Abstract
Subversion or appropriation of cellular signal transduction pathways is a common strategy employed by viruses to promote an environment within infected cells that supports the viral replicative cycle. Using subsets of 3T3 murine fibroblasts previously shown to differ in their ability to support myxoma virus (MV) replication, we investigated the role of host serine-threonine kinases (STKs) as potential mediators of the permissive phenotype. Both permissive and nonpermissive 3T3 cells supported equivalent levels of virion binding, entry, and early virus gene expression, indicating that MV tropism in 3T3 cells was not determined by receptor-mediated entry. In contrast, late virus gene expression and viral DNA replication were selectively compromised in restrictive 3T3 cells. Addition of specific protein kinase inhibitors, many of which shared the ability to influence the activity of the STKs p21-activated kinase 1 (PAK-1) and Raf-1 attenuated MV replication in permissive 3T3 cells. Western blot detection of the phosphorylated forms of PAK-1 (Thr423) and Raf-1 (Ser338) confirmed activation of these kinases in permissive cells after MV infection or gamma interferon treatment, but the activated forms of both kinases were greatly reduced or absent in restrictive 3T3 cells. The biological significance of these activations was demonstrated by using the autoinhibitory domain of PAK-1 (amino acids 83 to 149), expression of which reduced the efficiency of MV infection in permissive 3T3 cells concurrent with a decrease in PAK-1 activation. In comparison, overexpression of a constitutively active PAK-1 (T423E) mutant increased MV replication in restrictive 3T3 cells. These observations suggest that induced signaling via cellular STKs may play important roles in determining the permissiveness of host cells to poxvirus infection.
During the course of evolution, viruses have acquired multiple mechanisms to manipulate host responses, including the ability to inhibit apoptosis, dysregulate cytokine production, or downregulate the host antiviral immune response (reviewed in references 1, 2, and 35). A central component of each of these strategies is the ability to manipulate signaling pathways that regulate cellular communication, either by delivering viral genes and proteins with signaling potential into infected cells or by activating cell surface receptors with innate signaling functions during the processes of virion binding and entry (reviewed in reference 16). These interactions between virus and host, beginning with virion contact at the cell membrane, primarily involve processes that control endogenous pathways such as the cell cycle, as well as immune response mechanisms. The ultimate goal of these manipulations is the creation of an intracellular environment that promotes productive virus infection. Thus, the interplay between the virus and elements within cell signaling networks has important consequences for viral tropism and the pathogenic effects of viral infections.
Among viruses, poxviruses are particularly adept at micromanipulating the host responses to infection, a property facilitated by large genomes that encode numerous immunomodulatory proteins (2, 27, 30, 40). This extensive coding capacity also includes many proteins with the potential to influence host cell signal transduction and promote viral replication. For example, the early stages of vaccinia virus infection are characterized by activation of signaling kinases (p42MAPK and p44MAPK) and the transcription factor, ATF1, which leads to activation of c-fos and subsequent mitogenic signals that are essential for viral replication (10). Similarly, phosphorylation of membrane proteins in the extracellular enveloped form of vaccinia virus (EEV) by src family kinases promotes viral spread by mimicking the signaling pathways normally associated with actin polymerization at the cell membrane (13, 14, 46). Although EEV has not been shown to activate specific kinase signaling pathways, entry into target cells of the intracellular mature form of the virus (IMV) induces a signaling cascade involving Rac, protein kinase C (PKC), and tyrosine phosphorylation events (24). The virulence of many poxviruses, including vaccinia virus, myxoma virus (MV), and Shope fibroma virus, is also dependent on growth factor homologues that exploit the ErbB signaling network to promote activation of the host cell cycle conducive to viral replication (43). Conversely, poxvirus-encoded intracellular proteins also act to inhibit host antiviral mechanisms. For example, the molluscum contagiosum protein, MC159L, inhibits Fas signaling and activation of NF-κB, central signaling elements in pathways that regulate inflammation and apoptosis (15, 38). Cowpox virus and many other orthopoxviruses also encode proteins that interfere with NF-κB activity, including cytokine and chemokine receptor homologues and inhibitors of caspases and PKR (8, 9, 18, 25, 32). In addition, the vaccinia virus VH1 protein, a dual-specific phosphatase, blocks the gamma interferon (IFN-γ) signaling cascade by dephosphorylating the signal transducer and activator of transcription 1 (STAT-1) (29), while the A46R and A52R proteins of the virus suppress signal transduction through interleukin-1 (IL-1) and toll-like receptors (4). Thus, like many other virus families, poxviruses employ diverse mechanisms to skew normal intracellular signaling pathways and facilitate virus replication and spread.
MV is a Leporipoxvirus species that is the causative agent of myxomatosis, a lethal disease of European rabbits that presents with extensive fulminating lesions, immune dysfunction, and secondary bacterial infections of the respiratory tract (12). Although MV exhibits strict species specificity for the rabbit (30), it was observed previously that certain clones of 3T3 murine fibroblasts that expressed human CD4 together with one of several different human chemokine receptors were fully permissive for MV replication (23). This permissive phenotype could be reversed with herbimycin A, a broad-spectrum inhibitor of tyrosine kinases, but not with pertussis toxin, an inhibitor of G protein-coupled receptor signaling. Because the virus failed to replicate in a related 3T3 clone that expressed only CD4 but lacked chemokine receptors, it was proposed that MV uses chemokine receptors for infection in a manner different from primate lentiviruses. Recent studies have suggested that adsorption of MV particles to permissive, but not restrictive, 3T3 cells bearing CCR5 receptors rapidly activated Tyr kinase signal transduction that facilitated downstream signaling events leading to full productive infection (26).
In an attempt to investigate further the apparent linkage between virus tropism and signaling, MV replication was assessed in a broad spectrum of 3T3 cells transfected with a variety of receptors. The present study reports that permissiveness for MV infection was not uniquely correlated with ectopic chemokine receptor expression at the cell surface per se, but rather was linked to the inducible signaling properties of the various 3T3 transfectants. In restrictive 3T3 cells, the block to MV replication occurred after virion binding, entry, and early gene expression rather than by any reduction in receptor-mediated binding or entry. Analysis with a panel of protein kinase inhibitors suggested significant differences between permissive and restrictive cells in the ability to activate specific serine-threonine kinases (STKs), most notably PAK-1. Transfection of permissive cells with the PAK-1 autoinhibitory domain inhibited productive MV infection, while expression in restrictive cells of constitutively active PAK-1 was associated with increased MV replication. These findings further support a role for the activation of host cell signaling cascades in permissive MV replication.
MATERIALS AND METHODS
Cells.
Two NIH 3T3 murine fibroblast cell lines that exhibit different tropisms for poxvirus infection, 3T3.CD4 and 3T3.CD4.neo, were used in the present study as parental members of the various permissive and restrictive 3T3 clone families, respectively. Of note, the 3T3.CD4.neo cell line was incorrectly identified as the 3T3.T4.pmx control in a previous study (23). Both cell types were generated from 3T3 parental cells by stable transfection with different plasmids expressing human CD4 and can be distinguished by the unique presence of a neomycin resistance cassette in the restrictive 3T3.CD4.neo variant (summarized in Fig. 1). Additional 3T3-derived cell lines were generated by stable transfection of the appropriate 3T3.CD4 or 3T3.CD4.neo cell line with human chemokine receptors (23). All 3T3 cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Burlington, Canada) supplemented with 10% fetal calf serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. 3T3 and 3T3.CD4 cells were obtained from American Type Culture Collection (Manassas, Va.) and the AIDS Respository (National Institutes of Health), respectively. All other variants were obtained from D. Littman and C. Arendt (Skirball Institute, N.Y.).
