Abstract
The Tmevp3 locus controls the load of Theiler's virus RNA during persistent infection of the mouse central nervous system (CNS). We identified a candidate gene at this locus, Tmevpg1, by using a positional cloning approach. Tmevpg1 and its human ortholog, TMEVPG1, are expressed in the immune system and encode what appears to be a noncoding RNA. They are located in a cluster of cytokine genes that includes the genes for gamma interferon and one or two homolog of interleukin-10. We now report that Tmevpg1 is expressed in CNS-infiltrating immune cells of resistant B10.S mice, but not in those of susceptible SJL/J mice, following inoculation with Theiler's virus. The pattern of expression of Tmevpg1 is the same in B10.S mice and in SJL/J mice congenic for the resistant B10.S haplotype of Tmevp3. Nineteen polymorphisms were identified when the Tmevpg1 genes of B10.S and SJL/J mice were compared. Interestingly, Tmevpg1 is down regulated after in vitro stimulation of murine CD4+ or CD8+ splenocytes, whereas Ifng is up regulated. Similar patterns of expression of TMEVPG1 and IFNG were observed in human NK cells and CD4+ and CD8+ T lymphocytes. Therefore, Tmevpg1 is a strong candidate gene for the Tmevp3 locus and may be involved in the control of Ifng gene expression.
After intracranial inoculation, the DA strain of Theiler's virus replicates for approximately 2 weeks in neurons of the mouse brain and spinal cord, regardless of the mouse's genetic background. Some genetically resistant mice clear the infection at this stage. Others, which are susceptible to persistence of the infection, remain infected for life (26). However, in this case, the virus does not persist in neurons. Instead, it is found in glial cells of the white matter of the spinal cord. Persistent infection of the white matter induces chronic inflammation and primary demyelination similar to those seen in multiple sclerosis (25). Susceptibility to viral persistence varies greatly among inbred strains of mice. The viral genome load during persistent infection is controlled mainly by the H2D class I gene (4-6, 24). However, the SJL/J strain is the only inbred strain among 16 examined for which the viral genome load is greater than that predicted by its H2s haplotype (13). Studies of bone marrow chimeras of the SJL/J and B10.S strains, which both bear an H2s haplotype, showed that susceptibility loci with major effects on persistence are expressed in cells of the immune system (3). Some non-H2 susceptibility loci were mapped by using a backcross and congenic mice between the SJL/J and B10.S strains. Two susceptibility loci, Tmevp2 and Tmevp3, were located on chromosome 10 close to the Ifng locus (8, 12). However, immunological studies indicated that the Ifng gene does not explain the effects of the Tmevp2 or the Tmevp3 locus (30). Instead, the Tmevpg1 gene was recently identified by positional cloning of the Tmevp3 locus. It is located telomeric to a cluster of cytokines, which includes the Ifng and IL-22/Il-Tif genes (36). Tmevpg1 has six exons and appears to encode a noncoding RNA. It is expressed at a low level in the spleen and thymus, and occasionally in the liver and kidneys, of B10.S mice but never in the central nervous system (CNS) of these animals. Tmevpg1 has a human ortholog, TMEVPG1, with homologies to the mouse gene only in exon 1 and the region surrounding it. In the present paper, we show that Tmevpg1 is a strong candidate gene for the Tmevp3 locus and analyze its expression in the immune system.
MATERIALS AND METHODS
Mice and viral infection.
SJL/J mice were purchased from Janvier (Le Genest-St-Isle, France). C1 and C2 SJL/J congenic mice and B10.S mice were bred at the Pasteur Institute animal facility. The C1 and C2 SJL/J congenic mice were named SJL.B10-D10Mit70-D10Mit122 and SJL.B10.D10Mit180-D10Mit74, respectively, in a previous paper (8). The DA strain of Theiler's virus was produced by transfection of BHK-21 cells with the pTM762 plasmid as described elsewhere (27, 29). Three- to 4-week-old anesthetized mice were inoculated intracranially with 104 PFU of the DA strain in 40 μl of phosphate-buffered saline. Mice were sacrificed at 0, 3, 6, 9, and 45 days postinoculation (p.i.), depending on the experiment.
Purification of murine CD4+ and CD8+ splenocytes.
Spleens were teased to dissociate splenocytes. Red blood cells were lysed with Gey solution. Cells were labeled with magnetic microbeads coated with anti-CD4+ or anti-CD8+ antibodies in accordance with the manufacturer's (Miltenyi Biotech GmbH) recommendations and separated on an Automacs device (Miltenyi Biotech GmbH) by using the possel program. The purity of the separation was assessed by fluorescence-activated cell sorter analysis of whole splenocytes and enriched and negative fractions. Lymphocytes in the enriched fraction contained 70 to 80% CD4+ or CD8+ cells.
Purification of human NK+, CD4+, and CD8+ T lymphocytes.
