Abstract
The destruction of the immune system by progressive loss of CD4 T cells is the hallmark of AIDS. CCR5-dependent (R5) human immunodeficiency virus type 1 (HIV-1) isolates predominate in the early, asymptomatic stages of HIV-1 infection, while CXCR4-dependent (X4) isolates typically emerge at later stages, frequently coinciding with a rapid decline in CD4 T cells. Lymphocyte killing in vivo primarily occurs through apoptosis, but the importance of apoptosis of HIV-1-infected cells relative to apoptosis of uninfected bystander cells is controversial. Here we show that in human lymphoid tissues ex vivo, apoptosis of uninfected bystander CD4 T cells is a major mechanism of lymphocyte depletion caused by X4 HIV-1 strains but is only a minor mechanism of depletion by R5 strains. Further, X4 HIV-1-induced bystander apoptosis requires the interaction of the viral envelope glycoprotein gp120 with the CXCR4 coreceptor on CD4 T cells. These results emphasize the contribution of bystander apoptosis to HIV-1 cytotoxicity and suggest that in association with a coreceptor switch in HIV disease, T-cell killing evolves from an infection-restricted stage to generalized toxicity that involves a high degree of bystander apoptosis.
Gradual depletion of CD4 T cells is the hallmark of disease progression in AIDS (14). Both decreased thymic production and increased destruction of CD4 T cells are thought to play a role in CD4 depletion (25, 37). In vivo and in vitro data have shown that CXCR4-dependent (X4) human immunodeficiency virus type 1 (HIV-1) strains cause more pronounced depletion of CD4 T cells than CCR5-dependent (R5) strains (11, 23, 24, 29, 32, 33, 41, 44, 45). The higher cytopathogenicity of X4 strains is due at least in part to their wider range of susceptible target cells (11, 23, 24, 29, 32, 33, 41, 44, 45). CXCR4 is expressed on nearly all CD4 T lymphocytes, whereas only about 15 to 30% express detectable levels of CCR5 on the cell surface (7, 23).
Apoptosis of CD4 T cells has been proposed as a key mechanism underlying CD4 T-cell depletion in vivo (1, 34, 47), but the relative contributions of HIV-1-induced killing of productively infected cells as opposed to uninfected bystander cells remain highly controversial (8, 15, 17, 27, 39). To study HIV-1-induced cell death, we performed infections of ex vivo human lymphoid tissue cultures. This highly relevant system is permissive for HIV-1 infection independent of exogenous stimulation by mitogens or interleukin-2 and maintains its natural cytokine milieu, cellular activation status, and cell-to-cell interactions (19). Critically, these are all factors which have been shown to be crucial for the determination of HIV-1-induced cell death (10, 21, 26, 30, 35, 42).
We investigated the relative contributions of apoptosis in productively infected cells and apoptosis of uninfected bystander CD4 T cells to overall lymphocyte depletion. We observed massive HIV-1-induced apoptosis of bystander CD4 T cells following infections with X4 viruses but detected little bystander killing following infection with R5 viruses. Furthermore, bystander killing was critically dependent on the interaction of X4 gp120 with the chemokine receptor CXCR4 on CD4 T cells. These results provide important insights regarding the mechanism underlying the higher virulence of X4 viruses in vivo.
MATERIALS AND METHODS
Preparation of viral stocks.
NL4-3 was a gift from Malcolm Martin, and LAI was a gift from Keith Peden; both were obtained via the AIDS Research and Reference Program, Division of AIDS, National Institute of Allergy and Diseases, National Institutes of Health. The molecular clone 49-5 was a gift from Bruce Chesebro. The molecular clones LAI, NL4-3, and 49-5 were expanded by standard calcium phosphate transfection of 293T cells as described earlier (20). The primary isolates 1/85 and 7/86 were gifts from Ruth Connor (11) and were expanded by infection of heterologous peripheral blood mononuclear cells (PBMC). PBMC from four different donors were isolated from whole-blood buffy coats (Stanford Blood Bank, Palo Alto, Calif.) by Ficoll-Histopaque density gradient centrifugation (Histopaque 1077; Sigma, St. Louis, Mo.) and cultured for 48 h in medium (RPMI 1640; Mediatech, Washington, D.C.) supplemented with 10% fetal bovine serum (FBS) (Gemini Biological Products) and phytohemagglutinin (1 μg/ml) (Sigma). The PBMC were then washed and cultured in RPMI medium with 10% FBS and recombinant human interleukin-2 (5 U/ml) (Roche Molecular Biochemicals, Indianapolis, Ind.) for 2 days longer before they were infected overnight with HIV-1 at a multiplicity of infection of 0.05. Virus-containing supernatant was collected, and the cell number was adjusted to 2 × 106/ml at 3-day intervals. Production of virus was determined with an anti-p24 enzyme-linked immunosorbent assay (ELISA) (NEN, Boston, Mass.).
Culture and infection of human lymphoid tissues ex vivo.
Human noninflammatory tonsil tissue (provided by the National Disease Research Interchange [Philadelphia, Pa.] or the Kaiser Hospitals in San Francisco, South San Francisco, and San Rafael, Calif.) was removed during routine tonsillectomy. To prepare dispersed human lymphoid ex vivo cultures, tonsil tissue was mechanically dispersed by cutting tissue in 2- to 3-mm blocks and passing them through 70- and 40-μm cell strainers. Cells were washed in phosphate-buffered saline, and 2 × 106 isolated cells were plated in 96-well U-bottom plates in a final volume of 200 μl. Culture medium (RPMI 1640 supplemented with 15% FBS, 1% nonessential amino acids [Mediatech], 1 mM sodium pyruvate [Mediatech], 2 mM l-glutamine [Invitrogen, Carlsbad, Calif.], 2.5 U of amphotericin B [Invitrogen] per ml, 100 μg of gentamicin [Invitrogen] per ml, and 100 μg of ampicillin [Sigma] per ml) was changed every 3 days without dispersing the pellet. Human lymphoid tissue blocks were prepared as described previously (13, 19, 23, 24, 32, 41, 44). Within 24 h of preparation tissue blocks were inoculated with HIV-1 at 60 50% tissue culture infective doses (TCID50) per block, and dispersed lymphoid cultures were infected at 80 TCID50 per well. The TCID50 was determined by terminal dilution of the virus stocks in quadruplicate on heterologous PBMC (41).
Assessment of CD4 depletion and measurement of apoptosis.
