Abstract
A mutant human immunodeficiency virus (HIV) envelope protein (Env) with an engineered disulfide bond between the gp120 and gp41 subunits (SOS-Env) was expressed on cell surfaces. With the disulfide bond intact, these cells did not fuse to target cells expressing CD4 and CCR5, but the fusion process did advance to an intermediate state: cleaving the disulfide bond with a reducing agent after but not before binding to target cells allowed fusion to occur. Through the use of an antibody directed against CCR5, it was found that at the intermediate stage, SOS-Env had associated with coreceptors. Reducing the disulfide bond after this intermediate had been reached resulted in hemifusion at low temperature and fusion at physiological temperature. The addition of C34 or N36, peptides that prevent six-helix bundle formation, at the hemifused state blocked the fusion that would have resulted after raising the temperature. Thus, Env has not yet folded into six-helix bundles after hemifusion has been achieved. Because SOS-Env binds CCR5, it is suggested that the conformational changes in wild-type Env that result from this binding cause disengagement of gp120 from gp41 in the region of the engineered bond. It is proposed that this disengagement is the event that directly frees gp41 to undergo the conformational changes that lead to fusion. The intermediate state achieved prior to reduction of the disulfide bond was stable. The capture of this configuration of Env could yield a suitable antigen for vaccine development, and it may also be a target for pharmacological intervention against HIV-1 entry.
For many viruses, the fusion protein is assembled from three identical monomers. Each monomer is posttranslationally cleaved into two subunits, yielding a surface subunit (SU) and a transmembrane subunit (TM). The two subunits are tightly abutted. The fusion proteins of human immunodeficiency virus (HIV), influenza virus, Ebola virus, and respiratory syncytial virus, to name but a few, all follow this pattern. In each case, SUs bind to receptors on target cells and TMs induce membrane fusion; fusion allows the viral nucleocapsid to enter the cell's cytosol (12, 21, 26). A widely held view is that, prior to fusion, TMs are in a metastable state because they are physically clamped in place by SUs. During fusion, the TMs are released from the SU clamp and are then free to undergo the conformational changes that carry the fusion process forward, leading to infection (5, 7, 40). In the specific case of HIV-1 Env (gp160), SU (gp120) and TM (gp41) are tightly but not covalently associated (1, 39). Binding of gp120 to its receptor (CD4) induces conformational changes in gp120 that then allow it to couple to a coreceptor (chemokine receptor) on the target cell membrane (3, 26, 44). Refolding of gp41 ensues, culminating in membrane fusion.
The HIV-1 Env glycoproteins are important antigens for vaccine development, but an obstacle to the evaluation of native forms of Env as immunogens has been the instability of the Env trimers (6). In one approach to stabilizing Env trimers, cysteine substitutions were made to engineer a disulfide bond (referred to as the SS-bond) that covalently links the gp120 subunit and gp41 subunit of each monomer (4, 37, 38). The most effective position found for the disulfide bond is between residue 501 within the C5 domain of gp120 and residue 605 of gp41, which is immediately adjacent to the loop created by the sole intramolecular disulfide bond within this subunit. When expressed as a soluble gp140 protein (which contains only the ectodomain of gp41), this mutant Env, known as SOS, retains the antigenic properties of wild-type Env (4), suggesting that the gp41 ectodomain is properly folded. Although the soluble SOS gp140 protein is unstable and readily dissociates to a monomeric form (38), additional mutagenesis within gp41 can stabilize SOS gp140 as trimers (37).
In contrast to the instability of soluble SOS gp140, the full-length version of SOS-Env is stable when expressed on the cell surface. Here we show that full-length SOS-Env binds CD4 and a coreceptor (CCR5), but this does not lead to fusion, probably because gp41 remains clamped to gp120 by the engineered SOS disulfide bond. However, when a reducing agent is added to the coreceptor-bound form of SOS-Env, fusion occurs. We propose that the conformational changes in wild-type Env induced by coreceptor binding frees gp41 from the clamp of gp120, allowing gp41 to then reconfigure and cause the lipid rearrangements of hemifusion and fusion.
MATERIALS AND METHODS
Reagents.
Dithiothreitol (DTT), bovine serum albumin, and chlorpromazine (CPZ) were purchased from Sigma Chemical Co. (St. Louis, Mo.). The HIV-1 gp41 C34 peptide (residues 628 to 661), soluble CD4 ectodomain (sCD4; from Norbert Schülke), and monoclonal antibodies against gp120 (2G12; from Hermann Katinger) and against CCR5 (2D7) were obtained from the AIDS Research and Reference Reagent Program, National Institutes of Health. The peptide N36 (residues 546 to 581) was a generous gift from Min Lu (Cornell Medical College). Pooled serum from HIV-1-infected patients was kindly provided by Gregory Spear (Rush University, Chicago, Ill.). All fluorescent dyes were from Molecular Probes (Eugene, Oreg.).
WT-Env and SOS-Env expression.
pSV7d expression vectors bearing SOS-Env or wild-type Env (WT-Env) genes (JR-FL strain) were kindly provided by J. Binley (Scripps Research Institute, La Jolla, Calif.). Env was expressed in HEK 293T cells by cotransfecting with the HIV-1 rev-bearing pCDNA3 plasmid (Invitrogen, Carlsbad, Calif.) at a 2:1 Env/Rev ratio, with a standard calcium phosphate method. Surface expression of SOS-Env and WT-Env was quantified by flow cytometry with monoclonal antibody 2G12 and fluorescein isothiocyanate-conjugated goat anti-human immunoglobulin secondary antibody. The fusion proteins were expressed at low densities through the pSV7d plasmid. SOS-Env was almost completely but not totally proteolytically cleaved into 120-kDa and 40-kDa subunits, as observed in reducing gels. In nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the uncleaved and the cleaved but disulfide-linked SOS-Env cannot be distinguished because they migrate with the same mobility.