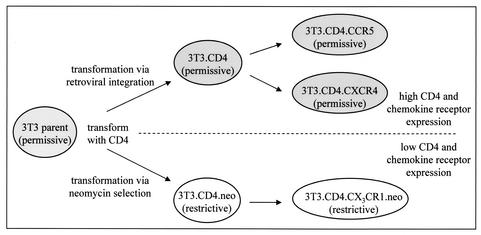
FIG. 1.
Summary of permissive and restrictive 3T3 cell subsets. Cell lines permissive for (shaded) or restrictive to (white) MV replication were derived from NIH 3T3 murine fibroblasts by stable transfection with plasmids expressing human CD4. Additional cell lines that retained the parental phenotype were generated following transfection of permissive 3T3.CD4 cells with the human chemokine receptors CCR5 or CXCR4 and transfection of restrictive 3T3.CD4.neo cells with CX3CR1.
Viruses and infection conditions.
Two derivatives of MV (strain Lausanne) that were created by intergenic insertion of a marker cassette were used for infection studies. vMyxlac, which contains a β-galactosidase cassette driven by the late MV promoter, was shown previously to infect permissive, but not restrictive, 3T3 cells (23). vMyxgfp, which was constructed by methods described previously (33), contains a green fluorescent protein (GFP) cassette inserted between open reading frames M135R and M136R of the MV genome. The GFP cassette was driven by a synthetic vaccinia virus early/late promoter described previously (7). Both viruses were propagated and titrated by focus formation on baby green monkey kidney (BGMK) cells as described previously (33). Cultures of 3T3 cells were incubated with the indicated multiplicity of infection (MOI) of either virus for 1 h at 37°C, after which infected cells were washed to remove excess virus and cultured in normal media until used in subsequent experiments. For β-galactosidase expression studies, cells infected with vMyxlac were washed with phosphate-buffered saline (PBS) at various time points postinfection (p.i.), fixed for 5 min in neutral buffered formalin (PBS and 3.7% formaldehyde), and incubated for 4 h at 37°C with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-gluronic acid) staining solution (100 μg of X-Gal per ml, 500 μM each potassium ferrocyanide and ferricyanide, 200 μM MgCl2 in PBS). For each experimental condition, the mean number of positive (blue) cells or foci was counted in six high-powered fields from triplicate wells. For plaque morphology and multistep growth curve studies, cells were infected with vMyxlac at a low MOI (0.01 PFU per cell) and cultured for 2 to 4 days before staining. For virion binding assays, cells were adsorbed with MV (MOI of 10) at 4°C for 30 min, washed with PBS, and immediately harvested. Harvested cells were subjected to three freeze-thaw cycles followed by sonication, and cell lysates were titrated as described above. All binding experiments with MV were performed with IMV particles isolated from infected cells.
Drug inhibition studies.
For each inhibitor, confluent cultures of 3T3 cells in six-well plates were preincubated for 1 h at 37°C with the working concentration of the drug. The inhibitor solution was then removed, and the cells were washed and infected with MV (approximately 400 focus-forming units [FFU] per well). After 1 h, excess virus was removed, and cells were cultured for 48 h in media containing the specific inhibitor. Cultures infected in the absence of inhibitor served as controls. The number of foci present in wells infected with vMyxlac or vMyxgfp was assessed by β-galactosidase assay or fluorescent microscopy, respectively. The efficiency of infection was determined by comparing the number of foci present in infected wells treated with the inhibitor to the number of foci evident in untreated, infected controls, where a value of 1.0 indicates no inhibition of infection. All inhibitors tested were purchased from Calbiochem (Mississauga, Canada) and initially reconstituted according to the manufacturer's directions in either dimethyl sulfoxide (Sigma, Oakville, Canada) or water. Working concentrations of each drug were prepared by dilution in DMEM and used as follows unless otherwise stated: 10 μM (each) GF109203X, KN-93, H-7, K-252a, AG490, AG17, AG1478, and SB203580; 30 μM (each) LY294002, BAPTA {N,N′-[1,2-ethanediylbis-(oxy-2,1-phenylene)]bis[N-carboxymethyl)glycine]}, and genistein; 30 nM okadaic acid; 1 μM herbimycin A; 200 ng of rapamycin per ml; and 1 μg of pertussis toxin per ml. Dose-response experiments were performed for each drug found to influence MV replication at several different time points p.i.
Transient transfection and analysis of the role of PAK-1 in 3T3 cell phenotype.
Approximately 5 × 105 3T3 cells were seeded in six-well plates (2-ml volume) and cultured to achieve 90% confluency. For each well, 4 μg of plasmid DNA was mixed with 10 μl of Lipofectamine 2000 reagent (Invitrogen) in 0.5 ml of Opti-MEM medium (Invitrogen) and transfected by incubation at 37°C for 12 h. Cells were washed twice with PBS and then cultured in normal medium for 24 h before being infected with MV (MOI of 1) as described above. Untransfected cells and cells transfected with specific plasmid lacking exogenous sequences (mock) served as controls. A similar protocol was used to transfect cells adhered to microscope slides with appropriate adjustments made for differences in cell number.
To assess the role of PAK-1 in the permissive phenotype, sequences encoding the autohibitory domain (amino acids [aa] 82 to 142) of PAK-1 (inPAK) were subcloned into a pDs2Red-C2 vector to generate a red fluorescent protein (RFP)-tagged variant of the domain. Plasmid containing inPAK sequences was obtained from A. Baur (University of Erlingen, Erlingen, Germany) (45). The pDs2Red-C2 plasmid, encoding a region of PAK-1 with no known inhibitory properties (nonPAK; aa 21 to 64), was used as a control. The numbers of cells expressing PAK sequences (RFP positive), infected by MV (GFP positive), or concurrently infected and expressing PAK sequences (both RFP and GFP positive) were determined by fluorescence microscopy at 24 and 48 h p.i. (hpi).
To assess the role of PAK-1 in the nonpermissive phenotype, microscope slides were seeded with 3T3.CD4.neo cells and transfected with a pCMV6M vector encoding myc-tagged wild-type PAK-1 (PAK-WT) or a constitutively active PAK-1 mutant (PAK-T423E). Both PAK-WT and PAK-T423E expression vectors were obtained from J. Chernoff (Fox Chase Cancer Center, Philadelphia, Pa.) (37). Expression of both PAK-WT and PAK-T423E was confirmed by Western blot analyses of parallel cultures with a monoclonal antibody (Invitrogen; 1:3,000) capable of detecting the myc epitope conjugated to each protein. At 24 h following infection with MV, slides were incubated for 5 min at room temperature with PBS containing 0.5% Tween 20 and 5% fetal bovine serum and washed twice with PBS. Washed cells were incubated for 20 min with monoclonal anti-myc antibody, washed twice, and then incubated for 20 min with a rhodamine red-conjugated goat anti-mouse immunoglobulin G (IgG) secondary antibody (Jackson Immunoresearch, West Grove, Pa.; 1:200) to detect the anti-myc primary antibody. Cells expressing PAK sequences and/or infected by MV were identified by fluorescence microscopy as described above.
Western blot analysis.
Cultured cells were lysed in buffer containing 10 mM Tris (pH 4.0), 10 mM NaCl, 3 mM MgCl2, and 0.5% Tween 20; sonicated to solubilize proteins; and cleared by centrifugation. Protein levels were quantified with a protein assay kit (Bio-Rad, Mississauga, Canada), and 25 μg of each sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Separated proteins were transferred to nitrocellulose and blocked with 5% skim milk in TBST (25 mM Tris-buffered saline, 0.5% Tween 20). Primary antibodies were diluted in 5% milk-TBST and incubated with membranes for 1 h at room temperature. Membranes were washed and incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies diluted 1:3,000 in 5% milk-TBST. Immunoreactive proteins were detected by chemiluminescence (Perkin-Elmer, Boston, Mass.). Loading of equal amounts of protein from each sample was confirmed by detection of the gene coding for the housekeeping protein, actin. To monitor viral protein expression, 3T3 cultures were mock infected or infected with vMyxlac (MOI of 10) in the presence (40 μg/ml) or absence of cytosine arabinoside (AraC; Sigma). Cells were harvested at 2 and 16 hpi and assessed for the presence of MV M-T7 or SERP-1, viral proteins that were expressed from early and late promoters, respectively. Monoclonal antibodies against Raf-1 (1:1,000) and pRaf-1 (1:500; Ser338) and polyclonal antibodies against actin (1:2,500), PAK-1 (1:1,000), and pPAK (1:500, Thr423) were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.). Goat anti-mouse, goat anti-rabbit, and donkey anti-goat IgG secondary antibodies were obtained from Jackson Immunoresearch.