CD4 depletion and apoptosis with annexin V-staining, activation of caspase-3, or the breakdown of the mitochondrial membrane potential were determined as described previously (29). To analyze apoptosis and infection of cells simultaneously, cells were first stained with annexin V-phycoerythrin, washed extensively, fixed for 30 min at room temperature in 1% paraformaldehyde, washed again extensively, and stained with fluorescein isothiocyanate (FITC)-conjugated antibody directed to p24 (clone KC57; Beckman Coulter, Miami, Fla.) and the appropriate surface markers in 0.1% saponin. Determination of caspase-3 activation combined with simultaneous p24 assessment was done with the CaspaTag caspase activity kit (Intergen, Purchase, N.Y.). Briefly, cells were incubated at 37°C for 1 h in FAM-peptide-FMK solution, fixed, and stained with monoclonal antibodies directed to CD4, CD3, and p24 as described above.
Isolation and coculture of CD4-enriched, CFSE-labeled cells.
To isolate CD4-enriched cells from uninfected human ex vivo lymphoid cultures, cultures were stained with FITC-conjugated antibodies directed to CD8, CD19, and CD14 (all from Becton Dickinson) and incubated with anti-FITC MicroBeads (Miltenyi Biotec, Bergisch-Gladbach, Germany). CD4 cells were enriched by using the depletion mode on an autoMACS bead sorter (Miltenyi Biotec). CD8− CD14− CD19− cells were stained with 10 μM 5 (and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes) for 15 min at 37°C. A total of 150,000 CFSE-positive cells (more than 90% CD4 T cells) were pretreated for at least 4 h at 37°C with 250 nM AMD3100, 5 μM zidovudine (AZT) (Sigma), or a 50-μg/ml concentration of one of the following antibodies: anti-CXCR4 (clone 12G5) (Becton Dickinson), anti-CCR5 (clone 2D7) (Becton Dickinson), or anti-gp120 (clone 1101) (Immunodiagnostics, Woburn, Mass.). In parallel, human ex vivo lymphoid cultures were infected with 200 TCID50 of the indicated virus per well and cultured for 6 days. On day 6, viral replication was assessed by using an anti-p24 ELISA. Pretreated, CFSE-labeled, uninfected cells were then added to the respective infected human ex vivo lymphoid cultures containing about 500,000 CD4 T cells in 2 × 106 total cells. The medium was changed every 3 days and supplemented with the indicated inhibitor. At 6 days after the start of the coculture, apoptosis in CFSE-positive CD4 T cells was measured by using annexin V staining. An aliquot of these cells was used to determine infection by intracellular p24 staining.
Preparation of culture supernatants and transfer to uninfected ex vivo human lymphoid cultures.
Human ex vivo lymphoid cultures were infected with 200 TCID50 of the indicated viral strain per well. At days 6 and 9 after infection, supernatants of the infected cultures were harvested, centrifuged at 2,300 × g for 10 min at 4°C, and sterile filtered to remove all contaminating cells. Cleared supernatants were diluted 1:1 with fresh medium, and the p24 concentration was determined by using an anti-p24 ELISA and adjusted to 45 ng/ml. Parallel uninfected cultures of the same donor were pretreated for 4 h with 5 μM AZT, and the medium was than removed and replaced with diluted supernatants. Cells were cultured for an additional 6 days in the presence or absence of AZT. At 6 days after the initial supernatant transfer, apoptosis in the CD3+ CD8+ and CD3+ CD8− cells was assessed by annexin V staining. An aliquot of these cells was used to determine infection by intracellular p24 staining.
In situ hybridization and TUNEL staining.
Ex vivo human lymphoid cultures were harvested 8 days after infection, fixed for 30 min at room temperature with 1% paraformaldehyde, and stained with FITC-labeled anti-CD3 antibody. CD3+ cells were isolated as described above, pelleted, and resuspended in 2.5% agarose. After embedding of the cell clots in paraffin, sample sections were subjected to HIV-1 RNA in situ hybridization as described previously (16). The terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay was performed with the Apotag peroxidase kit (Intergen). Tissue sections were deparaffinized in xylene, hydrated, and pretreated with proteinase K (Dako, Carpinteria, Calif.) at room temperature for 25 min. The terminal deoxynucleotidyl transferase enzyme was diluted 1:10 in reaction buffer and applied to the sections for 1 h at 37°C in a humid chamber. Detection was performed with an antidigoxigenin conjugate and a diaminobenzidine peroxidase substrate (Sigma). Slides were counterstained with 25% hematoxylin (Shandon, Pittsburgh, Pa.) and dehydrated, and cover slips were applied with Permount (Fisher, Pittsburgh, Pa.).
RESULTS
Apoptosis and depletion of CD4 T cells by X4 HIV-1 strains.
Apoptosis likely represents an important molecular mechanism underlying CD4 T-cell destruction (1, 15, 38, 47). To quantify levels of apoptosis in dispersed ex vivo human lymphoid cultures, we monitored several established markers: accessibility of phosphatidylserine to annexin V at the cell surface, activation of caspase-3, and the breakdown of the mitochondrial membrane potential.
