Abstract
Interferon-γ (IFN-γ) is critical for defense against pathogens, but the molecules that mediate its antimicrobial responses are largely unknown. IGTP is the prototype for a family of IFN-γ-regulated genes that encode 48-kDa GTP-binding proteins that localize to the endoplasmic reticulum. We have generated IGTP-deficient mice and found that, despite normal immune cell development and normal clearance of Listeria monocytogenes and cytomegalovirus infections, the mice displayed a profound loss of host resistance to acute infections of the protozoan parasite Toxoplasma gondii. By contrast, IFN-γ receptor-deficient mice have increased susceptibility to all three pathogens. Thus, IGTP defines an IFN-γ-regulated pathway with a specialized role in antimicrobial resistance.
Interferon-γ (IFN-γ) is an important cytokine for control of infectious agents and regulation of the immune system (1–3). Mice that lack production of IFN-γ or the IFN-γ receptor have decreased defense against parasites, bacteria, viruses, and tumors (4–6). IFN-γ is thought to exert its effects largely by activation of IFN-γ-responsive genes, over 200 of which have been identified (7). However, for most of these genes, their contribution in mediating the effects of IFN-γ is unknown.
One recently identified IFN-γ-regulated gene is IGTP (8). It is representative of a growing family of at least six genes that include LRG-47 (9), IRG-47 (10), TGTP/Mg21 (11, 12), IIGP (13), and GTPI (13). The functions of these genes are unknown, but each is expressed at high levels in both immune and nonimmune cells after exposure to IFN-γ, and each encodes a 47- to 48-kDa protein that contains GTP-binding sequences (8). Several of these proteins, including IGTP (14), LRG-47, and TGTP, localize to the endoplasmic reticulum (ER) of cells, suggesting that they may be involved in the processing or trafficking of immunologically relevant proteins, such as antigens or cytokines.
Here, we investigated the potential involvement of IGTP in IFN-γ-mediated immunity by creating IGTP-deficient mice through gene targeting. Despite normal resistance to Listeria monocytogenes and murine cytomegalovirus, these mice displayed a profound lack of resistance to infections of the parasite Toxoplasma gondii, demonstrating that IGTP is an essential mediator of specialized antimicrobial activities of IFN-γ.
Materials and Methods
IGTP Gene Targeting.
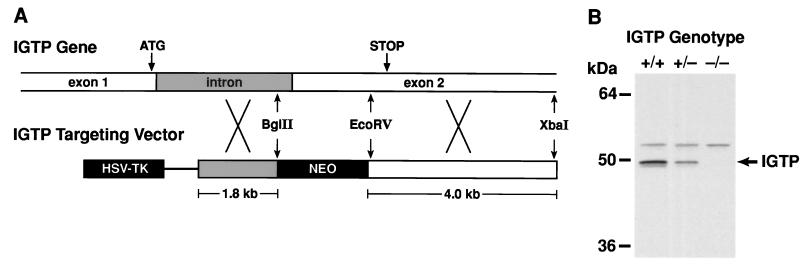
An IGTP targeting vector (Fig. 1A) was constructed from a 6-kb XbaI IGTP gene fragment, from which 0.9 kb of the IGTP gene corresponding to intronic sequence and codons 13–240 (8) were deleted and replaced with pGKneoBpA. In the vector, these sequences were flanked by pGKtkBpA (15, 16). The targeting vector was electroporated into CJ7 embryonic stem (ES) cells (15, 16), and homologous recombinants were selected by Southern blotting of BamHI-digested DNA with a 5′ external IGTP probe (an 0.18-kb BamHI–NcoI fragment of the IGTP cDNA). Using these cells and established procedures, IGTP-deficient mice were generated (15, 16). Two lines of IGTP-deficient mice were established from separate targeted ES cell lines, and the behavior of the two mouse lines was indistinguishable in subsequent studies. All experiments were performed by using 1- to 4-mo-old mice on a C57BL/6 × 129Sv genetic background. The mice were maintained in a specific pathogen-free facility.
Figure 1.
Targeting the IGTP gene. (A) An IGTP replacement targeting vector was prepared from the indicated IGTP gene fragments and used to generate IGTP-deficient mice. (B) Thymic protein from mice of the indicated genotypes was resolved by 10% SDS/PAGE and used for Western blotting with anti-C-terminal IGTP antisera. The positions of selected molecular weight markers are shown at the left.
