Abstract
The interaction of the T cell receptor (TCR) with peptide in the binding site of the major histocompatibility complex molecule provides the basis for T cell recognition during immune surveillance, repertoire development, and tolerance. Little is known about the extent to which repertoire selection is influenced directly by variation of the structure of the class I heavy chain. We find that the 2C TCR, normally positively selected in the context of the Kb molecule, is minimally selected into the CD8 lineage in the absence of antigen-processing genes. This finding underscores the importance of peptides in determining the positive-selecting class I ligands in the thymus. In contrast, Kbm3, a variant class I molecule that normally exerts a negative selection pressure on 2C-bearing T cells, positively selects 2C transgenic T cells into the CD8 lineage in an antigen-processing gene-deficient environment. These findings indicate that structural changes in the heavy chain can have direct influence in T cell recognition, from which we conclude that the nature of TCR interaction with class I heavy chain influences the array of TCRs selected during development of the functional adult repertoire.
Since it was recognized that class I major histocompatibility complex (MHC) molecules presenting short (8- to 10-aa) peptides serve as the ligand for the T cell receptor (TCR), the nature and role of the bound peptide have been the focus of much investigation. A number of models have been advanced to explain the nature of the epitopes recognized by developing T cells during thymic selection (reviewed in ref. 1). Central to these models is the extent to which self peptide serves as a critical element in the formation of the selecting antigen. Recent structural studies confirmed that the TCR make direct contact with the peptide bound by MHC molecules (2, 3). Mice that have a targeted disruption in the transporter associated with antigen-processing (TAP) genes, which are required for transporting most peptides into the endoplasmic reticulum for binding by class I, are deficient in antigen presentation and in the development of class I-restricted T cells (4). These observations demonstrate the importance of peptide as a key structural element of the class I complex. Furthermore, experiments with fetal thymic organ cultures from TAP-deficient mice document the role of specific peptides in positive selection of transgenic TCRs (5, 6).
Although it is clear that peptide is specifically recognized in the positive selection of these cells, it is difficult to ascertain the importance of direct interactions between the TCR and the class I heavy chain in the selection process. It has been shown that a limited repertoire of functional cytotoxic T lymphocytes (CTLs) is produced in TAP-deficient mice (7); however, the structure adopted by the selecting class I epitopes present in the thymus during selection in TAP-deficient mice remains unclear. Furthermore, recent studies have demonstrated that a single-peptide/MHC complex is sufficient to select at least a partially diverse T cell repertoire (8–10). These observations indicate that the complete complement of peptides available during selection is likely not necessary to develop a complete repertoire. Insight into the molecular basis of positive selection can be found in the examination of the crystal structure of a TCR engaged with a class I/peptide ligand. The structure of the 2C TCR complexed with Kb/dEV8, which is thought to be the positively selecting epitope for 2C (11), shows that 77% of the surface area contacted by the TCR was comprised by the heavy chain and only 23% by peptide (2). This study was followed by the estimate that two-thirds of the energy of interaction of this TCR with the original immunogen, Ld/QL9, is directed at the heavy chain (12). These observations would suggest that structural variation in the heavy chain may be a crucial feature in the thymic development of a functional repertoire.
We found that functional 2C transgenic T cells can be positively selected in a TAP-deficient mouse expressing Kbm3 as a transgene, providing evidence for recognition of peptide-deficient class I molecules by a T cell. These findings are consistent with affinity models for thymic selection and have implications for the relative importance of class I heavy chain in determining the nature of direct TCR-mediated recognition. During the course of this analysis, we also found that 2C-positive mice from our facility that express Kbm3 from either an endogenous or a transgenic locus develop nonfunctional 2C T cells in the periphery that display an anergic phenotype.
Materials and Methods
Mice.