RESULTS
MV replication in 3T3 cell subsets is independent of ectopic chemokine receptor expression.
Previously, MV was reported to productively infect a spectrum of murine 3T3 fibroblast cell lines that expressed human CD4 together with one of several human chemokine receptors, while 3T3 cells expressing CD4 alone, derived from a cell clone designated 3T3.T4.pMX, were nonpermissive (23). However, when these experiments were extended to include a broader number of 3T3-derived transfectants, significant discrepancies were observed. All 3T3 cell subtypes investigated were found to fall into two distinct phenotypes: cells that were permissive to MV replication and produced foci upon infection and cells that were nonpermissive to infection (Fig. 1). Moreover, the phenotype correlated with the parental CD4-expressing 3T3 clone that was used to generate each cell line. All permissive cell lines identified were found to be derived from the parent cell line, 3T3.CD4, which was transfected with the CD4-expressing plasmid, MLV-MX-CD4. On the other hand, the nonpermissive cell lines, 3T3.CD4.neo (3T3.T4.pMX) and 3T3.CD4.CX3CR1.neo, were uniquely derived from 3T3 cells transfected with the CD4-expressing plasmid, CD4-IRES.neo. In addition, the original nontransfected parental 3T3 cell line (American Type Culture Collection) supported MV infection despite the lack of either human CD4 or ectopic chemokine receptors (Fig. 1). These results suggested that the restrictive phenotype uniquely arose during the derivation of the 3T3.CD4.neo clone and was independent of the ectopic expression of specific transfected receptors.
MV binding, entry, and early gene expression are comparable in permissive and restrictive 3T3 cell lines.
The impaired replication of MV in restrictive 3T3.CD4.neo cells compared to that in permissive 3T3.CD4-derived cells was confirmed following a low-MOI infection. Infection of 3T3.CD4.neo cells with MV was nonproductive, yielding virtually no progeny virus, whereas viral growth in 3T3.CD4 cells was typical of a fully productive viral infection (Fig. 2A). In order to investigate further the potential role of cell surface molecules that mediate the restrictive phenotype of the 3T3.CD4.neo clones, the binding of MV particles to a variety of permissive (Fig. 2B, lanes 1 to 3) and restrictive (Fig. 2B, lanes 4 and 5) 3T3 cells was assessed. Following adsorption at 4°C to prevent internalization, no difference was observed in the binding of MV to any of the cells tested (Fig. 2B), indicating that cell surface levels of neither chemokine receptors nor CD4 influenced viral adsorption.
FIG. 2.
Properties of MV replication in 3T3 cell subsets. (A) Permissive 3T3.CD4 (open circles) and restrictive 3T3.CD4.neo (solid circles) cells were infected with MV at an MOI of 0.01 PFU per cell, and virus was collected at various days p.i. titrated on BGMK cells. Foci were not detected in MV-infected restrictive 3T3 cell lines. Titers are expressed as log PFU/106 cells and represent the mean ± standard deviation of triplicate wells. (B) MV IMV particles (MOI of 10) were adsorbed to permissive (black bars) 3T3, 3T3.CD4, and 3T3.CD4.CXCR4 cells and restrictive (white bars) 3T3.CD4.neo and 3T3.CD4.CX3CR1.neo cells by incubation at 4°C, and virus bound after 30 min was titrated on BGMK cells. No differences between cell lines were observed. Titers are expressed as log PFU per milliliter and represent the mean ± standard deviation of triplicate wells. (C) Restrictive 3T3.CD4.neo (lanes 1, 3, and 5) and permissive 3T3.CD4.CCR5 (lanes 2, 4, and 6) cells were infected with MV (MOI of 10) in the presence (+) or absence (−) of 40 μg of cytosine arabinoside (AraC) per ml, and proteins were collected at 2 and 16 hpi. Representative Western blots to detect early (αT7) and late (αSerp-1) viral proteins are shown.
To determine at which stage postentry the block in MV infection of restrictive cells occurred, restrictive 3T3.CD4.neo cells and permissive 3T3.CD4-derived cells were infected with MV, and the expression of representative early and late viral proteins was assessed by Western blot analyses at 2 and 16 hpi. No difference between permissive and restrictive cells in the expression of the early viral protein M-T7 was detected (Fig. 2C, upper panel, lanes 1 and 2), whereas synthesis of SERP-1, a late MV protein, was reduced dramatically in restrictive 3T3.CD4.neo cells compared to permissive 3T3.CD4-derived cell lines (Fig. 2C, lower panel, lanes 3 and 4). Expression of SERP-1, but not M-T7, was blocked fully in both permissive and nonpermissive cell types following treatment with the DNA synthesis inhibitor AraC (Fig. 2C, lanes 5 and 6). Quantification of viral DNA by dot blot hybridization confirmed that MV DNA levels in infected permissive cells increased gradually with time, while the abundance of viral DNA in MV-infected 3T3.CD4.neo cells, initially detected at 2 hpi, declined over the same period (data not shown). These findings further support the conclusion that the blockade of MV replication in restrictive 3T3.CD4.neo-derived cells is independent of the expression of any specific transfected chemokine receptors. Moreover, this replication defect occurs at an intracellular stage following virion binding, entry, and early gene expression, but prior to the later events of DNA replication and late gene expression.
Inhibitors of intracellular signaling kinases alter the permissiveness of 3T3 cells to MV infection.
Recent reports have suggested that the abilities of MV to alter host signal transduction pathways, such as by inducing tyrosine phosphorylation events, differ between permissive and restrictive 3T3 cell lines and may influence infectivity (23, 26). Given this potential link between the intracellular signaling machinery and MV tropism, the capacity of various signaling inhibitors to alter the tropism of MV infections in 3T3 cell lines was investigated. As shown in Fig. 3A and Table 1, several inhibitors were found to significantly reduce the ability of permissive 3T3.CD4-derived cells to support productive MV infection. Consistent with other studies (23, 26), the tyrosine kinase inhibitors genistein and herbimycin A decreased MV replication and either reduced the size and number of foci or completely inhibited viral replication expression (Fig. 3A and Table 1). Similar results were observed with the tyrphostin tyrosine kinase inhibitors AG490, AG17, and AG1478 (Fig. 3A), supporting a putative role for cellular tyrosine phosphorylation events in MV infection. However, this inhibitory effect was not limited to tyrosine kinase inhibitors. Okadaic acid and LY294002, which target protein phosphatases and phosphotidyinositol-3′-kinase, respectively, also decreased MV replication (Fig. 3A). The STK inhibitor K-252a inhibited replication, but, interestingly, no effect was observed with another STK inhibitor, H-7 (Fig. 3A). Of particular significance was the finding that U0126, an inhibitor of mitogen-activated protein kinase (MAPK)/ERK kinases 1 and 2(MEK-1 and -2) that attenuates vaccinia virus infection, increased MV replication in permissive cell lines more than threefold (Table 1) and even converted restrictive 3T3.CD4.neo cells into the permissive phenotype for MV infection (Table 1). Similarly, the restrictive phenotype of 3T3.CD4.neo cells was also reversed by AG1478, although the latter inhibitor reduced focus size on permissive 3T3 cells (Table 1). None of the other kinase inhibitors investigated altered MV replication (Fig. 3A). To ensure that the decreased viral replication did not simply reflect generalized metabolic defects caused by cell death, a standardized cell proliferation assay was used to monitor mitochondrial function between groups, with no differences detected between untreated and drug-treated cells at the inhibitor concentrations used (data not shown).