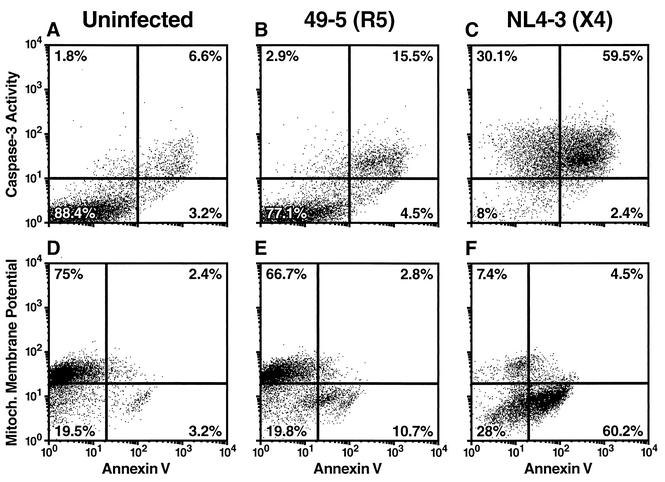
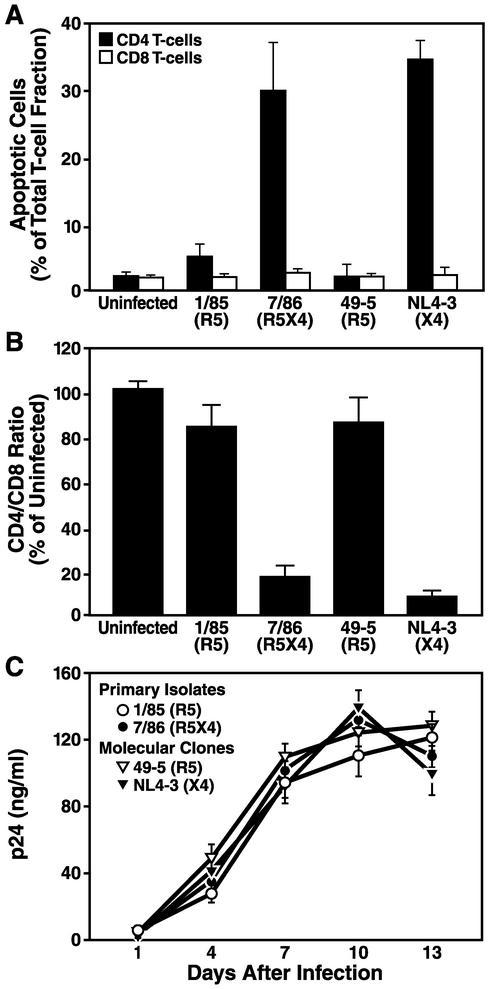
Infection of ex vivo human lymphoid cultures with the prototypic X4 molecular clone NL4-3 greatly increased the number of cells displaying apoptotic markers (Fig. 1C and F) compared to uninfected control cultures (Fig. 1A and D). In striking contrast, after infection with the R5 molecular clone 49-5, which differs from NL4-3 only in the coreceptor-determining V3 loop of gp120 (48), only a minority of CD4 T cells showed these apoptotic markers (Fig. 1B and E). Consistent with the effects seen for these two molecular clones, an R5X4 primary isolate (7/86), but not an R5 primary isolate (1/85) derived from the same patient (11), induced widespread apoptosis of CD4 T cells (Fig. 2A). Apoptosis of CD4 T cells in the context of X4 infections of human dispersed lymphoid cultures ex vivo was followed by marked depletion of CD4 T cells (Fig. 2B). In contrast, R5 strains caused only mild depletion relative to uninfected controls. Notably, R5 and X4 strains replicated with comparable kinetics (Fig. 2C). Similar results were observed in experiments using human lymphoid tissue blocks (data not shown). It is noteworthy that neither the R5 nor the X4 HIV-1 strains caused significant apoptosis of CD8 T cells (Fig. 2A) or B cells (data not shown) in human lymphoid histocultures.
FIG. 1.
X4 but not R5 HIV-1 induces massive apoptosis among CD4 T cells. Human lymphoid cultures ex vivo were infected for 12 days with the R5 viral strain 49-5 (B and E) or the X4 viral strain NL4-3 (C and F) or kept uninfected (A and D). Apoptosis of CD4 T cells was determined independently by annexin V staining (A to F), caspase-3 activity (A to C), and depolarization of the mitochondrial membrane (D to F). Presented are flow cytometry dot plots from a typical experiment from among three independent experiments using different donor tissues.
FIG. 2.
X4 but not R5 HIV-1 induces pronounced apoptosis and depletion among CD4 T cells. Human lymphoid cultures ex vivo were infected with the R5 strain 1/85 or 49-5, the R5X4 strain 7/86, or the X4 strain NL4-3 or kept uninfected. (A) At day 12, apoptosis among CD4 and CD8 T cells was determined by using annexin V staining. (B) CD4 T-cell depletion displayed as CD4/CD8 ratio. (C) Viral replication was monitored in the same infections by assessing the p24 concentration in the culture medium at days 1, 4, 7, 10, and 13 after infection, using an anti-p24 ELISA. Shown are the mean values (n = 3) with standard errors of the means from a representative experiment from among six experiments with different donor tissue.
Extensive apoptosis of bystander CD4 T cells by X4 HIV-1.
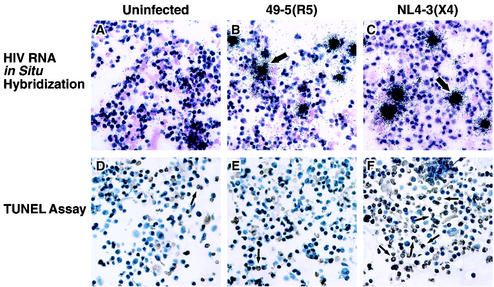
To dissect the possible contributions of infection-dependent apoptosis and bystander apoptosis to CD4 T-cell depletion in human lymphoid cultures ex vivo, we analyzed markers for HIV-1 infection and apoptosis in parallel. First, we scored productive infection by in situ hybridization for viral RNA transcripts (16) (Fig. 3A to C) and measured apoptosis by labeling DNA breaks by using the TUNEL method (Fig. 3D to F). Cultures inoculated with the R5 strain 49-5 showed a low level of apoptosis and productive infection (Fig. 3B and E). In striking contrast, samples infected with the X4 strain NL4-3 exhibited widespread apoptosis (Fig. 3F), while the number of productively infected T cells was low and comparable to those in samples infected with 49-5 (compare Fig. 3B and C). Indeed, the number of apoptotic cells in NL4-3-infected tissue exceeded by far the number of productively infected cells (Fig. 3C and F), thereby suggesting that most of the apoptotic cells in X4 HIV-1-infected cultures were not productively infected.
FIG. 3.
Apoptosis exceeds by far the infection level in NL4-3-infected lymphoid cultures ex vivo. CD3+ T cells were isolated from human lymphoid cultures ex vivo infected for 7 days with 49-5 (B and E) or NL4-3 (C and F) or from uninfected controls (A and D). (A to C) Viral infection was determined by HIV-1 RNA in situ hybridization. Representative infected cells are indicated by arrows. (D to F) Apoptosis was determined in parallel samples by using the TUNEL assay. Apoptotic cells are characterized by brown staining, and representative apoptotic cells are indicated by arrows.