The TAP-deficient mice used in this study were obtained from Anton Berns (Netherlands Cancer Institute, Amsterdam). The Kbm3 transgenic mice were produced in the Mayo immunogenetics mouse colony (directed by Chella David, Mayo Clinic, Rochester, MN). The transgene was constructed by replacing exons 4–8 of a genomic clone of Kb with a BamHI fragment carrying exons 4–8 of Dd. This manipulation introduced a serologic epitope on the α3 domain of the encoded class I molecule for the purpose of monitoring protein expression. The construct was then injected as a 5.9-kb EcoRI-ScaI fragment into SWRxB10.M F2 embryos. Expression and transmission of this transgene could then be followed with mAb, 34–2–12, which is specific for the α3 domain of Dd (13). A line that expressed the transgene at levels comparable to those of endogenous class I loci in the thymus, spleen, and peripheral blood was selected for use in these studies. This line was crossed either to a C57BL/6 2C transgenic TAP−/− mice followed by a further backcross to obtain TAP−/− homozygous mice or to 2C transgenic H-2b homozygous mice followed by intercrossing to obtain H-2b homozygous mice. Human β2-microglobulin (hβ2m) transgenic mice were obtained from Chella David and have been previously described (14). These mice were bred to 2C transgenic Kbm3 transgenic H-2b homozygous mice and backcrossed to obtain H-2b homozygous mice triple transgenic for 2C, hβ2m, and Kbm3, or they were bred to 2C transgenic TAP−/− mice and backcrossed to obtain TAP−/− homozygous mice double transgenic for 2C and hβ2m.
Cytotoxicity Assays.
Cytotoxicity assays were performed as previously described (15). Briefly, T cells isolated from 2C transgenic mice were stimulated weekly with lethally irradiated BALB/c splenic stimulator cells in the presence of rat Con A supernatant and rIL-2. CTL were tested 11 days after stimulation. Five-hour 51Cr release assays were performed with 3 × 103 targets per well. Percent specific lysis was calculated as follows: {(experimental lysis-spontaneous lysis)/(maximum release-spontaneous lysis)} × 100. In experiments where blocking antibody is included in the lysis assay, antibody was added to a concentration of 200 μg/ml.
Antibodies, Tetramers, and Flow Cytometry.
Monoclonal antibodies used in these studies were 1B2 specific for 2C TCR (16) and 34–2-12 specific for the Dd epitope tag for the Kbm3 transgene (13). 1B2 binding was detected by using an FITC-conjugated goat anti-mouse IgG (BioSource International, Camarillo, CA). 2C T cells were also detected by flow cytometry by using Kb tetramers harboring the peptide SIYRYYGL, which is a known high-affinity ligand for 2C (13). The class I tetramers are assembled in vitro from heavy chain and β2m produced in bacteria according to published protocols (17). The avidin molecules in the complexes were labeled with phycoerythrin (PE). Tetramers were used in addition to 1B2 for detection of 2C, because they had brighter fluorescence and gave greater separation of the TCR peaks. Anti-CD4 antibodies (Becton Dickinson) were directly conjugated to red613, and anti-CD8 antibodies (Becton Dickinson) were conjugated with FITC (if tetramer was a costain) or PE (if 1B2 was a costain). Flow cytometry was performed on a FACScan (Becton Dickinson).
Results and Discussion
T cell selection occurs via a direct moderate affinity interaction between the TCR and the class I complex in the thymus (1). Alloreactive T cells are part of the antigen-specific repertoire; however, their frequency is actually several orders of magnitude higher than the frequency of T cells specific for pathogen-derived antigens. Although the phenomenon of allorecognition is an important problem in transplantation immunology, it can also serve as a valuable experimental tool in the study of T cell interactions with class I MHC.
One well-characterized alloreactive T cell clone, DK, expresses the 2C TCR and was originally derived from an H-2b mouse immunized with splenocytes from an H-2d mouse (16). The immunizing ligand for this TCR has been identified as the Ld molecule presenting the allopeptide, p2Ca (18). Other specific ligands for 2C have been identified recently, including specific peptides bound to Kb as well as to the Kb mutant, Kbm3 (11, 19). We were interested in extending our understanding of the recognition patterns of 2C by performing cytotoxicity assays directed against T2 cells expressing transfected genes for the class I molecules, Ld and Kbm3. T2 is a human lymphoid cell line that has lost its TAP genes and is deficient in the presentation of endogenously derived peptides. Class I molecules on the surface of T2 cells are devoid of high-affinity peptide but will easily take up exogenously added peptide (20). We found that when 2C-expressing CTL were highly activated, T2 target cells bearing either Ld or Kbm3 were lysed in the absence of exogenously added peptide. On several occasions, we noted that addition of irrelevant peptides that were capable of binding the Ld or Kbm3 target molecules tended to inhibit lysis of the T2 cells (data not shown). We surmised that the 2C CTL has modest affinity for these functionally empty Ld and Kbm3 molecules. However, when these same T cells were less activated, specific peptides needed to be added to the target cells before the T cells would lyse them (11). Because the conditions leading to the two different activation states are not defined, this in vitro system was not a practical approach for investigating the ability of the 2C TCR to recognize functionally empty class I molecules.