Immunoprecipitation and SDS-PAGE analysis.
Proteins on the cell surfaces were labeled with sulfo-NHS-LC-biotin according to the manufacturer's instructions (Pierce Biotechnology Inc., Rockford, Ill.). Briefly, an equal number of biotinylated cells in each sample were incubated in DTT-containing or DTT-free phosphate-buffered saline and were then washed and lysed in 200 to 300 μl of NP-40-SDS-sodium deoxycholate buffer supplemented with a protease inhibitor cocktail (Sigma) for 30 min on ice. Lysates were clarified by centrifugation, and Env was immunoprecipitated overnight at 4°C with pooled HIV-1 patient sera in samples containing equal amounts of total cellular protein. The immune complexes were incubated with protein A-agarose beads for 4 h at 4°C, and then the beads were washed extensively and boiled in 2× SDS-PAGE sample loading buffer with and without 2-mercaptoethanol. The samples were fractionated by SDS-PAGE, transferred to a nitrocellulose filter, blocked with bovine serum albumin-dry milk, and incubated with streptavidin-conjugated horseradish peroxidase (Pierce), and gp160 and gp120 bands were detected with an ECL kit (Amersham-Pharmacia Biotech, Little Chalfont, Bucks, United Kingdom).
Cell labeling and fusion assay.
The target 3T3.CD4.CCR5 cells were loaded with CMAC (7-amino-4-chloromethylcoumarin, blue emission) (31). The effector 293T cells transfected with HIV-1 Env were loaded with the cytoplasmic dye calcein acetoxymethyl ester (calcein AM, green emission). Fusion was quantified by fluorescence video microscopy after incubation at 37°C (31). The extent of fusion was defined as the ratio of fused cells (positive for both dyes) to the sum of fused cells and the number of effector-target cell pairs that did not fuse. In separate experiments, both lipid and content mixing was quantified with target cells colabeled with CMAC and the membrane dye DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate, orange emission). All experiments were carried out at least three times (usually four to six independent experiments were performed); the average extent of fusion and the standard error of the mean were plotted.
Reduction of the SS-bond.
Unless otherwise indicated, the following three protocols were used to reduce the SS-bond and induce fusion: (i) continuous incubation with 0.5 mM DTT for 2 h at 37°C; (ii) treatment of the effector cells with 25 mM DTT for 10 min at 37°C, followed by coincubation with target cells for 1 h at 37°C under nonreducing conditions; and (iii) postbinding reductive cleavage, performed after incubating effector and target cells for 1 h at 37°C, followed by treatment with 25 mM DTT for 10 min, always at a temperature that does not permit fusion (23°C), and then by additional incubation for 30 to 45 min at 37°C under nonreducing conditions. In some experiments, SOS cells were incubated with 25 μg of sCD4 per ml for 20 min at 37°C before being treated with 25 mM DTT (while maintaining sCD4) for 10 min at 37°C and incubated with target cells.
Characterization of committed state.
The ability of 2D7 antibody to inhibit fusion was tested by adding the antibody either just prior to or after coincubation of SOS and target cells. In the first protocol, about 105 SOS cells were mixed with an equal number of target cells and incubated for 1 h at 37°C in the presence of 10 μg of 2D7. In the second case, the antibody was added after 1 h of cell coincubation and was allowed to bind for 30 min. In both protocols, 2D7-treated cells were subjected to DTT reduction, washed, incubated for 30 min at 37°C, and assayed for fusion. In control experiments, wild-type Env-expressing cells were coincubated with target cells at 18°C for 2.5 h (to create a temperature-arrested intermediate), and 2D7 was added either at the beginning of incubation or after 2 h of coincubation.
To test whether membrane continuity (hemifusion) was established at the committed state, the cells were treated with 0.5 mM CPZ for 1 min at 23°C. To ascertain whether SOS gp41 is folded into a six-helix bundle structure at hemifusion, the inhibitory peptides C34 (0.4 μM) and N36 (25 μM) were added at room temperature and allowed to bind for 15 min, and the temperature was again raised to 37°C.
RESULTS
SOS-Env can induce fusion if its bond is broken.
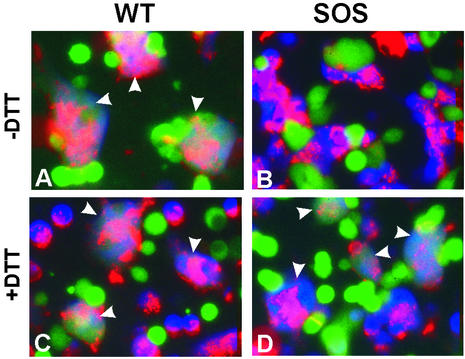
Effector cells expressing wild-type Env were loaded with an aqueous fluorescent dye and coincubated with target cells expressing CD4 and CCR5 that had been labeled with both an aqueous and a lipid fluorescent probe. The extent of fusion was quantified by monitoring content and lipid mixing. Fusion was efficient at 37°C (Fig. 1A). In contrast, there was no lipid or content redistribution within 2 h at 37°C when cells expressing SOS-Env were mixed with target cells (Fig. 1B), even though SOS-Env and WT-Env were expressed at comparable levels on cell surfaces, as measured by flow cytometry (data not shown). We tested whether the SS-bond was the reason SOS-Env did not promote fusion. When SOS cells and target cells were mixed and then coincubated at 37°C in the presence of the reducing agent DTT at 0.5 mM, fusion resulted (Fig. 1D). Fusion was mediated by WT-Env at this concentration of DTT (Fig. 1C). Thus, despite the presence of multiple, naturally occurring disulfide bonds within Env, CD4, and CCR5 (27), a low concentration of DTT can be used to cleave the engineered SS-bond of SOS-Env without appreciably compromising the normal functions of WT-Env, CD4, or CCR5. In fact, adding a high concentration (25 mM) of DTT for a short time (10 min) subsequent to coincubating the cells led to the same level of fusion as did a prolonged exposure to a low concentration of DTT (Fig. 2 and 3).
