blood and GALT of healthy control individuals and HIV-infected subjects (Fig. 1). Because GALT samples were pooled and disrupted by collagenase digestion prior to analysis, it was not possible to determine the absolute number of lymphocytes per unit area. Therefore, we determined the ratio of CD4+ to CD8+ lymphocytes in PBMC and GALT by staining with monoclonal antibodies to CD3, CD4, and CD8 and gating on viable lymphocytes, as determined by forward versus side scatter. Lymphocytes isolated by this method represent a mixture of LPL and IEL, and include both CD4+ and CD8+ T cells. In healthy controls, CD4+/CD8+ T-cell ratios in blood and GALT were similar, and they were greater than 1.0 in both compartments (means of 2.3 in blood and 2.2 in GALT [n = 5]) (Fig. 1). There was a strong positive correlation between CD4+/CD8+ ratios in blood and GALT (r2 = 0.969; P = 0.002).
FIG. 1.
CD4+/CD8+ T-cell ratios in blood and GALT. Values were determined by four-color flow cytometry. Results shown are for 5 healthy controls (left) and 10 HIV-positive individuals (right). Linear regression analysis was used to generate lines and curves with a 95% confidence interval.
Dramatic depletion of CD4+ T cells was observed in blood and GALT of most HIV-positive individuals (mean CD4+/CD8+ ratio, 0.4 [n = 10]; range, 0.1 to 0.9). The difference in mean CD4+/CD8+ ratios between HIV-positive and control individuals was highly significant for both blood and GALT (P < 0.001 [10 HIV-positive individuals versus five controls]). Of note is that only two HIV-positive patients had GALT CD4+/CD8+ ratios of greater than 0.5. Patient G01 was on combination antiretroviral therapy, with a plasma viral load of <50 copies/ml and >800 CD4+ T cells/μl (Table 1). The second, patient G02, was naive to antiretroviral drugs and had been clinically healthy since seroconversion (17 months prior to biopsy). Four patients demonstrated slightly greater CD4+ T-cell depletion in GALT than in PBMC; however, overall there was a strong positive correlation between CD4+/CD8+ ratios in blood and GALT (r2 = 0.852; P = 0.0001) (Fig. 1).
GALT CD8+ T cells are mainly T-cell receptor αβ memory cells with a partially activated phenotype.
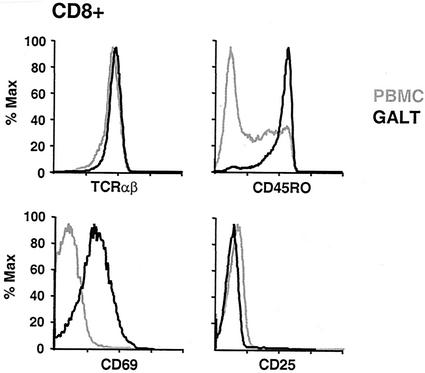
Greater than 90% of CD8+ T cells in GALT, as in PBMC, expressed the T-cell receptor αβ isoform (Fig. 2). A minority (<20%) of GALT T cells expressed the TCR γδ isoform; however, these were primarily CD4−/CD8−, as previously described (31, 32, 60) (not shown). Consistent with the interpretation that GALT T cells have a memory phenotype, the majority of GALT CD8+ T cells expressed CD45RO, while in general fewer than 50% of blood CD8+ T cells expressed this marker. This was true for HIV-infected individuals (P = 0.015 [blood versus GALT; n = 5]) and healthy controls (P = 0.013 [blood versus GALT; n = 3]) (Fig. 2).
FIG. 2.
Phenotype of GALT CD8+ T cells. PBMC and GALT from HIV-positive individuals and controls (not shown) were stained with monoclonal antibodies to the αβ T-cell receptor (top left), CD45RO isoform (top right), CD69 antigen (bottom left), and CD25 (bottom right). The results shown are gated on CD8+ T cells and are typical of those for HIV-positive patients and controls. Grey lines indicate PBMC, and black lines indicate GALT cells.
Relative to blood lymphocytes, an increased percentage of GALT CD8+ T cells expressed the early activation marker CD69 (Fig. 2). It was recently reported that the level of CD69 expression by human IEL was comparable to that by 48- to 72-h phytohemagglutinin-stimulated PBMC blasts (54). However, GALT T cells did not express another marker typical of activation, the interleukin-2 receptor α chain CD25 (Fig. 2) (67). These observations were similar for healthy and HIV-infected individuals and are consistent with previously reported findings for human intestinal lymphocytes (31, 49). Murine CD8+ IEL have also been reported to exhibit a memory phenotype with partial activation, expressing CD69 but not CD25 (67).
Trafficking of antigen-specific CD8+ T cells to GALT.
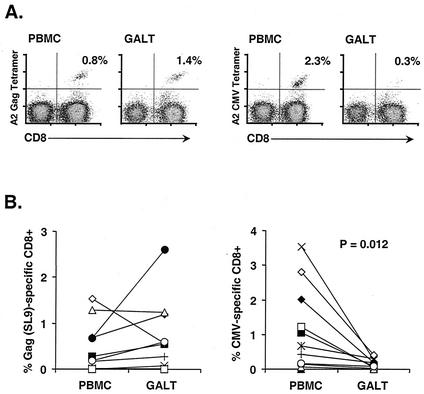
Studies with mice and nonhuman primates have demonstrated that antiviral CD8+ T cells are capable of localization and persistence in mucosal tissues (35, 36, 47, 64). However, little is known of HIV-specific T-cell responses in anatomical compartments other than peripheral blood. To determine the magnitude of the HIV-specific CD8+ T-cell response in GALT, we used MHC class I tetramer staining and flow cytometry. Of the 11 HIV-seropositive individuals analyzed, 10 were positive for the HLA-A*0201 allele. Preliminary screening identified seven patients whose peripheral blood CD8+ T cells recognized the HLA-A*0201-restricted Gag SL9 epitope (Gag aa 77 to 85) (Table 1; Fig. 3A). Direct ex vivo analysis of GALT mononuclear cells revealed that all seven subjects also had SL9-specific CD8+ T cells in rectal tissue. In addition, one patient appeared to have a minor population of SL9-specific CD8+ T cells in GALT, although staining of PBMC did not show a well-defined tetramer binding population (patient G05) (Table 1). Interestingly, in six of seven patients the frequency of SL9-specific, tetramer binding CD8+ T cells in GALT was equal to or greater than that in PBMC (Table 1; Fig. 3B). Patient G13 presented an exception to this trend, with 0.57% of GALT CD8+ T cells specific for SL9 and 1.53% of blood CD8+ T cells recognizing this epitope. Interestingly, this patient had begun antiretroviral therapy 6 days prior to biopsy. There was no correlation between the frequency of SL9-specific CD8+ T cells and plasma viral load or CD4+ T-cell count (data not shown).