To evaluate the hypothesis that the 2C TCR indeed recognizes peptide-deficient allogeneic molecules, we used an in vivo strategy. Current models of thymic selection hold that whether a given T cell is selected to populate the periphery depends on the affinity of the TCR for the class I molecules expressed in the thymus; TCRs with high affinity for class I are negatively selected, those with no appreciable affinity for class I die by neglect, and only those with modest affinity are positively selected (1). It was previously shown that 2C-expressing T cells are negatively selected by the Kb variant, Kbm3 (21). On the basis of our in vitro finding that 2C can recognize functionally empty Kbm3 molecules, we predicted that Kbm3 could alter the previously described thymic selection pattern for 2C-bearing thymocytes in a TAP-deficient mouse. Whereas high-affinity interactions result in negative selection, the modest reactivity observed in vitro by using TAP-deficient cells suggested the possibility that Kbm3 might positively select T cells expressing the 2C TCR in a TAP-deficient environment. We therefore generated a Kbm3 transgenic mouse line. The reason Kbm3 had to be introduced as a transgene is that TAP is tightly linked with the class I genes of the MHC, and it would be difficult to breed the K locus away from the TAP genes. An epitope-tagged chimeric Kbm3 transgene was constructed so that the α3 domain as well as the transmembrane and cytoplasmic domains were derived from Dd, providing a defined serological epitope to identify the transgene in the presence of the otherwise serologically related Kb molecule. Numerous studies have shown that α3 polymorphisms have little effect on antigen presentation or T cell interaction with class I molecules (22–25).
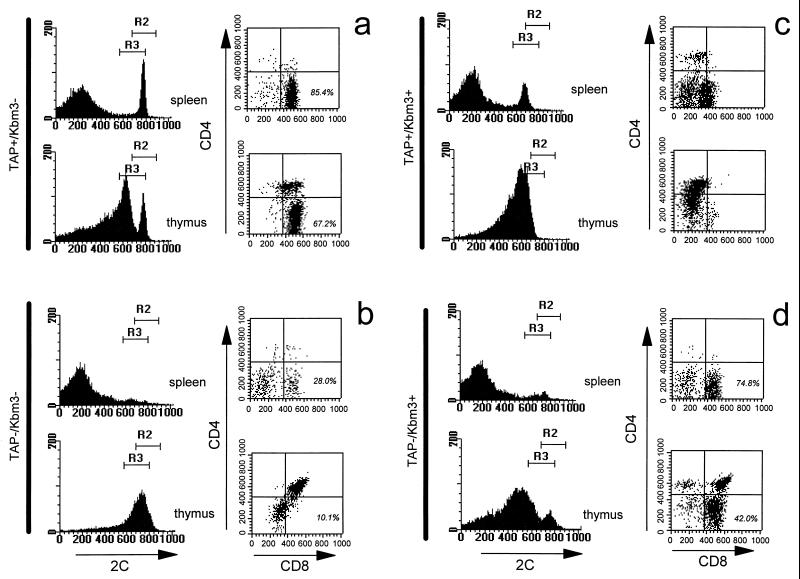
As has been previously shown, 2C T cells are positively selected on Kb in a C57BL/6J background (Fig. 1a). However, in TAP−/− mice, which are otherwise identical to C57BL/6J mice, 2C-bearing T cells are largely arrested at the double-positive stage in the thymus, and a greatly reduced number of 2C+CD8+ cells are detected in the periphery (Fig. 1b). However, the introduction of Kbm3 in a TAP-deficient background results in 2C-bearing T cells progressing onto the CD8 single positive stage and going on to populate the periphery (Fig. 1d). This suggests that there is a biologically significant affinity of 2C for Kbm3 in the absence of normal peptide loading.