FIG. 1.
Cell-cell fusion induced by WT-Env (left panels) and SOS-Env (right panels). Effector cells expressing WT-Env or SOS-Env were loaded with calcein (green), and target cells were loaded with CMAC (blue) and colabeled with the membrane dye DiI (red). Cell fusion was examined after coincubation for 2 h at 37°C in the absence (A and B) or in the presence (C and D) of 0.5 mM DTT. Fused cells are marked by arrowheads.
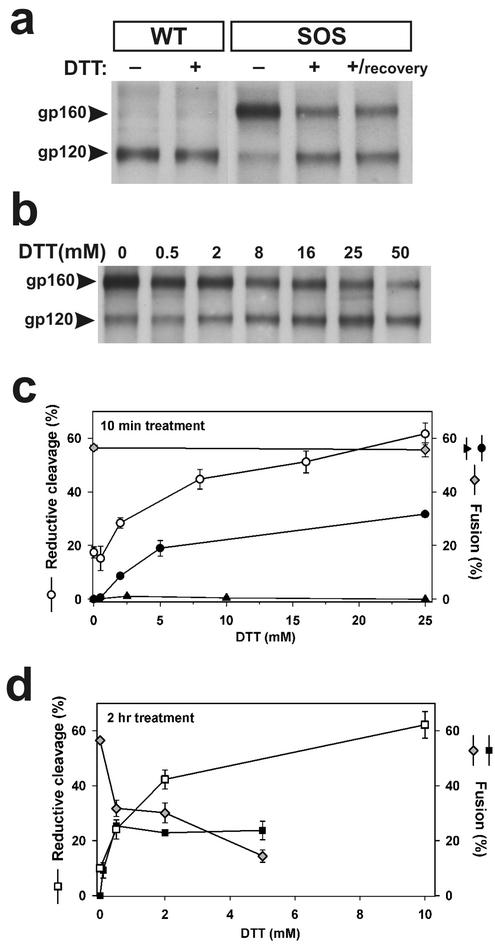
FIG. 2.
Reductive cleavage of SS-bond and fusion activity of SOS-Env. (a) Wild-type (lanes 1 and 2) and SOS (lanes 3 to 5), treated or not treated with 25 mM DTT (10 min at 37°C), were assayed either immediately after reduction (lanes 1 to 4) or after a 2-h incubation in phosphate-buffered saline (lane 5). (b) Dose dependence of the reductive cleavage of SS-bonds by DTT. (c) The extent of reductive cleavage of SOS as a function of DTT concentration determined from gels (as in b) as the ratio of total gp120 to the sum of gp120 and gp160 (open circles). In parallel experiments, the extent of fusion was quantified for SOS cells treated with DTT either before (solid triangles) or after (solid circles) incubation with target cells. The extent of fusion after cells expressing WT-Env were exposed to DTT for 10 min was the same as in the absence of the exposure (gray diamonds). (d) The extent of fusion between cells for WT-Env (gray diamonds) and SOS-Env (solid squares) as a function of DTT concentration continuously maintained for 2 h. The increase in the extent of SS-bond reduction with DTT concentration is shown (open squares).
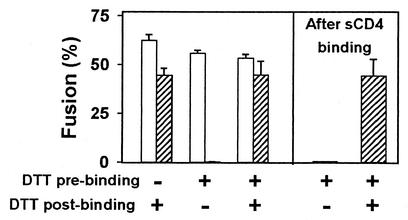
FIG. 3.
SOS-Env is activated by DTT applied after but not before binding to target cells for 1 h at 37°C. The extent of fusion induced by SOS-Env (hatched bars) and WT-Env (open bars) is shown for different DTT treatment protocols. In all cases, 25 mM DTT was maintained for 10 min at 37°C. In separate experiments, SOS cells were incubated with 25 μg of sCD4 per ml before the SS-bond was reduced with DTT (last two bars).
We verified that for SOS-Env expressed on the cell surface, the SS-bond was reduced by DTT. Under nonreducing conditions, almost all WT-Env was cleaved into its subunits, as determined by the absence of a 160-kDa band and the presence of a 120-kDa band in SDS-PAGE (Fig. 2a). Wild-type Env was not affected by treating cells with 25 mM DTT. For SOS-Env, the majority (≈85%) of gp120 and gp41 were coupled by SS-bonds, and the bonds were stable in the presence of denaturing concentrations of SDS. Treating the SOS cells with DTT (25 mM for 10 min at 37°C) before lysing them efficiently reduced the SS-bonds (Fig. 2a, lane 4). In separate experiments, SOS cells treated with DTT were incubated for 2 h under nonreducing conditions to test whether the reduced SS-bond would reform; it did not (Fig. 2a, lane 5). The extent of reduction of the SS-bond increased with increasing DTT concentration (Fig. 2b). The concentration of DTT that cells could tolerate and the duration of time that cells could be incubated with DTT were limited because of cytotoxic effects, but even under extreme conditions, about 30% of the SOS-Env did not separate into gp120 and gp41 monomers. About one-half of these 160-kDa bands were due to SOS-Env that was not proteolytically cleaved: a small but definite 160-kDa band was apparent on reducing gels even though all disulfide bonds should have been broken (data not shown). Thus, a small but not zero fraction of the SS-bonds was resistant to reduction by DTT.
For fusion to occur, SS-bond must be reduced after SOS cells have bound to target cells.