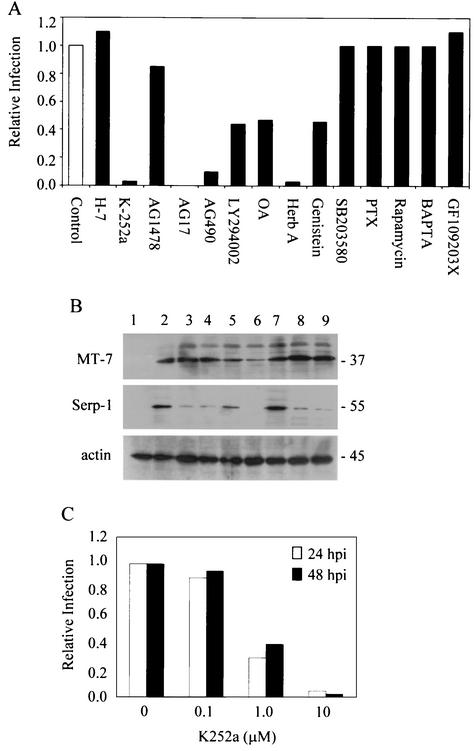
FIG. 3.
Effect of signaling inhibitors on MV replication in 3T3 cells. (A) Permissive 3T3.CD4 cells were infected with vMyxlac (400 PFU per well) in the absence (control) or presence of various kinase inhibitors, and the foci were detected by β-galactosidase assay at 48 hpi. Values are expressed relative to the number of foci in control wells (white bar; no inhibition = 1) and represent data from six fields in each of triplicate wells. Inhibitor concentrations are as stated in Materials and Methods. PTX, pertussis toxin; Herb A, herbimycin A; OA, okadaic acid. (B) Representative Western blots demonstrating detection of early (MT-7) and late (Serp-1) MV proteins at 16 hpi in permissive 3T3.CD4 cells treated with genistein (lane 3), herbimycin A (lane 4), AG490 (lane 5), AG17 (lane 6), U0126 (lane 7), LY294002 (lane 8), or K-252a (lane 9). Uninfected cells (lane 1) and cells infected in the absence of inhibitor (lane 2) served as controls. Detection of the housekeeping protein actin ensured equal loading of samples. Values to the right indicate protein size in kilodaltons. (C) 3T3.CD4 cells were infected with vMyxgfp (MOI of 0.01) in the presence of K-252a (0 to 10 μM), and GFP-positive cells were detected at 24 and 48 hpi by fluorescence microscopy. The numbers of infected cells in treated cultures relative to untreated control wells (six fields in each of triplicate wells) are represented.
TABLE 1.
Effect of select signaling inhibitors on PAK and Raf
| Inhibitora | Targetb | Effect on MV replicationc
|
Effect on kinase expression or activityd
|
||
|---|---|---|---|---|---|
| Permissive | Restrictive | PAK-1 | Raf-1 | ||
| H-7 | STKs | n/c | n/c | n/a | n/a |
| K-252A | STKs | dec, sf | n/c | − | − |
| AG1478 | EGFR tyrosine kinase | dec, sf | inc, f | − | − |
| AG17 | PDGF tyrosine kinase | dec | n/c | − | − |
| AG490 | JAK-2 | dec, sf | n/c | − | |
| LY294002 | PI3 kinase | dec, sf | n/c | − | |
| Okadaic acid | PP1/PP2A | dec, sf | n/c | − | |
| Herbimycin A | Tyrosine kinases | dec, sf | n/c | − | − |
| Genistein | Tyrosine kinases | dec, sf | n/c | − | − |
| U0126 | MEK-1/2 | inc, f | inc, f | + | |
SB203580, GF109203X, rapamycin, BAPTA-AM, and pertussis toxin did not influence the permissiveness of 3T3 cell lines and have no reported effect on PAK.
EGFR, epidermal growth factor receptor; PDGF, platelet-derived growth factor receptor; JAK, janus kinase; PI3, phosphotidylinositol-3′; PP1/ PP2A, protein phosphatases 1 and 2A; MEK, MAPK/ERK kinase.
Inhibitors increased (inc), decreased (dec), or did not change (n/c) MV replication. MV focus morphology was either normal (f) or smaller (sf).
Inhibitors either increase (+), decrease (−), or do not affect (n/a) kinase activity or expression. Blank entries indicate that the inhibitor has not been reported to affect PAK or Raf activity.
To determine if the signaling inhibitors that attenuated MV infection of permissive 3T3 cells targeted viral replication at the same stage as the blockade observed in nonpermissive cells, early and late viral protein expression levels were compared in treated 3T3 cells at 16 hpi. As shown in Fig. 3B, the tyrosine kinase inhibitors genistein (lane 3) and herbimycin A (lane 4), LY294002 (lane 8), and the STK inhibitor K-252a (lane 9) all decreased expression of the late MV protein SERP-1, but did not affect levels of the MT-7 early protein in permissive 3T3 cells. In contrast, the tyrphostins AG490 and AG17 inhibited both early and late gene expression, suggesting an earlier abort of the virus infection (Fig. 3B, lanes 5 and 6). Of interest, U0126, which reversed the restrictive phenotype and increased MV replication in permissive cells (Table 1), also increased SERP-1 expression in permissive 3T3 cells (Fig. 3B, lane 7).
The finding that inhibitors of several different classes of cell signaling molecules were able to partially reverse the permissiveness of 3T3.CD4-derived cells suggested that multiple signal transduction pathways may contribute to the differences in the phenotype of the 3T3 cell lines. However, as shown in Table 1, all of the inhibitors found to influence MV replication shared the common ability to modulate signaling through pathways containing one or more STKs, particularly Raf-1 and p21-activated kinase (PAK-1). Of greatest interest was the finding that that the PAK-1 inhibitor, K-252a (48), abrogated MV replication in 3T3 cells at the same stage as that observed in restrictive cells, while H-7, an STK inhibitor that does not affect either PAK-1 or Raf-1 (48), had no effect on viral infection. Moreover, the inhibitory effect of K-252a was dose dependent and was consistently observed over the 48-h assay period (Fig. 3C). In addition, Western blot analyses revealed a concurrent decline in the abundance of activated PAK-1 in permissive 3T3 cells following treatment with K-252a (data not shown). As a result, subsequent experiments focused on determining whether PAK-1/Raf-1 STKs might regulate MV infection in the 3T3 cell lines.
MV infection differentially alters STK expression and activation in permissive and restrictive 3T3 cells.
The PAK signaling pathway has been implicated previously in the infectivity of some viruses, most notably human immunodeficiency virus type 1 (HIV-1), based on observations of increased PAK activation and the subsequent phosphorylation of its downstream effectors in infected cells (5, 31). Since activation of both PAK-1 and Raf-1 involves phosphorylation of specific residues within the proteins, differences in the activation of these STKs were assessed by Western blot analysis following infection of 3T3-derived cell lines with MV. Prior to infection, constitutive expression of both PAK-1 and Raf-1, but not their activated forms, was evident in permissive 3T3-CD4 cells (Fig. 4A, lane 1). Following MV infection, however, a moderate increase in the levels of total PAK-1 protein, concurrent with a rapid induction of the Thr423-phosphorylated active form of the kinase (pPAK-1), was detected (Fig. 4A, lanes 2 to 6). A similar increase in Raf-1 protein levels was observed in infected cultures, accompanied by a marked induction of the Ser338-phosphorylated form (pRaf-1) of the protein (Fig. 4A). Of note, phosphorylation of Raf-1 on Ser338 is associated with activation of the kinase through the Ras-independent, PAK-dependent pathway (22). Although low levels of constitutive PAK-1 were detected in restrictive 3T3.CD4.neo cells, infection with MV produced minimal changes in protein levels and only a transient activation of PAK-1 that was not sustained beyond 15 min p.i. (Fig. 4A, lane 8). In contrast, neither the activated form nor the unactivated form of Raf-1 was detectable in infected or uninfected cultures of restrictive 3T3.CD4.neo cells (Fig. 4A, lanes 7 to 12).