Figure 1.
Kbm3 positively selects 2C T cells into the CD8 single-positive compartment. Flow cytometric analysis of the selection profiles for the 2C transgenic T cells in TAP-positive and -negative as well as Kbm3-positive and -negative mice is indicated. Total splenocytes or thymocytes were isolated and stained with a Kb/SIYR tetramer, as shown by the histograms. The dot plots show the CD4 CD8 profiles of the cells expressing high 2C. All dot plots are collected through region 2 (R2) of the histograms except for the TAP-positive/Kbm3-positive mice, which are collected through region 3 (R3) because of their lower 2C expression levels. The percent of gated cells that are CD8-single positive is not shown for the TAP-positive Kbm3-positive mouse, because these cells straddle the quadrant boundary.
We also found that a number of 2C T cells can make it into the periphery in TAP-positive Kbm3 mice with identical results obtained regardless of whether the Kbm3 molecule was expressed from its endogenous locus or from the transgene locus (Fig. 1c and data not shown). Although the relative abundance of these cells varied from mouse to mouse, interestingly, these cells always had reduced TCR and CD8 expression levels (Fig. 1c). Given that Kbm3 and 2C interact with moderate affinity (26), and that numerous studies have shown that CD8 expression levels can alter the fate of responding T cells (27, 28), we conclude that the surviving T cells have down modulated their affinity for the selecting ligand by virtue of the fact that they have reduced TCR and CD8 levels at the cell surface. This pattern of TCR and CD8 expression would then be consistent with affinity models for thymic selection. These peripheral T cells express CD8α, and β chains with both chains decreased at the cell surface and at similar ratios to one another, as observed in C57BL/6J splenic T cells (data not shown).
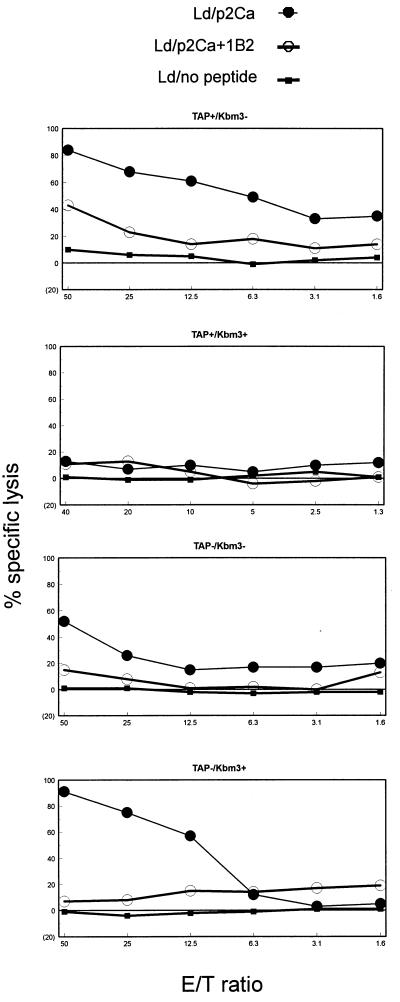
To address the question of whether the 2C-expressing T cells selected in TAP-positive Kbm3 mice were functional, we stimulated them with allogeneic (BALB/c) splenocytes and assessed their ability to lyse Ld-expressing cells in vitro. We chose BALB/c spleen cells as stimulators, because Ld is the original and strongly antigenic stimulus for the 2C TCR. We used the human lymphoid TAP-deficient cell line, T2, transfected with Ld as targets. We observed that T cells from H-2b and from TAP-negative Kbm3 mice could lyse allogeneic targets when they were loaded with exogenously added antigenic peptides. The lysis was 2C mediated because the anti-2C clonotypic antibody, 1B2, could block the killing (Fig. 2). Because the 2C transgene does not mediate complete exclusion of endogenous TCR expression, the extent to which 1B2 blocks lysis was an indication of the extent to which killing could be attributed to 2C T cells. Residual killing would be attributed to non-2C T cells. Because CTL lysis was blocked by 1B2 in all cases, we conclude that cytotoxicity was mediated by the 2C TCR. The 2C cells selected in the TAP-positive Kbm3 mice were found to be unresponsive against the defined 2C epitope, even though these mice harbored a considerably larger number of 2C T cells than TAP-negative Kbm3 mice (Figs. 1 and 2). This unresponsive phenotype is consistent with the “anergic” profile of these T cells displaying markedly reduced levels of TCR and CD8 expression.