FIG. 3.
MHC class I tetramer staining of HIV- and CMV-specific CD8+ T cells from blood and GALT. (A) Representative results for an HIV-positive individual. Plots are gated on CD3+ lymphocytes. Numbers in the upper right corner of each plot represent the percentage of CD8+ T cells bound by HLA-A*0201-HIV Gag SL9 (left) or CMV pp65 (right) tetramers. (B) Combined results of MHC class I tetramer staining for peripheral blood and GALT from 10 HLA-A*0201-positive, HIV-infected individuals. Each patient is represented by a different symbol, and lines between symbols identify PBMC and GALT samples from a single individual.
PBMC from eight patients were screened with the HIV-1 Pol IV9 tetramer (Pol aa 476 to 484). Of these, two patients (G04 and G22) had substantial populations of IV9-specific CD8+ T cells in blood. In Patient G04, IV9-specific CD8+ T cells were distributed in GALT (1.00%) and blood (0.85%) at frequencies similar to those for SL9-specific cells (Table 1). Thus, in this individual, both populations appeared to be present at slightly higher frequencies in GALT than in blood. Insufficient GALT cells were isolated from patient G22 to perform Pol staining in addition to other assays.
All 11 HIV-positive subjects were also seropositive for CMV as demonstrated by enzyme-linked immunosorbent assay. Screening of PBMC with MHC class I tetramers revealed that 10 of 10 HLA-A*0201-positive individuals had detectable responses to the CMV pp65 epitope (aa 495 to 503, NLVPMVATV) (Fig. 3A). CMV can be an important pathogen of the gastrointestinal tract in AIDS patients (9), and CMV-specific CD8+ T cells have been detected in rectal tissue from HIV-positive individuals (51). Surprisingly, the relative frequencies of HIV and CMV-specific populations differed markedly in GALT. A striking reduction in the frequency of CMV-specific CD8+ T cells relative to PBMC was observed in GALT from all 10 patients (P = 0.012) (Fig. 3B). Thus, in this group of chronically HIV-infected individuals without symptoms of active gastrointestinal disease, the frequency of HIV-specific CD8+ T cells was increased in GALT relative to PBMC, while that of CMV-specific CD8+ T cells was markedly decreased. The simplest model to account for this observation is that many HIV-specific CD8+ T cells are primed within intestinal lymphoid tissue, and these mucosally primed cells preferentially return to GALT effector sites in the epithelium and the lamina propria (39). Accordingly, the frequency of HIV- and CMV-specific CD8+ T cells detected in rectal mucosa may be linked to the extent of HIV and CMV viral replication in GALT.
GALT T cells express mucosal trafficking markers.
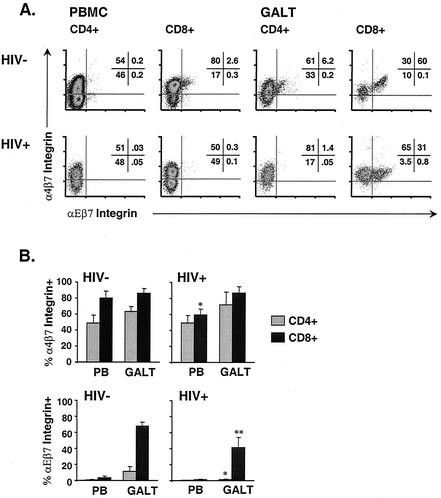
Integrins α4β7 and αΕβ7 have been implicated in T-cell trafficking to GALT. Integrin α4β7 is expressed on circulating lymphocytes that have been primed in, and will ultimately return to, mucosal tissues (66). α4β7 integrin interacts with MAdCAM-1 on high endothelial venules, facilitating rolling and arrest prior to extravasation (reviewed in reference 66). Recent evidence suggests that T cells primed in mesenteric lymph nodes begin expressing this integrin within 2 days of exposure to antigen (6). Expression is maintained on lymphocytes that have returned to mucosal tissue. In peripheral blood of two healthy individuals, means of 48.9% of CD4+ and 80.1% of CD8+ T cells expressed this marker (Fig. 4). In GALT of the same healthy controls, α4β7 integrin was present on 63.5% of CD4+ and 86.3% of CD8+ T cells.
FIG. 4.
Expression of β7 integrins in blood and GALT. (A) Expression of integrins α4β7 and αΕβ7 on CD4+ and CD8+ T cells from blood and GALT of HIV-negative (top) and HIV-positive (bottom) individuals. Numbers indicate the percentage of CD4+ or CD8+ lymphocytes in each quadrant. (B) Mean (bar height) and standard deviation (error bars) α4β7 and αΕβ7 levels for HIV-infected individuals and controls as stated in the text. Top, α4β7 expression. ∗, P = 0.014 for blood CD8+ T cells from six HIV-positive versus two HIV-negative individuals. Bottom, αΕβ7 expression. ∗ and ∗∗, P = 0.003 for GALT CD4+ T cells and P = 0.004 for GALT CD8+ T cells, respectively, from six HIV-positive versus four HIV-negative individuals.
