Abstract
To investigate the role of the ORF47 protein kinase of varicella-zoster virus (VZV), we constructed VZV recombinants with targeted mutations in conserved motifs of ORF47 and a truncated ORF47 and characterized these mutants for replication, phosphorylation, and protein-protein interactions in vitro and for infectivity in human skin xenografts in the SCID-hu mouse model in vivo. Previous experiments showed that ROka47S, a null mutant that makes no ORF47 protein, did not replicate in skin in vivo (J. F. Moffat, L. Zerboni, M. H. Sommer, T. C. Heineman, J. I. Cohen, H. Kaneshima, and A. M. Arvin, Proc. Natl. Acad. Sci. USA 95:11969-11974, 1998). The construction of VZV recombinants with targeted ORF47 mutations made it possible to assess the effects on VZV infection of human skin xenografts of selectively abolishing ORF47 protein kinase activity. ORF47 mutations that resulted in a C-terminal truncation or disrupted the DYS kinase motif eliminated ORF47 kinase activity and were associated with extensive nuclear retention of ORF47 and IE62 proteins in vitro. Disrupting ORF47 kinase function also resulted in a marked decrease in VZV replication and cutaneous lesion formation in skin xenografts in vivo. However, infectivity in vivo was not blocked completely as long as the capacity of ORF47 protein to bind IE62 protein was preserved, a function that we identified and mapped to the N-terminal domain of ORF47 protein. These experiments indicate that ORF47 kinase activity is of critical importance for VZV infection and cell-cell spread in human skin in vivo but suggest that it is the formation of complexes between ORF47 and IE62 proteins, both VZV tegument components, that constitutes the essential contribution of ORF47 protein to VZV replication in vivo.
Varicella-zoster virus (VZV) is a ubiquitous human alphaherpesvirus that causes varicella (chicken pox), establishes latency in sensory ganglia, and can reactivate to cause herpes zoster. The pathogenesis of primary VZV infection involves inoculation of respiratory mucosa, initiation of a cell-associated viremia, and the appearance of widely distributed vesicular skin lesions (1, 4). T lymphocytes appear to be a major target cell for VZV viremia, and these migrating cells have the capacity to transport the virus to epidermal and dermal cells (15, 19). VZV skin lesions contain high titers of infectious virus, which is transmissible to other susceptible individuals in the population.
VZV ORF47 encodes the ORF47 protein, which has the characteristic amino acid sequence and functions of a serine/threonine protein kinase and has homologies to herpes simplex virus (HSV) UL13 and cellular casein kinase II (CKII) (5, 6, 12, 27, 34). The ORF47 protein is dispensable for VZV replication in vitro, as shown in studies of ROka47S, a recombinant virus described by Heineman and Cohen, in which ORF47 transcription was blocked by a stop codon mutation (8). In contrast, the ORF47 protein is essential for VZV infection of differentiated human T-cell and skin xenografts in the SCID-hu model of VZV infection in vivo (21). Thus, ORF47 protein is required for viremia and cutaneous replication, which are the critical events in the pathogenesis of primary VZV infection.
The ORF47 protein autophosphorylates, and it phosphorylates the major immediate-early transactivating protein encoded by ORF62/ORF71, designated the IE62 protein (14, 26); the IE63 protein (12); gE, which is the predominant VZV glycoprotein (11); and the ORF32 gene product (30). By analogy with HSV UL13, other viral and cellular substrates are probably modified by ORF47 protein kinase (31). As tegument components, ORF47 and ORF62 proteins are delivered simultaneously into the cytoplasm of the infected cell after uncoating. The IE62 protein initiates transcription from its own and all other VZV gene promoters examined thus far, acting synergistically with several viral and cellular transactivating proteins (9, 13, 14, 23, 25, 26, 28, 29, 36). The VZV ORF47 protein is predicted to bind to IE62 protein, because it phosphorylates IE62 protein. Since the ORF47 and IE62 proteins are also components of the VZ virion tegument, their physical interaction may contribute to tegument formation. VZV gE is essential for replication (18) and requires differential phosphorylation to mediate its two functions of cell fusion and intracellular trafficking to the Golgi for virion assembly (11). The efficient replication of the ROka47S mutant in cell cultures suggests that ORF47 protein-mediated phosphorylation of the IE62 protein and gE and ORF47-IE62 protein binding are not essential for VZV infectivity in vitro. In the absence of ORF47 kinase, CKII phosphorylates gE sufficiently to permit VZV cell-cell spread (11). In contrast, cell-cell spread and virion synthesis cannot occur in intact host tissues in vivo in the absence of ORF47 protein (21).
Having established that the ORF47 protein is essential in vivo (21), we investigated whether its kinase activity accounts for the essential role of ORF47 as a virulence determinant. To address this question, we constructed VZV recombinants with ORF47 mutations that were designed to disrupt kinase activity. Recombinant viruses with mutations that altered conserved kinase motifs or resulted in a C-terminal truncation of ORF47 protein were characterized for phosphorylating activity, protein complex formation, and intracellular localization of ORF47 and IE62 proteins in vitro and for infectivity in human skin in the SCID-hu mouse model.
The ORF47 protein kinase mutations were designed by analysis of the ORF47 sequence to identify predicted kinase domains. By sequence comparison, the ORF47 protein appears to contain the 11 kinase subdomains typical for all members of the large serine/threonine kinase family and the sequences that distinguish it from tyrosine kinases (5, 6). The central core of the catalytic subdomains starts with subdomain VI, which corresponds to amino acid residue 240 in the ORF47 protein. Within the central core subdomains of kinases, conserved motifs (containing invariant amino acids) are essential for catalytic functioning. In subdomain VII, for example, the highly conserved DFG motif is necessary for ATP binding. The invariant aspartate in this motif is believed to orient the γ phosphate of ATP for substrate transfer by chelating the activating Mg2+ ions. Point mutations at this site have been shown to abolish kinase activity in v-fps, a viral tyrosine kinase (22). In the ORF47 protein, the corresponding putative ATP-binding site reads DYS, with phenylalanine and glycine being very conservative changes from tyrosine and serine, respectively. The APE motif is another highly conserved sequence in subdomain VIII. This site, which corresponds to PPE in the ORF47 protein, along with upstream sequences within the same subdomain, is thought to be important for the tertiary structure of the kinase domain (5, 6). Mutational analysis has shown that all three amino acids in the APE motif are essential for kinase function in v-src (2). Exchange of a conserved amino acid, lysine 169, in the ORF47 protein, which is upstream of the central core kinase domains in subdomain II, reduced ORF47 autophosphorylation in vitro by almost 50% (12).