Figure 2.
2C+ CD8+ T cells selected by Kbm3 in a TAP-deficient environment are functional CTL precursors. Cytotoxic function of cells derived from transgenic mice. The specific lysis of T2 targets expressing the Ld alloantigen unloaded or loaded with p2Ca, with and without blocking by the 2C anticlonotypic antibody, 1B2, as a function of effector (E)-to-target (T) ratio. 1B2 blocking indicates the extent of the killing that can be attributed specifically to 2C-bearing CTLs. All mice are H-2b homozygous and carry the 2C transgene as well as the indicated TAP and/or Kbm3 genes.
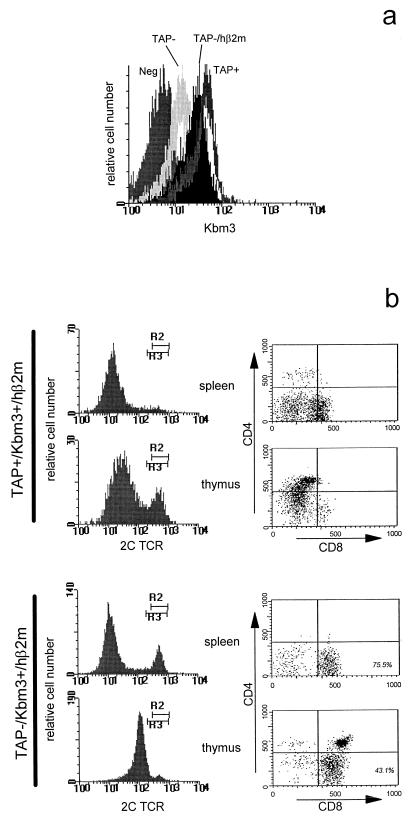
Avidity models for thymic selection hold that the density of TCR ligands in the thymus is a major determinant of whether a given T cell is positively or negatively selected (1). We considered the possibility that 2C is positively selected in a TAP-negative Kbm3 mouse, because there is a lower expression level of the negatively selecting antigen than in a TAP-positive background. It is well established that hβ2m has higher affinity for mouse class I heavy chains than does mouse β2m (29), and it has been shown that expression of hβ2m in TAP-deficient mice can partially restore class I expression levels and augment selection signals in the thymus (30). We bred a hβ2m transgene into our Kbm3 transgenic mice and confirmed that it rescued expression levels of class I in TAP-deficient mice to approximately 50% of the levels found in the TAP-positive mice (Fig. 3a). Despite this increased level of Kbm3 expression in the thymus, we observed no difference in the 2C selection patterns in hβ2m transgenic TAP deficient mice (Fig. 3b), indicating that structural elements of the Kbm3 molecule are responsible for the positive selection of 2C T cells and not the expression level.
Figure 3.
Increased expression of Kbm3 in a TAP-deficient environment does not influence the selection of 2C T cells. Cell profiles of hβ2m transgenic mice. (A) Flow cytometric analysis of total thymocytes derived from TAP-deficient Kbm3-transgenic mice that either do or do not express a hβ2m transgene. (B) Histograms show 2C expression levels in total splenocytes or thymocytes as monitored with 1B2, and the dot plots show the CD4 CD8 profiles of the cells expressing high 2C. The gating and percentage representation for these cells is the same as described for Fig. 1. Comparison of these profiles with their counterparts from Fig. 1 reveals no significant differences.