In contrast, αΕβ7 integrin (CD103) is expressed by fewer than 5% of peripheral blood lymphocytes but by 30 to 40% of LPL and 80 to 90% of IEL (7) (Fig. 4). Expression of αΕβ7 is believed to be important for tethering of IEL, which are primarily CD8+ T cells, to adjacent intestinal epithelial cells (IEC) (7). This interaction involves binding of αΕβ7 to its ligand, E-cadherin, on epithelial cells. In GALT from four healthy controls, αΕβ7 integrin was expressed by means of 11.2% of CD4+ and 68.3% of CD8+ T cells (Fig. 4). Greater than 95% of GALT lymphocytes expressing αΕβ7 also expressed α4β7.
Expression of β7 integrins in HIV-infected subjects suggests subtle alterations in CD8+ T-cell trafficking to GALT.
Expression of α4β7 integrin by GALT T cells was similar in healthy controls and HIV-infected individuals. However, HIV-infected individuals had fewer circulating α4β7+ CD8+ T cells than healthy controls (means of 59.1 versus 80.1% [P = 0.014] for six HIV-positive patients and two controls) (Fig. 4B). This result suggests two possible interpretations: first, priming of antigen-specific CD8+ T cells in mucosal tissues may be somewhat impaired in chronically HIV-infected individuals; second, induction of α4β7 integrin expression on mucosally primed CD8+ T cells may be slightly reduced due to impaired signaling.
While the differences observed in α4β7 integrin expression were relatively minor, GALT T cells from HIV-infected patients showed a striking reduction in αΕβ7 integrin expression. Mean expression was 1.4% on GALT CD4+ T cells and 41.1% on GALT CD8+ T cells (P = 0.003 and 0.004, respectively, for six HIV-positive patients versus four controls) (Fig. 4B). The apparent depletion of CD4+ T cells expressing αΕβ7 integrin suggests that these cells serve as targets for HIV infection. The reduction of αΕβ7 integrin expression on GALT CD8+ T cells suggests that tethering of CD8+ IEL to adjacent epithelial cells may be impaired during HIV infection. This defect might reduce the ability of circulating CD8+ T cells to remain in GALT, resulting in eventual local impairment of CD8+ T-cell mediated immune surveillance.
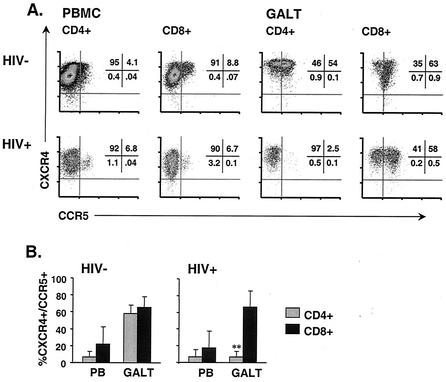
Depletion of GALT CD4+ T cells expressing CXCR4 and CCR5.
The particular susceptibility of lamina propria CD4+ T cells to HIV infection is believed to be related to two factors: expression of viral coreceptors CXCR4 and CCR5 and activation status (i.e., CD69+, HLA-DR+, and CD38+) (26, 46, 65). This enhanced susceptibility of LPL to HIV infection, compared to PBMC, likely accounts for the rapid depletion of lamina propria CD4+ T cells during acute infection of rhesus macaques with SIVmac (65).
In three healthy control individuals, greater than 90% of blood T cells expressed CXCR4 but only a minority expressed CCR5 (means of 6.9 and 21.8% for CD4+ and CD8+ cells, respectively) (Fig. 5) (69). As previously reported, the majority of GALT T cells expressed both chemokine receptors (3); 59.3% of GALT CD4+ and 75.9% of GALT CD8+ T cells from uninfected controls expressed CCR5. Furthermore, GALT T cells stained with antibodies to CXCR4 showed greater mean fluorescence intensity, suggesting greater receptor density, than blood T cells (Fig. 5A).
FIG. 5.
Expression of CXCR4 and CCR5 in blood and GALT. (A) Expression of chemokine receptors CXCR4 and CCR5 on CD4+ and CD8+ T cells from blood and GALT of HIV-negative (top) and HIV-positive (bottom) individuals. Numbers indicate the percentages of CD4+ or CD8+ lymphocytes in each quadrant. (B) Mean (bar height) and standard deviation (error bars) values for HIV-infected individuals and controls as stated in the text. ∗∗, P < 0.001 for GALT CD4+ T cells expressing CXCR4+ and CCR5+ from seven HIV-positive versus three HIV-negative individuals.
In seven HIV-positive individuals, the percentage of mucosal CD8+ T cells expressing CCR5 was similar to that observed in controls (mean of 75.1%) (Fig. 5B) (3). However, the population of CXCR4+/CCR5+ GALT CD4+ T cells observed in healthy controls was nearly absent in HIV-positive individuals (mean of 12%; P < 0.001 for seven HIV-positive patients versus three healthy controls) (Fig. 5B). This observation supports the earlier finding that GALT CD4+ T cells expressing both HIV coreceptors are preferentially depleted.
Differential trafficking of antigen-specific CD8+ T cells to GALT.
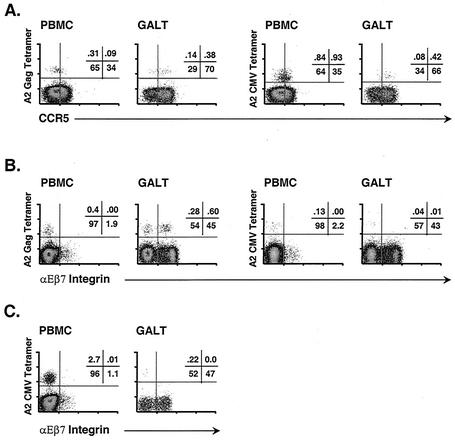
The high percentage of CCR5+ T cells in GALT suggests a role for CCR5 ligands (i.e., MIP-1α, MIP-1β, and RANTES) in chemotaxis to GALT during an inflammatory response (24). Furthermore, it was previously reported that MIP-1α, MIP-1β, and RANTES are expressed at high levels in GALT of HIV-positive individuals and patients with inflammatory bowel disease (44). To elucidate the basis for differential trafficking of HIV- and CMV-specific CD8+ T cells, we assessed expression of CCR5 on tetramer binding CD8+ T cells from blood and GALT of four patients: G04, G12, G13, and G25. As shown in Fig. 6A for patient G04, antigen-specific CD8+ T cells in blood included both CCR5− and CCR5+ subsets. However, in GALT, the majority of HIV- and CMV-specific CD8+ T cells expressed CCR5. There were no consistent, significant differences in CCR5 expression between the two populations. Thus, while chemokine-mediated chemotaxis may contribute to the presence of antigen-specific, CCR5+ T cells in GALT, it cannot explain the retention of HIV- but not CMV-specific CD8+ T cells in intestinal tissue.