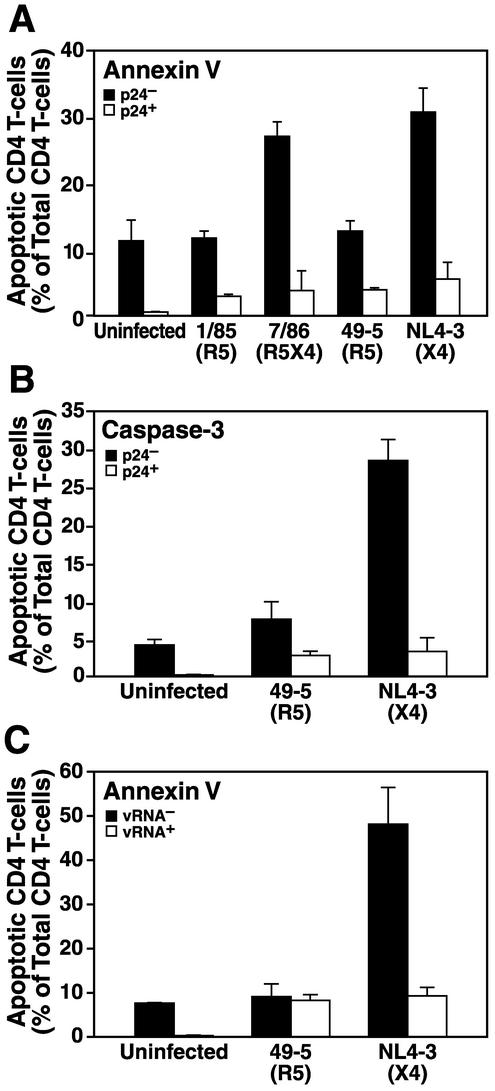
To discriminate unambiguously between apoptosis of productively infected and bystander cells, it is crucial to analyze simultaneously apoptosis and infection on a single-cell level. Therefore, we performed flow cytometric analysis of samples costained for intracellular HIV-1 p24 capsid antigen, to monitor productive infection, and for annexin V binding or caspase-3 activation, two sensitive markers for apoptosis. Infection of human lymphoid cells ex vivo with either R5 or X4 strains resulted in low numbers of productively infected (p24-positive) apoptotic CD4 T cells (Fig. 4A and B). In contrast, infection with the X4 strains NL4-3 and 7/86, but not the R5 strains 49-5 and 1/85, induced high levels of apoptosis among bystander (p24-negative) CD4 T cells (Fig. 4A and B). Furthermore, the majority of apoptotic cells induced by X4 strains in these cultures were observed in the p24-negative fraction. Infection of human lymphoid tissue blocks rather than dispersed cultures yielded indistinguishable results (data not shown). We confirmed these results by using a flow cytometry-based approach to identify infected cells by fluorescence in situ hybridization with a cocktail of fluorescently labeled probes directed against the HIV-1 gag and pol transcripts (40) (Fig. 4C). Collectively, these results strongly support the hypothesis that X4 viruses have the ability to induce apoptosis among a large fraction of bystander CD4 T cells. These experiments, however, could not exclude the possibility that infected cells in an early phase of the replication cycle may be triggered to undergo apoptosis, since HIV-1 infection was scored based on viral markers (gag and pol) that are expressed only late in the viral life cycle.
FIG. 4.
X4 but not R5 strains induce extensive apoptosis in bystander CD4 T cells. Human lymphoid cultures ex vivo were infected for 7 days with the R5 strain 1/85 or 49-5, the R5X4 strain 7/86, the X4 strain NL4-3, or kept uninfected. Apoptosis in productively infected and uninfected CD4 T cells was determined by either annexin V staining (A and C) or caspase-3 activity (B). Intracellular p24 (A and B) or viral RNA (C) was used to determine viral infection. Error bars indicate standard errors of the means.
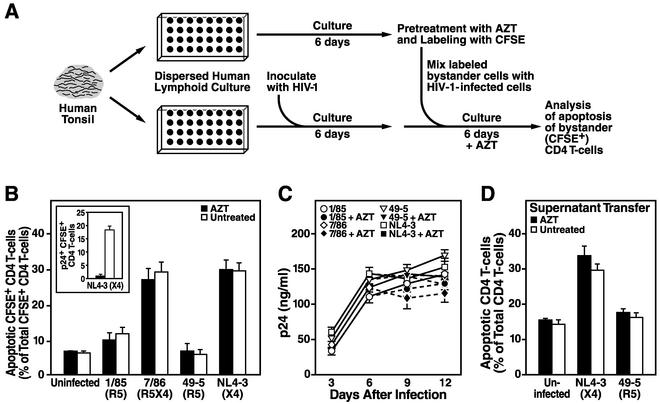
To address this question, we designed an experimental strategy that allowed us to study the fate of bystander CD4 T cells in the context of an infected human lymphoid culture in an unambiguous fashion (Fig. 5A). First, dispersed human lymphoid cultures were challenged with HIV-1 and cultivated for 6 days, thereby allowing for a substantial infection to develop (Fig. 5C). Second, CD4 T cells were isolated from uninfected cultures of the same donor, treated with the viral reverse transcriptase inhibitor AZT, and labeled with the fluorescent dye CFSE (Fig. 5A). A total of 150,000 of these uninfected bystander CD4 T cells were then added to the infected cultures and cocultured in the continuous presence of AZT to prevent their infection. Since AZT did not block viral output from the previously infected cells from the primary culture, the level of viral p24 antigen in these remixed cultures remained high and comparable to that in cultures not treated with AZT (Fig. 5C).
FIG. 5.
Bystander CD4 T cells undergo apoptosis when cocultured with X4 HIV-infected cells or upon exposure to cell-free supernatants from X4 HIV-infected cultures. (A) Schematic representation of the experimental setup. CD4 T cells were isolated from uninfected ex vivo human lymphoid cultures and labeled with CFSE. A total of 150,000 CFSE-labeled CD4-enriched cells (more than 90% CD4 T cells) were added to human lymphoid cultures ex vivo from the same donor, which had been infected for 6 days with the indicated HIV-1 strains. Cells were cultured in the presence or absence of the viral reverse transcriptase inhibitor AZT (5 μM) for 6 days longer. The medium was changed every 3 days, and AZT was readded to the respective cultures. (B) After 6 days of coculture, apoptosis in CFSE-positive CD4 T cells was determined by using annexin V staining. In parallel, infection of the CFSE-positive CD4 T cells was analyzed by intracellular p24 staining (inset). (C) Viral replication was monitored in the same cultures by assessing the p24 concentration in the culture supernatant at days 3, 6, 9, and 12 after infection by using an anti-p24 ELISA. AZT was added at days 6 and 9 to the indicated samples. Shown are the mean values (n = 3) with standard errors of the means from a representative experiment from among at least three experiments with different donor tissue. (D) Culture supernatants of human lymphoid cultures ex vivo infected with the R5 strain 49-5 or the X4 strain NL4-3 were collected at days 6 and 9, sterile filtered, and diluted 1:1 with fresh medium. The concentration of p24 in the diluted supernatants was determined by using an anti-p24 ELISA and adjusted to 45 ng/ml, and the supernatants were added to uninfected cultures of cells from the same donor. The cells were cultured for an additional 6 days in the presence or absence of 5 μM AZT, and apoptosis was determined by using annexin V staining. Shown are the mean values (n = 3) with standard errors of the means from a representative experiment from among two experiments with different donor tissue.
After 6 days of coculture, levels of apoptosis in the CFSE-positive CD4 T cells were determined. Regardless of the absence or continuous presence of AZT, a large fraction of CFSE-positive cells within X4-infected but not R5-infected cultures exhibited apoptosis (Fig. 5B). As a positive control for the antiviral potency of AZT in these experiments, its presence completely protected CFSE-positive CD4 T cells from becoming productively infected over the course of the 6-day observation period following remixing (Fig. 5B [inset] and data not shown).