In the present experiments, ORF47 mutations that resulted in a C-terminal truncation or disrupted the DYS kinase motif abolished ORF47 kinase activity and were associated with extensive nuclear retention of ORF47 and IE62 proteins in vitro. Disrupting ORF47 kinase function resulted in a marked decrease in VZV replication and cutaneous lesion formation in skin xenografts in vivo. However, infectivity was not blocked completely as long as binding of the ORF47 protein and the IE62 protein, which was mapped to the N-terminal domain of the ORF47 protein, was preserved. These experiments indicate that ORF47 kinase activity is of critical importance for VZV infection and cell-cell spread in human skin in vivo, but it is the interaction between the two VZV tegument proteins, the ORF47 protein and the IE62 protein, that defines the essential contribution of the ORF47 protein to VZV replication in vivo.
MATERIALS AND METHODS
Construction of mutations in ORF47.
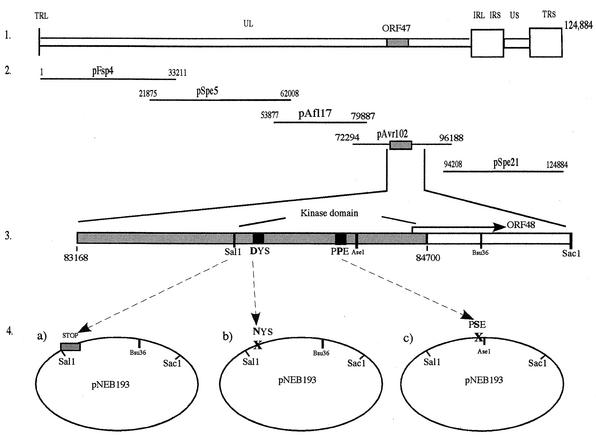
A modification of a previously described cosmid transfection system divided the largest of the original cosmids, pPme2, into two overlapping cosmids, pAf17l and pAvr102, facilitating mutations of ORF47 (17, 26a). The first step in making VZV ORF47 mutant viruses was to introduce targeted mutations into ORF47 in the cosmid pAvr102, which was the fourth of five overlapping VZV DNA fragments cloned into SuperCos 1 cosmid vectors (Stratagene, La Jolla, Calif.) (10, 26a). A SalI-AscI fragment (12 kb) was excised from pAvr102 and cloned into pNEB193. A 2.5-kb SalI-SacI fragment was subcloned and used as a template for PCRs to introduce the mutations. Three mutations were made, including a truncation of ORF47, designated ORF47ΔC, which terminated ORF47 at the start of the sequence for the kinase domain in the 3′ half of ORF47, directly behind a unique SalI site at nucleotide 799; an ORF47 D-N point mutation at nucleotide 847 that replaced aspartic acid with asparagine in the conserved DYS kinase motif; and an ORF47 P-S point mutation at nucleotide 997 which replaced the second conserved proline in the PPE motif with serine. To make ORF47ΔC, the sense primer included the SalI restriction sequence preceded by eight random nucleotides, a Flag Tag sequence, a stop sequence with stop codons in all three frames, an introduced PacI restriction site, and 36 bases of the ORF47 sequence directly following the SalI site (5′-GGCGCGCCACGTCGACGATTACAAGGACGATGACGATAAGTAATTAGCTGATTAATTAAAACTTTGCCTCGTTGGAAATAACCACAGCAGTAATC-3′). The corresponding antisense primer was placed 1,815 bp after the start of ORF47 and located in ORF48 at a Bsu36I site unique to the 2.5-kb SalI-SacI fragment and had the following sequence: 5′-CGGCTCCTGAGGCTGTGTGGACGTCTTAAAG-3′. The resulting 1,014-bp PCR product was cloned via SalI and Bsu36I into the 2.5-kb fragment, replacing the original sequence.
The D-N mutant was made using the relative proximity of the SalI site. A sense primer was constructed that spans the area from the SalI site to the DYS region and changes the codon from aspartic acid to aspartate (5′-TTTTCTTAACGTCGACAACTTTGCCTCGTTGGAAATAACCACAGCAGTAATCGGAAACTATAGCCTAG-3′). The same antisense primer and cloning technique was used as described for ORF47ΔC. The P-S mutant was constructed with an antisense primer that changed the respective codon and included a downstream unique AseI site (5′-GGCCCGTTCCATTAATATAATCAAGCAAGATTTCAGAGGGTTGGTTTG-3′). This primer was paired with a sense primer located at the SalI site (5′-TTTTCTTAACGTCGACAACTTTGCCTCG-3′), producing a 250-bp fragment that was cloned via SalI and AseI into the 2.5-kb fragment. All ORF47 mutations were returned into the pAvr cosmid by way of the cloned SalI-AscI fragment. The PCR primers were manufactured by OPERON Technologies, Inc. (Alameda, Calif.).
Recombinant viruses.
Recombinant viruses were isolated by transfection of human melanoma cells with mutated pAvr cosmid and the four intact cosmids (10, 26a). Melanoma cells were maintained in tissue culture medium (MEM; Mediatech, Washington, D.C.) supplemented with 10% fetal calf serum (Tissue Culture Biologicals, Tulare, Calif.), nonessential amino acids, and antibiotics. VZV recombinants rOks47ΔC, rOka47D-N, rOka47P-S, and rOka were generated using cosmids pSpe5, pSpe21, and pFsp4 of vaccine Oka origin; pAvr102 and pAfl17 were of parent Oka origin. To confirm that the ORF47 mutations had been transferred correctly, genomic DNA was isolated from cells infected with each mutant and segments containing the ORF47 gene were amplified by PCR, cloned into the TOPO cloning vector (Invitrogen, Carlsbad, Calif.), and sequenced (using an Applied Biosystems automated sequencing apparatus [model 373A, version 2.0.1S]) with T3 and T7 primers. Viruses were passaged to human embryonic lung fibroblasts (HEL) and stored at −80°C in fetal calf serum with 10% dimethyl sulfoxide for SCID-hu experiments. The ROka47S null mutant was kindly provided by Jeffrey Cohen, National Institutes of Health (8).
The replication kinetics and peak titers of rOka, rOka47ΔC, rOka47D-N, and rOka47P-S were assessed by growth curve assays. Melanoma cells were seeded in a six-well plate and infected with an inoculum of 2 × 103 PFU each. Wells were trypsinized on days 1, 2, 3, 4, and 5, and titers were determined in an infection focus assay (7, 19).
Immunoprecipitation and Western blotting.