FIG. 6.
Antigen-specific CD8+ T cells express lymphocyte trafficking markers. (A) Expression of CCR5 by HIV Gag (left)- and CMV (right)-specific CD8+ T cells in blood and GALT of patient G04. (B) Expression of αΕβ7 integrin by HIV Gag (left)- and CMV (right)-specific CD8+ T cells in blood and GALT of patient G25. (C) Expression of αΕβ7 integrin by CMV-specific CD8+ T cells in blood and GALT of patient G05.
We next assessed expression of αΕβ7 integrin on HIV- and CMV-specific CD8+ T cells in GALT and PBMC from patients G05, G10, G22, and G25. Patients G22 and G25 had relatively high frequencies of Gag-specific T cells in GALT and blood but few CMV-specific CD8+ T cells in either compartment. In GALT from patients G22 and G25, respectively, 30 and 60% of HIV-specific CD8+ T cells expressed αΕβ7 integrin; however, few if any CMV-specific CD8+ T cells expressed αΕβ7 (Fig. 6B). Patients G05 and G10 had very high frequencies of CMV-specific CD8+ T cells in blood (i.e., >1%). However, even in these patients, few CMV-specific CD8+ T cells expressed αΕβ7 in GALT (Fig. 6C). Taken together, these results suggest that HIV-specific CD8+ T cells present at a moderately high frequency in GALT likely underwent initial priming within mucosal inductive sites, inducing expression of homing receptors that enabled their localization and retention in GALT. Accordingly, if mucosal trafficking of antigen-specific T cells is driven by local viral replication, these data suggest ongoing, active replication of HIV, but not CMV, in GALT of these patients. Additional studies to assess HIV and CMV replication in tissues should help to clarify this issue.
DISCUSSION
These studies demonstrate that GALT is the site of an active CD8+ T-cell response during chronic HIV infection. In the relatively healthy, chronically infected individuals studied, we observed a similar or increased frequency of HIV-specific CD8+ T cells in GALT and blood, with maximal responses in the range of 1 to 3% of CD8+ T cells. These findings are similar to those reported for rhesus macaques experimentally infected with SIVmac, although the frequencies of antigen-specific CD8+ T cells are lower than those detected in simian GALT following acute infection (47, 64). It has been suggested that HIV-specific CD8+ T cells may fail to home to tissue reservoirs for viral replication, notably lymph nodes, because of decreased expression of CCR7 and CD62L (8). In contrast, our results demonstrate the capacity of HIV-specific CD8+ T cells to localize to GALT and suggest that an inability to home to this important site of viral replication is not a major factor in immune failure.
Although both HIV- and CMV-specific CD8+ T cells were detected in GALT, CMV-specific cells were found at much lower frequency in rectal mucosa than in peripheral blood of most patients. This finding was somewhat surprising in light of recent studies in mice, which demonstrated long-term persistence of CD8+ T cells specific for vesicular stomatitis virus, Listeria monocytogenes, and vaccinia virus in mucosal tissues (35, 36). CD8+ T cells specific for both HIV and CMV in rectal tissue expressed CCR5, suggesting a role for CCR5 ligands in chemotaxis to intestinal mucosa. The chemokines MIP-1α, MIP-1β, and RANTES are expressed at high levels in GALT of patients with both HIV infection and inflammatory bowel disease (44). Thus, CCR5-mediated chemotaxis may play an important role in CD8+ T-cell trafficking to GALT during HIV infection and other inflammatory conditions. Two alternative explanations should also be considered. First, the possibility exists that T cells upregulate CCR5 expression after migrating to GALT, perhaps as a consequence of exposure to locally produced cytokines that may upregulate CCR5 expression (18, 70). Second, T cells localized to GALT have a CD45RO+ memory phenotype. Accordingly, expression of CCR5 in these cells might be related to the T-cell differentiation state rather than to trafficking per se (13, 59, 68).
CCR5 expression cannot account for the differential retention of HIV- and CMV-specific CD8+ T cells in GALT, since expression of this receptor was common to HIV- and CMV-specific T cells in the patients tested. Although the complex homing patterns of antigen-specific T cells remain incompletely understood, current dogma suggests that most activated T cells preferentially survey the original site of infection (27, 39). Thus, T cells primed in mucosal inductive sites express integrins that direct their homing to mucosal effector sites (39). In mice, CD8+ T cells primed in mesenteric lymph nodes begin expressing the α4β7 integrin within 2 days of antigen exposure (6). Thus, the trafficking of HIV-specific CD8+ T cells to GALT might be explained by local priming in response to ongoing HIV replication in GALT. The selective retention of HIV-specific T cells in GALT (Table 1; Fig. 3), coupled with the expression patterns of αΕβ7 integrin by HIV- and CMV-specific CD8+ T cells (Fig. 6), appears to support this hypothesis.
None of the patients studied had evidence of active CMV disease, although all were CMV seropositive. The major tissue reservoir for CMV during latent infection is not known, but recent work with mice suggests that capillary endothelial cells and monocyte/macrophage progenitors in bone marrow may be important sites (21, 22). CMV can be an important gastrointestinal pathogen in advanced HIV disease (9). To further address the trafficking patterns of CMV-specific T cells, it will be of interest to study patients with clinical evidence of active CMV disease and to determine whether some patients have evidence of ongoing CMV replication in GALT.
The most direct effect of HIV infection on GALT is depletion of CD4+ T cells (11, 48, 62), particularly CXCR4+/CCR5+ memory cells and those expressing αΕβ7 integrin. Among the chronically infected individuals studied here, only two had CD4+/CD8+ T-cell ratios of greater than 0.5 in GALT. One of these patients reported high compliance on combination antiretroviral therapy, with complete suppression of plasma viremia. These findings warrant further investigation and suggest that GALT CD4+ T cells are relatively well maintained (or restored) in patients with suppressed viremia due to combination therapy (37). It will also be of interest to study GALT T-cell populations in long-term nonprogressors.