In a similar experiment, we tested whether supernatants from R5 or X4 HIV-infected cultures that were transferred to uninfected parallel cultures can cause apoptosis in the uninfected cells. Six days of culture of uninfected cells in the presence of supernatant derived from cultures infected with the X4 virus NL4-3 resulted in increased apoptosis in these cells regardless of the presence or absence of AZT (Fig. 5D). In contrast, supernatant from cultures infected with the R5 virus 49-5 had no significant effect on the uninfected cells (Fig. 5D).
These results demonstrate that the unique ability of an X4 virus infection to induce widespread apoptosis in human lymphoid histoculture is not dependent on the productive infection of these CD4 T cells, since a reverse transcriptase inhibitor did not protect the majority of cells from undergoing apoptosis in this context despite potent inhibition of viral spread. Furthermore, the supernatant transfer experiment shows that direct contact of the bystander cells with the infected cells is not required for X4 HIV-induced bystander apoptosis.
Mechanism of bystander apoptosis by X4 HIV-1.
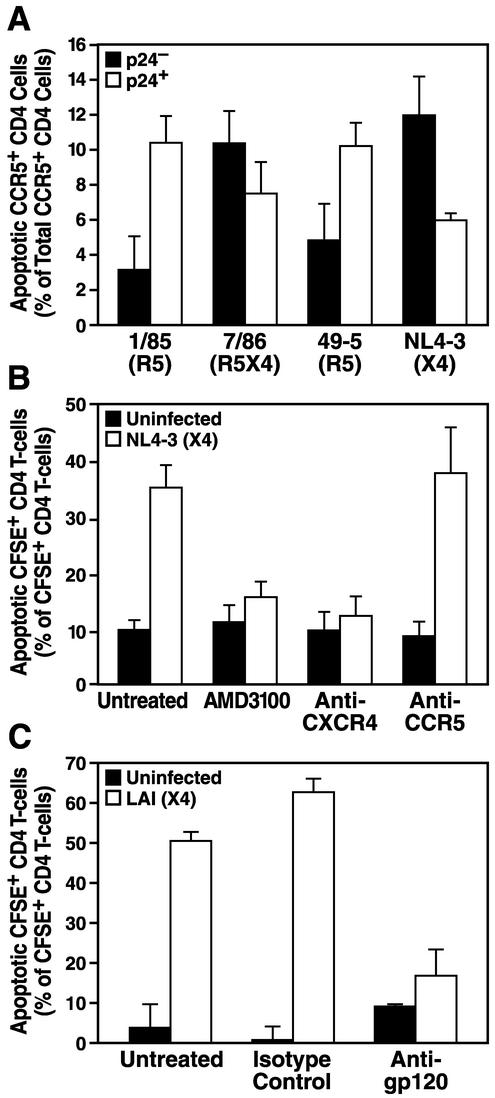
We next sought to explore the mechanism of this X4-specific phenotype. The pronounced bystander apoptosis induced by X4 strains could not be attributed to general cytotoxicity due to a higher concentration of viral particles in X4 virus-infected cultures, since R5 and X4 viruses showed similar replication kinetics and peak p24 concentrations (Fig. 2C) (24, 29, 32, 41, 44). It was important to determine whether the low level of apoptosis detected within R5 virus infections in human lymphoid histocultures was entirely a reflection of the low frequency of CCR5+ CD4 T cells (24, 29, 32, 41, 44) or represented a failure of R5 virus infections to induce bystander apoptosis in CCR5+ T cells. We found that among CCR5+ T cells in R5-infected cultures, nearly all apoptotic cells were productively infected (p24 positive) (Fig. 6A). Consistent with this observation, no significant levels of apoptosis were found among CCR5− CD4 T cells following R5 virus infections (data not shown). In contrast, X4 virus infections caused widespread apoptosis in both the CCR5+ (Fig. 6A) and CCR5− (data not shown) subsets, predominantly in bystander CD4 T cells within each of these subsets. Collectively, these analyses revealed significant bystander killing by X4 viruses, while killing by R5 viruses was restricted to the infected subset of CCR5+ CD4 cells.
FIG. 6.
HIV-1-induced bystander apoptosis critically depends on the gp120-CXCR4 interaction. (A) Human lymphoid cultures ex vivo were infected for 7 days with the R5 strain 1/85 or 49-5, the R5X4 strain 7/86, or the X4 strain NL4-3 or were kept uninfected. Apoptosis among CCR5+ CD4 cells was determined by annexin V staining, and infection was monitored simultaneously by intracellular p24 staining. (B and C) Uninfected, CFSE-labeled, CD4-enriched cells were cocultured with human lymphoid cultures ex vivo infected for 6 days with the X4 HIV-1 strain NL4-3 (B) or LAI (C) in the presence of the CXCR4-specific inhibitor AMD3100, an anti-CXCR4 antibody, or an anti-CCR5 antibody (B) or an anti-gp120 antibody (C). After 6 days of coculture, apoptosis in CFSE-positive CD4 T cells was determined by using annexin V staining. Shown are the mean values (n = 3) with standard errors of the means from a representative experiment from among three experiments with different donor tissues.
Since infections with isogenic X4 and R5 viruses differing only in their coreceptor-determining V3 loops had such dramatically different effects on the viability of bystander CD4 T cells, we hypothesized that the interaction of the viral envelope with the specific cellular chemokine receptor might play a significant role. To test this hypothesis, we employed the same experimental system described above to study the effects of coreceptor-specific drugs and antibodies on uninfected CFSE-labeled CD4 T cells in infected histocultures. Treatment of CFSE-labeled cells with the bicyclam AMD3100, which specifically blocks the binding of X4 HIV-1 to CXCR4 (12, 43), or with the anti-CXCR4 monoclonal antibody 12G5 completely prevented bystander killing by an X4 virus (Fig. 6B). In contrast, treatment with the anti-CCR5 monoclonal antibody 2D7, which is of the same isotype as 12G5, had no protective effect against the high levels of bystander apoptosis seen in untreated controls. Likewise, treatment with a neutralizing anti-X4 gp120 antibody eliminated bystander apoptosis by the X4 virus LAI (Fig. 6C). Taken together, these data demonstrate that binding of viral envelope gp120 to CXCR4 is required for the induction of high levels of apoptosis by X4 HIV-1 strains among bystander CD4 T cells in human lymphoid cultures ex vivo.