The possibility exists that the α3 polymorphisms between Dd and Kb or structural differences between class I complexes formed with murine β2m vs. hβ2m will result in diminished affinity for the CD8 coreceptor. If this were the case, it could also lead to a situation where positive selection of 2C would be allowed in the presence of Kbm3. Although the measured affinities of CD8 for numerous mouse and human class I alleles have been found to be largely invariant (31, 32), we chose to assemble tetramers with murine and hβ2m and with and without the α3 polymorphisms and measured their affinity for 2C. We found that tetramers utilizing murine β2m and differing only in α3 had virtually identical affinities for 2C T cells (41.5 nM and 45.7 nM for Kbm3 and Kbm3-Dd tetramers, respectively). However tetramers bearing hβ2m and Kbm3-Dd actually had a higher affinity (1.1 nM) for 2C T cells. We conclude that the affinities of the Kbm3 molecules used in this study are not diminished by experimental manipulations, and we therefore rule out the possibility that these structural elements allowed positive selection of 2C, because they resulted in lower-affinity ligands for the TCR/CD8 receptor complex.
A number of recent reports have favored an avidity model for positive selection on the basis of results obtained where class I expression levels were modulated with TAP and β2m mutants (5, 6, 33, 34). A very interesting report from Cook et al. describes how they set out to determine whether a lower level of expression of the Ld molecule, which was achieved by crossing in mutations in the Ld and β2m genes, can positively select transgenic 2C TCR-bearing T cells. They found that when they reached an expression level of 2% of wild type by expressing one functional copy of the Ld locus and homozygous deletion of β2m, T cells expressing the 2C receptor were positively selected. This finding was interpreted as evidence for an avidity model of selection but failed to address the following considerations. First, a specific peptide required for the formation of a negatively selecting epitope might not be represented in the repertoire of peptides displayed by the small number of class I molecules expressed by a β2m-deficient thymus. Second, a conformation adopted by heavy chain in the presence of β2m, which formed the negatively selecting epitope, might not have formed in the absence of β2m (35). Third, a different subset of peptides might be bound by the class I heavy chain complexed to β2m compared with those bound by the same heavy chains in the absence of β2m (36), resulting in the disappearance of the negatively selecting epitope. Consideration of these points reminds us of the qualitative features of the elements that shape the repertoire. Although thymic selection is likely influenced to some extent by determinant density, our finding that a negatively selecting class I molecule can positively select a T cell when devoid of high-affinity peptide argues that the class I heavy chain has a strong direct influence on TCR binding and will therefore influence TCR repertoire selection.
There is increasing evidence that, because the TCR has interacted with the MHC over evolutionary time, the structure of the TCR has been shaped to have a high likelihood of recognizing MHC-determined epitopes (37, 38). Furthermore, it has been rationalized that TCRs must have wide crossreactivity to ensure a comprehensive response to pathogens (39). It is interesting to consider that detailed studies of 2C binding found that the majority of the strength of the interaction comes from contacts made with the class I heavy chain. This may be particular to 2C because of the short uncharged CDR3s of this TCR in comparison to other receptors, exemplified by the anti-TAX/HLA-A2 TCRs (40). Whether 2C is rare in its relative interactions with heavy chain and peptide will require the extensive study of more TCR/class I complexes. However, because a tremendous diversity of peptides and other molecules can be presented by members of the MHC superfamily, it can be reasoned that the nature of the bound peptides should be of limited significance in the molding of the conserved aspects of TCR structure.
The demonstration that TCRs can have appreciable affinity for structural variants of the class I heavy chain (and also likely structural variants of class II molecules) has important implications, particularly with respect to allorecognition. During selection of the TCR repertoire, heavy-chain interactions with the receptor contribute to the affinity required to activate the positive selection program. The greater the affinity of the TCR for the MHC molecule, the more likely that peptides exist that push the affinity of the interaction to a level leading to negative selection in the biological milieu. Consequently, surviving receptors will likely have moderate to low affinity for the peptide-deficient MHC molecules. In the current study, we show that the self-restricting element Kb inefficiently supports positive selection in a peptide-deficient TAP-negative environment. However, in the case of allorecognition, TCRs that have high affinity for the MHC molecule have a greater probability of encountering bound peptides that contribute sufficient additional interactions with the TCR to support the initiation of signals leading to an alloresponse. The prediction is that allorecognition will depend less on the structure of the bound peptide than self-restricted recognition, which is consistent with recent mathematical modeling of thymocyte selection (41). In experimental support of this hypothesis, we have shown that the alloantigen Kbm3 supports positive selection efficiently in the absence of TAP-dependent peptides (Fig. 1), and that a self peptide that binds both Kb and Kbm3 (42) is antigenic in the case of Kbm3, where the TCR has higher affinity for the heavy chain.