Peripheral blood lymphocytes (PBL) were isolated from the buffy coat of healthy volunteer blood donors by Ficoll separation, magnetically labeled by using CD4+ T-cell, CD8+ T-cell, and NK cell isolation kits (Miltenyi Biotech GmbH), and separated on an Automacs device (Miltenyi Biotech GmbH) by using the deplete program. The purity of the separation was assessed by fluorescence-activated cell sorter analysis of whole PBL and enriched and negative fractions. Lymphocytes in the enriched fraction contained 70 to 80% CD4+, CD8+, or CD56+ cells.
Cell culture.
Cells were cultured in RPMI medium-10% fetal calf serum with 50 ng of phorbol 12-myristate 13-acetate (PMA; Sigma) per ml and 1.5 μM ionomycin (Sigma) at a density of 0.5 × 106/ml. Cells were harvested after the indicated time, pelleted, and stored at −80°C. Human CD4+ and CD8+ T cells and murine CD8+ splenocytes were washed three times after 24 h of culture and then cultured further without PMA or ionomycin for 48 h.
PCR amplification.
Extraction of total RNA, reverse transcription (RT)-PCR amplification, and the protocol for hybridization of amplified DNA have been described previously for the mouse Tmevpg1 and human TMEVPG1 genes (36). Briefly, total RNA was extracted from tissues with guanidinium thiocyanate (14), DNase treated, and reverse transcribed with random hexamer primers. Similar methods were used for cultured cells, except that the total RNA was extracted by the RNeasy kit (Qiagen, France). Tmevpg1 expression was analyzed by PCR amplification (40 cycles) of a 178-bp fragment with primers in exons 1 and 2. To study TMEVPG1 expression, a 233-bp product was amplified with primers located in exons 1 and 3. The primers are separated by more than 30 kb in the mouse genome and by 16,610 bp in the human genome. The specificity of the amplification was confirmed by hybridization with a 32P-labeled internal probe. PCR products were also cloned and sequenced. The sequence of the B10.S Tmevpg1 RT-PCR product was identical to that predicted from the genomic sequences. A 281-bp fragment of mouse Ifng was amplified by PCR with 5′-AAGTTCTGGGCTTCTCCTCC-3′ as the forward primer and 5′-GACTTCAAAGAGTCTGAGGT-3′ as the reverse primer. After a denaturation step of 2 min at 94°C, 25 to 40 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 15 s were performed. The sequence of the internal probe was 5′-TGCCAGTTCCTCCAGATATC-3′. A 294-bp fragment of human IFNG was amplified by PCR with 5′-GGTTCTCTTGGCTGTTACTGC-3′ as the forward primer and 5′-GTCATCTCGTTTCTTTTTGTTGCT-3′ as the reverse primer. After a denaturation step of 2 min at 94°C, 25 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s were performed. The sequence of the internal probe was 5′-GGTCATTCAGATGTAGCGGA-3′.
RESULTS
Tmevpg1 expression pattern.
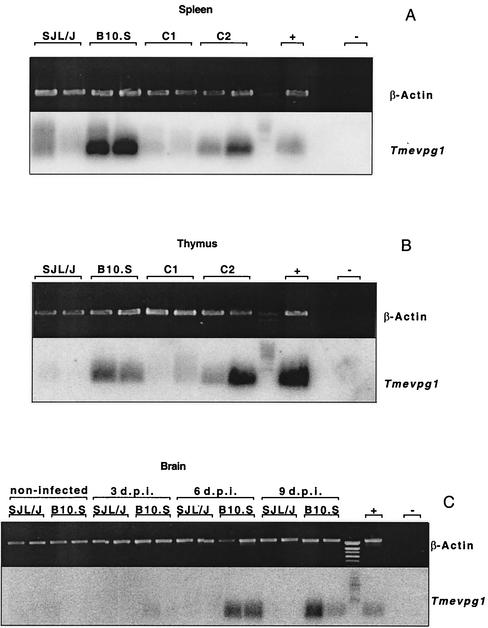
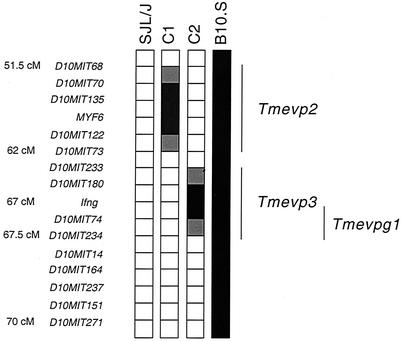
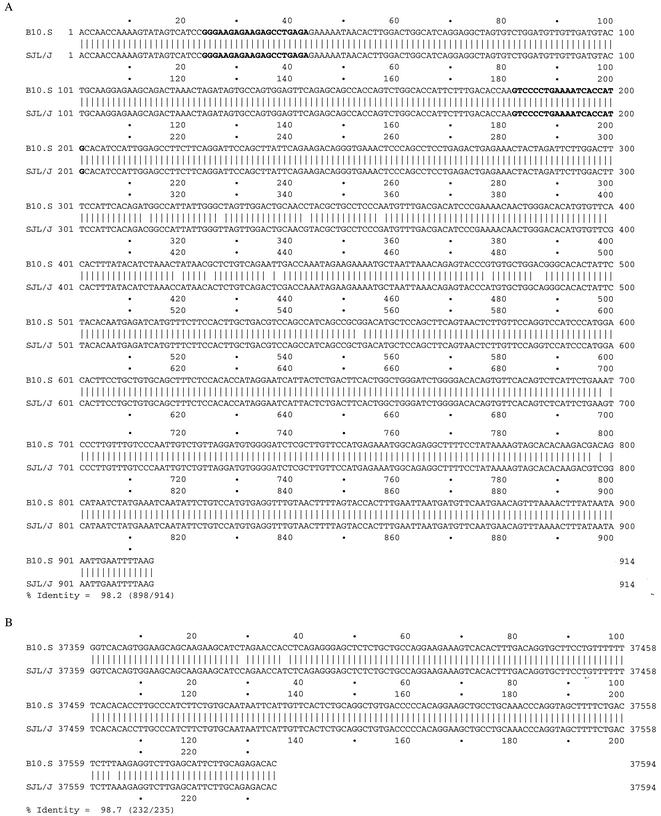