Polyclonal anti-ORF47 protein antiserum was made by cloning the first 300 bp of ORF47 into pGEX-2T (Pharmacia Biotech, Milwaukee, Wis.) by PCR with primers containing BamHI and EcoRI. Protein expression was induced, and the purified glutathione S-transferase-fusion peptide was used for rabbit immunization (33). Rabbit immunization was performed at Josman, LLC (Napa, Calif.). Rabbit serum was collected before and after immunization and stored at −20°C. For immunoprecipitation experiments, melanoma cells infected with rOka, rOka47ΔC, rOka47D-N, and rOka47P-S were lysed with radioimmunoprecipitation assay buffer (50 mM Tris [pH 8], 150 mM NaCl, 1% IGEPAL CA-360 [Sigma], 0.1% sodium dodecyl sulfate [SDS] [Bio-Rad], 0.5% deoxycholic acid [Sigma]) containing a Complete Mini tablet (Roche, Inc.) in one 1-ml volume per T-75 flask. ROka47S was used as a negative control (kindly provided by Jeffrey Cohen, National Institutes of Health). The infected cell lysates were precleared with rabbit preimmune serum (4 μl) plus protein A Sepharose CL-4B beads (36 μl) (Pharmacia, Inc.) for 30 min at 4°C. The supernatant was incubated overnight with rabbit anti-ORF47 antiserum (4 μl)-protein A Sepharose (36 μl). Samples were washed three times with Hepes-NaCl-Triton-glycerol (HNTG) buffer, boiled in sample buffer, and separated by SDS-polyacrylamide gel electrophoresis (PAGE) in 7.5% gels. Proteins were transferred to Immobilon membranes. IE62 was detected on the membrane by incubation with a polyclonal anti-IE62 antiserum (kindly provided by Paul Kinchington, University of Pittsburgh).
Cellular CKII expression was detected by Western blot analysis of lysates of melanoma cells, primary human lung cells (human embryonic lung and MRC-5 cells), and skin xenografts. Positive control CKII was purchased from Upstate Biotechnology, Inc., Lake Placid, N.Y., and anti-CK II antibody was a generous gift from Michael Dahmer, University of California, Davis.
Kinase assays.
Melanoma cells were infected with rOka, rOka47ΔC, rOka47D-N, or rOka47P-S and harvested with radioimmunoprecipitation assay buffer; lysates were precleared, incubated with ORF47 antiserum overnight at 4°C, and washed three times with HNTG buffer. The HNTG supernatant was removed completely, and 1 ml of kinase buffer (20 mM HEPES [pH 7.5], 10 mM MgCl2, 100 μM orthovanadate, 2 mM dithiothreitol) was added. Beads were spun down, and the kinase buffer was replaced with 30 μl of kinase reaction buffer (containing 20 μM cold ATP and 200 μCi of [γ-32P]ATP/μl in kinase buffer) per sample. Samples were incubated for 20 min at 30°C and boiled after the addition of 6× SDS sample buffer. Samples were separated by SDS-PAGE, and gels were blotted as described above. A photographic film (Kodak) was used to detect the radioactive signals localized on the filter. After the radioactive signal decayed, the filter was probed with polyclonal anti-IE62 antibody.
Immunofluorescence.
Infected melanoma cells were fixed in 2% formaldehyde-0.1% Triton for 1 h after 20, 30, and 42 h of infection. Cells were washed five times in phosphate-buffered saline (PBS) for 5 min, blocked with 5% donkey serum in PBS for 30 min, and incubated overnight with murine anti-IE62 monoclonal antibody and rabbit anti-ORF47 polyclonal antiserum. After five washes with PBS, the secondary antibodies, Texas Red-coupled anti-mouse and fluorescein isothiocyanate-coupled anti-rabbit antibodies (Jackson ImmunoResearch, Inc.), were added for 1 h and shielded from light. After five PBS washes, coverslips were mounted with Vectashield (Vector Laboratories, Inc.) and stored in the dark. Imaging was performed at the Cell Sciences Imaging Facility, Stanford, Calif., with a MultiProbe 2010 laser confocal microscope (Molecular Dynamics, Sunnyvale, Calif.).
Infection of human skin xenografts in the SCID-hu mouse model.
Skin implants were engrafted in male homozygous C.B-17 scid/scid mice (20). Human fetal tissues were obtained with informed consent according to federal and state regulations. Animals were cared for according to guidelines of the Animal Welfare Act PL 94-279 and the Stanford University Administrative Panel on Laboratory Animal Care. Implants were harvested weekly from 2 to 4 weeks after inoculation or mock infection; tissues were analyzed by infection focus assays, immunohistochemistry, and sequencing of ORF47 in recovered viruses (19, 20). Using polyclonal human anti-VZV immunoglobulin G (19), VZV proteins were detected in skin sections.
RESULTS
Construction of rOka recombinants with ORF47 mutations.
The ORF47 gene consists of a 1,533-bp sequence located in the unique long region of the VZV genome. Two point mutants (D-N and P-S) and one truncated version (ΔC) of ORF47 were generated as detailed in Materials and Methods and Fig. 1. Expected ORF47 sequences in recombinant viruses were confirmed by sequencing the full-length PCR products (data not shown).
FIG. 1.
Schematic illustration of the construction of ORF47 mutants. (Panel 1) VZV genome, showing the localization of the ORF47 gene. (Panel 2) Five-cosmid system used to produce mutant viruses. ORF47 is located in pAvr102. (Panel 3) Schematic depiction of ORF47 indicating the coding sequence of the kinase domain in the 3′ half of the gene and the conserved kinase motifs that were targets for point mutations (shown in amino acid single-letter code). (Panel 4) A 2.5-kb SalI-SacI fragment subcloned into pNEB193 (via a 12-kb SalI-AscI fragment cloned into pNEB193; step not shown) containing the 3′ half of ORF47 and downstream sequences. This plasmid was used to produce the ORF47 mutations as follows: (a) insertion of STOP codons at the beginning of the kinase domain code, yielding the ORF47ΔC construct; (b) point mutation in the DYS motif, yielding the ORF47D-N construct; and (c) point mutation in the PPE motif, yielding the ORF47P-S construct. All mutants were constructed via PCR using the SalI restriction site as a sense and mutagenesis primer and a Bsu36I site 1,014 bp downstream of SalI as a location for the antisense primer (a and b) or an AseI site located 250 bp downstream of the SalI site and very close to the PPE motif as a location for the antisense and mutagenesis primer (c). All mutations were reinserted into pAvr102 via the SalI-AscI construct.
Replication of rOka47 mutants in vitro.
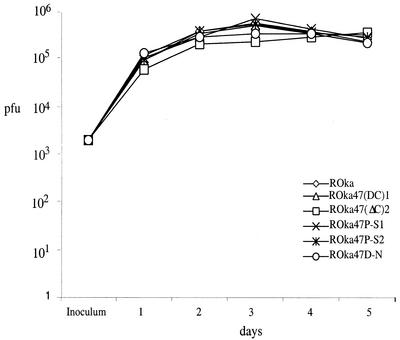
The rOka47ΔC, rOka47D-N, and rOka47P-S viruses were indistinguishable from rOka in growth kinetics, virus yield, and plaque morphology in melanoma cells (Fig. 2). All recombinants grew to approximately 5 × 105 PFU by day 3. These results were expected, based upon previous observations with ROka47S, in which blocking ORF47 expression had no effect on VZV replication in cell culture (8, 21).