DISCUSSION
It is likely that multiple mechanisms contribute to the HIV-1-associated loss of CD4 T cells in HIV-1-infected individuals. The early hypothesis that destruction of productively infected cells is the major mechanism underlying CD4 T-cell loss in vivo (28, 31, 34, 47, 50) has been difficult to reconcile with newer findings that only 0.001 to 1% of CD4 T cells in lymph nodes, tonsils, and blood from HIV-1-infected individuals harbor virus (3, 5, 22, 46). In this context, a number of mechanisms for HIV-1-associated bystander killing have been proposed (2, 4, 6, 8-10, 15, 18, 21, 27, 35, 39, 49), but the controversy as to their relevance is ongoing (25, 51).
The present work sought to elucidate the significance of these alternative mechanisms in a relevant ex vivo human lymphoid culture system. In contrast to other experimental models, these cultures allow the analysis of apoptosis in a natural environment with preserved cytokine composition and cellular complexity and an unaltered cellular activation status. Furthermore, instead of studying apoptosis induced by recombinant proteins or the overexpression of single viral gene products, we measured cell death in the context of HIV-1 infections at a very low multiplicity of infection, thereby mimicking the natural infection process as closely as possible. We used two different versions of the ex vivo human lymphoid culture system: the original model first described by Margolis and coworkers (13, 19, 23, 24, 32, 41, 44), using tissue blocks, as well as a modified version of it developed by Goldsmith and coworkers (29, 32), using dispersed cultures, that allows more experimental flexibility. So far, we have not observed significant differences between these experimental models concerning viral replication and infection as well as depletion and apoptosis of CD4 T cells.
Key aspects of the present analysis of bystander apoptosis are the careful discrimination of productively infected and uninfected cells and the simultaneous assessment of markers of apoptosis on a single-cell basis. We found that X4 HIV-1 strains have a potent capacity to kill uninfected bystander CD4 T cells. Remarkably, bystander apoptosis made a much higher contribution to the depletion of CD4 T cells than did apoptosis of productively infected cells. Importantly, the reverse transcriptase inhibitor AZT, which exerted a profound antiviral effect, did not prevent the majority of CD4 T cells from undergoing apoptosis in the context of an X4 virus infection. This experiment shows that bystander cells, the infection of which is prevented by AZT, nonetheless undergo apoptosis upon exposure to viral envelope gp120 antigen (see below). Collectively, these findings support the notion that the majority of CD4 T cells undergoing apoptosis are true bystander cells that do not harbor HIV-1 DNA or newly synthesized viral proteins.
Interestingly, we found that the ability to trigger high levels of bystander apoptosis in human lymphoid tissue was a nearly exclusive characteristic of X4 and R5X4 viruses. In contrast, apoptosis induced by R5 strains was restricted largely to productively infected CCR5+ CD4 T cells. The relative inability of R5 strains to induce significant bystander killing may be one explanation for the fact that CD4 T-cell depletion is modest in most HIV-1-infected individuals during the early phase of infection, when R5 strains predominate. Furthermore, this finding provides a causal mechanism that may underlie the temporal association between the emergence of X4 strains and markedly increased depletion of CD4 T cells in vivo.
Mechanistically, based upon several lines of experimental evidence, bystander apoptosis in ex vivo human cultures appeared to be dependent on the interaction of viral gp120 with CD4 and CXCR4. First, bystander apoptosis was not detected in CXCR4-expressing CD8 T or B cells, suggesting that expression of CD4 on the target cell is a prerequisite. Second, bystander killing in CD4 T cells was completely prevented by the addition of the CXCR4 inhibitor AMD3100 or an anti-CXCR4 monoclonal antibody, both of which do not interfere with the engagement of gp120 with CD4. Thus, signaling through CD4 alone is not sufficient, but interaction with CXCR4 is required for the induction of bystander apoptosis. Third, an anti-gp120 monoclonal antibody eliminated the induction of apoptosis by an X4 virus, demonstrating that the binding of CXCR4 to gp120, rather than its natural ligand stromal cell-derived factor-1α or another, unknown cellular factor, triggered apoptosis in CD4 T cells. However, we cannot rule out the possibility that further cellular or viral factors either are required or have an enhancing effect on X4 HIV-1-induced bystander apoptosis, although gp120 is necessary and appears to underlie the restriction to CXCR4+ CD4 T cells. The supernatant transfer experiment in Fig. 5D showed that apoptosis of bystander CD4 T cells does not require direct contact between infected cells and the uninfected bystander cells that subsequently undergo apoptosis. Further experiments will be needed to clarify whether the conformation of gp120 (e.g., soluble or virion bound) plays a role in triggering bystander killing. We can exclude with certainty that free, unintegrated HIV-1 cDNAs were responsible for induction of apoptosis (36) in bystander CD4 T cells, since the viral replication cycle could not progress beyond reverse transcription in the presence of AZT.
In principle, the cellular source of a virus could influence its capacity to induce bystander apoptosis. R5 viruses can replicate in CCR5-expressing CD4 T cells as well as in macrophages, whereas the X4 strains used in this study replicate exclusively in CD4 T cells. We have previously demonstrated that in ex vivo human lymphoid cultures approximately 50% of the viral output of R5 viruses originates from CD4 T cells (13). Thus, the complete absence of bystander apoptosis by R5 viruses cannot be accounted for by a lack of CD4 T-cell-derived R5 virus. Also, as shown in Fig. 3B and C, the numbers of productively infected CD4 T cells did not vary significantly between R5 and X4 virus infections.
In summary, these studies demonstrate the significant contribution of HIV-1-induced bystander killing to the overall cytopathicity of X4 HIV-1 strains. Our key experimental observation of HIV-1-induced bystander apoptosis is consistent with earlier observations made in clinical specimens from AIDS patients (8, 15). We further demonstrate that bystander apoptosis is an almost exclusive characteristic of X4 HIV-1 strains and is mediated by the binding of X4 Env to CXCR4 on CD4 T cells. These results clarify that X4 strains exert a profound cytopathic effect on a much wider range of target cells via their particular capacity to induce bystander apoptosis. This combination of direct, infection-dependent T-cell killing during the early, R5 HIV-1-dominated stages of infection and broad, indirect T-cell destruction during late, often X4 HIV-1-dominated stages of infection provides an important paradigm for the natural history of HIV-1 disease. Furthermore, based on these concepts, it is plausible that inhibition of bystander killing by CXCR4-specific compounds would have a protective effect on CD4 T-cell depletion beyond their immediate effects on viral spread and systemic viral load and thereby significantly delay disease progression.
Acknowledgments
We thank the members of the surgical staff at Kaiser Hospitals (San Rafael, San Francisco, and South San Francisco, Calif.) for generous assistance in obtaining posttonsillectomy samples. Special thanks go to Heather Gravois, John Carroll, Jack Hull, and Chris Goodfellow for their assistance in the preparation of the manuscript. We thank Cecil Fox, Peggy Chin, Jo Dee Fish, and Scott Furlan for excellent technical assistance and Prerana Jayakumar, Dan Eckstein, and especially Jason Kreisberg for valuable discussions.