We studied Tmevpg1 expression in the spleens (Fig. 1A) and thymuses (Fig. 1B) of 3- to 4-week-old uninfected B10.S, SJL/J, and C1 and C2 SJL/J congenic mice by RT-PCR. A DNA band of approximately 180 bp was amplified from B10.S, but not SJL/J, samples. Even if the intensity of the DNA band was low for some B10.S samples (data not shown) and a smear was detected on occasion in SJL/J samples (for example, in the first lane of Fig. 1A), Tmevpg1 expression was strikingly different in these two mouse strains. These patterns of Tmevpg1 expression segregated differently between the two SJL congenic lines. The C1 congenic mice had a pattern of expression similar to that of SJL/J mice, and the C2 congenic mice had a pattern of expression similar to that of B10.S mice (Fig. 1A and B). Figure 2 shows the genotype of these congenic mice for the Tmevp2 and Tmevp3 loci. Interestingly, Tmevpg1 is of the SJL/J haplotype in the C1 line and of the B10.S haplotype in the C2 line (F. Levillayer and J. F. Bureau, unpublished results). Therefore, in the two SJL congenic mice, the expression pattern of the Tmevpg1 gene depends on the haplotype of both the Tmevpg1 gene and the Tmevp3 locus. The promoter region and the six exons of Tmevpg1 were amplified from SJL/J and B10.S genomic DNA, sequenced, and compared. Nineteen polymorphisms were found in this 1,149-bp sequence (Fig. 3).
FIG. 1.
Expression of the Tmevpg1 gene in mice. Expression of the Tmevpg1 and β-Actin genes in the spleens (A) and thymuses (B) of uninfected SJL/J, B10.S, and C1 and C2 congenic mice and in the brains (C) of SJL/J and B10.S mice at 0, 3, 6, and 9 days p.i. (d.p.i.) with Theiler's virus. Two mice of each strain were studied, one male and one female. +, positive control; −, negative control (water).
FIG. 2.
Genotype of the telomeric part of chromosome 10 of SJL/J, B10.S, and C1 and C2 congenic mice. Open squares, regions from the SJL/J parent; solid squares, regions from the B10.S parent; hatched squares, a genetic segment where a crossover occurred. Markers indicating polymorphisms between the SJL/J and B10.S parents are on the left. The lines at the right indicate the positions of the Tmevp2 and Tmevp3 loci. The Tmevpg1 gene is located between the Ifng and D10Mit234 markers. Genetic positions, except for those of the D10Mit74, D10Mit234, and D10Mit14 markers, were obtained from http://www.informatics.jax.org/. cM, centimorgans.
FIG. 3.
Polymorphisms in the Tmevpg1 gene sequences of the SJL/J and B10.S strains. The SJL/J and B10.S genomic sequences of the six exons (A) and the 235-bp region of the promoter that is conserved between the mouse and human genomes (B) were compared by using DNA Strider (CEA, Gif-sur-Yvette, France). In panel A, the sequences of the primer pair used to study Tmevpg1 expression are in bold characters. In panel B, the positions are those of the sequence of BAC 13C18.
The expression of the Tmevpg1 gene was studied during the acute phase of infection by Theiler's virus of B10.S and SJL/J mice. The level of expression of the Tmevpg1 gene in the spleens and thymuses of B10.S mice did not change during infection (data not shown). Expression in the brains of B10.S mice was detected at 6 and 9 days p.i. No expression was detected in the brains of SJL/J mice at any time p.i. (Fig. 1C). Similar results were obtained in the spinal cord (data not shown).