In most patients, the extents of CD4+ T-cell depletion in blood and GALT were similar. Rhesus macaques infected with SIVmac undergo profound depletion of GALT CD4+ T cells within 2 weeks of infection, during which time peripheral blood CD4+ T-cell counts remain relatively stable (63, 65). However, little is known about the kinetics of CD4+ T-cell depletion in human GALT; thus, our findings may be more typical of chronic HIV infection. Other significant effects of HIV infection on GALT T cells include decreased expression of αΕβ7 integrin by CD8+ T cells. Although antigen-specific CD8+ T cells remained capable of localizing to GALT in the patients studied, decreased expression of α4β7 integrin by blood CD8+ T cells, coupled with decreased expression of αΕβ7 integrin within GALT, might be predicted to reduce the efficiency of mucosal antigen-specific T-cell responses over time. In this regard, it will be of interest to determine whether decreased expression of β7 integrins correlates with disease progression.
These findings demonstrate the feasibility of using rectal biopsy tissue to study immune responses to HIV and other pathogens in humans. Much work remains to fully characterize the mechanisms of induction, trafficking patterns, and effector pathways utilized by mucosal CD8+ T cells. Although MHC class I tetramers provide a rapid, quantitative assessment of CD8+ T-cell frequency, their use remains limited to a short list of immunodominant epitopes. Recent studies suggest that the overall HIV-specific CD8+ T-cell response is not adequately predicted by the response to a single epitope (5). Accordingly, it will be important to extend these studies to other epitopes, using methods such as cytokine flow cytometry and ELISpot, in order to provide a more complete picture of mucosal immunity to HIV. Because lymphoid tissues harbor a majority of the body's lymphocytes, including CD4+ T cells, which are highly susceptible to HIV infection, additional studies of GALT and other mucosal sites will be of great relevance to our understanding of viral pathogenesis.
Acknowledgments
This research was supported by the National Institutes of Health (NIH) through the University of California Center for AIDS Research (CFAR) (NIH grant P30-AI27763). B.L.S. was the recipient of UCSF-GIVI CFAR Developmental Award 2483SC. Salary support was provided by NIH grants R21 AI-47746 and R21 NS-44976 to B.L.S. D.F.N. is an Elizabeth Glaser Scientist supported by the Elizabeth Glaser Pediatric AIDS Foundation.
We thank Marty Bigos, Director of the Gladstone Flow Cytometry Core, for helpful discussions and guidance on flow cytometry experiments. We acknowledge Michael Briskin, Millenium Pharmaceuticals, for providing Act-1 monoclonal antibody. We are grateful to Graham S. Ogg, Oxford University, for supplying MHC class I tetrameric complexes. We thank the study volunteers for their participation and the staff of the General Clinical Research Center (GCRC), San Francisco General Hospital, for expert assistance. We acknowledge Douglas Black (the Positive Health Program, San Francisco General Hospital) and Diane Schmidt (Gladstone Institute of Virology and Immunology) for advice and assistance with patient enrollment. We also thank Miles Davenport (University of New South Wales) for stimulating discussions of lymphocyte trafficking.
REFERENCES
- 1.Agace, W. W., J. M. Higgins, B. Sadasivan, M. B. Brenner, and C. M. Parker. 2000. T-lymphocyte-epithelial-cell interactions: integrin alpha(E)(CD103)beta(7), LEEP-CAM and chemokines. Curr. Opin. Cell Biol. 12:563-568. [DOI] [PubMed] [Google Scholar]
- 2.Agace, W. W., A. I. Roberts, L. Wu, C. Greineder, E. C. Ebert, and C. M. Parker. 2000. Human intestinal lamina propria and intraepithelial lymphocytes express receptors specific for chemokines induced by inflammation. Eur. J. Immunol. 30:819-826. [DOI] [PubMed] [Google Scholar]
- 3.Anton, P. A., J. Elliot, M. A. Poles, I. M. McGowan, J. Matud, L. E. Hultin, K. Grovit-Ferbas, C. R. Mackay, I. S. Y. Chen, and J. V. Giorgi. 2000. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS 14:1761-1765. [DOI] [PubMed] [Google Scholar]
- 4.Belyakov, I. M., J. D. Ahlers, B. Y. Brandwein, P. Earl, B. L. Kelsall, B. Moss, W. Strober, and J. A. Berzofsky. 1998. The importance of local mucosal HIV-specific CD8(+) cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. J. Clin. Investig. 102:2072-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betts, M. R., J. P. Casazza, B. A. Patterson, S. Waldrop, W. Trigona, T. M. Fu, F. Kern, L. J. Picker, and R. A. Koup. 2000. Putative immunodominant human immunodeficiency virus-specific CD8+ T-cell responses cannot be predicted by major histocompatibility complex class I haplotype. J. Virol. 74:9144-9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell, D. J., and E. C. Butcher. 2002. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T-cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195:135-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cepek, K. L., S. K. Shaw, C. M. Parker, G. J. Russell, J. S. Morrow, D. L. Rimm, and M. B. Brenner. 1994. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature 372:190-193. [DOI] [PubMed] [Google Scholar]
- 8.Chen, G., P. Shankar, C. Lange, H. Valdez, P. R. Skolnik, L. Wu, N. Manjunath, and J. Lieberman. 2001. CD8 T cells specific for human immunodeficiency virus, Epstein-Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood 98:156-164. [DOI] [PubMed] [Google Scholar]