FIG. 2.
Replication of VZV in melanoma cells. Melanoma cells were inoculated on day 0 with 2 × 103 PFU of rOka or rOka mutants. Two rOka47ΔC and rOka47P-S mutants were generated and tested independently. Aliquots were harvested daily for 5 days, and infectious foci were determined by titration on melanoma cell monolayers. Each time point represents the mean of results for at least three wells.
Replication of rOka47 mutants in human skin xenografts.
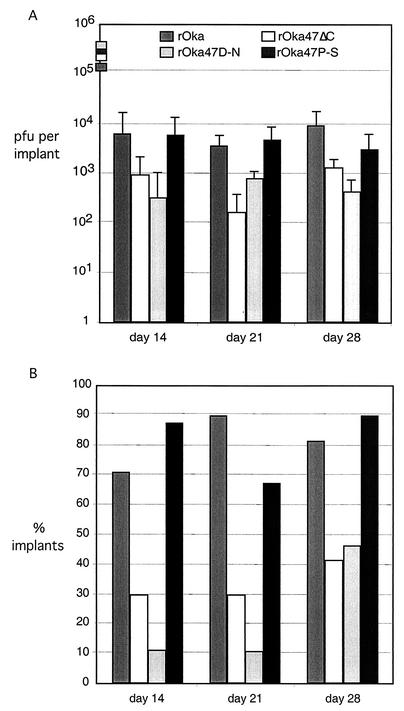
The rOka and rOka47P-S viruses replicated to close to 104 PFU per implant at day 14 after inoculation; rOka47P-S titers remained equivalent to those of rOka at days 21 and 28 (Fig. 3A). In contrast, growth of rOka47ΔC and rOka47D-N was decreased and was delayed significantly. On day 14, infectious virus was recovered from only 3 of 10 implants inoculated with rOka47ΔC; the mean titer for positive implants was 103 PFU per implant; and 1 of 10 implants inoculated with rOka47D-N yielded infectious virus on day 14, with a mean titer of 5 × 102 PFU in this implant. On days 21 and 28, rOka47ΔC and rOka47D-N titers remained approximately 10% or less of those in skin xenografts inoculated with rOka (Fig. 3A). Over all time points, infectious virus was recovered from 82% (23/28) of rOka-infected implants and 83% (25/30) of rOka47P-S-infected implants. Conversely, viral growth was detected in only 32% (10/31) of implants inoculated with rOka47ΔC and 22% (7/32) of those inoculated with rOka47D-N (Fig. 3B). The expected ORF47 sequence was confirmed for all mutants recovered from skin implants (data not shown).
FIG. 3.
Replication of VZV ORF47 mutants in skin xenografts in SCID-hu mice. Skin tissue was infected with rOka, rOka47ΔC, rOka47D-N, and rOka47P-S grown in HEL cells. (A) Samples were harvested after 14, 21, and 28 days, and virus was titrated on melanoma monolayers. Each time point represents the mean number of plaques from two experiments, with four to six implants per experiment. Implants that did not contain infectious virus were excluded from the average. Inocula (shown as boxes on the y axis) are given as PFU/10 μl, which represents the amount used to infect each implant. (B) Percentages of implants that contained virus. The average of all implants in two experiments was computed. A total of 28 implants were infected with rOka, 31 implants were infected with rOka47ΔC, 32 implants were infected with rOka47D-N, and 30 implants were infected with rOka47P-S.
Immunohistologic analyses of rOka47 mutants in human skin xenografts.
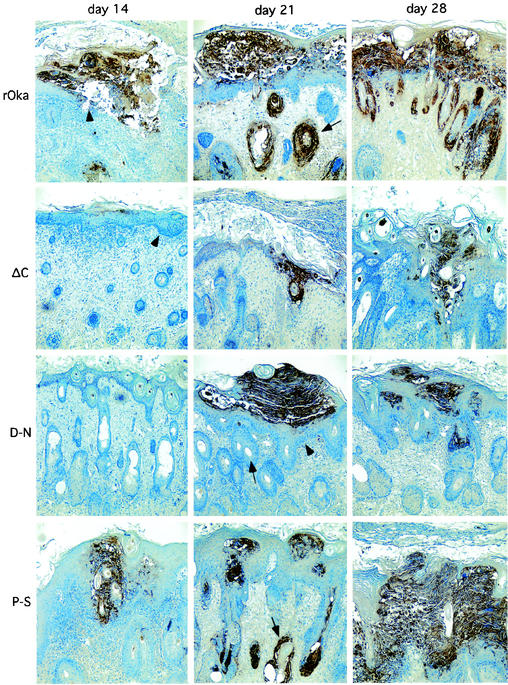
Infection with rOka and rOkaORF47P-S resulted in cutaneous lesions by day 14, which appeared in the epidermis and progressed through the dermis-epidermis junction (basement membrane) (Fig. 4). In contrast, rOka47ΔC infection progressed much more slowly, involving only the epidermal layer at day 14; VZV-infected cells were not detected at day 14 after inoculation of rOka47D-N. By day 21, rOka and rOka47P-S had produced extensive lesions at the epidermal surface, disrupted the basement membrane extensively, and infected multiple hair follicles located on the dermal side of the dermis-epidermis junction. In contrast, rOka47ΔC and rOka47D-N generated small lesions, infection did not penetrate the basement membrane, and hair follicles were not infected. By day 28, all of these cytopathologic differences were even more pronounced; the rOka and rOka47P-S viruses had spread throughout the epidermal layer and into many hair follicles, and large sections of the basement membrane had disappeared. In contrast, rOka47ΔC and rOka47D-N infection remained highly localized without penetrating the basement membrane. Infection with rOka47ΔC had some disruptive effect on the cells of the basement membrane, which were enlarged and less well ordered, but no virus-infected cells were detected in the dermis. rOka47D-N was detected in a single hair follicle on the dermal side of the basement membrane (Fig. 4).
FIG.4.
Immunohistologic staining of skin xenografts. Paraffin sections of xenografts collected at days 14, 21, and 28 were stained with human polyclonal VZV antiserum to visualize skin lesions (×10 magnification). Xenograft infected with rOka shows extended lesions, dissolution of the basement membrane layer (arrowhead), and infected hair follicles at days 21 (arrow) and 28. Xenografts infected with rOka47ΔC (ΔC) and rOka47D-N (D-N) show smaller lesions, the basement membrane remained intact (arrowheads, ΔC [day 14] and D-N [day 21]), and hair follicles were not infected (arrow, D-N). One single hair follicle shows infection with rOka47D-N at day 28. Xenografts infected with rOka47P-S (P-S) have larger lesions and show dissolution of the basement membrane. Multiple hair follicles were infected (arrow, P-S [day 21]).