Thus, the Tmevpg1 gene is expressed in the spleens and thymuses of uninfected B10.S mice and in their brains and spinal cords during the acute phase of Theiler's virus infection. No expression of the Tmevpg1 gene is observed in SJL/J mice. Therefore, Tmevpg1 is a candidate gene for the Tmevp3 locus because its expression depends on the haplotype of both the Tmevpg1 gene and the Tmevp3 locus.
The Tmevpg1 gene is expressed in peripheral immune cells.
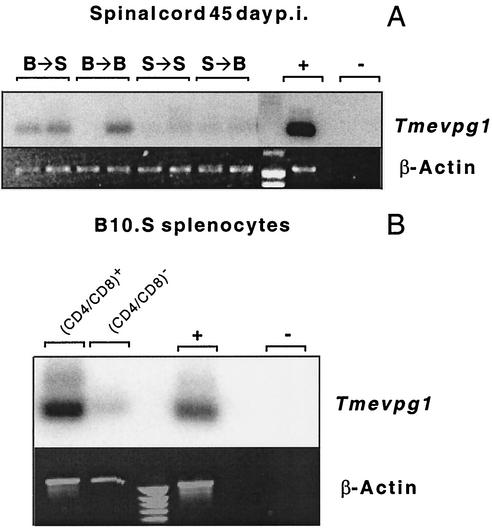
The expression of Tmevpg1 in the CNSs of Theiler's virus-infected mice could be due to the infiltration by peripheral immune cells or to the activation of CNS-resident cells such as microglia. To distinguish between these possibilities, we used bone marrow chimeras of SJL/J and B10.S mice and took advantage of the fact that Tmevpg1 is expressed in B10.S mice and not in SJL/J mice (3). In these experiments, if Tmevpg1 is expressed in infiltrating immune cells, its level of expression will correlate with the B10.S haplotype of bone marrow cells. In contrast, if Tmevpg1 is expressed in activated CNS-resident cells, its level of expression will correlate with the B10.S haplotype of the recipient mice. Figure 4A shows that chimeras reconstituted with B10.S bone marrow expressed Tmevpg1 at 45 days p.i. in the spinal cord whereas chimeras reconstituted with SJL/J bone marrow did not. Therefore, the Tmevpg1 gene is expressed mainly in infiltrating immune cells and not in CNS-resident cells during chronic infection by Theiler's virus.
FIG. 4.
Expression of the Tmevpg1 gene in immune cells. (A) Expression of the Tmevpg1 and β-Actin genes in the spinal cords of bone marrow chimeras of SJL/J and B10.S mice at 45 days p.i. with Theiler's virus. B → S, SJL/J mice reconstituted with B10.S bone marrow; B → B, B10.S mice reconstituted with B10.S bone marrow; S → S, SJL/J mice reconstituted with SJL/J bone marrow; S → B, B10.S mice reconstituted with SJL/J bone marrow. Two mice were studied in each case. (B) Expression in B10.S splenocytes. +, positive control; −, negative control (water).
We also studied the expression of Tmevpg1 in different subsets of ex vivo splenocytes from B10.S mice. Tmevpg1 was expressed at a higher level in CD4+ or CD8+ splenocytes than in CD4− and CD8− splenocytes (Fig. 4B). All of these data indicate that the Tmevpg1 gene is expressed in peripheral immune cells of B10.S mice.
The Tmevpg1 gene is expressed in nonstimulated murine CD4+ and CD8+ splenocytes.
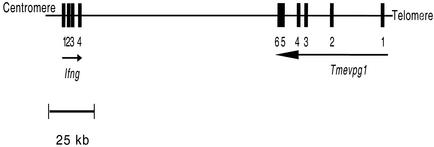
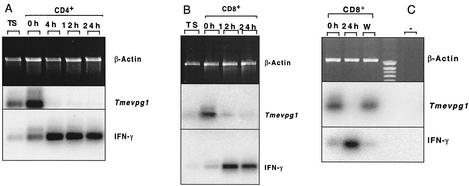
The Tmevpg1 and Ifng genes are transcribed on opposite DNA strands (Fig. 5), suggesting that their expression may depend on each other. To examine this possibility, the expression of both genes was studied by RT-PCR in CD4+ and CD8+ B10.S splenocytes after stimulation with PMA and ionomycin (Fig. 6). As expected, a 178-bp band specific for Tmevpg1 was amplified from unstimulated CD4+ and CD8+ splenocytes. The intensity of this band decreased considerably, or it disappeared completely, after 4 to 24 h of activation with PMA and ionomycin. In CD8+ splenocytes, the band reappeared at the original level 48 h after removal of PMA and ionomycin by washing (Fig. 6C). In contrast, an intense 281-bp band specific for Ifng was amplified from cells stimulated with PMA and ionomycin. The faint Ifng band detected in nonstimulated CD4+ or CD8+ splenocytes was probably due to the activation of these cells by anti-CD4+ or anti-CD8+ antibodies during purification. Therefore, the Tmevpg1 and Ifng genes are expressed in the same subsets of splenocytes and the pattern of expression of the Tmevpg1 gene during stimulation is a mirror image of that of the Ifng gene.