FIG. 4.
Pak-1 and Raf-1 expression in MV-infected 3T3 cells. (A) Representative Western blots showing detection of PAK-1 and Raf-1 in cell lysates from 3T3.CD4 (lanes 1 to 6) and 3T3.CD4.neo (lanes 7 to 12) cultures at various time points (minutes) after MV infection (MOI of 5). Kinase activation is demonstrated by detection of specific phosphorylated forms of PAK-1 (Thr423) and Raf-1 (Ser338). Equal sample loading was confirmed by detection of the gene coding for the housekeeping protein actin. (B and C) Detection of activated PAK-1 and Raf-1 in additional permissive 3T3.CD4.CCR5 (lanes 1 to 5) and 3T3.CD4.CXCR4 (lanes 6 to 10) cells and restrictive 3T3.CD4.CX3CR1.neo (lanes 11 to 15) cells after MV infection (B) or stimulation with 100 ng of IFN-γ per ml (C).
To determine whether this phenomenon was a property of all 3T3 cells of a given lineage, activation of PAK-1 and Raf-1 was assessed following infection of other 3T3 clones. Like the parent 3T3.CD4 cell line, MV infection induced sustained activation of PAK-1 and Raf-1 in permissive 3T3.CD4.CCR5 and 3T3.CD4.CXCR4 clones (Fig. 4B, lanes 1 to 10). Similarly, restrictive 3T3.CD4.CX3CR1.neo cells exhibited the same profile as 3T3.CD4.neo cells (Fig. 4B, lanes 11 to 15). The activated form of PAK-1 was detected immediately after exposure to the virus and rapidly declined, but activated pRaf-1 was not detectable at any time point in restrictive 3T3.CD4.neo-derived clones (Fig. 4B, lanes 11 to 15). These results indicated that the abilities of MV to activate STKs dramatically differ between permissive and restrictive 3T3 cell lines. Moreover, like infectivity, the parent 3T3 cell line from which each clone was derived determined this STK response profile, rather than the expression of either ectopic chemokine receptors or CD4.
The question of whether the differences in STK activation observed between permissive and restrictive 3T3 cell lines represented a phenotype inherent to each cell line was addressed by investigating activation of PAK-1 and Raf-1 following treatment with IFN-γ. Stimulation of permissive 3T3.CD4.CCR5 and 3T3.CD4.CXCR4 cells produced the same rapid and sustained induction of phosphorylated PAK-1 and Raf-1 that characterized MV infection of these cells (Fig. 4C, lanes 1 to 10), with little or no change in total protein levels (data not shown). Also consistent with the infection results, cytokine treatment produced minimal activation of PAK-1 in restrictive 3T3.CD4.CX3CR1.neo cells (Fig. 4C, lanes 11 to 15). However, differences in activation of Raf-1 in permissive 3T3 cells were observed. Although MV infection failed to induce any activation of Raf-1 in 3T3.CD4.neo and 3T3.CD4.CX3CR1.neo cells, pRaf-1 (Ser338) was detectable after IFN-γ treatment of both cell lines (Fig. 4C, lanes 12 and 13) (data not shown). Thus, IFN-γ, but not MV infection, induced activation of Raf-1 in restrictive 3T3 cells to a lesser degree and for a shorter duration than in permissive cell lines.
To investigate further the differences in expression and activation of PAK-1 and Raf-1 in permissive and restrictive 3T3 cell lines, expression of these kinases in response to either MV infection or IFN-γ treatment was assessed by reverse transcription PCR (RT-PCR). Although PAK-1 mRNA levels were increased from basal levels by IFN-γ treatment to a greater extent than by MV infection, both permissive 3T3.CD4 cells and restrictive 3T3.CD4.neo cells responded to each stimulus in a similar manner (data not shown). Similarly, MV infection and IFN-γ stimulation induced Raf-1 mRNA expression in both cell types, although mRNA levels were slightly elevated in 3T3.CD4 cultures compared to 3T3.CD4.neo cells (data not shown). Thus, permissive and restrictive 3T3 cells were not distinguishable at the level of inducible PAK-1 or Raf-1 gene expression.
Inhibition of PAK-1 attenuates MV infection of permissive 3T3 cells.
The preceding data suggested a link between the activation of host cell STKs following MV infection and the capacity for the virus to replicate in 3T3 cells. This relationship was investigated further by using a dominant-negative form of the autoinhibitory domain of PAK-1 (inPAK) that has been shown to inhibit PAK-1 activation and block subsequent phosphorylation of its substrates (45). As shown in Fig. 5A, MV-infected permissive 3T3.CD4 cells transfected with inPAK exhibited decreased induction of the activated, phosphorylated form of PAK-1 compared to the level of induction in untransfected control cultures (lane 2 versus lane 3), whereas expression of the noninhibitory domain (nonPAK) did not influence PAK-1 phosphorylation (lane 5 versus lane 6). To confirm that expression of inPAK in permissive cells blocked infection by MV, RFP-tagged versions of both the inhibitory and noninhibitory domains were constructed by subcloning into the pDSRed2-C1 vector. Following transfection, permissive 3T3.CD4 cells were infected with vMyxgfp, an MV mutant expressing GFP, and cultures were assessed for the presence of yellow cells (expressing both RFP and GFP). Cultures that were neither infected nor transfected (Fig. 5B, panel i), only infected with vMyxgfp (Fig. 5B, panel ii), or only transfected with tagged inPAK (Fig. 5B, panel iii) served as controls. Analysis of 3T3 cultures transfected with inPAK at 24 h following infection revealed numerous productively infected cells expressing GFP (Fig. 5B, panel iv) and transfected cells expressing RFP (Fig. 5B, panel v). However, yellow cells indicative of inPAK-RFP expression concurrent with vMyxgfp infection were seldom detected when the two fields were overlaid (Fig. 5B, panel vi, and C). In comparison, yellow fluorescent cells were abundant following infection of cells transfected with either the noninhibitory nonPAK domain (Fig. 5B, panel ix, and C) or empty vector (mock, Fig. 5C), indicating that cells expressing the inPAK inhibitor were no longer permissive to MV infection. These results supported a positive, obligatory role for PAK-1 signaling in establishing permissiveness for viral replication.
FIG.5.
Expression of inPAK inhibits MV replication in permissive 3T3 cells. (A) Representative Western blots showing detection of activated, phosphorylated PAK-1 in uninfected 3T3.CD4 cells (lanes 1 and 4) and MV-infected (MOI of 5) 3T3.CD4 cells that were nontransfected (lanes 3 and 6) or transiently transfected with the PAK-1 autoinhibitory domain (inPAK, lane 2) or a noninhibitory PAK-1 domain (nonPAK, lane 5). Expression of inPAK, but not nonPAK, decreased MV-induced activation of PAK-1. (B) Representative fluorescence microscopy images of inPAK (iv, v, and vi)- or nonPAK (vii, viii, and ix)-transfected 3T3.CD4 cells at 24 h following infection with vMyxgfp (MOI of 1). Red and green cells indicate transfected and MV-infected cells, respectively, while yellow cells following merging of red and green fields indicate concurrent infection and transfection. Images obtained following merging of red and green fields from uninfected and nontransfected cultures (i), cultures only infected (ii), and cultures only transfected (iii) are included as controls. A prominent cell that is both infected and transfected with inPAK is indicated by an arrowhead. Original magnification, ×10. (C) Graphical representation of the number of cells concurrently infected and transfected with inPAK, nonPAK, or vector lacking exogenous sequences (mock). Values represent the mean number of yellow cells ± standard deviation (SD) from six fields in each of triplicate wells once the red and green fields were merged.