Protein and RNA Analyses.
For Western blot analysis, protein lysates were prepared from cells or tissues, separated by 10% SDS/PAGE, and blotted with anti-IGTP antibodies, as described previously (8). For Northern blot analysis, 15-μg total RNA samples were separated on 1.2% agarose/formaldehyde gels and blotted with mouse IGTP and human glyceraldehyde-3-phosphate dehydrogenase cDNA probes, as described previously (8).
T. gondii Infection.
Mice were injected i.p. with 0.5 ml PBS containing 20 cysts of the avirulent ME49 strain of T. gondii, which had been prepared from the brains of infected C57BL/6 mice. The mice were monitored daily.
For ex vivo cytokine analysis, single-cell spleen cell and peritoneal exudate cell (PEC) cultures were prepared from infected mice, and red cells were removed from the spleen cells by using ACK lysing buffer (BioWhittaker). The spleen cells and PECs were then cultured in 96-well plates, at 8 × 105 and 4 × 105 cells per well, respectively, in 200 μl RPMI medium 1640 (Life Technologies, Rockville, MD) supplemented with 10% (vol/vol) FBS (Life Technologies). When indicated, cell cultures were stimulated with 10 mg/ml plate-bound anti-CD3 (PharMingen) or 10 mg/ml STAg (soluble tachyzoite antigen), which had been prepared from sonicated RH parasites as previously described (17). Conditioned media were collected 72 hr later for determination of IFN-γ and IL-12 p40 levels by using sandwich ELISA as described previously (18).
Sera were prepared from blood that was collected at the time of sacrifice, allowed to clot, and then centrifuged at 6,000 rpm for 10 min.
L. monocytogenes Infection.
The mice were inoculated i.p. with different doses of L. monocytogenes EGD strain (kindly provided by K. Elkins, U.S. Food and Drug Administration). Health and survival of the mice were monitored daily for at least 14 days. For experiments involving the measurement of serum cytokine levels, bacterial loads in the spleen and liver, or IGTP expression levels, the mice were inoculated i.p. with 1,000 bacteria, and the sera and relevant tissues were isolated 3 days later. Bacterial counts were determined by excising spleens and livers sterilely, homogenizing portions of the organs in PBS, and plating serial dilutions of the homogenate on LB agar plates. Colony counts were assessed the following day, and the total bacterial load per organ was calculated.
Viral Infection.
Murine cytomegalovirus (MCMV) (19) and Ebola virus infection (20), and the subsequent hepatic analysis and cytokine ELISA were performed as described in detail previously.
Results
To target the IGTP gene, we constructed a targeting vector in which the majority of the IGTP protein coding sequence was replaced by a neomycin resistance gene (Fig. 1A). Using this vector, we generated IGTP-deficient mice that produced no detectable IGTP protein in all tested tissues, including thymus (Fig. 1B). These mice displayed no obvious physical or behavioral abnormalities; they were fertile; and the targeted IGTP allele segregated with near Mendelian ratios (data not shown). In addition, complete histological analysis of the major organs indicated nothing remarkable, and FACS analysis revealed no changes in immune cell development, as assessed by examining T cell (CD3+, CD4+, CD8+, CD25+), B cell (B220+), natural killer (NK) cell (DX5+), granulocyte (Mac1+, 8C5+), macrophage (Mac1+, 8C5−, DX5−), and erythroid (TER119+) cell markers in the thymus, spleen, bone marrow, and lymph nodes (data not shown).
To examine host defense against pathogens in the IGTP-deficient mice, we challenged them with several infectious agents, including the protozoan parasite T. gondii, the bacterium L. monocytogenes, and MCMV. Resistance to each of these pathogens is crucially dependent on host production of IFN-γ; mice lacking IFN-γ or the IFN-γ receptor demonstrate markedly increased susceptibility to each of them (6, 18, 19, 21, 22). In the present studies, we found that infection of wild-type mice with any of the three pathogens dramatically increased IGTP expression in liver and spleen (Fig. 2). However, despite the uniformly increased IGTP levels seen in wild-type mice, IGTP-deficient mice displayed severely compromised defense specifically against T. gondii, but not against the bacterium or virus.