This work was supported by NIH grants (CA86814 and AI43695) to M.A.G. and by the J. David Gladstone Institutes.
REFERENCES
- 1.Ameisen, J. C., and A. Capron. 1991. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol. Today 12:102-105. [DOI] [PubMed] [Google Scholar]
- 2.Badley, A. D., D. Dockrell, M. Simpson, R. Schut, D. H. Lynch, P. Leibson, and C. V. Paya. 1997. Macrophage-dependent apoptosis of CD4+ T lymphocytes from HIV-infected individuals is mediated by FasL and tumor necrosis factor. J. Exp. Med. 185:55-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagasra, O., S. P. Hauptman, H. W. Lischner, M. Sachs, and R. J. Pomerantz. 1992. Detection of human immunodeficiency virus type 1 provirus in mononuclear cells by in situ polymerase chain reaction. N. Engl. J. Med. 326:1385-1391. [DOI] [PubMed] [Google Scholar]
- 4.Berndt, C., B. Mopps, S. Angermuller, P. Gierschik, and P. H. Krammer. 1998. CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4 (+) T cells. Proc. Natl. Acad. Sci. USA 95:12556-12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biberfeld, P., K. J. Chayt, L. M. Marselle, G. Biberfeld, R. C. Gallo, and M. E. Harper. 1986. HTLV-III expression in infected lymph nodes and relevance to pathogenesis of lymphadenopathy. Am. J. Pathol. 125:436-442. [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco, J., E. Jacotot, C. Cabrera, A. Cardona, B. Clotet, E. De Clercq, and J. A. Este. 1999. The implication of the chemokine receptor CXCR4 in HIV-1 envelope protein-induced apoptosis is independent of the G protein-mediated signalling. AIDS 13:909-917. [DOI] [PubMed] [Google Scholar]
- 7.Bleul, C. C., L. Wu, J. A. Hoxie, T. A. Springer, and C. R. Mackay. 1997. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc. Natl. Acad. Sci. USA 94:1925-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbonari, M., A. M. Pesce, M. Cibati, A. Modica, L. Dell'Anna, G. D'Offizi, A. Angelici, S. Uccini, A. Modesti, and M. Fiorilli. 1997. Death of bystander cells by a novel pathway involving early mitochondrial damage in human immunodeficiency virus-related lymphadenopathy. Blood 90:209-216. [PubMed] [Google Scholar]
- 9.Clerici, M., A. Sarin, J. A. Berzofsky, A. L. Landay, H. A. Kessler, F. Hashemi, C. W. Hendrix, S. P. Blatt, J. Rusnak, M. J. Dolan, R. L. Coffman, P. A. Henkart, and G. M. Shearer. 1996. Antigen-stimulated apoptotic T-cell death in HIV infection is selective for CD4+ T cells, modulated by cytokines and effected by lymphotoxin. AIDS 10:603-611. [DOI] [PubMed] [Google Scholar]
- 10.Clerici, M., A. Sarin, R. L. Coffman, T. A. Wynn, S. P. Blatt, C. W. Hendrix, S. F. Wolf, G. M. Shearer, and P. A. Henkart. 1994. Type 1/type 2 cytokine modulation of T-cell programmed cell death as a model for human immunodeficiency virus pathogenesis. Proc. Natl. Acad. Sci. USA 91:11811-11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donzella, G. A., D. Schols, S. W. Lin, J. A. Este, K. A. Nagashima, P. J. Maddon, G. P. Allaway, T. P. Sakmar, G. Henson, E. De Clercq, and J. P. Moore. 1998. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 4:72-77. [DOI] [PubMed] [Google Scholar]
- 13.Eckstein, D. A., M. P. Sherman, M. L. Penn, P. S. Chin, C. M. De Noronha, W. C. Greene, and M. A. Goldsmith. 2001. HIV-1 Vpr enhances viral burden by facilitating infection of tissue macrophages but not nondividing CD4+ T cells. J. Exp. Med. 194:1407-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fauci, A. S. 1988. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science 239:617-622. [DOI] [PubMed] [Google Scholar]
- 15.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129-134. [DOI] [PubMed] [Google Scholar]
- 16.Fox, C. H., and M. Cottler-Fox. 1993. In situ hybridization for detection of HIV RNA, p. 12.8.1-12.8.21. In W. Strober (ed.), Current protocols in immunology. Wiley, New York, N.Y. [DOI] [PubMed]
- 17.Gandhi, R. T., B. K. Chen, S. E. Straus, J. K. Dale, M. J. Lenardo, and D. Baltimore. 1998. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J. Exp. Med. 187:1113-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geleziunas, R., W. Xu, K. Takeda, H. Ichijo, and W. C. Greene. 2001. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410:834-838. [DOI] [PubMed] [Google Scholar]
- 19.Glushakova, S., B. Baibakov, L. B. Margolis, and J. Zimmerberg. 1995. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat. Med. 1:1320-1322. [DOI] [PubMed] [Google Scholar]
- 20.Goldsmith, M. A., M. T. Warmerdam, R. E. Atchison, M. D. Miller, and W. C. Greene. 1995. Dissociation of the CD4 downregulation and viral infectivity enhancement functions of human immunodeficiency virus type 1 Nef. J. Virol. 69:4112-4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gougeon, M. L., H. Lecoeur, A. Dulioust, M. G. Enouf, M. Crouvoiser, C. Goujard, T. Debord, and L. Montagnier. 1996. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J. Immunol. 156:3509-3520. [PubMed] [Google Scholar]
- 22.Gratton, S., R. Cheynier, M. J. Dumaurier, E. Oksenhendler, and S. Wain-Hobson. 2000. Highly restricted spread of HIV-1 and multiply infected cells within splenic germinal centers. Proc. Natl. Acad. Sci. USA 97:14566-14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grivel, J. C., and L. B. Margolis. 1999. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat. Med. 5:344-346. [DOI] [PubMed] [Google Scholar]
- 24.Grivel, J. C., M. L. Penn, D. A. Eckstein, B. Schramm, R. F. Speck, N. W. Abbey, B. Herndier, L. Margolis, and M. A. Goldsmith. 2000. Human immunodeficiency virus type 1 coreceptor preferences determine target T-cell depletion and cellular tropism in human lymphoid tissue. J. Virol. 74:5347-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grossman, Z., M. Meier-Schellersheim, A. E. Sousa, R. M. Victorino, and W. E. Paul. 2002. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat. Med. 8:319-323. [DOI] [PubMed] [Google Scholar]
- 26.Herbein, G., U. Mahlknecht, F. Batliwalla, P. Gregersen, T. Pappas, J. Butler, W. A. O'Brien, and E. Verdin. 1998. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature 395:189-194. [DOI] [PubMed] [Google Scholar]