Expression of constitutively activated PAK-1 promotes MV infection of restrictive 3T3 cells.
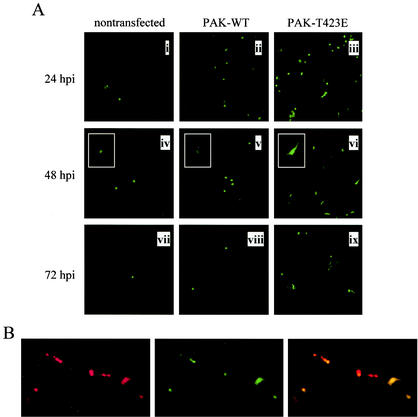
Since inhibition of PAK-1 activity attenuated MV replication in permissive 3T3 cells, we next investigated the potential for activation of PAK-1 to influence the restrictive 3T3 phenotype. Compared to nontransfected controls or cells overexpressing wild-type PAK-1 (PAK-WT), MV-infected cells were more abundant in 3T3.CD4.neo cell cultures transfected with a constitutively active PAK-1 mutant, PAK-T423E (Fig. 6A), over a 3-day time course. Moreover, foci suggestive of viral spread between cells were evident by 72 hpi in PAK-T423E-transfected cultures (Fig. 6A, panel ix), but absent in the other groups. Of note, GFP-positive cells in PAK-T423E cultures presented with larger cell bodies and more prominent processes (Fig. 6A, panel vi, inset), while infected cells in nontransfected and PAK-WT-transfected cultures were smaller and rounded in appearance. To determine if infection of 3T3.CD4.neo cells by MV correlated with expression of activated PAK-1, cells were immunostained at 24 hpi with red fluorescent antibody capable of recognizing the myc epitope on PAK-T423E. As shown in Fig. 6B, expression of PAK-T423E (red cells, left panel) was closely correlated with MV infection (green cells, middle panel) when the two fields were overlaid (yellow cells, right panel). Taken together, these findings suggest that exogenous activation of PAK-1 has the ability to reverse the restrictive 3T3 phenotype and promote MV replication.
FIG. 6.
Expression of PAK-T423E promotes MV replication in restrictive 3T3 cells. (A) Representative fluorescence microscopy images of nontransfected 3T3.CD4.neo cells (i, iv, and vii) and 3T3.CD4.neo cells transiently transfected with wild-type PAK-1 (PAK-WT; ii, v, and vii) or constitutively active PAK-1 (PAK-T423E; iii, vi, and ix) 24, 48, and 72 h following infection with vMyxgfp (MOI of 1). GFP-positive cells in cultures expressing PAK-T423E were more abundant and presented with larger cell bodies than those of other groups (insets). Original magnification, ×10; inset magnification, ×20. (B) PAK-T423E expression in transfected 3T3.CD4.neo cells was assessed by serial immunostaining with rhodamine red-conjugated antibody. Green and red cells indicate MV-infected (middle panel) and PAK-T423E-transfected (left panel) cells, respectively, while yellow cells (right panel) indicate concurrent infection and transfection. Original magnification, ×20.
DISCUSSION
Previously, it has been shown that poxvirus infection of mammalian cells is associated with dramatic and rapid cell signaling events that induce the activation of distinct species of cellular signaling molecules, including JAKs and STATs (26), MAPK/ERKs (10), and rac/rho (24). The results of the present study also support a role for induced host cell signaling elements such as PAK-1 and Raf-1 as important determinants in the cell tropism of MV. PAK-1 is a member of a family of closely related STKs that act as downstream effectors for small GTP-binding proteins, such as p21cdc42 (reviewed in reference 19). In response to diverse environmental stimuli, PAK-1 initiates kinase cascades that underlie numerous pathways, including motility and cytoskeletal rearrangements, activation of transcription factors, and both pro- and antiapoptotic processes. Moreover, PAK-1 also represents a point of convergence for signals from several small GTPases, including ras, rac, and rho, allowing for the cooperative regulation of cell proliferation, differentiation, and transformation (19). Similarly, the Raf-1 proto-oncogene is expressed ubiquitously in mammalian cells and is an essential component of the Ras/Raf/MEK/ERK signaling module that transduces signals for cell differentiation, proliferation, transformation, and apoptosis (reviewed in reference 22). Raf-1, which requires phosphorylation at both Ser and Tyr for activation, also represents a point of convergence for Tyr and Ser-Thr phosphorylation cascades (22). Thus, the ability to interact with these kinases would enable poxviruses to co-opt multiple pathways that could promote an intracellular environment permissive to replication.
A potential role for STKs such as PAK-1 in virus infection is not without precedent. Several viruses, including herpes simplex virus type 2 (HSV-2) and HIV and simian immunodeficiency virus (SIV), have been shown to usurp the PAK pathway to promote viral replication. For example, the US3 gene of HSV-2 encodes a PAK homologue that mediates morphological changes in host cells that are essential for replication (28). Like PAK, the US3 gene product possesses STK activity and is regulated by p21cdc42 and rac1 (28). The primate lentivirus (HIV, SIV) accessory protein, Nef, forms a multiprotein signaling complex that activates PAK and leads to phosphorylation of its downstream effectors (5, 31, 45). Modulation of the activity of cellular kinases by this complex leads to enhanced viral infectivity and has been shown to be associated with the antiapoptotic function of the protein (45). Moreover, preliminary studies in our laboratory have indicated parallels between MV and vaccinia virus in their abilities to replicate in 3T3 subsets that may share dependence on PAK-1 activity. Thus, STKs may be a common element in the pathogenesis of many different viruses.
The exact mechanisms by which STKs may influence viral replication remain unclear. Like HSV-2, MV has sufficient coding capacity to encode a PAK homologue, and in fact, the virus is predicted to encode several proteins with potential kinase activity (6). Therefore, the results obtained in the present study could reflect the activity of a viral kinase, rather than cellular PAK-1. However, our results are more consistent with a mechanism in which MV infection both induces and requires activation of cellular STKs, which in turn mediate a signaling cascade that promotes some early aspect of viral replication. The present results indicate clearly that the block to MV replication in restrictive 3T3 cell lines coincides temporally with core uncoating, DNA replication, and/or the transition from early to late gene expression. Because the only identifiable difference between permissive and restrictive 3T3 cells is the CD4-encoding plasmid with which they were transfected, it remains likely that the differences in poxvirus susceptibility between the two 3T3.CD4 lineages are due to the clonal uniqueness of the 3T3.CD4.neo cell lines. For example, the CD4-IRES-neo vector may have integrated into a locus essential for MV replication, such as a gene that expresses a regulator of the PAK-Raf pathway. Unfortunately, plasmid integration in stable transfectants is random, hindering attempts to replicate this phenomenon. However, our results do not suggest an inability to express PAK-1 or Raf-1 in restrictive 3T3 cells, since both permissive and restrictive cell lines express PAK-1 and Raf-1 proteins and can activate them in response to specific cytokine stimulation. Rather, the finding that overexpression of constitutively active PAK-1, but not wild-type PAK-1, promoted MV replication in restrictive 3T3 cells suggests a defect in the pathways that regulate PAK-1 activation. Future studies will concentrate on determining the potential role played by regulatory molecules located upstream and downstream of the PAK-1 signaling cascade.