One might reasonably conclude that DTT allows fusion to proceed by simply breaking the SS-bond, rendering SOS-Env identical to the native state of WT-Env. But we found that for DTT treatment to lead to fusion, SOS-Env must have already undergone some of the conformational changes known to occur for WT-Env after it has bound to receptors and coreceptors. This was shown by experiments in which SOS cells were treated with DTT before being mixed with target cells. Because the reduced SS-bond does not readily reform (Fig. 2a), this DTT treatment should render SOS-Env fusogenic if DTT does no more than cleave the SS-bond. SOS cells treated with varied concentrations of DTT did not fuse to target cells when coincubated at 37°C (Fig. 2c, solid triangles). Thus, when SS-bonds were cleaved (≈60%, Fig. 2c, open circles) prior to binding to target cells, SOS-Env still did not induce fusion. In contrast, when SOS cells and target cells were first coincubated at 37°C and only afterward exposed to DTT, fusion was efficient (Fig. 2c, solid circles).
SDS-PAGE analysis showed that SS-bonds were reduced when DTT was added subsequent to mixing and incubating SOS cells and target cells (data not shown), but this biochemical method cannot pinpoint the small percentage of SOS-Env that is in contact with target cells. Because DTT allows fusion after SOS cells have been bound to target cells, we can conclude that DTT breaks the SS-bonds of the critical Envs, the ones that actually bind to the target cell. In fact, the extent of fusion observed when DTT was added only after binding to target cells (Fig. 2c, solid circles) correlated with the extent of reduction of the SS-bonds by pretreatment (open circles), indicating that once SOS-Env binds to the target cell, reduction of the SS-bonds of SOS-Env in the contact region permits fusion. Once the SS-bond was reduced subsequent to binding, SOS-Env retained its ability to cause fusion even after being exposed to an oxidizing environment. After removal of the DTT, 50 mM oxidized glutathione was added at a temperature that does not permit fusion to occur. Raising the temperature to 37°C led to fusion (data not shown), indicating that, once broken, the majority of the engineered SS-bonds did not reform.
Although the addition of DTT to SOS cells prior to target cell binding fails to allow fusion despite breaking SS-bonds, it does not inactivate SOS-Env's ability to induce fusion. Whereas SOS cells treated with DTT did not fuse to target cells (Fig. 2c and Fig. 3, second hatched bar), adding DTT after binding these cells did lead to the full extent of fusion (Fig. 3, third hatched bar). In contrast, wild-type Env-induced fusion was not significantly affected by any of the DTT treatment protocols (Fig. 3, open bars). Thus, the effects of DTT in promoting fusion can be attributed to its breakage of the SS-bond rather than reduction of naturally occurring disulfide bonds within Env or reduction of any of the disulfide bonds in CD4 or CCR5.
The fraction of the SS-bonds reduced by DTT increased with concentration when the reducing agent was continuously present for 2 h (Fig. 2d, open squares). But the extent of SOS-induced cell-cell fusion leveled off, reaching its maximum level at ≈0.5 mM DTT (Fig. 2d, solid squares). It is possible that naturally occurring disulfide bonds were broken in the continuous presence of high concentrations of DTT, and this deleterious reduction of natural bonds competed with the increased fusion one would expect after SS-bonds are broken. In fact, the continuous presence of a high concentration of DTT did decrease wild-type Env-induced fusion (Fig. 2d, gray diamonds), almost certainly due to reduction of natural disulfide bonds of CD4, CCR5, and/or Env. In contrast, a short (10-min) exposure to a high (25 mM) concentration of DTT did not reduce the extent of fusion (Fig. 2c, gray diamonds). Any disulfide bonds that were broken by DTT probably reformed quickly after removal of the reducing agent.
SOS binds to its receptor/coreceptor and undergoes conformational changes.
Binding of WT-Env and SOS gp140 to a soluble receptor (sCD4) exposes a cryptic epitope that overlaps the coreceptor binding site (4, 22, 25, 36). It therefore seemed possible that adding sCD4 to SOS cells would induce conformational changes in full-length SOS-Env to the extent that DTT treatment would now render the SOS cells fusion competent. Again, fusion did not occur (Fig. 3, next-to-last bar). However, adding DTT after the SOS cells (with bound sCD4) had been coincubated with target cells led to the full extent of fusion (Fig. 3, last bar), showing that binding of sCD4 did not cause any inactivation of SOS-Env. These experiments also indicate that breaking of the engineered SS-bond will not allow fusion to occur unless SOS-Env has already engaged CCR5.
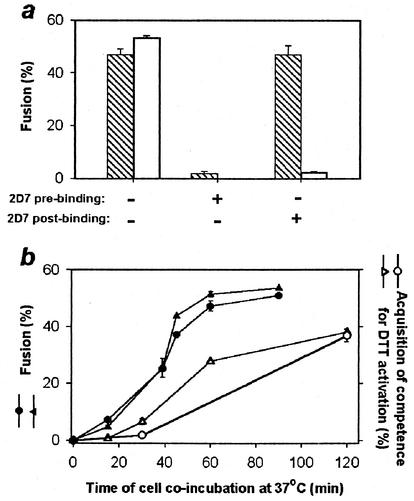
We further tested whether SOS-Env associated with CCR5 by adding, at various stages, the 2D7 antibody, which inhibits fusion by binding to the second extracellular loop of CCR5 (42). There was almost no fusion when the antibody was continuously present during a 1-h coincubation of SOS cells and target cells (Fig. 4a, second hatched bar). But the full extent of fusion occurred if 2D7 was added after coincubating the cells (Fig. 4a, third hatched bar). When SOS cells were first treated with DTT and then coincubated with target cells, here too the addition of 2D7 did not block fusion upon subsequent DTT treatment (data not shown). Thus, SOS-Env engaged both CD4 and CCR5 independently of whether the SS-bond was intact. (This is consistent with the prior conclusion, with monoclonal antibody 17b, that complexes of sCD4 and SOS gp140 expose coreceptor binding sites [4].) In contrast, 2D7 did inhibit fusion when added at a WT-Env intermediate that was arrested by suboptimal temperature (Fig. 4a, third open bar), showing that the temperature-arrested WT-Env state has not completed association with chemokine receptors (15, 31). In short, the SOS-Env intermediate state which is captured by the presence of the SS-bond is further advanced in the fusion process than the forms of WT-Env that have been trapped through a suboptimal temperature.