- 9.Cheung, T. W., and S. A. Teich. 1999. Cytomegalovirus infection in patients with HIV infection. Mt. Sinai J. Med. 66:113-124. [PubMed] [Google Scholar]
- 10.Chui, D. W., and R. L. Owen. 1994. AIDS and the gut. J. Gastroenterol. Hepatol. 9:291-303. [DOI] [PubMed] [Google Scholar]
- 11.Clayton, F., G. Snow, S. Reka, and D. P. Kotler. 1997. Selective depletion of rectal lamina propria rather than lymphoid aggregate CD4 lymphocytes in HIV infection. Clin. Exp. Immunol. 107:288-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couedel-Courteille, A., R. Le Grand, M. Tulliez, J. Guillet, and A. Venet. 1997. Direct ex vivo simian immunodeficiency virus (SIV)-specific cytotoxic activity detected from small intestine intraepithelial lymphocytes of SIV-infected macaques at an advanced stage of infection. J. Virol. 71:1052-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davenport, M. P., M. C. Grimm, and A. R. Lloyd. 2000. A homing selection hypothesis for T-cell trafficking. Immunol. Today 21:315-317. [DOI] [PubMed] [Google Scholar]
- 14.Dwinell, M. B., N. Lugering, L. Eckmann, and M. F. Kagnoff. 2001. Regulated production of interferon-inducible T-cell chemoattractants by human intestinal epithelial cells. Gastroenterology 120:49-59. [DOI] [PubMed] [Google Scholar]
- 15.Fackler, O. T., M. Schafer, W. Schmidt, T. Zippel, W. Heise, T. Schneider, M. Zeitz, E. O. Riecken, N. Mueller-Lantzsch, and R. Ullrich. 1998. HIV-1 p24 but not proviral load is increased in the intestinal mucosa compared with the peripheral blood in HIV-infected patients. AIDS 12:139-146. [DOI] [PubMed] [Google Scholar]
- 16.Guy-Grand, D., and P. Vassalli. 2002. Gut intraepithelial lymphocyte development. Curr. Opin. Immunol. 14:255-259. [DOI] [PubMed] [Google Scholar]
- 17.Hel, Z., J. Nacsa, B. Kelsall, W. P. Tsai, N. Letvin, R. W. Parks, E. Tryniszewska, L. Picker, M. G. Lewis, Y. Edghill-Smith, M. Moniuszko, R. Pal, L. Stevceva, J. D. Altman, T. M. Allen, D. Watkins, J. V. Torres, J. A. Berzofsky, I. M. Belyakov, W. Strober, and G. Franchini. 2001. Impairment of Gag-specific CD8+ T-cell function in mucosal and systemic compartments of simian immunodeficiency virus mac251- and simian-human immunodeficiency virus KU2-infected macaques. J. Virol. 75:11483-11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwasaki, M., T. Mukai, P. Gao, W. R. Park, C. Nakajima, M. Tomura, H. Fujiwara, and T. Hamaoka. 2001. A critical role for IL-12 in CCR5 induction on T cell receptor-triggered mouse CD4(+) and CD8(+) T cells. Eur. J. Immunol. 31:2411-2420. [DOI] [PubMed] [Google Scholar]
- 19.Kim, C. H., E. J. Kunkel, J. Boisvert, B. Johnston, J. J. Campbell, M. C. Genovese, H. B. Greenberg, and E. C. Butcher. 2001. Bonzo/CXCR6 expression defines type 1-polarized T-cell subsets with extralymphoid tissue homing potential. J. Clin. Investig. 107:595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klavinskis, L. S., L. A. Bergmeier, L. Gao, E. Mitchell, R. G. Ward, G. Layton, R. Brookes, N. J. Meyers, and T. Lehner. 1996. Mucosal or targeted lymph node immunization of macaques with a particulate SIVp27 protein elicits virus-specific CTL in the genito-rectal mucosa and draining lymph nodes. J. Immunol. 157:2521-2527. [PubMed] [Google Scholar]
- 21.Koffron, A. J., M. Hummel, B. K. Patterson, S. Yan, D. B. Kaufman, J. P. Fryer, F. P. Stuart, and M. I. Abecassis. 1998. Cellular localization of latent murine cytomegalovirus. J. Virol. 72:95-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo, K., H. Kaneshima, and E. S. Mocarski. 1994. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc. Natl. Acad. Sci. USA 91:11879-11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotler, D. P., S. Reka, A. Borcich, and W. J. Cronin. 1991. Detection, localization, and quantitation of HIV-associated antigens in intestinal biopsies from patients with HIV. Am. J. Pathol. 139:823-830. [PMC free article] [PubMed] [Google Scholar]
- 24.Kunkel, E. J., and E. C. Butcher. 2002. Chemokines and the tissue-specific migration of lymphocytes. Immunity 16:1-4. [DOI] [PubMed] [Google Scholar]
- 25.Kunkel, E. J., J. J. Campbell, G. Haraldsen, J. Pan, J. Boisvert, A. I. Roberts, E. C. Ebert, M. A. Vierra, S. B. Goodman, M. C. Genovese, A. J. Wardlaw, H. B. Greenberg, C. M. Parker, E. C. Butcher, D. P. Andrew, and W. W. Agace. 2000. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lapenta, C., M. Boirivant, M. Marini, S. M. Santini, M. Logozzi, M. Viora, F. Belardelli, and S. Fais. 1999. Human intestinal lamina propria lymphocytes are naturally permissive to HIV-1 infection. Eur. J. Immunol. 29:1202-1208. [DOI] [PubMed] [Google Scholar]
- 27.Lefrancois, L., and D. Masopust. 2002. T cell immunity in lymphoid and non-lymphoid tissues. Curr. Opin. Immunol. 14:503-508. [DOI] [PubMed] [Google Scholar]
- 28.Lefrancois, L., C. M. Parker, S. Olson, W. Muller, N. Wagner, and L. Puddington. 1999. The role of beta7 integrins in CD8 T cell trafficking during an antiviral immune response. J. Exp. Med. 189:1631-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehner, T., Y. Wang, M. Cranage, L. A. Bergmeier, E. Mitchell, L. Tao, G. Hall, M. Dennis, N. Cook, R. Brookes, L. Klavinskis, I. Jones, C. Doyle, and R. Ward. 1996. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat. Med. 2:767-775. [DOI] [PubMed] [Google Scholar]