Our results are also consistent with a significant role for the induced activation of Tyr kinases in MV infection, as shown by the ability of several Tyr kinase inhibitors, but not pertussis toxin, to reverse the permissive phenotype (23, 26; this paper). For example, a Tyr phosphorylation cascade involving signaling molecules such as JAK-2 and STAT-1 was recently reported to be induced by MV infection of permissive 3T3 cells and posited to be triggered by adsorption of virions to the cell surface (26). Thus, it is conceivable that productive infection of 3T3 cells by MV involves coordinated upregulation of both Tyr kinases and STKs. For example, activation of Raf-1 requires cooperative Tyr and Ser phosphorylation mediated by src family kinases and PAK-1, respectively (22). Similarly, Raf-1 activation has also been shown to involve STATs and JAKs (36, 41, 47), elements of the Tyr phosphorylation cascade reported in MV-infected permissive 3T3 cells (26). Cooperation between STKs and Tyr kinases to promote poxvirus infection has been demonstrated for vaccinia virus, where entry of the IMV form of the virus triggers PKC phosphorylation, Rac 1 activation, and Tyr phosphorylation (24). Notably, Rac 1 is also an important regulator of PAK activity (19). Further studies with specific signaling inhibitors might shed light on which of the observed activations are critical for permissive viral infection.
Our results indicate that MV-induced activation of STKs is independent of ectopically expressed chemokine receptors. However, it should also be emphasized that our inability to confirm a direct linkage between permissiveness and ectopic chemokine receptor expression does not preclude the involvement of endogenous G protein-coupled receptors in signaling following poxvirus replication. In earlier reports, MV infection of permissive 3T3 cells was independent of G protein-coupled signaling and did not require the signaling capacity of chemokine receptors, specifically CCR5, to be intact (23, 26). Although earlier studies have shown that CCR5 antibodies and ligands can inhibit MV infection of permissive cells lines, these results were specific to the anti-CCR5 antibody used and were observed only in cells stably transfected with human CCR5. Thus, the earlier observations may reflect proreplication intracellular signaling events that were independent of interaction with specific receptors.
Perhaps the most unexpected aspect of our results is the observation that U0126 and the tyrphostin AG1478 were able to reverse the restrictive phenotype of 3T3.CD4.neo cell lines. Although the mechanism by which AG1478 achieves this effect remains uncertain, the effect of U0126 can be rationalized in the context of the PAK-Raf pathway. Raf activity is normally constrained by an autoinhibitory feedback loop; thus, activation of Raf-1 requires that the inhibitory state maintaining the balance between activation and autoinhibition be removed (22). U0126 has been shown to block the activity of one of these constraints, the kinase suppressor KSR-1, thereby increasing Raf-1 expression and activation (44). This mechanism also explains the ability of U0126 to markedly increase MV replication in both restrictive and permissive cells. In contrast, SB203580, an inhibitor of in vivo but not in vitro Raf-1 activity (17), did not alter the permissive phenotype.
Regardless of the mechanism, the results of the present study support a critical role for the cellular PAK-1/Raf-1 signaling pathways in the efficiency of MV replication. Similar dependencies on host kinase-regulated signaling pathways have been reported for other viruses, including primate lentiviruses (5, 20), Borna virus (34), influenza virus (34), hepatitis B virus (21), papillomavirus (3), murine leukemia virus (11, 39), reovirus (42), and herpesviruses (49), further emphasizing the importance of understanding how viruses micromanipulate the host signal transduction machinery to achieve permissive conditions favoring productive virus replication.
Acknowledgments
This work was supported by the Canadian Institutes for Health Research (CIHR). G.M. holds a Canada Research Chair in Molecular Virology. J.B.J. holds a Robarts Research Institute Fellowship award.
We thank J. Chernoff, A. Baur, D. Littman, and C. Arendt for their generosity in supplying reagents.
REFERENCES
- 1.Alcami, A., and U. H. Koszinowski. 2000. Viral mechanisms of immune evasion. Mol. Med. Today 6:365-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett, J. W., J. X. Cao, S. Hota-Mitchell, and G. McFadden. 2001. Immunomodulatory proteins of myxoma virus. Semin. Immunol. 13:73-84. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Bassat, H., S. Rosenbaum-Mitrani, Z. Hartzstark, R. Levitzki, M. Chaouat, Z. Shlomai, B. Y. Klein, N. Kleinberger-Doron, A. Gazit, R. Tsvieli, and A. Levitzki. 1999. Tyrphostins that suppress the growth of human papilloma virus 16-immortalized human keratinocytes. J. Pharmacol. Exp. Ther. 290:1442-1457. [PubMed] [Google Scholar]
- 4.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. O'Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, A., X. Wang, E. Sawai, and C. Cheng-Mayer. 1999. Activation of the PAK-related kinase by human immunodeficiency virus type 1 Nef in primary human peripheral blood lymphocytes and macrophages leads to phosphorylation of a PIX-p95 complex. J. Virol. 73:9899-9907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron, C., S. Hota-Mitchell, L. Chen, J. Barrett, J. X. Cao, C. Macaulay, D. Willer, D. Evans, and G. McFadden. 1999. The complete DNA sequence of myxoma virus. Virology 264:298-318. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti, S., J. R. Sisler, and B. Moss. 1997. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques 23:1094-1097. [DOI] [PubMed] [Google Scholar]
- 8.Chang, H. W., J. C. Watson, and B. L. Jacobs. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89:4825-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies, M. V., O. Elroy-Stein, R. Jagus, B. Moss, and R. J. Kaufman. 1992. The vaccinia virus K3L gene product potentiates translation by inhibiting double-stranded-RNA-activated protein kinase and phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J. Virol. 66:1943-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Magalhaes, J. C., A. A. Andrade, P. N. Silva, L. P. Sousa, C. Ropert, P. C. Ferreira, E. G. Kroon, R. T. Gazzinelli, and C. A. Bonjardim. 2001. A mitogenic signal triggered at an early stage of vaccinia virus infection: implication of MEK/ERK and protein kinase A in virus multiplication. J. Biol. Chem. 276:38353-38360. [DOI] [PubMed] [Google Scholar]
- 11.Esther, A., S. Iftach, and P. Esther. 1994. Inhibition of Moloney murine leukemia virus replication by tyrphostins, tyrosine kinase inhibitors. FEBS Lett. 341:99-103. [DOI] [PubMed] [Google Scholar]
- 12.Fenner, F., and F. Ratcliffe. 1965. Myxomatosis. Cambridge University Press, Cambridge, United Kingdom.