FIG. 4.
DTT activates SOS-Env after SOS-Env engages coreceptor of the target cell. (a) Extent of fusion induced by SOS-Env (cross-hatched bars) and by WT-Env (open bars) after the inhibitory 2D7 antibody was added to SOS cells and target cells either pre- or postbinding. (b) Kinetics of wild-type Env-induced fusion (solid symbols) and formation of a DTT-activatable state as a function of time of coincubating SOS cells with target cells (open symbols). Cells expressing SOS-Env and WT-Env were either treated (open and solid circles, respectively) or not treated (open and solid triangles, respectively) with DTT prior to incubation with target cells.
We measured the time course for formation of the DTT-activatable state to assess how much the SS-bond hindered SOS-Env conformational changes. SOS cells (either treated with DTT or not) and target cells were coincubated for various times and treated with DTT at 23°C, a temperature that does not allow Env to induce fusion, and the extent of fusion at 37°C was then measured. The extent of fusion increased slowly with incubation time for both the untreated (Fig. 4b, open triangles) and DTT-treated SOS cells (open circles). Fusion was faster for cells expressing WT-Env (solid symbols) than for SOS-Env reaching the intermediate state. In short, the conformational changes that SOS-Env undergoes when bound to target cells allow DTT to activate fusion, but the presence of the SS-bond slows down these changes. At this point in the fusion process, the gp120 and gp41 subunits are held by the SS-bond and are on the verge of separation. We refer to this arrested intermediate state as a “committed” intermediate.
Reducing SS-bonds results spontaneously in hemifusion.
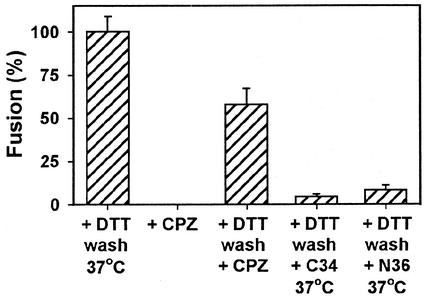
Hemifusion is the merger of contacting membrane monolayer leaflets to form a single membrane, known as a hemifusion diaphragm, that continues to separate aqueous compartments. Because lipid dye redistributes poorly, if at all, at early states of hemifusion that transit to fusion (8, 9), hemifusion might have occurred at the committed intermediate despite the absence of lipid (DiI) spread (Fig. 1B). Chlorpromazine (CPZ) is a membrane-permeating amphipathic agent that destabilizes hemifusion diaphragms, thereby promoting aqueous dye spread (8, 30, 32). We used CPZ to test whether hemifusion has occurred at the committed state. The addition of CPZ did not induce dye spread between cells captured at the committed intermediate (Fig. 5, second bar), showing that the committed intermediate occurs prior to hemifusion. When the SS-bond at the committed intermediate was reduced at 23°C, the addition of CPZ induced aqueous dye transfer (Fig. 5, third bar). In other words, gp41 spontaneously reconfigures to cause hemifusion upon disengagement from gp120. In some experiments, reducing the SS-bond resulted in a significant amount of fusion at 23°C before the addition of CPZ.
FIG. 5.
Characterization of fusion intermediate arrested by SS-bonds. SOS and target cells arrested at the committed state were exposed to 0.5 mM CPZ either before (second bar) or after (third bar) DTT treatment. Alternatively, cells captured at the committed state and treated with DTT were allowed to bind the C34 (0.4 μM) or N36 (25 μM) peptide and then brought back to the optimal temperature (fourth and fifth bars, respectively).
Synthetic peptides derived from the N- and C-terminal repeat regions of gp41 strongly inhibit HIV-1 infection and Env-mediated fusion by preventing gp41 from folding into six-helix bundles (11, 13, 14, 18, 26, 34, 41). The gp41 of WT-Env does not fold into six-helix bundles prior to pore formation (31), and folding into bundles is not complete until after a pore forms (29). We showed that after creating the committed intermediate and reducing the SS-bond to allow hemifusion, six-helix bundles had not yet formed; allowing N-terminal (N36) and C-terminal (C34) synthetic peptides to bind to SOS-Env at the hemifusion state prevented the fusion that would otherwise occur when the temperature was raised to 37°C (Fig. 5, last two bars). Thus, the TM subunit of SOS-Env has not folded into a six-helix bundle either at the committed state or at hemifusion.
DISCUSSION
It is known for influenza virus (17, 23) and suspected for several other viruses (2, 10, 16, 35) that the SU subunit of the fusion protein clamps the TM subunit and that disengagement of the subunits releases the clamp, allowing the TM to spontaneously undergo a “spring-loaded” transition that results in fusion. It has been theorized that this clamp and spring-loaded mechanism, leading directly to infection, is a general feature of viral fusion proteins (7). The present study provides the first evidence that such a mechanism occurs for HIV-1 Env. We have found that breaking the SS-bond at the committed state spontaneously leads to hemifusion. Because the SS-bond connects the C5 of gp120 to the loop of gp41, separation of these regions is the event that allows gp41 of SOS-Env to fully reconfigure (Fig. 6). For WT-Env, it is known that hydrophobic interactions between these gp120 and gp41 regions are particularly important for keeping the subunits attached to each other (20, 28, 33, 43). We propose that for WT-Env, binding to chemokine receptors causes C5 of gp120 to disengage from the loop of gp41. This disengagement, along with separation of gp120 and gp41 at other points of contact, fully releases the clamp, allowing gp41 to reconfigure to induce hemifusion and fusion.