Figure 2.
Induction of IGTP expression by different pathogens. Pairs of wild-type mice (B and C), or wild-type and IGTP-deficient mice (A), were inoculated with 1,000 L. monocytogenes for 3 days (A), 105 plaque-forming units MCMV for 36 or 72 hr (B), or 20 cysts ME49 strain T. gondii for 5 days (C). Protein lysates were prepared from the indicated tissues or cells and used for Western blotting with an anti-IGTP antibody. The positions of selected molecular weight markers are indicated at the left.
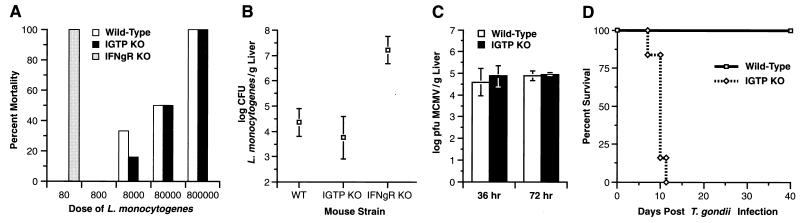
In the L. monocytogenes studies, all IGTP-deficient and wild-type mice inoculated with 800 or fewer bacteria survived the challenge, whereas all IFN-γ-deficient mice receiving 80 bacteria uniformly succumbed within 4–5 days (Fig. 3A). At higher innocula, the IGTP-deficient and wild-type mice showed similar mortality rates (Fig. 3A). Furthermore, splenic and hepatic bacterial loads 3 days after inoculation with 1,000 bacteria were about equivalent in IGTP-deficient and wild-type mice, but were increased 3–4 logs in IFN-γ receptor-deficient mice (Fig. 3B).
Figure 3.
Differential susceptibility of IGTP-deficient mice to pathogens. (A) Wild-type, IGTP-deficient (IGTP KO), or IFN-γ receptor-deficient (IFNgR KO) mice were inoculated i.p. with the indicated doses of L. monocytogenes, and mortality was assessed over a 14-day period. (B) Wild-type, IGTP-deficient, or IFN-γ receptor-deficient mice were inoculated i.p. with 1,000 L. monocytogenes; after 3 days, the number of bacteria in the liver was determined. (C) Wild-type or IGTP-deficient mice were inoculated with 105 plaque-forming units MCMV i.p.; after 36 or 72 h, the number of virus in the liver was determined. (D) Wild-type and IGTP-deficient mice were inoculated i.p. with 20 cysts ME49 strain T. gondii. The cumulative mortality of the indicated numbers of IGTP-deficient mice (◊) or wild-type mice (□) was followed for 40 days after infection. The graph is representative of three experiments.
In the MCMV studies, IGTP-deficient and wild-type mice had comparable hepatic viral loads (Fig. 3C), as well as similar incidences of focal hepatic necrosis (data not shown), at both 36 and 72 hr following MCMV inoculation. Conversely, it has been shown previously that IFN-γ-deficient mice have marked increases in both hepatic viral titers and hepatic necrosis after MCMV infection (19, 22). In addition, the IGTP-deficient mice displayed undiminished cytotoxic T lymphocyte activity and NK cell cytolytic activity (data not shown). We also challenged the IGTP-deficient mice with Ebola virus, but they showed neither altered susceptibility to Ebola nor a defect in their ability to be immunized against it (data not shown).
In striking contrast to the bacterial and viral studies, the IGTP-deficient mice showed a complete inability to restrict acute T. gondii infection. All IGTP-deficient mice inoculated i.p. with 20 cysts of the parasite died within 8–12 days, whereas wild-type mice survived the infection (Fig. 3D). This pronounced susceptibility to the parasite mimicked that previously reported for IFN-γ-deficient mice, which died within the same time frame (18), and it suggested that IGTP was essential for IFN-γ-dependent host resistance to T. gondii.
To directly assess the ability of the mice to restrict parasite replication, we inoculated IGTP-deficient and wild-type mice with T. gondii and measured parasitic loads at 5 days postinfection. By microscopic examination, 19 +/− 5% (SEM) of PECs from IGTP-deficient mice contained parasites, compared with 0.05 +/− 0.05% of those from wild-type mice, indicating that immune defense was not sufficient to prevent spread of the parasite.