FIG. 5.
Genomic organization of the murine Tmevpg1 and Ifng genes on chromosome 10. Solid squares, exons; arrows, 5′-to-3′ orientation of a gene. No gene has been identified between Tmevpg1 and Ifng. This map is based on data from http://www.ensembl.org/Mus_musculus/
FIG. 6.
Expression of the murine Tmevpg1 and Ifng genes in CD4+ (A) and CD8+ (B and C) splenocytes. Hours after activation by PMA and ionomycin are indicated. TS, total splenocytes; W, 48 h after removal of PMA and ionomycin and washing; +, positive control; −, negative control (water); IFN-γ, gamma interferon.
Pattern of expression of TMEVPG1 and IFNG genes in human NK cells and CD4+ and CD8+ T lymphocytes.
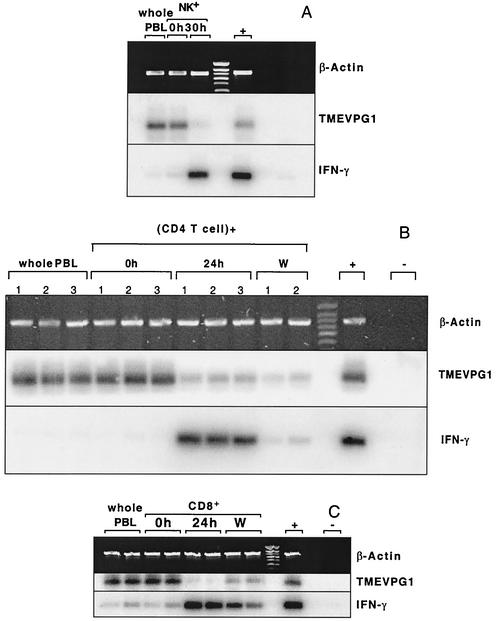
The expression of the TMEVPG1 and IFNG genes was studied in NK cells, CD4+ T lymphocytes, and CD8+ T lymphocytes from human peripheral blood (Fig. 7). A 233-bp band specific for TMEVPG1 was amplified from nonstimulated NK cells and CD4+ and CD8+ T lymphocytes. The intensity of this band decreased considerably after the stimulation of these lymphocytes with PMA and ionomycin. The expression of TMEVPG1 reappeared in CD8+ T lymphocytes 72 h after removal of PMA and ionomycin and washing. A 294-bp band specific for IFNG was amplified from all of the stimulated samples, as expected. The intensity of this band decreased considerably after washing. Therefore, TMEVPG1 is expressed in the three subsets of unstimulated lymphocytes. Furthermore, the pattern of expression of TMEVPG1 during stimulation is a mirror image of that of IFNG.
FIG. 7.
Expression of human TMEVPG1 and IFNG in NK cells (A), CD4+ T lymphocytes (B), and CD8+ T lymphocytes (C). Two and three samples from different healthy volunteers were studied for CD4+ and CD8+ T lymphocytes, respectively. Hours after activation by PMA and ionomycin are indicated. W, 48 h after removal of PMA and ionomycin and washing; +, positive control; −, negative control (water); IFN-γ, gamma interferon.
DISCUSSION
The murine Tmevpg1 gene and its human ortholog, TMEVPG1, were identified recently during the positional cloning of Tmevp3, a locus that controls the difference in the viral genome load between SJL/J and B10.S mice (36). Both genes are located in a cluster of cytokine genes that includes the gamma interferon gene (16, 22, 36). Previous studies showed that Tmevpg1 is expressed mainly in the spleen and thymus and that its human ortholog is expressed in PBL. In the present study, we tested the possibility that Tmevpg1 is a candidate gene for the Tmevp3 locus and investigated the expression of this gene in the immune system.
Tmevpg1 is expressed in the spleens and thymuses of B10.S mice and in the CNSs of these mice after infection with Theiler's virus. In contrast, no expression was detected in SJL/J mice. Studies of SJL/J mice congenic for the B10.S haplotype of the Tmevp2 or Tmevp3 locus showed that expression of Tmevpg1 depends on the haplotype of both the Tmevp3 locus and the Tmevpg1 gene. Therefore, the Tmevpg1 gene is a strong candidate gene for the Tmevp3 locus. The frequency of polymorphisms in the promoter and the six exons of Tmevpg1 of the SJL/J and B10.S strains is 0.0165. This frequency is consistent with that obtained by studying BAC 13C18, which contains the first two exons of Tmevpg1. Six microsatellite markers out of eight tested were polymorphic between these two strains (F. Bihl, F. Levillayer, and J. F. Bureau, unpublished results). Published data showed a high rate of polymorphism in this region, suggesting that it may contain a quantitative trait locus such as Tmevp3 (10, 37). Studies are under way to identify polymorphisms responsible for the difference in the expression of Tmevpg1 in the SJL/J and B10.S strains.
