Abstract
We generated an ORF65 deletion mutant by using a cosmid system constructed from the genome of a low-passage clinical isolate (P-Oka). Using the SCID-hu mouse model, we demonstrated that the ORF65 protein is dispensable for viral replication in skin and T cells, which are critical host cell targets during primary varicella-zoster virus infection.
Varicella-zoster virus (VZV) is a human herpesvirus that causes varicella (chickenpox) as the primary infection, establishes latency in sensory nerve ganglia, and may reactivate as herpes zoster (shingles) (1, 2). Open reading frame (ORF) 65 of VZV is one of four genes located in the short unique region of the genome and is homologous to Us9 in herpes simplex virus type 1 and the other alphaherpesviruses (3, 4, 7-9, 12, 16, 18). VZV ORF65 is predicted to encode an 11-kDa protein with 20% serine and threonine residues and a hydrophobic carboxyl terminus (6). Cohen et al. demonstrated that a partial deletion of ORF65 in a recombinant vaccine Oka strain was dispensable for viral replication in melanoma cells (5). The role of VZV ORF65 protein in vivo has not been reported.
In previous work, Moffat et al. found that the vaccine Oka strain was attenuated in its growth in skin xenografts compared with the low-passage clinical isolate P-Oka (15). P-Oka was isolated from a varicella lesion and used to develop the attenuated vaccine Oka strain (17). To introduce mutations into a VZV genome from a wild-type virus, we made a cosmid system for VZV by using DNA derived from the P-Oka clinical isolate. This cosmid system was used to construct a complete deletion of ORF65. We evaluated the effects of the complete deletion of ORF65 on viral replication in vitro and in vivo by using a SCID-hu mouse model for VZV infection.
Generation of P-Oka cosmids and ORF65 deletion mutants.
P-Oka was isolated from a child with varicella, passaged six times in human fibroblasts, and stored at −70°C (17). The virus was amplified an additional four times, and viral DNA was prepared (10). P-Oka cosmid clones were prepared as described previously (11). Two independently derived P-Oka cosmids with deletions of ORF65 were constructed as shown in Fig. 1. Human melanoma cells were transfected with intact P-Oka cosmids and one of two ORF65 deletion cosmids as previously described (13). Cell monolayers were split at a ratio of 1:3 when confluent. Plaques appeared 8 to 10 days following transfection. The absence of the ORF65 coding region was verified by PCR, restriction enzyme digestion, and sequencing of the recombinant P-Oka (rP-Oka) viruses minus ORF65 (rP-OkaΔ65). The rP-Oka virus was verified by restriction enzyme digestion (data not shown).
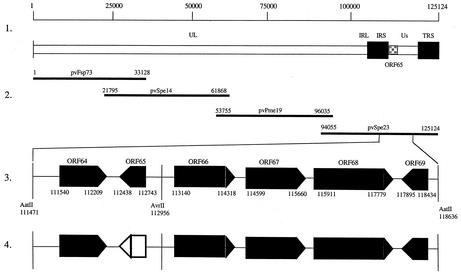
FIG. 1.
Construction of P-Oka cosmid vectors with full deletion of VZV ORF65. Line 1 shows a schematic diagram of the P-Oka VZV genome and the location of ORF65 in the unique short region. Line 2 depicts the overlapping segments of the VZV genome used to construct the P-Oka VZV cosmids. Line 3 shows the AatII fragment which includes ORF65. Line 4 indicates the deleted region (open box), resulting in cosmid pvSpe23Δ65.
VZV protein expression by rP-Oka and rP-OkaΔ65.
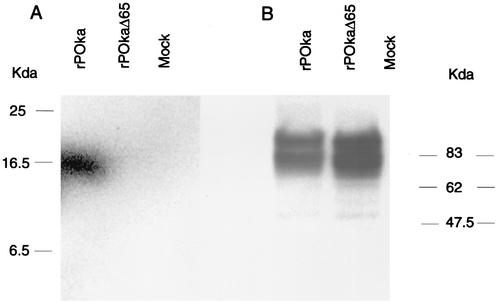
Immunoblot analyses were done to characterize viral protein expression in rP-Oka and rP-OkaΔ65. As shown in Fig. 2A, ORF65 protein was detected in rP-Oka-infected cells as a unique band at 16 kDa but not in rP-OKaΔ65-infected cells. Cohen et al. reported that the ORF65 protein is posttranslationally modified to a 16-kDa protein by casein kinase II in a recombinant vaccine Oka strain (5). Expressions of VZV glycoprotein E (gE) were equivalent in rP-Oka- and rP-OkaΔ65-infected cells (Fig. 2B).