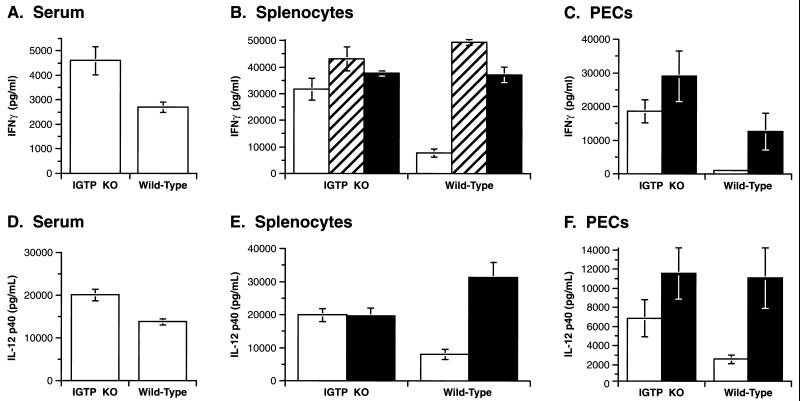
We also measured IL-12 p40 and IFN-γ levels at 5 days postinfection; increased production of these cytokines is absolutely required to restrict T. gondii infection (18, 21, 23). In sera, both IL-12 and IFN-γ levels were slightly elevated in infected IGTP-deficient mice, compared with infected wild-type mice (Fig. 4 A and D). From splenocytes and PECs isolated from infected IGTP-deficient mice and cultured in vitro, there was also slightly increased IFN-γ and IL-12 production; these levels increased when the cells were stimulated with anti-CD3 or a Toxoplasma antigen mixture (STAg), to levels comparable to those secreted by infected wild-type cells (Fig. 4 B, C, E, and F). Therefore, the IGTP-deficient mice responded to T. gondii infection with a robust IFN-γ response, implying that their defect in controlling T. gondii was distal to IFN-γ. The slightly increased levels in IL-12 and IFN-γ were probably only a result of the persistent infection.
Figure 4.
Undiminished IL-12 p40 and IFN-γ production in T. gondii-infected IGTP-deficient mice. Mice were inoculated i.p. with 20 cysts ME49 strain T. gondii. Five days postinoculation of IGTP-deficient or wild-type mice, IL-12 p40 and IFN-γ levels were determined in sera (A and D) and the conditioned media of cultures splenocytes (B and E) or PECs (C and F). Splenocytes and PECs were incubated in control medium (open bars), medium supplemented with anti-CD3 antibody (striped bars), or medium supplemented with a soluble Toxoplasma antigen mixture, STAg (filled bars). SD for groups of four mice are shown.
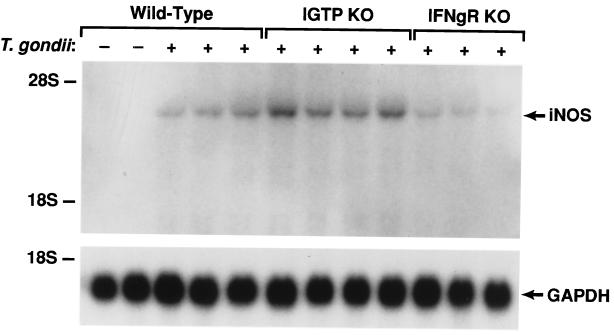
In the IGTP-deficient mice, we also examined production of nitric oxide (NO), an important effector of macrophage-based killing. Studies with mice deficient in inducible NO synthase (iNOS), the enzyme that generates NO, have shown that NO is essential for resistance to chronic T. gondii infections, but not acute infections (24). We found that IGTP-deficient mice showed large increases in hepatic iNOS mRNA levels 8 days after T. gondii infection; the iNOS mRNA levels in infected wild-type mice were also increased, but not to the same extent (Fig. 5). However, in infected IFN-γ receptor-deficient mice, iNOS levels were increased only slightly (Fig. 5). Higher iNOS levels in the IGTP-deficient mice, relative to those in wild-type mice, may have been because of the uncontrolled infection. However, the retention of a marked increase in iNOS levels in IGTP-deficient mice following infection suggested that their lack of host resistance to T. gondii was not simply a result of decreased NO production.
Figure 5.