Abbreviations
- MHC
major histocompatibility complex
- TCR
T cell receptor
- TAP
transporter associated with antigen processing
- CTL
cytotoxic T lymphocyte
- β2m
β2-microglobulin
- hβ2m
human β2m
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Jameson S C, Hogquist K A, Bevan M J. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 2.Garcia K C, Degano M, Stanfield R L, Brunmark A, Jackson M R, Peterson P A, Teyton L, Wilson I A. Science. 1996;274:209–219. [PubMed] [Google Scholar]
- 3.Garboczi D N, Ghosh P, Utz U, Fan Q R, Biddison W E, Wiley D C. Nature (London) 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 4.Van Kaer L, Ashton-Rickardt P G, Ploegh H L, Tonegawa S. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 5.Sebzda E, Wallace V A, Mayer J, Yeung R S, Mak T W, Ohashi P S. Science. 1996;263:1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 6.Ashton-Rickardt P G, Bandeira A, Delaney J R, Van Kaer L, Pircher H P, Zinkernagel R M, Tonegawa S. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 7.Aldrich C J, Ljunggren H G, Van Kaer L, Ashton-Rickardt P G, Tonegawa S, Forman J. Proc Natl Acad Sci USA. 1994;91:6525–6528. doi: 10.1073/pnas.91.14.6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ignatowicz L, Kappler J, Marrack P. Cell. 1996;84:521–529. doi: 10.1016/s0092-8674(00)81028-4. [DOI] [PubMed] [Google Scholar]
- 9.Surh C D, Lee D S, Fung-Leung W P, Karlsson L, Sprent J. Immunity. 1997;7:209–219. doi: 10.1016/s1074-7613(00)80524-5. [DOI] [PubMed] [Google Scholar]
- 10.Liu C P, Parker D, Kappler J, Marrack P. J Exp Med. 1997;186:1441–1450. doi: 10.1084/jem.186.9.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tallquist M D, Yun T J, Pease L R. J Exp Med. 1996;184:1017–1026. doi: 10.1084/jem.184.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manning T C, C J, Schlueter C J, Brodnicki T C, Parke E A, Speir J A, Garcia K C, Teyton L, Wilson I A, Kranz D M. Immunity. 1998;8:413–425. doi: 10.1016/s1074-7613(00)80547-6. [DOI] [PubMed] [Google Scholar]
- 13.Margulies D H, Evans G A, Flaherty L, Seidman J G. Nature (London) 1982;295:168–170. doi: 10.1038/295168a0. [DOI] [PubMed] [Google Scholar]
- 14.Kievits F, Ivanyi P, Krimpenfort P, Berns A, Ploegh H L. Nature (London) 1987;329:447–449. doi: 10.1038/329447a0. [DOI] [PubMed] [Google Scholar]
- 15.Pullen J K, Hunt H D, Horton R M, Pease L R. J Immunol. 1989;143:1674–1679. [PubMed] [Google Scholar]
- 16.Kranz D M, Sherman D H, Sitkovsky M V, Pasternack M S, Eisen H N. Proc Natl Acad Sci USA. 1984;81:573–577. doi: 10.1073/pnas.81.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 18.Udaka K, Tsomides T J, Eisen H N. Cell. 1992;69:989–998. doi: 10.1016/0092-8674(92)90617-l. [DOI] [PubMed] [Google Scholar]
- 19.Rammensee H G, Falk K, Rötzschke O. Annu Rev Immunol. 1993;11:213–244. doi: 10.1146/annurev.iy.11.040193.001241. [DOI] [PubMed] [Google Scholar]
- 20.Hosken N A, Bevan M J. Science. 1990;248:367–370. doi: 10.1126/science.2326647. [DOI] [PubMed] [Google Scholar]
- 21.Sha W C, Nelson C A, Newberry R D, Pullen J K, Pease L R, Russell J H, Loh D Y. Proc Natl Acad Sci USA. 1990;87:6186–6190. doi: 10.1073/pnas.87.16.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen H, Wraith D, Pala P, Askonas B, Flavell R A. Nature (London) 1984;309:279–281. doi: 10.1038/309279a0. [DOI] [PubMed] [Google Scholar]