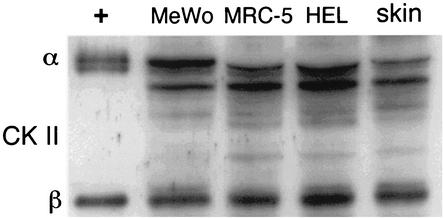
Since CKII can complement ORF47 kinase function, we examined whether the impaired replication of rOka47ΔC and rOka47D-N in skin xenografts was related to a difference in CKII expression in skin cells versus cells grown in cultures in vitro. When CKII was assessed by Western blot analysis of uninfected skin xenografts, uninfected melanoma cells, and human lung fibroblasts (HEL and MRC-5 cells), CKII detection was equivalent in lysates of skin cells and cells grown in cultures (Fig. 5).
FIG. 5.
Expression of CKII in cell cultures and SCID-hu skin xenografts. Lane 1, CKII α and β chain (Upstate Biotechnology, Inc.) (+); lane 2, melanoma cells (MeWo); lane 3, MRC-5 cells; lane 4, HEL cells; lane 5, skin xenograft cells. Lane 1 was used as positive control. All other lanes showed similar levels of CKII expression.
Immunoprecipitation and ORF47 kinase experiments.
Based upon the observed differences in the pathogenicity of rOka47 mutants in vivo, further investigations of ORF47 kinase function and ORF47 protein binding to IE62 protein were done in melanoma cells infected with the rOka47ΔC, rOka47D-N, and rOka47P-S mutants in vitro. A series of immunoprecipitation and kinase assays demonstrated that the binding of ORF47 and IE62 proteins was mediated by the N-terminal region of the ORF47 protein, whereas phosphorylation depended upon the kinase domain located in the C-terminal region and, specifically, on the DYS motif at residues 282 to 284.
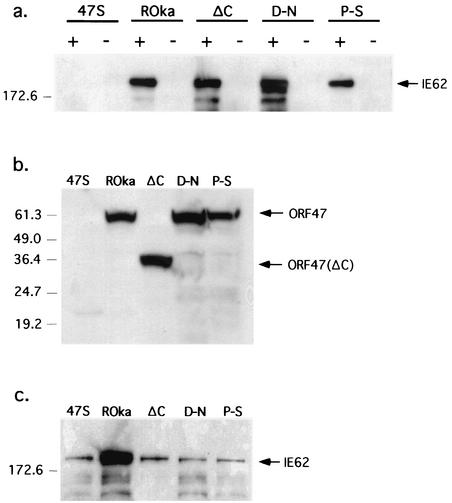
Melanoma cells were infected with ROka47S, rOka, rOka47ΔC, rOka47D-N, or rOka47P-S, cell lysates were prepared, and ORF47 protein and associated proteins were precipitated with ORF47 antiserum. Complex formation between ORF47 and IE62 proteins was detected by Western blotting in cells infected with rOka, rOka47ΔC, rOka47D-N, and rOka47P-S (Fig. 6a). No complex formation occurred in cells infected with ROka47S, a recombinant virus that expresses only the first 165 amino acids of ORF47 protein due to the insertion of a STOP codon (8); ROka47S (kindly provided by Jeffrey Cohen, National Institutes of Health) was used as a negative control. Expression of the full-length ORF47 kinases and the ΔC-truncated protein was documented as a 30-kDa band (Fig. 6b), and testing for IE62 protein expression confirmed equivalent infection levels in all lysates (Fig. 6c). ORF47 protein binding to IE62 protein was preserved in both point mutants and the truncation mutant but was not observed with the ROka47S control.
FIG. 6.
Immunoprecipitation of IE62 protein with ORF47 antiserum from melanoma cells infected with rOka or rOka ORF47 mutants. (a) Melanoma cells were infected with ROka47S (47S), rOka, rOka47ΔC (ΔC), rOka47D-N (D-N), or rOka47P-S (P-S). Infected cell lysates were incubated with ORF47 antiserum (+) or preimmune serum (−) and subjected to SDS-PAGE. A Western blot filter was probed with IE62 antiserum. A band slightly above the 172.6-kDa marker (which corresponds to the 175-kDa weight of IE62) was detected in lanes rOka, ΔC, D-N, and P-S (containing the ORF47 antiserum) but not in the lanes containing the ROka47S virus (kindly provided by J. Cohen, National Institutes of Health). (b and c) Western blot of total lysates. As a control, 20-μl portions of the infected cell lysate described above were directly subjected to SDS-PAGE. The filters were probed with ORF47 antiserum (b) or IE62 antiserum (c).
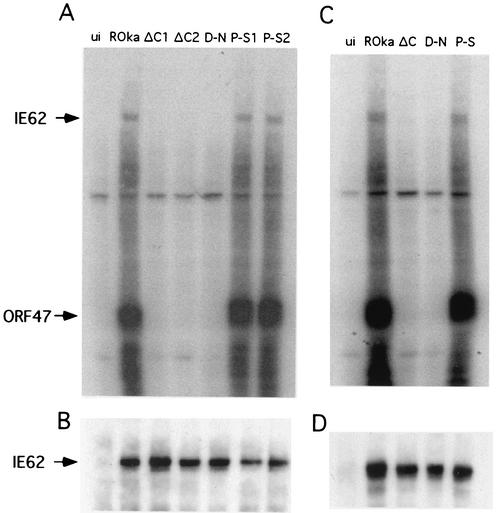
To investigate whether mutant ORF47 proteins phosphorylate IE62 protein and autophosphorylate, melanoma cells were infected with rOka, rOka47ΔC, rOka47D-N, and rOka47P-S; cell lysates were then immunoprecipitated with ORF47 antiserum and evaluated by in vitro kinase assays. The intact ORF47 protein from rOka-infected cells and the ORF47P-S protein phosphorylated IE62 protein and autophosphorylated in vitro, whereas the ORF47ΔC and ORF47D-N mutant proteins did neither (Fig. 7A). As the second step in these experiments, the filter was probed with IE62 antiserum, again confirming preservation of IE62 protein binding for all of the three mutant forms of ORF47 protein (Fig. 7B).
FIG. 7.
ORF47 protein kinase assay. (A) Melanoma cells were left uninfected (ui) or were infected with rOka, with both rOka47ΔC mutants (ΔC1 and ΔC2), with rOka47D-N (D-N), or with both rOka47P-S mutants (P-S1 and P-S2). A kinase assay was performed, lysates were subjected to SDS-PAGE, and proteins were transferred to membrane and autoradiographed. Phosphorylated bands of IE62 and ORF47 were determined by size. (B) After the radioactive signal decayed, the filter was probed with IE62 antiserum to show equal amounts of IE62 in all samples except uninfected cells. (C) rOka, rOka47ΔC, rOka47D-N, and rOka47P-S were cultured in melanoma cells after 28 days of growth in human skin xenografts in SCID-hu mice. Cell lysates were subjected to kinase assays as described above. (D) IE62 equal binding control.