Undiminished iNOS production in T. gondii-infected IGTP-deficient mice. Wild-type, IGTP-deficient (IGTP KO), and IFN-γ receptor-deficient (IFNgR KO) mice were inoculated i.p. with 20 cysts ME49 strain T. gondii. Eight days postinoculation, hepatic mRNA was prepared and used for sequential Northern blotting with iNOS and glyceraldehyde-3-phosphate dehydrogenase probes. Positions of the major ribosomal RNA species are indicated as determined from the stained gel.
Discussion
IFN-γ regulates a broad range of antimicrobial responses against protozoa, bacteria, and viruses. The studies presented here indicate that IGTP is an essential component of an IFN-γ-regulated pathway that mediates host resistance to a subset of pathogens including protozoa such as T. gondii.
Little is known about the molecular function of IGTP. It is a GTP-binding protein that is membrane bound and localizes predominantly to the ER (8, 14). It is expressed at high levels in many IFN-γ-stimulated cells, including immune cells such as macrophages, T cells, and B cells, and nonimmune cells, such as fibroblasts and hepatocytes (8). Therefore, in the context of host resistance, IGTP could function by regulating immune cell function, or alternatively, by providing an antimicrobial activity in all cells, including nonimmune cells. As to the former, although we have not examined immune cell function exhaustively, we found no evidence that IGTP regulates any specific immune cell activity. For instance, in vivo production of IL-12 p40, IFN-γ, and NO by IGTP-deficient mice was not decreased following acute T. gondii infection; cytotoxic T lymphocyte and NK cell killing in IGTP-deficient cells was not reduced; and IGTP-deficient splenocytes showed undiminished proliferative responses to T cell and B cell mitogens (data not shown). Furthermore, the observation that IGTP-deficient mice display normal resistance to L. monocytogenes argues that IGTP does not result in generalized immune deficiency or dysregulation.
Thus, it seems more likely that IGTP provides a more generalized effect against T. gondii in many types of cells, not only in immune cells. In fact, it has been shown recently that IFN-γ-induced elements are required in both immune cells and nonimmune cells for normal host resistance to T. gondii (25). This is in contrast to resistance to L. monocytogenes, in which IFN-γ action is only required in immune cells (25). IGTP is expressed in immune and nonimmune cells, and it may well provide critical elements of host resistance to T. gondii within both cell types. Following invasion of host cells, T. gondii establishes itself within a parasitophorous vacuole that is somewhat sequestered from the endocytic and exocytic machinery of the host cells. Parasite growth then occurs within the vacuole (26). We have proposed previously that, as a GTP-binding protein within the ER, IGTP may regulate vesicular transport within the cell (14). It is possible that through regulation of vesicular movement to the parasitophorous vacuole, IGTP controls parasite clearance. It is intriguing to suggest that this involves the targeted transport of IFN-γ-induced toxic mediators to the vacuole.
IGTP is only one in a family of IFN-γ-induced GTPases that includes LRG-47 (9), IRG-47 (10), TGTP/Mg21 (11, 12), IIGP (13), and GTPI (13); however, the role of the related proteins in in vivo host resistance has not been addressed. It is possible that they may operate within the same pathway, or, alternatively, that they may regulate different antimicrobial pathways that are all controlled by IFN-γ. The marked phenotype of the IGTP-deficient mice suggests that the latter may be true and that the different genes evolved to mediate distinct antimicrobial responses. Consistent with this idea, in vitro studies have shown that the related protein TGTP possesses specific antiviral activity, in that its overexpression in fibroblasts can limit plaque formation by vesicular stomatitis virus, but not by a herpes virus (27).
The immune mechanisms that regulate resistance to protozoan infections, such as those of T. gondii, are poorly understood. The identification of IGTP as the first down-stream mediator of IFN-γ that is required for acute resistance to T. gondii in vivo is, therefore, significant. Toxoplasmosis is a serious clinical concern, particularly in the case of AIDS patients, and current treatments are limited by adverse drug effects (15). Future studies should define the nature of IGTP's actions and explore the possibility of manipulating them to develop novel therapies against increasingly prevalent human T. gondii infections.
Acknowledgments
We thank Richard Frederickson for preparation of the figures and Ave Cline and Michelle Reed for manuscript preparation. This research was sponsored in part by the National Cancer Institute, Department of Health and Human Services, under Advanced BioScience Laboratories Contract NO1-CO-46000.