Studies of bone marrow chimeras of SJL/J and B10.S mice and of the expression of Tmevpg1 in CD4+ and CD8+ splenocytes showed that the Tmevpg1 gene is expressed in peripheral immune cells. This pattern of expression is in agreement with the fact that susceptibility loci with a major effect when the SJL/J and B10.S mice are compared are expressed in the hematopoietic system (3).
The Tmevpg1 gene and its human ortholog are expressed in immune cells only when they are not stimulated. The fact that TMEVPG1 is expressed in human NK and T lymphocytes strongly suggests, by analogy, that murine splenocytes expressing Tmevpg1 are T lymphocytes. The expression in nonstimulated lymphocytes may be explained by the presence of two Ets consensus transcription factor recognition sites in the TMEVPG1 and Tmevpg1 promoters (7, 36). Indeed, Ets1 is expressed in resting T cells in an unphosphorylated form that can bind to DNA and activate transcription. Ets1 is quickly phosphorylated after T-cell stimulation and becomes inactive. Studies of Ets1 knockout mice have confirmed that this transcription factor plays a role in the survival of mature resting T cells (11, 33).
Expression of Tmevpg1 in the CNS following Theiler's virus infection suggests that the gene is expressed in infiltrating immune cells. As discussed above, Tmevpg1 is expressed only in unstimulated CD4+ and CD8+ splenocytes. Therefore, a portion of these CNS-infiltrating immune cells may not be activated. The Vβ TcR repertoire present in the CNS during Theiler's infection is consistent with this interpretation: some of the T cells that infiltrate the CNS at either 21 or 45 days p.i. are not being expanded, as shown by the Gaussian distribution of the length of their CDR3 (32, 34).
Many noncoding RNAs have been discovered recently during the annotation of whole genome sequences (17). Their functions are poorly understood, but some of them, such as Xist and H19, are associated with gene silencing (15, 35). We speculate that TMEVPG1 may down-regulate the expression of IFNG because (i) both genes are expressed in the same subset of lymphocytes (i.e., CD4+, CD8+, and NK), (ii) they have opposite expression patterns following stimulation, and (iii) the Tmevpg1 gene and its human ortholog encode a noncoding RNA. Recently published data are consistent with this hypothesis. The monoallelic expression of the interleukin-4 and Ifng genes depends on transcriptional regulation elements and chromatin rearrangements that extend far beyond the size of these two genes (1, 2, 9, 31), suggesting that the transcriptional regulation occurs at multiple levels. Hutchins et al. suggested that activating and silencing signals integrate to provide spatially and temporally restricted expression patterns for cytokines such as interleukin-4 (21). Effectors of Ifng expression have been recently identified (20). In contrast, the silencing process is poorly understood (21, 23) although we know that it is associated with the DNA methylation pattern (18, 19, 28, 38). Silencing of Ifng could also implicate a noncoding RNA such as the one encoded by the Tmevpg1 gene.
In conclusion, the Tmevpg1 gene has different patterns of expression in susceptible SJL/J and resistant B10.S mice and is a good candidate gene for the Tmevp3 locus. The molecular mechanism of this difference is being investigated. Elucidation of this mechanism may help us to understand the function of this noncoding RNA and its role in susceptibility to persistent infection by Theiler's virus.
Acknowledgments
We acknowledge S. Aubagnac for the gift of mRNA from bone marrow chimeras, F. Levillayer and F. Levy-Acobas for technical assistance, M. Lamari and P. Bierling, Etablissement Français du Sang d'Ile de France, for the generous gift of human blood samples, and F. Lemonnier, Lucile Mollet, and Leah Sartorius for discussion.
This work was supported by grants from the Institut Pasteur Fondation, the Centre National de la Recherche Scientifique, and the Association pour la Recherche sur la Sclérose en Plaques. S.V. is the recipient of scholarships from the Fondation pour la Recherche Médicale and the Association pour la Recherche sur la Sclérose en Plaques. P.-S.R. was supported by a grant from Assistance Publique Hopitaux de Paris.