To document that this functional analysis of ORF47 protein domains was valid and relevant to VZV pathogenesis in vivo, the ORF47 kinase assay was also performed using rOka, rOka47ΔC, rOka47D-N, and rOka47P-S viruses that were recovered 28 days after infection of skin xenografts. These experiments showed that the mutant ORF47 kinases ORF47ΔC and ORF47D-N did not phosphorylate IE62 protein or autophosphorylate, whereas intact ORF47 protein and the ORF47P-S point mutant phosphorylated IE62 and ORF47 proteins (Fig. 7C). This result indicates that the slow-growing rOka47ΔC and rOka47D-N viruses did not acquire any kinase gain-of-function mutation during replication in skin in vivo, which might have restored the capacity of their respective ORF47 proteins to phosphorylate IE62 or to autophosphorylate. ORF47 protein binding to IE62 protein was again demonstrated for all mutants by probing with IE62 monoclonal antibody (Fig. 7D).
Localization of ORF47 and IE62 proteins in infected cells in vitro.
To further evaluate the effects of ORF47 mutations, the intracellular localization of ORF47 protein and IE62 protein was examined at 20, 30, and 42 h after infection of melanoma cells with rOka and rOka47 mutants. As shown in representative examples (Fig. 8), ORF47 protein localized to the cytoplasm and was expressed quite extensively on plasma membranes in cells infected with rOka but was detected in only a few nuclei within syncytia formed by rOka-infected cells. In contrast, ORF47 protein was detected in the nuclei of most cells infected with rOka47ΔC or rOka47D-N as well as in the cytoplasm, indicating nuclear import and retention of ORF47 protein when kinase domains were disrupted. Like intact ORF47 protein, ORF47P-S protein was not detected in nuclei of cells infected with rOka47P-S. These patterns of ORF47 protein distribution were observed at all time points in cells infected with rOka and the rOka47ΔC, rOka47D-N, and rOka47P-S mutants.
FIG.8.
Confocal analysis of melanoma cells infected with rOka and rOka ORF47 mutants for expression of ORF47 and IE62 proteins. Melanoma cells were infected with rOka, rOka47ΔC (ΔC), rOka47D-N (D-N), or rOka47P-S (P-S) for 30 h. ORF47 kinase was detected with a secondary fluorescein isothiocyanate-conjugated antibody (green), and IE62 was detected with a secondary Texas Red-conjugated antibody (red). Images of each row were merged. Colocalizations of ORF47 kinase and IE62 appear as yellow.
The intracellular distribution of IE62 protein was also altered dramatically in cells infected with rOka47ΔC and rOka47D-N, with IE62 protein being almost exclusively nuclear at all time points. In contrast, IE62 protein was present in cytoplasm as well as nuclei at 20, 30, and 42 h after infection with rOka and rOka47P-S (Fig. 8). IE62 protein is also cytoplasmic at these times in cells infected with the ROka47S null mutant (data not shown) (13). Thus, preserving the binding of ORF47 and IE62 proteins while abolishing ORF47 kinase activity was associated with an aberrant nuclear retention of ORF47 and IE62 proteins. The changes in cellular localization of these critical VZV proteins were not associated with altered production of infectious virus in cells infected with rOka47ΔC or rOka47D-N in vitro (Fig. 2), whereas viral replication was significantly reduced in differentiated human skin cells in vivo (Fig. 3).
DISCUSSION
The construction of VZV recombinants with targeted mutations in the ORF47 gene and evaluation of these rOka47 mutants in vitro and in human skin xenografts in vivo indicate that the ORF47 protein has two important and distinct functions, which are kinase activity and IE62 protein binding. Experiments with the ROka47S stop codon mutant demonstrated that ORF47 protein is essential for skin infection (21). Since kinase activity was its defined function, the requirement of ORF47 protein for replication in vivo was attributed to its phosphorylation of viral and (perhaps) cellular protein substrates. We report now that rOka47 mutants with disrupted ORF47 kinase function retain the capacity to replicate in human skin, albeit with a markedly delayed and diminished growth phenotype. Since the ORF47 protein made by rOka47ΔC (which is truncated at 266 amino acids) binds IE62 protein, we suggest that ORF47 protein complex formation with IE62 protein, and perhaps with other proteins, is an essential function. This prediction is consistent with a model of VZV tegument formation in which ORF47 protein binds to IE62 protein (and perhaps to other tegument proteins encoded by ORFs 4, 9, 10, and 63) (reviewed in reference 35). Based on this model, we suggest that the N terminus of the ORF47 protein recruits the IE62 protein to the intracellular site of virion assembly and/or binds to the IE62 protein as a structural component of the tegument. If ORF47 protein were only acting as a kinase, abolishing its capacity to phosphorylate IE62 protein should have made its formation of complexes with IE62 protein irrelevant. In contrast, preserving these interactions was associated with some VZV replication in skin, whereas no infection occurred in the absence of ORF47 protein in vivo. Thus, we conclude that ORF47 protein has a function, mapped to its N terminus and independent of its kinase activity, which is necessary and sufficient for viral growth in vivo, and we suggest that this function involves binding to IE62 protein. Further, defining these two independent contributions of ORF47 protein to VZV pathogenesis required evaluation of ORF47 mutants in vivo in the SCID-hu model, because ORF47 protein is completely dispensable in vitro.
While ORF47 protein kinase activity was not essential in vivo, blocking kinase function severely restricted VZV infectivity in skin xenografts. Constructing VZV recombinant viruses with ORF47 mutations confirmed that kinase activity maps to the C terminus, as indicated in transient expression experiments, and established that the predicted kinase motif, DYS, is necessary for autophosphorylation and phosphorylation of IE62 protein. Most importantly, rOka47D-N experiments demonstrated that the DYS motif is the essential kinase domain required for the characteristic replication of VZV in differentiated human skin cells within the intact tissue microenvironment in vivo. The conserved DFG motif (DYS in ORF47 protein) is invariant in virtually all kinases and is considered necessary for phosphate transfer from ATP to the substrate; ORF47 kinase activity was completely abolished by the D-N substitution. Mutation of the conserved motif, APE (PPE in ORF47 protein), to construct rOka47P-S had no effect on ORF47 kinase function or on VZV infectivity in vivo. Because the role of subdomain VIII appears to be to ensure the stability of protein folding, single residue changes, even within the conserved central motif, may not necessarily generate a protein impaired in kinase activity (5, 6). Our experiments suggest that the proline-serine exchange did not alter the tertiary structure of ORF47 protein significantly. rOka47ΔC expressed a truncated ORF47, which might have retained some kinase function because sequence analysis predicts that the ORF47 kinase domain starts at amino acid 132 (http://smart.embl-heidelberg.de/) (16, 32). However, the core of the kinase domain, containing the putative ATP binding site, resides in the C terminus, and truncated ORF47 protein did not mediate phosphorylation. Of particular interest, whereas CKII compensates for ORF47 kinase activity in vitro (11), little infectious virus was recovered from skin xenografts infected with rOka47ΔC or rOka47D-N, despite the presence of CKII.