Abbreviations
- ER
endoplasmic reticulum
- PEC
peritoneal exudate cell
- MCMV
murine cytomegalovirus
- NK cell
natural killer cell
- iNOS
inducible NO synthase
References
- 1.Stark G R, Kerr I M, Williams B R G, Silverman R H, Schreiber R D. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 2.Boehm U, Klamp T, Groot M, Howard J C. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 3.Billiau A. Adv Immunol. 1996;62:61–130. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan D H, Shanlaran V, Dighe A S, Stockert E, Aguet M, Old L J, Schreiber R D. Proc Natl Acad Sci USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 6.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinjernagel R M, Aguet M. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 7.Der S D, Zhou A, Williams B R G, Silverman R H. Proc Natl Acad Sci USA. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor G A, Jeffers M, Largaespada D A, Jenkins N A, Copeland N G, Vande Woude G F. J Biol Chem. 1996;271:20399–20405. doi: 10.1074/jbc.271.34.20399. [DOI] [PubMed] [Google Scholar]
- 9.Sorace J M, Johnson R J, Howard D L, Drysdale B E. J Leukocyte Biol. 1995;58:477–484. doi: 10.1002/jlb.58.4.477. [DOI] [PubMed] [Google Scholar]
- 10.Gilly M, Wall R. J Immunol. 1992;148:3275–3281. [PubMed] [Google Scholar]
- 11.Carlow D A, Marth J, Clark-Lewis I, Teh H-S. J Immunol. 1995;154:1724–1734. [PubMed] [Google Scholar]
- 12.Lafuse W P, Brown D, Castle L, Zwilling B S. J Leukocyte Biol. 1995;57:477–483. doi: 10.1002/jlb.57.3.477. [DOI] [PubMed] [Google Scholar]
- 13.Boehm U, Guethlein L, Klamp T, Ozbek K, Schaub A, Futterer A, Pfeffer K, Howard J C. J Immunol. 1998;161:6715–6723. [PubMed] [Google Scholar]
- 14.Taylor G A, Stauber R, Rulong S, Hudson E, Pei V, Pavlakis G N, Resau J H, Vande Woude G F. J Biol Chem. 1997;272:10639–10645. doi: 10.1074/jbc.272.16.10639. [DOI] [PubMed] [Google Scholar]
- 15.Porter S B, Sande M A. N Engl J Med. 1992;327:1643–1648. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 16.Bonin, A., Reid, S. W. & Tessarollo, L. (2000) Methods Mol. Biol., in press. [DOI] [PubMed]
- 17.Grunvald E, Chiaramonte M, Hieny S, Wysocka M, Trincieri G, Vogel S N, Gazinelli R T, Sher A. Infect Immun. 1996;64:2010–2018. doi: 10.1128/iai.64.6.2010-2018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scharton-Kersten T M, Wynn T A, Denkers E Y, Bala S, Grunvald E, Hieny S, Gazzinelli R T, Sher A. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 19.Orange J S, Wang B, Terhorst C, Biron C A. J Exp Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bray M, Davis K, Geisbert T, Schmaljohn C, Huggins J. J Infect Dis. 1998;178:651–661. doi: 10.1086/515386. [DOI] [PubMed] [Google Scholar]
- 21.Gazzinelli R T, Wysocka M, Hayashi S, Denkers E Y, Hieny S, Caspar P, Trinchieri G, Sher A. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 22.Pomeroy C, Delong D, Clabots C, Riciputi P, Filice G A. J Lab Clin Med. 1998;132:124–133. doi: 10.1016/s0022-2143(98)90007-5. [DOI] [PubMed] [Google Scholar]
- 23.Gazzinelli R T, Hieny S, Wynn T, Wolf S, Sher A. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scharton-Kersten T M, Yap G, Magram J, Sher A. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yap G S, Sher A. J Exp Med. 1999;189:1083–1091. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sibley L D. Semin Cell Biol. 1993;4:335–344. doi: 10.1006/scel.1993.1040. [DOI] [PubMed] [Google Scholar]
- 27.Carlow D A, Teh S-J, Huang-Sia T. J Immunol. 1998;161:2348–2355. [PubMed] [Google Scholar]