FIG. 2.
Immunoblot analysis of ORF65 expression in vitro. ORF65 protein was detected with a polyclonal rabbit serum against ORF65 (A); VZV gE was detected with a monoclonal anti-gE antibody (B).
Replication of rP-Oka and rP-OkaΔ65 in vitro.
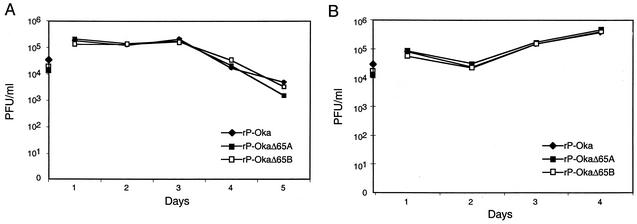
Viral replication was assessed by plaque assay (Fig. 3A). Growth kinetics of rP-Oka and rP-OkaΔ65 viruses in melanoma cells were comparable at days 1 to 5. These observations are consistent with those of Cohen et al., who reported that a partial deletion of ORF65 in a vaccine recombinant did not alter replication in melanoma cells (5). The growth kinetics of rP-Oka and rP-OkaΔ65 mutants in Vero cells were also identical (Fig. 3B). Plaque size, an indicator of cell-cell spread, was comparable for rP-Oka and rP-OkaΔ65 viruses in Vero cells as well. The mean plaque size of rP-OkaΔ65 was 0.73 ± 0.23 mm (mean ± 2 standard deviations), compared to 0.74 ± 0.25 mm for rP-Oka. Complete deletion of ORF65 from rP-Oka had no effect on VZV replication in melanoma and Vero cells and did not alter plaque size in vitro.
FIG. 3.
Growth kinetics of rP-Oka and rP-OkaΔ65. Infected cell monolayers were harvested on days 1, 2, 3, 4, and 5 after inoculation, and the amount of infectious virus was determined by titration on melanoma cells (A) and Vero cells (B).
rP-Oka and rP-OkaΔ65 replication in vivo.
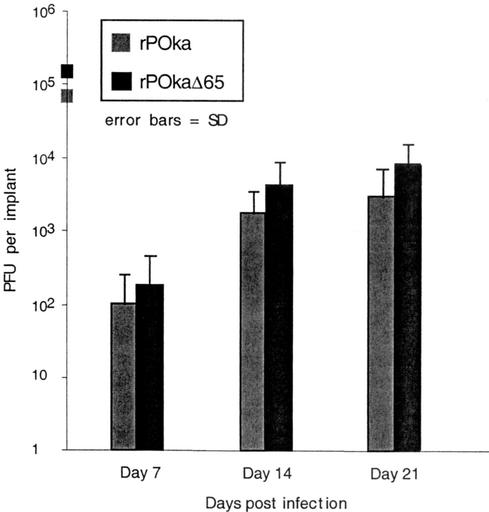
The SCID-hu mouse model for VZV infection has been described previously (14-16). Care and use of mice in this study complied with the Animal Welfare Act and were approved by Stanford University. SCID-hu mice with human skin xenografts or thymus and liver (thy/liv) xenografts were inoculated with rP-Oka and rP-OkaΔ65 and evaluated for VZV replication at 7, 14, and 21 days (skin) or 10 and 18 days (thy/liv) after infection (human tissues were provided by Advanced Bioscience Resources [Alameda, Calif] and were obtained in accordance with state and federal regulations). At days 7 and 14, >80% of rP-Oka- and rP-OkaΔ65-infected skin implants yielded infectious virus (Fig. 4). Viral replication peaked on day 21, with 7.4 × 103 PFU/implant for rP-Oka and 2.2 × 104 PFU/implant for rP-OkaΔ65. VZV protein expressions in rP-Oka- and rP-OkaΔ65-infected skin cell lysates, assessed by immunoblotting using a high-titer polyclonal serum for VZV proteins, were equivalent. SCID-hu thy/liv implants were evaluated at 10 and 18 days postinfection. The levels of viral replication at day 10 were equivalent, with mean viral titers of 3.9 × 104 PFU/implant for rP-Oka-infected implants and 1.4 × 104 PFU/implant for rP-OkaΔ65-infected implants. At day 18 postinfection, rP-Oka- and rP-OkaΔ65-infected implants showed marked necrosis and lymphocyte depletion. Virus remained detectable in only two of six rP-Oka-infected implants and one of four rP-OkaΔ65-infected implants. The mean viral yield for rP-Oka-infected implants at day 18 was 6.2 × 103 PFU/implant. The rP-OkaΔ65-infected implant yielded 1.1 × 103 PFU of infectious virus.