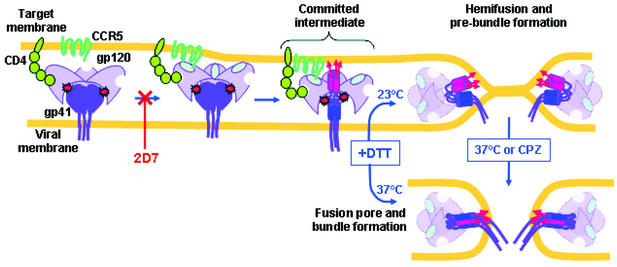
FIG. 6.
Model for membrane fusion mediated by SOS-Env. The SS-bonds between gp120 (light purple) and gp41 (dark purple) of SOS-Env are shown by red starbursts. CD4 is green, and CCR5 is dark cyan. The fusion peptides (red arrows) insert into the target cell membrane at the committed state. The amino-terminal trimeric coiled coil of gp41 is shown as three abutted red cylinders, and the carboxy-terminal heptad repeat regions are shown by blue cylinders. The gp120 subunits, after disengagement from gp41, are shown as being paler than before disengagement. Reduction of the SS-bonds allows gp41 to fold into a prebundle conformation and induce hemifusion at a temperature that is not permissive for fusion or to fold into a six-helix bundle and induce fusion pore formation at the optimal temperature.
From the state of advancement of the committed state, we were able to infer that gp41 has already undergone significant conformational changes at this state. We did this by comparing the committed state with an intermediate state of WT-Env that was arrested prior to hemifusion (by incorporating a lipid, lysophosphatidylcholine, into membranes) (31). Removing the arresting lysophosphatidylcholine did not yield hemifusion at 23°C (although subsequent raising of the temperature to 37°C led to full fusion). Relieving the arrest of the committed state does induce hemifusion, indicating that the lipid-arrested stage is less advanced than the committed state. At the lipid-arrested stage, Env can undergo all conformational changes that do not require membrane merger, which should include insertion of the fusion peptide into the target membrane. Thus, at the committed state, the fusion peptide should already be inserted in the target membrane (Fig. 6), anchoring gp41 to both membranes. Therefore, even though the committed state is the most advanced fusion-intermediate form of HIV-1 Env captured to date, the six-helix bundle has not yet formed at this stage (Fig. 5).
We previously showed that for CXCR4-dependent Env, some (perhaps all) of the copies of Env that participate in the formation and growth of a fusion pore have not yet folded into six-helix bundles, even after the pore has formed (29). In the present study, we have explicitly shown that for CCR5-dependent Env, the six-helix bundle has not yet formed after membranes have hemifused (Fig. 5). We proposed that the binding of the first and the second of the C-terminal segments of gp41 into the grooves of the central coiled coil (creating four- and five-helix bundles, respectively) is the event that induces hemifusion and fusion. In this model, the inhibitory peptides block fusion by competing with the formation of these late prebundle structures. It is only after full membrane continuity has been created by pore formation that the extremely stable six-helix bundle forms, and this final bundle structure stabilizes the open pore against closure (29).
The finding that SOS-Env can undergo significant conformational changes despite covalent linkage between its subunits is not surprising: binding of CD4 to uncleaved trimeric Env, where the subunits are obviously linked in one continuous gp160 chain, induces conformational changes that include the exposure of binding sites for inhibitory peptides (19, 24). Although the conformational changes that SOS-Env undergoes are probably similar to those of wild-type Env, the slow kinetics for reaching the committed state (Fig. 4b) indicates that the SS-bond did hinder protein movements.
Why would SOS-Env induce fusion when treated with DTT after binding to target cells but not before? We offer several possible explanations: Since some of the SS-bonds remain intact after treating SOS cells with DTT (Fig. 2), it is likely that not all copies of SOS-Env have the same conformation, and this could have a number of consequences. For example, the initially inaccessible SS-bonds may become accessible after the committed state is reached, and these are the copies of SOS-Env that induce fusion. Alternatively, SOS-Env that retains the SS-bonds may be inhibiting fusion in a dominant-negative fashion. Because SOS-Env with intact SS-bonds fully engages CCR5, dominant-negative inhibition would have to occur downstream of this engagement. The dominant-negative mechanism seems unlikely because coexpressing SOS-Env and WT-Env yielded cells that fused to target cells without DTT additions (data not shown).
Another explanation involves the obstruction of fusion by the formation of unnatural disulfide bonds. DTT-treated WT-Env cells were fusogenic, and therefore the introduced cysteines would be participating in any unnatural disulfide bonds that inhibited fusion. Because the gp120 subunits of SOS-Env treated with DTT were not disulfide bonded to other subunits (Fig. 2), the inhibitory disulfide bond between a cysteine of the SS-bond and a naturally occurring cysteine should occur either within the same subunit or between gp41 subunits. For untreated cells, engagement with chemokine receptors would strain SOS-Env to the point that reduction of the SS-bond would lead to hemifusion and fusion regardless of any unnatural bonds that may form.
It was not obvious, a priori, that DTT treatment could render SOS-Env fusogenic, because natural disulfide bonds might unavoidably be reduced and eliminate fusion. But fusion occurred for wild-type Env in the continuous presence of a low concentration of DTT or after a brief exposure to a high concentration of DTT, giving hope that the SS-bond could be reduced without deleterious side effects. Based on what was learned from the present study, the committed state was produced with pseudovirus expressing SOS-Env (4a) and here too, DTT treatment led to fusion.