The comparative analysis of the consequences of disrupting ORF47 kinase activity on VZV replication in vitro and in skin xenografts in vivo provides new insights about how the ORF47 protein contributes to VZV pathogenesis. Our experiments with rOka47ΔC and rOka47D-N mutants support the accumulating evidence that cell-cell spread of VZV depends upon cell fusion and syncytia formation but does not require complete virion assembly in vitro (11, 13). Conversely, experiments with these mutants in vivo indicate that cell-cell spread in intact human skin is highly dependent upon ORF47 kinase function. Further, based upon the failure to recover any infectious virus from skin xenografts infected with the ROka47S null mutant and upon the recovery of some infectious virus when the capacity of ORF47 protein to bind to IE62 protein was preserved, we suggest that complete virion assembly is required for VZV infection of human skin in vivo.
Our mutational analysis using VZV recombinants confirmed that ORF47 protein phosphorylates IE62 protein, which has been considered to reduce IE62 protein-mediated transactivation in the nucleus at early and late time points and enhance its accumulation at the cytoplasmic site of tegument formation. Experiments with the ROka47S stop codon mutant have shown no difference (13) or some delay in the translocation of IE62 protein from the nucleus to the cytoplasm (11), suggesting that the second VZV serine/threonine kinase (encoded by ORF66) or CKII substitutes to phosphorylate IE62 protein (11, 13). In our experiments, replication was not affected by the persistence of a predominantly nuclear localization of both IE62 and ORF47 proteins in cell cultures infected with rOka47ΔC or rOka47D-N. Nuclear expression of ORF47 protein was rare in rOka-infected syncytia but was quite pronounced in rOka47ΔC- and rOka47D-N-infected cells. The limited transport of IE62 protein from the nucleus to the cytoplasm in cells infected with rOka47ΔC or rOka47D-N may be secondary to increased transfer of nonphosphorylated ORF47 protein into the nucleus, where it binds but does not phosphorylate IE62 protein. Nevertheless, infectious virus yields were not reduced, indicating that only minimal cytoplasmic localization of IE62 and ORF47 proteins, presumed to be mediated by ORF66 kinase or CKII phosphorylation, is necessary for VZV replication in vitro. In contrast, inoculation of skin xenografts with rOka47ΔC or rOka47D-N suggests that such complementation provides very limited support for VZV replication in vivo, probably by allowing some cytoplasmic translocation of IE62 and ORF47 proteins and virion tegument assembly which does not occur in skin in the absence of ORF47 protein. The documentation that rOka47ΔC or rOka47D-N viruses recovered from skin xenografts remained “kinase dead” and did not phosphorylate IE62 protein supports the interpretation that the contribution of ORF47 protein to virion formation was the critical factor in permitting some replication in vivo.
The immediate phosphorylation of other virion tegument proteins, including IE62 protein, after viral entry to facilitate tegument disassembly is another possible function of ORF47 protein. HSV tegument disassembly has been shown to be regulated by phosphorylation of component proteins (24). Even if VZ virions could be assembled despite the blocking of ORF47 kinase activity, phosphorylation by ORF47 protein could be important for tegument disassembly. Cell-cell spread and replication of rOka47ΔC and rOka47D-N would not be affected in vitro, assuming that complete virion assembly is not required. However, slower tegument disassembly when ORF47 protein is made but its kinase activity is abolished could contribute to the delayed and restricted replication of rOka47ΔC and rOka47D-N mutants in differentiated skin cells in vivo.
In addition to its interactions with IE62 protein, both as a phosphorylation substrate and as a tegument component, disruption of ORF47 kinase function may have altered the intracellular trafficking of gE, which would be predicted to impair VZV replication in vivo. Kenyon et al. showed that ORF47 protein targets serines in the acidic cluster of gE for phosphorylation and causes gE recycling from plasma membranes to the trans-Golgi network (TGN), whereas CKII preferentially phosphorylates threonines and enhances gE association with plasma membranes (11). Trafficking of gE to the TGN, the putative site of virion assembly, was reduced substantially in cells infected with the ROka47S mutant (11), but increased trafficking of gE to plasma membranes enhanced syncytium formation and cell-cell spread, and infectious virus yields were not affected in vitro. Nevertheless, some low-affinity phosphorylation of serines in the gE acid cluster was mediated by CKII in ROka47S-infected cells and some gE localization to the TGN was observed (11). Given the presence of CKII in skin cells, these observations are consistent with the limited replication of rOka47ΔC and rOka47D-N in vivo, despite the blocking of ORF47 kinase function.
The VZV ORF47 protein is representative of homologous gene products that are conserved in all of the herpesviruses (3, 31). This evaluation of VZV recombinants with targeted mutations in ORF47 protein for effects on VZV replication in human skin in vivo in the SCID-hu model of VZV pathogenesis suggests that ORF47 protein and related gene products are likely to be important as both virion components and viral kinases.
Acknowledgments
This work was supported by grants AI20459 and AI36884 (A.M.A.) from the National Institute of Allergy and Infectious Diseases and by Developmental and Neonatal Biology Training grant T32 HD07249 (J.B.).
REFERENCES
- 1.Arvin, A. M. 2001. Varicella-zoster virus, p. 2731-2767. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott, Philadelphia, Pa.
- 2.Bryant, D., and J. T. Parsons. 1983. Site-directed point mutation in the src gene of Rous sarcoma virus results in an inactive src gene product. J. Virol. 45:1211-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen, J. I., and S. E. Straus. 2001. Varicella-zoster virus and its replication, p. 2707-2730. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott, Philadelphia, Pa.