REFERENCES
- 1.Agarwal, S., and A. Rao. 1998. Long-range transcriptional regulation of cytokine gene expression. Curr. Opin. Immunol. 10:345-352. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal, S., J. P. B. Viola, and A. Rao. 1999. Chromatin-based regulatory mechanisms governing cytokine gene transcription. J. Allergy Clin. Immunol. 103:990-999. [DOI] [PubMed] [Google Scholar]
- 3.Aubagnac, S., M. Brahic, and J.-F. Bureau. 2002. Bone marrow chimeras reveal non-H-2 hematopoietic control of susceptibility to Theiler's virus persistent infection. J. Virol. 76:5807-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubagnac, S., M. Brahic, and J. F. Bureau. 2001. Viral load increases in SJL/J mice persistently infected by Theiler's virus after inactivation of the β2m gene. J. Virol. 75:7723-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azoulay-Cayla, A., S. Dethlefs, B. Pérarnau, E. L. Larsson-Sciard, F. A. Lemonnier, M. Brahic, and J.-F. Bureau. 2000. H-2Db−/− mice are susceptible to persistent infection by Theiler's virus. J. Virol. 74:5470-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azoulay-Cayla, A., S. Syan, M. Brahic, and J. F. Bureau. 2001. Roles of the H-2Db and H-Kb genes in resistance to persistent Theiler's murine encephalomyelitis virus infection of the central nervous system. J. Gen. Virol. 82:1043-1047. [DOI] [PubMed] [Google Scholar]
- 7.Bassuk, A. G., and J. M. Leiden. 1997. The role of Ets transcription factors in the development and function of the mammalian immune system. Adv. Immunol. 64:65-104. [DOI] [PubMed] [Google Scholar]
- 8.Bihl, F., M. Brahic, and J.-F. Bureau. 1999. Two loci, Tmevp2 and Tmevp3, located on the telomeric region of chromosome 10, control the persistence of Theiler's virus in the central nervous system. Genetics 152:385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bix, M., and R. M. Locksley. 1998. Independent and epigenetic regulation of interleukin-4 alleles in CD4+ T cells. Science 281:1352-1354. [DOI] [PubMed] [Google Scholar]
- 10.Bonhomme, F., J.-L. Guénet, B. Dod, K. Moriwaki, and G. Bulfield. 1987. The polyphyletic origin of laboratory inbred mice and their rate of evolution. J. Linn. Soc. 30:51-58. [Google Scholar]
- 11.Bories, J. C., D. M. Willerford, D. Grevin, L. Davidson, A. Camus, P. Martin, D. Stehelin, and F. W. Alt. 1995. Increased T-cell apoptosis and terminal B-cell differentiation induced by inactivation of the Ets-1 proto-oncogene. Nature 377:635-638. [DOI] [PubMed] [Google Scholar]
- 12.Bureau, J.-F., X. Montagutelli, F. Bihl, S. Lefebvre, J.-L. Guénet, and M. Brahic. 1993. Mapping loci influencing the persistence of Theiler's virus in the murine central nervous system. Nat. Genet. 5:87-91. [DOI] [PubMed] [Google Scholar]
- 13.Bureau, J.-F., X. Montagutelli, S. Lefebvre, J.-L. Guénet, M. Pla, and M. Brahic. 1992. The interaction of two groups of murine genes determines the persistence of Theiler's virus in the central nervous system. J. Virol. 66:4698-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 15.ConstÂncia, M., B. Pickard, G. Kelsey, and W. Reik. 1998. Imprinting mechanisms. Genet. Res. 8:881-900. [DOI] [PubMed] [Google Scholar]
- 16.Dumoutier, L., E. Van Roost, G. Ameye, L. Michaux, and J.-C. Renauld. 2000. IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun. 1:488-494. [DOI] [PubMed] [Google Scholar]
- 17.Eddy, S. R. 2002. Computational genomics of noncoding RNA genes. Cell 109:137-140. [DOI] [PubMed] [Google Scholar]
- 18.Falek, P. R., S. Z. Ben-Sasson, and M. Ariel. 2000. Correlation between DNA methylation and murine IFN-γ and IL-4 expression. Cytokine 12:198-206. [DOI] [PubMed] [Google Scholar]
- 19.Fitzpatrick, D. R., K. M. Shirley, L. E. McDonald, H. Bielefeldt-Ohmann, G. F. Kay, and A. Kelso. 1998. Distinct methylation of the interferon γ (IFN-γ) and interleukin 3 (IL-3) genes in newly activated primary CD8+ T lymphocytes: regional IFN-γ expression are heritable in CD44highCD8+ T cells. J. Exp. Med. 188:103-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grogan, J. L., and R. M. Locksley. 2002. T helper cell differentiation: on again, off again. Curr. Opin. Immunol. 14:366-372. [DOI] [PubMed] [Google Scholar]
- 21.Hutchins, A. S., A. C. Mullen, H. W. Lee, K. J. Sykes, F. A. High, B. D. Hendrich, A. P. Bird, and S. L. Reiner. 2002. Gene silencing quantitatively controls the function of a developmental trans-activator. Mol. Cell 10:81-91. [DOI] [PubMed] [Google Scholar]
- 22.Knappe, A., S. Hör, S. Wittmann, and H. Fickenscher. 2000. Induction of a novel cellular homolog of interleukin-10, AK155, by transformation of T lymphocytes with herpesvirus saimiri. J. Virol. 74:3881-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, P. P., D. R. Fitzpatrick, C. Beard, H. Jessup, S. Lehar, K. W. Makar, M. Pérez-Melgosa, M. T. Sweeter, M. S. Schlissel, S. Nguyen, S. R. Cherry, J. H. Tsai, S. M. Tucker, W. M. Weaver, A. Kelso, R. Jaenisch, and C. B. Wilson. 2001. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15:763-774. [DOI] [PubMed] [Google Scholar]
- 24.Lin, X., L. R. Pease, and M. Rodriguez. 1997. Differential generation of class I H-2D- versus H-2K-restricted cytotoxicity against a demyelinating virus following central nervous system infection. Eur. J. Immunol. 27:963-970. [DOI] [PubMed] [Google Scholar]
- 25.Lipton, H., S. Miller, R. Melvold, and R. S. Fujinami. 1986. Theiler's murine encephalomyelitis virus (TMEV) infection in mice as a model for multiple sclerosis, p. 248-257. In A. L. Notkins and M. B. A. Oldstone (ed.), Concepts in viral pathogenesis, vol. 2. Springer Verlag, New York, N.Y.