SOS-Env offers a number of advantages for drug and vaccine design, and the capture of the committed state could aid such efforts. SOS-Env is inherently a more stable protein than WT-Env because the SS-bond covalently links the subunits. Env's conformational changes should progressively expose conserved epitopes buried in the native state and SOS-Env has undergone significant changes by the point of the committed state. The finding that the addition of DTT at this state allows fusion to occur suggests that SOS-Env can be stabilized in configurations that WT-Env passes through when inducing fusion. The committed state is a particularly stable intermediate and should withstand considerable biochemical manipulation. The form of Env at this state should expose epitopes that are not expressed in native forms of Env. If, at this state, the complex between SOS-Env, CD4, and perhaps CCR5 could be isolated or produced in a practical manner, it could provide a new configuration of Env for use as a vaccine antigen. The committed state could also serve as a target for identifying antiviral compounds with activity against later stages of the virus-cell fusion process.
Acknowledgments
We thank James Binley for providing the SOS plasmid and for stimulating discussions. Min Lu kindly provided the N36 peptide. Sofya Brener's excellent technical assistance is gratefully acknowledged.
This work was supported by NIH grants RO1 GM-27367, GM-54787, and AI-45463.
REFERENCES
- 1.Allan, J. S., J. E. Coligan, F. Barin, M. F. McLane, J. G. Sodroski, C. A. Rosen, W. A. Haseltine, T. H. Lee, and M. Essex. 1985. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science 228:1091-1094. [DOI] [PubMed] [Google Scholar]
- 2.Barnett, A. L., and J. M. Cunningham. 2001. Receptor binding transforms the surface subunit of the mammalian C-type retrovirus envelope protein from an inhibitor to an activator of fusion. J. Virol. 75:9096-9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 4.Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Binley, J. M., C. S. Cayanan, C. Wiley, N. Schülke, W. C. Olson, and D. R. Burton. 2003. Redox-triggered infection by disulfide-shackled human immunodeficiency virus type 1 pseudovirions. J. Virol. 77:5678-5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullough, P. A., F. M. Hughson, J. J. Skehel, and D. C. Wiley. 1994. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 371:37-43. [DOI] [PubMed] [Google Scholar]
- 6.Burton, D. R. 2002. Antibodies, viruses and vaccines. Nat. Rev. Immunol. 2:706-713. [DOI] [PubMed] [Google Scholar]
- 7.Carr, C. M., and P. S. Kim. 1993. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell 73:823-832. [DOI] [PubMed] [Google Scholar]
- 8.Chernomordik, L. V., V. A. Frolov, E. Leikina, P. Bronk, and J. Zimmerberg. 1998. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J. Cell Biol. 140:1369-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, F. S., R. M. Markosyan, and G. B. Melikyan. 2002. The process of membrane fusion: nipples, hemifusion, pores, and pore growth. Curr. Top. Membr. 52:501-529.
- 10.Damico, R. L., J. Crane, and P. Bates. 1998. Receptor-triggered membrane association of a model retroviral glycoprotein. Proc. Natl. Acad. Sci. USA 95:2580-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derdeyn, C. A., J. M. Decker, J. N. Sfakianos, X. Wu, W. A. O'Brien, L. Ratner, J. C. Kappes, G. M. Shaw, and E. Hunter. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 74:8358-8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doms, R. W. 2000. Beyond receptor expression: the influence of receptor conformation, density, and affinity in HIV-1 infection. Virology 276:229-237. [DOI] [PubMed] [Google Scholar]
- 13.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 14.Furuta, R. A., C. T. Wild, Y. Weng, and C. D. Weiss. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat. Struct. Biol. 5:276-279. [DOI] [PubMed] [Google Scholar]
- 15.Gallo, S. A., A. Puri, and R. Blumenthal. 2001. HIV-1 gp41 six-helix bundle formation occurs rapidly after the engagement of gp120 by CXCR4 in the HIV-1 Env-mediated fusion process. Biochemistry 40:12231-12236. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert, J. M., L. D. Hernandez, J. W. Balliet, P. Bates, and J. M. White. 1995. Receptor-induced conformational changes in the subgroup A avian leukosis and sarcoma virus envelope glycoprotein. J. Virol. 69:7410-7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godley, L., J. Pfeifer, D. Steinhauer, B. Ely, G. Shaw, R. Kaufmann, E. Suchanek, C. Pabo, J. J. Skehel, D. C. Wiley, et al. 1992. Introduction of intersubunit disulfide bonds in the membrane-distal region of the influenza hemagglutinin abolishes membrane fusion activity. Cell 68:635-645. [DOI] [PubMed] [Google Scholar]
- 18.Golding, H., M. Zaitseva, E. de Rosny, L. R. King, J. Manischewitz, I. Sidorov, M. K. Gorny, S. Zolla-Pazner, D. S. Dimitrov, and C. D. Weiss. 2002. Dissection of human immunodeficiency virus type 1 entry with neutralizing antibodies to gp41 fusion intermediates. J. Virol. 76:6780-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He, Y., R. Vassell, M. Zaitseva, N. Nguyen, Z. Yang, Y. Weng, and C. D. Weiss. 2003. Peptides trap the human immunodeficiency virus type 1 envelope glycoprotein fusion intermediate at two sites. J. Virol. 77:1666-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helseth, E., U. Olshevsky, C. Furman, and J. Sodroski. 1991. Human immunodeficiency virus type 1 gp120 envelope glycoprotein regions important for association with the gp41 transmembrane glycoprotein. J. Virol. 65:2119-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman, T. L., C. C. LaBranche, W. Zhang, G. Canziani, J. Robinson, I. Chaiken, J. A. Hoxie, and R. W. Doms. 1999. Stable exposure of the coreceptor-binding site in a CD4-independent HIV-1 envelope protein. Proc. Natl. Acad. Sci. USA 96:6359-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemble, G. W., D. L. Bodian, J. Rose, I. A. Wilson, and J. M. White. 1992. Intermonomer disulfide bonds impair the fusion activity of influenza virus hemagglutinin. J. Virol. 66:4940-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koshiba, T., and D. C. Chan. 2003. The prefusogenic intermediate of HIV-1 gp41 contains exposed C-peptide regions. J. Biol. Chem. 278:7573-7579. [DOI] [PubMed]