- 4.Grose, C. 1981. Variation on a theme by Fenner: the pathogenesis of chickenpox. Pediatrics 68:735-737. [PubMed] [Google Scholar]
- 5.Hanks, S. K., and T. Hunter. 1995. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576-596. [PubMed] [Google Scholar]
- 6.Hanks, S. K., A. M. Quinn, and T. Hunter. 1988. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241:42-52. [DOI] [PubMed] [Google Scholar]
- 7.Heineman, T. C., and J. I. Cohen. 1994. Deletion of the varicella-zoster virus large subunit of ribonucleotide reductase impairs growth of virus in vitro. J. Virol. 68:3317-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heineman, T. C., and J. I. Cohen. 1995. The varicella-zoster virus (VZV) open reading frame 47 (ORF47) protein kinase is dispensable for viral replication and is not required for phosphorylation of ORF63 protein, the VZV homolog of herpes simplex virus ICP22. J. Virol. 69:7367-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inchauspe, G., S. Nagpal, and J. M. Ostrove. 1989. Mapping of two varicella-zoster virus-encoded genes that activate the expression of viral early and late genes. Virology 173:700-709. [DOI] [PubMed] [Google Scholar]
- 10.Kemble, G. W., P. Annunziato, O. Lungu, R. E. Winter, T. A. Cha, S. J. Silverstein, and R. R. Spaete. 2000. Open reading frame S/L of varicella-zoster virus encodes a cytoplasmic protein expressed in infected cells. J. Virol. 74:11311-11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenyon, T. K., J. I. Cohen, and C. Grose. 2002. Phosphorylation by the varicella-zoster virus ORF47 protein serine kinase determines whether endocytosed viral gE traffics to the trans-Golgi network or recycles to the cell membrane. J. Virol. 76:10980-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenyon, T. K., J. Lynch, J. Hay, W. Ruyechan, and C. Grose. 2001. Varicella-zoster virus ORF47 protein serine kinase: characterization of a cloned, biologically active phosphotransferase and two viral substrates, ORF62 and ORF63. J. Virol. 75:8854-8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinchington, P. R., K. Fite, and S. E. Turse. 2000. Nuclear accumulation of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, is inhibited by phosphorylation mediated by the VZV open reading frame 66 protein kinase. J. Virol. 74:2265-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinchington, P. R., J. K. Hougland, A. M. Arvin, W. T. Ruyechan, and J. Hay. 1992. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J. Virol. 66:359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ku, C. C., J. A. Padilla, C. Grose, E. C. Butcher, and A. M. Arvin. 2002. Tropism of varicella-zoster virus for human tonsillar CD4+ T lymphocytes that express activation, memory, and skin homing markers. J. Virol. 76:11425-11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Letunic, I., L. Goodstadt, N. J. Dickens, T. Doerks, J. Schultz, R. Mott, F. Ciccarelli, R. R. Copley, C. P. Ponting, and P. Bork. 2002. Recent improvements to the SMART domain-based sequence annotation resource. Nucleic Acids Res. 30:242-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallory, S., M. Sommer, and A. M. Arvin. 1998. Analysis of the glycoproteins I and E of varicella-zoster virus (VZV) using deletional mutations of VZV cosmids. J. Infect. Dis. 178(Suppl. 1):S22-S26. [DOI] [PubMed] [Google Scholar]
- 18.Mo, C., E. E. Schneeberger, and A. M. Arvin. 2000. Glycoprotein E of varicella-zoster virus enhances cell-cell contact in polarized epithelial cells. J. Virol. 74:11377-11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moffat, J. F., M. D. Stein, H. Kaneshima, and A. M. Arvin. 1995. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J. Virol. 69:5236-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moffat, J. F., L. Zerboni, P. R. Kinchington, C. Grose, H. Kaneshima, and A. M. Arvin. 1998. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 72:965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moffat, J. F., L. Zerboni, M. H. Sommer, T. C. Heineman, J. I. Cohen, H. Kaneshima, and A. M. Arvin. 1998. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc. Natl. Acad. Sci. USA 95:11969-11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moran, M. F., C. A. Koch, I. Sadowski, and T. Pawson. 1988. Mutational analysis of a phosphotransfer motif essential for v-fps tyrosine kinase activity. Oncogene 3:665-672. [PubMed] [Google Scholar]
- 23.Moriuchi, M., H. Moriuchi, S. E. Straus, and J. I. Cohen. 1994. Varicella-zoster virus (VZV) virion-associated transactivator open reading frame 62 protein enhances the infectivity of VZV DNA. Virology 200:297-300. [DOI] [PubMed] [Google Scholar]
- 24.Morrison, E. E., Y. F. Wang, and D. M. Meredith. 1998. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J. Virol. 72:7108-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng, T. I., and C. Grose. 1992. Serine protein kinase associated with varicella-zoster virus ORF 47. Virology 191:9-18. [DOI] [PubMed] [Google Scholar]
- 26.Ng, T. I., L. Keenan, P. R. Kinchington, and C. Grose. 1994. Phosphorylation of varicella-zoster virus open reading frame (ORF) 62 regulatory product by viral ORF 47-associated protein kinase. J. Virol. 68:1350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Niizuma, T., L. Zerboni, M. H. Sommer, H. Ito, S. Hinchliffe, and A. M. Arvin. 2003. Construction of varicella-zoster virus recombinants from parent Oka cosmids and demonstration that ORF65 protein is dispensable for infection of human skin and T cells in the SCID-hu mouse model. J. Virol. 77:6062-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overton, H. A., D. J. McMillan, L. S. Klavinskis, L. Hope, A. J. Ritchie, and P. Wong-Kai-In. 1992. Herpes simplex virus type 1 gene UL13 encodes a phosphoprotein that is a component of the virion. Virology 190:184-192. [DOI] [PubMed] [Google Scholar]
- 28.Perera, L. P., J. D. Mosca, W. Ruyechan, and J. Hay. 1992. Regulation of varicella-zoster virus gene expression in human T lymphocytes. J. Virol. 66:5298-5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perera, L. P., J. D. Mosca, W. T. Ruyechan, G. S. Hayward, S. E. Straus, and J. Hay. 1993. A major transactivator of varicella-zoster virus, the immediate-early protein IE62, contains a potent N-terminal activation domain. J. Virol. 67:4474-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy, S. M., E. Cox, I. Iofin, W. Soong, and J. I. Cohen. 1998. Varicella-zoster virus (VZV) ORF32 encodes a phosphoprotein that is posttranslationally modified by the VZV ORF47 protein kinase. J. Virol. 72:8083-8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott, Philadelphia, Pa.
- 32.Schultz, J., F. Milpetz, P. Bork, and C. P. Ponting. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 95:5857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, D. B., and L. M. Corcoran. 1987. Expression and purification of glutathione-S-transferase fusion proteins, p. 16.17.11-16.17.15. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. John Wiley & Sons, Inc., Boston, Mass. [DOI] [PubMed]
- 34.Smith, R. F., and T. F. Smith. 1989. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J. Virol. 63:450-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spengler, M., N. Niesen, C. Grose, W. T. Ruyechan, and J. Hay. 2001. Interactions among structural proteins of varicella zoster virus. Arch. Virol. Suppl. 17:71-79. [DOI] [PubMed] [Google Scholar]
- 36.Tyler, J. K., and R. D. Everett. 1993. The DNA binding domain of the varicella-zoster virus gene 62 protein interacts with multiple sequences which are similar to the binding site of the related protein of herpes simplex virus type 1. Nucleic Acids Res. 21:513-522. [DOI] [PMC free article] [PubMed] [Google Scholar]