- 26.Lipton, H. L., J. Kratochvil, P. Sethi, and M. C. Dal Canto. 1984. Theiler's virus antigen detected in mouse spinal cord 2 1/2 years after infection. Neurology 34:1117-1119. [DOI] [PubMed] [Google Scholar]
- 27.McAllister, A., F. Tangy, C. Aubert, and M. Brahic. 1989. Molecular cloning of the complete genome of Theiler's virus, strain DA, and production of infectious transcripts. Microb. Pathog. 7:381-388. [DOI] [PubMed] [Google Scholar]
- 28.Melvin, A. J., M. E. McGurn, S. J. Bort, C. Gibson, and D. B. Lewis. 1995. Hypomethylation of the interferon-γ gene correlates with its expression by primary T-lineage cells. Eur. J. Immunol. 1995:426-430. [DOI] [PubMed] [Google Scholar]
- 29.Michiels, T., V. Dejong, R. Rodrigus, and C. Shaw-Jackson. 1997. Protein 2A is not required for Theiler's virus replication. J. Virol. 71:9549-9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monteyne, P., F. Bihl, F. Levillayer, M. Brahic, and J.-F. Bureau. 1999. The Th1/Th2 balance does not account for the difference of susceptibility of mouse strains to Theiler's virus persistent infection. J. Immunol. 162:7330-7334. [PubMed] [Google Scholar]
- 31.Mullen, A. C., F. A. High, A. S. Hutchins, H. W. Lee, A. V. Villarino, D. M. Livingston, A. L. Kung, N. Cereb, T.-P. Yao, S. Y. Yang, and S. L. Reiner. 2001. Role of T-bet in commitment of Th1 cells before IL-12-dependent selection. Science 292:1907-1910. [DOI] [PubMed] [Google Scholar]
- 32.Musette, P., J.-F. Bureau, G. Gachelin, P. Kourilsky, and M. Brahic. 1995. T lymphocyte repertoire in Theiler's virus encephalomyelitis: the nonspecific infiltration of the central nervous system of infected SJL/J mice is associated with a selective local T cell expansion. Eur. J. Immunol. 25:1589-1593. [DOI] [PubMed] [Google Scholar]
- 33.Muthusamy, N., K. Barton, and J. M. Leiden. 1995. Defective activation and survival of T cells lacking the Ets-1 transcription factor. Nature 377:639-642. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez, M., N. Prayoonwiwat, P. Zhou, and C. David. 1993. Expression of T cell receptor Vβ transcripts in central nervous system of mice susceptible and resistant to Theiler's virus-induced demyelination. J. Neuroimmunol. 47:95-100. [DOI] [PubMed] [Google Scholar]
- 35.Storz, G. 2002. An expanding universe of noncoding RNA. Science 296:1260-1263. [DOI] [PubMed] [Google Scholar]
- 36.Vigneau, S., F. Levillayer, H. Crespeau, L. Cattolico, B. Caudron, F. Bihl, C. Robert, M. Brahic, J. Weissenbach, and J.-F. Bureau. 2001. Homology between a 173-kb region from mouse chromosome 10, telomeric to the Ifng locus, and human chromosome 12q15. Genomics 78:206-213. [DOI] [PubMed] [Google Scholar]
- 37.Wade, C. M., E. J. Kulbokas III, A. W. Kirby, M. C. Zody, J. C. Mullikin, E. S. Lander, K. Lindblad-Toh, and M. J. Daly. 2002. The mosaic structure of variation in the laboratory mouse genome. Nature 420:574-578. [DOI] [PubMed] [Google Scholar]
- 38.Young, H. A., P. Ghosh, J. Ye, J. Lederer, A. Lichtman, J. R. Gerard, L. Penix, C. B. Wilson, A. J. Melvin, M. E. McGurn, D. B. Lewis, and D. D. Taub. 1994. Differentiation of the T helper phenotypes by analysis of the methylation state of the IFN-γ gene. J. Immunol. 153:3603-3610. [PubMed] [Google Scholar]