- 30.London, S. D., J. A. Cebra-Thomas, D. H. Rubin, and J. J. Cebra. 1990. CD8 lymphocyte subpopulations in Peyer's patches induced by reovirus serotype 1 infection. J. Immunol. 144:3187-3194. [PubMed] [Google Scholar]
- 31.Lundqvist, C., V. Baranov, S. Hammarstrom, L. Athlin, and M. Hammarstrom. 1995. Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int. Immunol. 7:1473-1487. [DOI] [PubMed] [Google Scholar]
- 32.Lundqvist, C., S. Melgar, M. M. Yeung, S. Hammarstrom, and M. L. Hammarstrom. 1996. Intraepithelial lymphocytes in human gut have lytic potential and a cytokine profile that suggest T helper 1 and cytotoxic functions. J. Immunol. 157:1926-1934. [PubMed] [Google Scholar]
- 33.Luster, A. D. 2001. Chemokines regulate lymphocyte homing to the intestinal mucosa. Gastroenterology 120:291-294. [DOI] [PubMed] [Google Scholar]
- 34.Markowitz, M., M. Vesanen, K. Tenner-Racz, Y. Cao, J. M. Binley, A. Talal, A. Hurley, X. Jin, M. R. Chaudhry, M. Yaman, S. Frankel, M. Heath-Chiozzi, J. M. Leonard, J. P. Moore, P. Racz, D. F. Nixon, and D. D. Ho. 1999. The effect of commencing combination antiretroviral therapy soon after human immunodeficiency virus type 1 infection on viral replication and antiviral immune responses. J. Infect. Dis. 179:527-537. [DOI] [PubMed] [Google Scholar]
- 35.Masopust, D., J. Jiang, H. Shen, and L. Lefrancois. 2001. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J. Immunol. 166:2348-2356. [DOI] [PubMed] [Google Scholar]
- 36.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 37.Mattapallil, J. J., Z. Smit-McBride, P. Dailey, and S. Dandekar. 1999. Activated memory CD4+ T helper cells repopulate the intestine early following antiretroviral therapy of simian immunodeficiency virus-infected rhesus macaques but exhibit a decreased potential to produce interleukin-2. J. Virol. 73:6661-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattapallil, J. J., Z. Smit-McBride, M. McChesney, and S. Dandekar. 1998. Intestinal intraepithelial lymphocytes are primed for gamma interferon and MIP-1β expression and display antiviral cytotoxic activity despite severe CD4+ T-cell depletion in primary simian immunodeficiency virus infection. J. Virol. 72:6421-6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mowat, A., and J. Viney. 1997. The anatomical basis of intestinal immunity. Immunol. Rev. 156:145-166. [DOI] [PubMed] [Google Scholar]
- 40.Murphey-Corb, M., L. A. Wilson, A. M. Trichel, D. E. Roberts, K. Xu, S. Ohkawa, B. Woodson, R. Bohm, and J. Blanchard. 1999. Selective induction of protective MHC class I-restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J. Immunol. 162:540-549. [PubMed] [Google Scholar]
- 41.Musey, L., Y. Hu, L. Eckert, M. Christensen, T. Karchmer, and M. J. McElrath. 1997. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J. Exp. Med. 185:293-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 43.Ogg, G. S., X. Jin, S. Bonhoeffer, P. Moss, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, A. Hurley, M. Markowitz, D. D. Ho, A. J. McMichael, and D. F. Nixon. 1999. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J. Virol. 73:797-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsson, J., M. A. Poles, A.-L. Spetz, J. Elliot, L. E. Hultin, J. V. Giorgi, J. Andersson, and P. A. Anton. 2000. Human immunodeficiency virus type 1 infection is associated with significant mucosal inflammation characterized by increased expression of CCR5, CXCR4 and β-chemokines. J. Infect. Dis. 182:1625-1635. [DOI] [PubMed] [Google Scholar]
- 45.Papadakis, K. A., J. Prehn, V. Nelson, L. Cheng, S. W. Binder, P. D. Ponath, D. P. Andrew, and S. R. Targan. 2000. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J. Immunol. 165:5069-5076. [DOI] [PubMed] [Google Scholar]
- 46.Poles, M. A., J. Elliott, P. Taing, P. A. Anton, and I. S. Chen. 2001. A preponderance of CCR5+ CXCR4+ mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J. Virol. 75:8390-8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitz, J. E., R. S. Veazey, M. J. Kuroda, D. B. Levy, A. Seth, K. G. Mansfield, C. E. Nickerson, M. A. Lifton, X. Alvarez, A. A. Lackner, and N. L. Letvin. 2001. Simian immunodeficiency virus (SIV)-specific cytotoxic T lymphocytes in gastrointestinal tissues of chronically SIV-infected rhesus monkeys. Blood 98:3757-3761. [DOI] [PubMed] [Google Scholar]
- 48.Schneider, T., H. U. Jahn, W. Schmidt, E. O. Riecken, M. Zeitz, R. Ullrich, et al. 1995. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Gut 37:524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider, T., R. Ullrich, C. Bergs, W. Schmidt, E. O. Riecken, M. Zeitz, et al. 1994. Abnormalities in subset distribution, activation, and differentiation of T cells isolated from large intestine biopsies in HIV infection. Clin. Exp. Immunol. 95:430-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shacklett, B. L., T. J. Beadle, P. A. Pacheco, J. H. Grendell, P. A. Haslett, A. S. King, G. S. Ogg, P. M. Basuk, and D. F. Nixon. 2000. Characterization of HIV-1-specific cytotoxic T lymphocytes expressing the mucosal lymphocyte integrin CD103 in rectal and duodenal lymphoid tissue of HIV-1-infected subjects. Virology 270:317-327. [DOI] [PubMed] [Google Scholar]
- 51.Shacklett, B. L., T. J. Beadle, P. A. Pacheco, J. H. Grendell, P. A. Haslett, A. S. King, G. S. Ogg, P. M. Basuk, and D. F. Nixon. 2000. Isolation of cytomegalovirus-specific cytotoxic T-lymphocytes from gut-associated lymphoid tissue (GALT) of HIV type 1-infected subjects. AIDS Res. Hum. Retroviruses 16:1157-1162. [DOI] [PubMed] [Google Scholar]