- 25.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaBranche, C. C., G. Galasso, J. P. Moore, D. P. Bolognesi, M. S. Hirsch, and S. M. Hammer. 2001. HIV fusion and its inhibition. Antiviral Res. 50:95-115. [DOI] [PubMed] [Google Scholar]
- 27.Leonard, C. K., M. W. Spellman, L. Riddle, R. J. Harris, J. N. Thomas, and T. J. Gregory. 1990. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J. Biol. Chem. 265:10373-10382. [PubMed] [Google Scholar]
- 28.Maerz, A. L., H. E. Drummer, K. A. Wilson, and P. Poumbourios. 2001. Functional analysis of the disulfide-bonded loop/chain reversal region of human immunodeficiency virus type 1 gp41 reveals a critical role in gp120-gp41 association. J. Virol. 75:6635-6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markosyan, R. M., F. S. Cohen, and G. B. Melikyan. 2003. HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation. Mol. Biol. Cell 14:926-938. [DOI] [PMC free article] [PubMed]
- 30.Melikyan, G. B., S. A. Brener, D. C. Ok, and F. S. Cohen. 1997. Inner but not outer membrane leaflets control the transition from glycosylphosphatidylinositol-anchored influenza hemagglutinin-induced hemifusion to full fusion. J. Cell Biol. 136:995-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melikyan, G. B., R. M. Markosyan, H. Hemmati, M. K. Delmedico, D. M. Lambert, and F. S. Cohen. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 151:413-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melikyan, G. B., R. M. Markosyan, M. G. Roth, and F. S. Cohen. 2000. A point mutation in the transmembrane domain of the hemagglutinin of influenza virus stabilizes a hemifusion intermediate that can transit to fusion. Mol. Biol. Cell 11:3765-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merat, R., H. Raoul, T. Leste-Lasserre, P. Sonigo, and G. Pancino. 1999. Variable constraints on the principal immunodominant domain of the transmembrane glycoprotein of human immunodeficiency virus type 1. J. Virol. 73:5698-5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munoz-Barroso, I., S. Durell, K. Sakaguchi, E. Appella, and R. Blumenthal. 1998. Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J. Cell Biol. 140:315-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinter, A., R. Kopelman, Z. Li, S. C. Kayman, and D. A. Sanders. 1997. Localization of the labile disulfide bond between SU and TM of the murine leukemia virus envelope protein complex to a highly conserved CWLC motif in SU that resembles the active-site sequence of thiol-disulfide exchange enzymes. J. Virol. 71:8073-8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salzwedel, K., E. D. Smith, B. Dey, and E. A. Berger. 2000. Sequential CD4-coreceptor interactions in human immunodeficiency virus type 1 Env function: soluble CD4 activates Env for coreceptor-dependent fusion and reveals blocking activities of antibodies against cryptic conserved epitopes on gp120. J. Virol. 74:326-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders, R. W., M. Vesanen, N. Schuelke, A. Master, L. Schiffner, R. Kalyanaraman, M. Paluch, B. Berkhout, P. J. Maddon, W. C. Olson, M. Lu, and J. P. Moore. 2002. Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J. Virol. 76:8875-8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulke, N., M. S. Vesanen, R. W. Sanders, P. Zhu, M. Lu, D. J. Anselma, A. R. Villa, P. W. Parren, J. M. Binley, K. H. Roux, P. J. Maddon, J. P. Moore, and W. C. Olson. 2002. Oligomeric and conformational properties of a proteolytically mature, disulfide-stabilized human immunodeficiency virus type 1 gp140 envelope glycoprotein. J. Virol. 76:7760-7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veronese, F. D., A. L. DeVico, T. D. Copeland, S. Oroszlan, R. C. Gallo, and M. G. Sarngadharan. 1985. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science 229:1402-1405. [DOI] [PubMed] [Google Scholar]
- 40.Weissenhorn, W., S. A. Wharton, L. J. Calder, P. L. Earl, B. Moss, E. Aliprandis, J. J. Skehel, and D. C. Wiley. 1996. The ectodomain of HIV-1 env subunit gp41 forms a soluble, alpha-helical, rod-like oligomer in the absence of gp120 and the N-terminal fusion peptide. EMBO J. 15:1507-1514. [PMC free article] [PubMed] [Google Scholar]
- 41.Wild, C. T., D. C. Shugars, T. K. Greenwell, C. B. McDanal, and T. J. Matthews. 1994. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc. Natl. Acad. Sci. USA 91:9770-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu, L., W. A. Paxton, N. Kassam, N. Ruffing, J. B. Rottman, N. Sullivan, H. Choe, J. Sodroski, W. Newman, R. A. Koup, and C. R. Mackay. 1997. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J. Exp. Med. 185:1681-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyatt, R., E. Desjardin, U. Olshevsky, C. Nixon, J. Binley, V. Olshevsky, and J. Sodroski. 1997. Analysis of the interaction of the human immunodeficiency virus type 1 gp120 envelope glycoprotein with the gp41 transmembrane glycoprotein. J. Virol. 71:9722-9731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]