- 27.Herbein, G., C. Van Lint, J. L. Lovett, and E. Verdin. 1998. Distinct mechanisms trigger apoptosis in human immunodeficiency virus type 1-infected and in uninfected bystander T lymphocytes. J. Virol. 72:660-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 29.Jekle, A., B. Schramm, P. Jayakumar, V. Trautner, D. Schols, E. de Clercq, J. Mills, S. M. Crowe, and M. A. Goldsmith. 2002. Coreceptor phenotype of natural human immunodeficiency virus with Nef deleted evolves in vivo, leading to increased virulence. J. Virol. 76:6966-6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katsikis, P. D., M. E. Garcia-Ojeda, J. F. Torres-Roca, I. M. Tijoe, C. A. Smith, and L. A. Herzenberg. 1997. Interleukin-1 beta converting enzyme-like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J. Exp. Med. 186:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowalski, M., L. Bergeron, T. Dorfman, W. Haseltine, and J. Sodroski. 1991. Attenuation of human immunodeficiency virus type 1 cytopathic effect by a mutation affecting the transmembrane envelope glycoprotein. J. Virol. 65:281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreisberg, J. F., D. Kwa, B. Schramm, V. Trautner, R. Connor, H. Schuitemaker, J. I. Mullins, A. B. van't Wout, and M. A. Goldsmith. 2001. Cytopathicity of human immunodeficiency virus type 1 primary isolates depends on coreceptor usage and not patient disease status. J. Virol. 75:8842-8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwa, D., J. Vingerhoed, B. Boeser-Nunnink, S. Broersen, and H. Schuitemaker. 2001. Cytopathic effects of non-syncytium-inducing and syncytium-inducing human immunodeficiency virus type 1 variants on different CD4+-T-cell subsets are determined only by coreceptor expression. J. Virol. 75:10455-10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurent-Crawford, A. G., B. Krust, S. Muller, Y. Riviere, M. A. Rey-Cuille, J. M. Bechet, L. Montagnier, and A. G. Hovanessian. 1991. The cytopathic effect of HIV is associated with apoptosis. Virology 185:829-839. [DOI] [PubMed] [Google Scholar]
- 35.Ledru, E., H. Lecoeur, S. Garcia, T. Debord, and M. L. Gougeon. 1998. Differential susceptibility to activation-induced apoptosis among peripheral Th1 subsets: correlation with Bcl-2 expression and consequences for AIDS pathogenesis. J. Immunol. 160:3194-3206. [PubMed] [Google Scholar]
- 36.Li, L., J. M. Olvera, K. E. Yoder, R. S. Mitchell, S. L. Butler, M. Lieber, S. L. Martin, and F. D. Bushman. 2001. Role of the non-homologous DNA end joining pathway in the early steps of retroviral infection. EMBO J. 20:3272-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCune, J. M. 2001. The dynamics of CD4+ T-cell depletion in HIV disease. Nature 410:974-979. [DOI] [PubMed] [Google Scholar]
- 38.Meyaard, L., S. A. Otto, R. R. Jonker, M. J. Mijnster, R. P. Keet, and F. Miedema. 1992. Programmed death of T cells in HIV-1 infection. Science 257:217-219. [DOI] [PubMed] [Google Scholar]
- 39.Miura, Y., N. Misawa, N. Maeda, Y. Inagaki, Y. Tanaka, M. Ito, N. Kayagaki, N. Yamamoto, H. Yagita, H. Mizusawa, and Y. Koyanagi. 2001. Critical contribution of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to apoptosis of human CD4+ T cells in HIV-1-infected hu-PBL-NOD-SCID mice. J. Exp. Med. 193:651-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson, B. K., V. L. Mosiman, L. Cantarero, M. Furtado, M. Bhattacharya, and C. Goolsby. 1998. Detection of HIV-RNA-positive monocytes in peripheral blood of HIV-positive patients by simultaneous flow cytometric analysis of intracellular HIV RNA and cellular immunophenotype. Cytometry 31:265-274. [DOI] [PubMed] [Google Scholar]
- 41.Penn, M. L., J. C. Grivel, B. Schramm, M. A. Goldsmith, and L. Margolis. 1999. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc. Natl. Acad. Sci. USA 96:663-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosok, B. I., J. E. Brinchmann, G. Stent, R. Bjerknes, P. Voltersvik, J. Olofsson, and B. Asjo. 1998. Correlates of apoptosis of CD4+ and CD8+ T cells in tonsillar tissue in HIV type 1 infection. AIDS Res. Hum. Retrovir. 14:1635-1643. [DOI] [PubMed] [Google Scholar]
- 43.Schols, D., S. Struyf, J. Van Damme, J. A. Este, G. Henson, and E. De Clercq. 1997. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J. Exp. Med. 186:1383-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schramm, B., M. L. Penn, R. F. Speck, S. Y. Chan, E. De Clercq, D. Schols, R. I. Connor, and M. A. Goldsmith. 2000. Viral entry through CXCR4 is a pathogenic factor and therapeutic target in human immunodeficiency virus type 1 disease. J. Virol. 74:184-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuitemaker, H., M. Koot, N. A. Kootstra, M. W. Dercksen, R. E. de Goede, R. P. van Steenwijk, J. M. Lange, J. K. Schattenkerk, F. Miedema, and M. Tersmette. 1992. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J. Virol. 66:1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simmonds, P., P. Balfe, J. F. Peutherer, C. A. Ludlam, J. O. Bishop, and A. J. Brown. 1990. Human immunodeficiency virus-infected individuals contain provirus in small numbers of peripheral mononuclear cells and at low copy numbers. J. Virol. 64:864-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terai, C., R. S. Kornbluth, C. D. Pauza, D. D. Richman, and D. A. Carson. 1991. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J. Clin. Investig. 87:1710-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toohey, K., K. Wehrly, J. Nishio, S. Perryman, and B. Chesebro. 1995. Human immunodeficiency virus envelope V1 and V2 regions influence replication efficiency in macrophages by affecting virus spread. Virology 213:70-79. [DOI] [PubMed] [Google Scholar]
- 49.Vlahakis, S. R., A. Algeciras-Schimnich, G. Bou, C. J. Heppelmann, A. Villasis-Keever, R. C. Collman, and C. V. Paya. 2001. Chemokine-receptor activation by env determines the mechanism of death in HIV-infected and uninfected T lymphocytes. J. Clin. Investig. 107:207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 51.Yang, Y., and J. D. Ashwell. 2001. Exploiting the apoptotic process for management of HIV: are we there yet? Apoptosis 6:139-146. [DOI] [PubMed] [Google Scholar]