- 13.Frischknecht, F., S. Cudmore, V. Moreau, I. Reckmann, S. Rottger, and M. Way. 1999. Tyrosine phosphorylation is required for actin-based motility of vaccinia but not Listeria or Shigella. Curr. Biol. 9:89-92. [DOI] [PubMed] [Google Scholar]
- 14.Frischknecht, F., V. Moreau, S. Rottger, S. Gonfloni, I. Reckmann, G. Superti-Furga, and M. Way. 1999. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature 401:926-929. [DOI] [PubMed] [Google Scholar]
- 15.Garvey, T. L., J. Bertin, R. M. Siegel, G.-H. Wang, M. J. Lenardo, and J. I. Cohen. 2002. Binding of FADD and caspase-8 to molluscum contagiosum virus MC159 v-FLIP is not sufficient for its antiapoptotic function. J. Virol. 76:697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greber, U. F. 2002. Signalling in viral entry. Cell Mol. Life Sci. 59:608-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall-Jackson, C. A., M. Goedert, P. Hedge, and P. Cohen. 1999. Effect of SB 203580 on the activity of c-Raf in vitro and in vivo. Oncogene 18:2047-2054. [DOI] [PubMed] [Google Scholar]
- 18.Hu, F. Q., C. A. Smith, and D. J. Pickup. 1994. Cowpox virus contains two copies of an early gene encoding a soluble secreted form of the type II TNF receptor. Virology 204:343-356. [DOI] [PubMed] [Google Scholar]
- 19.Jaffer, Z. M., and J. Chernoff. 2002. p21-activated kinases: three more join the Pak. Int. J. Biochem. Cell Biol. 34:713-717. [DOI] [PubMed] [Google Scholar]
- 20.Johnston, J. B., Y. Jiang, G. van Marle, M. B. Mayne, W. Ni, J. Holden, J. C. McArthur, and C. Power. 2000. Lentivirus infection in the brain induces matrix metalloproteinase expression: role of envelope diversity. J. Virol. 74:7211-7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein, N. P., M. J. Bouchard, L. H. Wang, C. Kobarg, and R. J. Schneider. 1999. Src kinases involved in hepatitis B virus replication. EMBO J. 18:5019-5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolch, W. 2000. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351:289-305. [PMC free article] [PubMed] [Google Scholar]
- 23.Lalani, A. S., J. Masters, W. Zeng, J. Barrett, R. Pannu, H. Everett, C. W. Arendt, and G. McFadden. 1999. Use of chemokine receptors by poxviruses. Science 286:1968-1971. [DOI] [PubMed] [Google Scholar]
- 24.Locker, J. K., A. Kuehn, S. Schleich, G. Rutter, H. Hohenberg, R. Wepf, and G. Griffiths. 2000. Entry of the two infectious forms of vaccinia virus at the plasma membane is signaling-dependent for the IMV but not the EEV. Mol. Biol. Cell 11:2497-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loparev, V. N., J. M. Parsons, J. C. Knight, J. F. Panus, C. A. Ray, R. M. Buller, D. J. Pickup, and J. J. Esposito. 1998. A third distinct tumor necrosis factor receptor of orthopoxviruses. Proc. Natl. Acad. Sci. USA 95:3786-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masters, J., A. A. Hinek, S. Uddin, L. C. Platanias, W. Zeng, G. McFadden, and E. N. Fish. 2001. Poxvirus infection rapidly activates tyrosine kinase signal transduction. J. Biol. Chem. 276:48371-48375. [DOI] [PubMed] [Google Scholar]
- 27.Moss, B., and J. L. Shisler. 2001. Immunology 101 at poxvirus U: immune evasion genes. Semin. Immunol. 13:59-66. [DOI] [PubMed] [Google Scholar]
- 28.Murata, T., F. Goshima, T. Daikoku, H. Takakuwa, and Y. Nishiyama. 2000. Expression of herpes simplex virus type 2 US3 affects the Cdc42/Rac pathway and attenuates c-Jun N-terminal kinase activation. Genes Cells 5:1017-1027. [DOI] [PubMed] [Google Scholar]
- 29.Najarro, P., P. Traktman, and J. A. Lewis. 2001. Vaccinia virus blocks gamma interferon signal transduction: viral VH1 phosphatase reverses Stat1 activation. J. Virol. 75:3185-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nash, P., J. Barrett, J. X. Cao, S. Hota-Mitchell, A. S. Lalani, H. Everett, X. M. Xu, J. Robichaud, S. Hnatiuk, C. Ainslie, B. T. Seet, and G. McFadden. 1999. Immunomodulation by viruses: the myxoma virus story. Immunol. Rev. 168:103-120. [DOI] [PubMed] [Google Scholar]
- 31.Nunn, M. F., and J. W. Marsh. 1996. Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J. Virol. 70:6157-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oie, K. L., and D. J. Pickup. 2001. Cowpox virus and other members of the orthopoxvirus genus interfere with the regulation of NF-kappaB activation. Virology 288:175-187. [DOI] [PubMed] [Google Scholar]
- 33.Opgenorth, A., K. Graham, N. Nation, D. Strayer, and G. McFadden. 1992. Deletion analysis of two tandemly arranged virulence genes in myxoma virus, M11L and myxoma growth factor. J. Virol. 66:4720-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Planz, O., S. Pleschka, and S. Ludwig. 2001. MEK-specific inhibitor U0126 blocks spread of Borna disease virus in cultured cells. J. Virol. 75:4871-4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ploegh, H. L. 1998. Viral strategies of immune evasion. Science 280:248-253. [DOI] [PubMed] [Google Scholar]
- 36.Sakatsume, M., L. F. Stancato, M. David, O. Silvennoinen, P. Saharinen, J. Pierce, A. C. Larner, and D. S. Finbloom. 1998. Interferon gamma activation of Raf-1 is Jak1-dependent and p21ras-independent. J. Biol. Chem. 273:3021-3026. [DOI] [PubMed] [Google Scholar]
- 37.Sells, M. A., U. G. Knaus, S. Bagrodia, D. M. Ambrose, G. M. Bokoch, and J. Chernoff. 1997. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 7:202-210. [DOI] [PubMed] [Google Scholar]
- 38.Shisler, J. L., and B. Moss. 2001. Molluscum contagiosum virus inhibitors of apoptosis: the MC159 v-FLIP protein blocks Fas-induced activation of procaspases and degradation of the related MC160 protein. Virology 282:14-25. [DOI] [PubMed] [Google Scholar]
- 39.Sklan, E. H., A. Gazit, and E. Priel. 2000. Inhibition of murine AIDS (MAIDS) development in C57BL/6J mice by tyrphostin AG-1387. Virology 278:95-102. [DOI] [PubMed] [Google Scholar]
- 40.Smith, G. L. 1999. Vaccinia virus immune evasion. Immunol. Lett. 65:55-62. [DOI] [PubMed] [Google Scholar]
- 41.Stancato, L. F., C. R. Yu, E. F. Petricoin III, and A. C. Larner. 1998. Activation of Raf-1 by interferon gamma and oncostatin M requires expression of the Stat1 transcription factor. J. Biol. Chem. 273:18701-18704. [DOI] [PubMed] [Google Scholar]
- 42.Strong, J. E., M. C. Coffey, D. Tang, P. Sabinin, and P. W. Lee. 1998. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 17:3351-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tzahar, E., J. D. Moyer, H. Waterman, E. G. Barbacci, J. Bao, G. Levkowitz, M. Shelly, S. Strano, R. Pinkas-Kramarski, J. H. Pierce, G. C. Andrews, and Y. Yarden. 1998. Pathogenic poxviruses reveal viral strategies to exploit the ErbB signaling network. EMBO J. 17:5948-5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, X., and G. P. Studzinski. 2001. Phosphorylation of raf-1 by kinase suppressor of ras is inhibited by “MEK-specific” inhibitors PD 098059 and U0126 in differentiating HL60 cells. Exp. Cell Res. 268:294-300. [DOI] [PubMed] [Google Scholar]
- 45.Wolf, D., V. Witte, B. Laffert, K. Blume, E. Stromer, S. Trapp, P. d'Aloja, A. Schurmann, and A. S. Baur. 2001. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat. Med. 7:1217-1224. [DOI] [PubMed] [Google Scholar]
- 46.Wolffe, E. J., A. S. Weisberg, and B. Moss. 2001. The vaccinia virus A33R protein provides a chaperone function for viral membrane localization and tyrosine phosphorylation of the A36R protein. J. Virol. 75:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia, K., N. K. Mukhopadhyay, R. C. Inhorn, D. L. Barber, P. E. Rose, R. S. Lee, R. P. Narsimhan, A. D. D'Andrea, J. D. Griffin, and T. M. Roberts. 1996. The cytokine-activated tyrosine kinase JAK2 activates Raf-1 in a p21ras-dependent manner. Proc. Natl. Acad. Sci. USA 93:11681-11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu, J. S., S. H. Chang, W. H. Chan, and H. C. Chen. 2001. Enzyme-linked immunosorbent assay for the determination of p21-activated kinase activity. J. Biochem. 129:243-251. [DOI] [PubMed] [Google Scholar]
- 49.Yura, Y., J. Kusaka, Y. Kondo, H. Tsujimoto, H. Yoshida, and M. Sato. 1995. Inhibitory effect of tyrphostin on the replication of herpes simplex virus type 1. Arch. Virol. 140:1181-1194. [DOI] [PubMed] [Google Scholar]