- 52.Shaw, S. K., and M. B. Brenner. 1995. The beta 7 integrins in mucosal homing and retention. Semin. Immunol. 7:335-342. [DOI] [PubMed] [Google Scholar]
- 53.Shibahara, T., J. N. Wilcox, T. Couse, and J. L. Madara. 2001. Characterization of epithelial chemoattractants for human intestinal intraepithelial lymphocytes. Gastroenterology 120:60-70. [DOI] [PubMed] [Google Scholar]
- 54.Sillett, H. K., J. Southgate, P. D. Howdle, and L. K. Trejdosiewicz. 1999. Expression of activation and costimulatory elements by human intestinal intraepithelial lymphocytes. Scand. J. Immunol. 50:52-60. [DOI] [PubMed] [Google Scholar]
- 55.Smith, P. D., C. H. Fox, H. Masur, H. S. Winter, and D. W. Alling. 1994. Quantitative analysis of mononuclear cells expressing human immunodeficiency virus type 1 RNA in esophageal mucosa. J. Exp. Med. 180:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smit-McBride, Z., J. J. Mattapallil, M. McChesney, D. Ferrick, and S. Dandekar. 1998. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 72:6646-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stevceva, L., B. Kelsall, J. Nacsa, M. Moniuszko, Z. Hel, E. Tryniszewska, and G. Franchini. 2002. Cervicovaginal lamina propria lymphocytes: phenotypic characterization and their importance in cytotoxic T-lymphocyte responses to simian immunodeficiency virus SIVmac251. J. Virol. 76:9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sydora, B. C., B. D. Jamieson, R. Ahmed, and M. Kronenberg. 1996. Intestinal intraepithelial lymphocytes respond to systemic lymphocytic choriomeningitis virus infection. Cell. Immunol. 167:161-169. [DOI] [PubMed] [Google Scholar]
- 59.Tomiyama, H., T. Matsuda, and M. Takiguchi. 2002. Differentiation of human CD8+ T-cells from a memory to memory/effector phenotype. J. Immunol. 168:5538-5550. [DOI] [PubMed] [Google Scholar]
- 60.Ullrich, R., H. L. Schieferdecker, K. Ziegler, E. O. Riecken, and M. Zeitz. 1990. Gamma-delta T cells in the human intestine express surface markers of activation and are preferentially located in the epithelium. Cell. Immunol. 128:619-627. [DOI] [PubMed] [Google Scholar]
- 61.Vajdy, M., R. Veazey, I. Tham, C. deBakker, S. Westmoreland, M. Neutra, and A. Lackner. 2001. Early immunologic events in mucosal and systemic lymphoid tissues after intrarectal inoculation with simian immunodeficiency virus. J. Infect. Dis. 184:1007-1014. [DOI] [PubMed] [Google Scholar]
- 62.Vajdy, M., R. S. Veazey, H. K. Knight, A. A. Lackner, and M. R. Neutra. 2000. Differential effects of simian immunodeficiency virus infection on immune inductive and effector sites in the rectal mucosa of rhesus macaques. Am. J. Pathol. 157:485-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Veazey, R., M. DeMaria, L. Chalifoux, D. Shvetz, D. Pauley, H. Knight, M. Rosenzweig, R. Johnson, R. Desrosiers, and A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 64.Veazey, R. S., M. C. Gauduin, K. G. Mansfield, I. C. Tham, J. D. Altman, J. D. Lifson, A. A. Lackner, and R. P. Johnson. 2001. Emergence and kinetics of simian immunodeficiency virus-specific CD8+ T cells in the intestines of macaques during primary infection. J. Virol. 75:10515-10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Veazey, R. S., I. C. Tham, K. G. Mansfield, M. DeMaria, A. E. Forand, D. E. Shvetz, L. V. Chalifoux, P. K. Sehgal, and A. A. Lackner. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 74:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Andrian, U. H., and C. R. Mackay. 2000. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343:1020-1034. [DOI] [PubMed] [Google Scholar]
- 67.Wang, H. C., Q. Zhou, J. Dragoo, and J. R. Klein. 2002. Most murine CD8(+) intestinal intraepithelial lymphocytes are partially but not fully activated T cells. J. Immunol. 169:4717-4722. [DOI] [PubMed] [Google Scholar]
- 68.Wills, M. R., G. Okecha, M. P. Weekes, M. K. Gandhi, P. J. Sissons, and A. J. Carmichael. 2002. Identification of naive or antigen-experienced human CD8+ T-cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8+ T-cell response. J. Immunol. 168:5455-5464. [DOI] [PubMed] [Google Scholar]
- 69.Yang, Y. F., M. Tomura, M. Iwasaki, T. Mukai, P. Gao, S. Ono, J. P. Zou, G. M. Shearer, H. Fujiwara, and T. Hamaoka. 2001. IL-12 as well as IL-2 upregulates CCR5 expression on T cell receptor-triggered human CD4+ and CD8+ T cells. J. Clin. Immunol. 21:116-125. [DOI] [PubMed] [Google Scholar]
- 70.Yang, Y. F., M. Tomura, M. Iwasaki, S. Ono, J. P. Zou, K. Uno, G. M. Shearer, H. Fujiwara, and T. Hamaoka. 2001. IFN-alpha acts on T-cell receptor-triggered human peripheral leukocytes to up-regulate CCR5 expression on CD4+ and CD8+ T cells. J. Clin. Immunol. 21:402-409. [DOI] [PubMed] [Google Scholar]
- 71.Zabel, B. A., W. W. Agace, J. J. Campbell, H. M. Heath, D. Parent, A. I. Roberts, E. C. Ebert, N. Kassam, S. Qin, M. Zovko, G. J. LaRosa, L. L. Yang, D. Soler, E. C. Butcher, P. D. Ponath, C. M. Parker, and D. P. Andrew. 1999. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J. Exp. Med. 190:1241-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zeitz, M., T. Schneider, and R. Ullrich. 1996. Mucosal HIV infection: a paradigm for dysregulation of the mucosal immune system, p. 421-436. In M. Kagnoff (ed.), Essentials of mucosal immunology. Academic Press, San Diego, Calif.