- 23.Ozato K, Evans G A, Shykind G, Margulies D H, Seidman J G. Proc Natl Acad Sci USA. 1983;80:2040–2043. doi: 10.1073/pnas.80.7.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiss C S, Evans G A, Margulies D H, Seidman J G, Burakoff S J. Proc Natl Acad Sci USA. 1983;80:2709–2712. doi: 10.1073/pnas.80.9.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroynowski I, Forman J, Goodenow R S, Schiffer S G, McMillan M, Sharrow S O, Sachs D H, Hood L. J Exp Med. 1985;161:935–952. doi: 10.1084/jem.161.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia K C, Tallquist M D, Pease L R, Brunmark A, Scott C S, Degano M, Stura E A, Peterson P A, Wilson I A, Teyton L. Proc Natl Acad Sci USA. 1997;94:13838–13843. doi: 10.1073/pnas.94.25.13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee N A, Loh D H, Lacy E. J Exp Med. 1992;175:1013–1025. doi: 10.1084/jem.175.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jameson S C, Hogquist K A, Bevan M J. Nature (London) 1994;369:750–752. doi: 10.1038/369750a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luscher M A, Newton B L, Barber B H. J Immunol. 1994;153:5068–5081. [PubMed] [Google Scholar]
- 30.Martien van Santen H, Woolsey A, Rickardt P G, Van Kaer L, Baas E J, Berns A, Tonegawa S, Ploegh H L. J Exp Med. 1995;181:787–792. doi: 10.1084/jem.181.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia K C, Scott C A, Brunmark A, Carbone F R, Peterson P A, Wilson I A, Teyton L. Nature (London) 1996;384:577–581. doi: 10.1038/384577a0. [DOI] [PubMed] [Google Scholar]
- 32.Wyer J R, Willcox B E, Gao G F, Gerth U C, Davis S J, Bell J I, van der Merwe P A, Jakobsen B K. Immunity. 1999;10:219–225. doi: 10.1016/s1074-7613(00)80022-9. [DOI] [PubMed] [Google Scholar]
- 33.Hogquist K A, Jameson S C, Heath W R, Howard J L, Bevan M J, Carbone F R. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 34.Cook J R, Wormstall E M, Hornell T, Russell J, Connolly J M, Hansen T H. Immunity. 1997;7:233–241. doi: 10.1016/s1074-7613(00)80526-9. [DOI] [PubMed] [Google Scholar]
- 35.Solheim J C, Cook J R, Hansen T H. Immunol Res. 1995;14:200–217. doi: 10.1007/BF02918217. [DOI] [PubMed] [Google Scholar]
- 36.Joyce S, Kuzushima K, Kepecs G, Angeletti R H, Nathenson S G. Proc Natl Acad Sci USA. 1994;91:4145–4149. doi: 10.1073/pnas.91.10.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janeway C A, Chervonsky A V, Sant'Angelo D. Curr Biol. 1997;7:R299–300. doi: 10.1016/s0960-9822(06)00142-4. [DOI] [PubMed] [Google Scholar]
- 38.Zerrahn J, Held W, Raulet D H. Cell. 1997;88:627–636. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]
- 39.Mason D. Immunol Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 40.Garboczi D N, Biddison W E. Immunity. 1999;10:1–7. doi: 10.1016/s1074-7613(00)80001-1. [DOI] [PubMed] [Google Scholar]
- 41.Detours V, Perelson A S. Proc Natl Acad Sci USA. 1999;96:5153–5158. doi: 10.1073/pnas.96.9.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tallquist M D, Weaver A J, Pease L R. J Immunol. 1998;160(2):802–809. [PubMed] [Google Scholar]