FIG. 4.
Replication of rP-Oka and rP-OkaΔ65 in skin implants. Viral titers of infected skin tissues recovered on days 7, 14, or 21 after infection were determined by plaque assay. Grey bars indicate the implants infected with rP-Oka; black bars indicate implants infected with rP-OkaΔ65. Differences are not significant (Student's t test, P > 0.05).
Immunohistochemical staining of rP-Oka- and rP-OkaΔ65-infected skin and thy/liv implants.
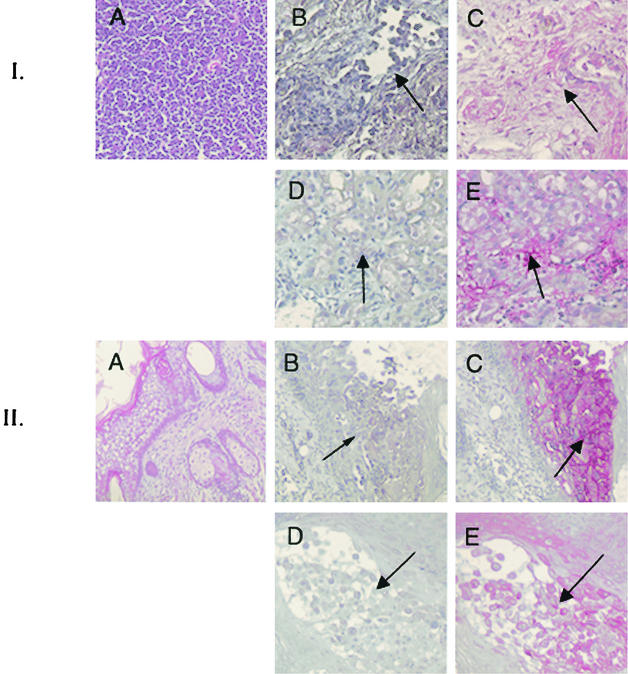
Immunohistochemical staining was performed as previously described (14) to determine whether lack of ORF65 protein expression was associated with differences in the cytopathic effects of VZV replication in infected tissues (14). rP-Oka- and rP-OkaΔ65-infected thy/liv implants showed equivalent cytopathic effects, including lymphocyte depletion and necrosis, at day 18 postinfection (Fig. 5, top set of five panels). As is characteristic of wild-type VZV infection of human skin, infection of skin implants with rP-Oka resulted in destruction of epidermal layers and penetration into dermal tissues by day 21 after infection (Fig. 5, bottom set of five panels). Skin infected with the ORF65 deletion mutant also showed evidence of deep penetration into dermal tissues.
FIG. 5.
Immunohistochemical staining of T-cell and skin implants infected with rP-Oka or rP-OkaΔ65. Representative sections from T-cell implants obtained 18 days after infection (top set of five panels) and from skin implants obtained 21 days after infection (bottom set of five panels) are shown. Panels depict a hematoxylin and eosin-stained uninfected cell control (A), rP-Oka-infected implants stained with nonimmune serum (B), rP-OkaΔ65-infected implants stained with nonimmune serum (D), rP-Oka-infected implants stained with a human polyclonal immune serum (C), and rP-OkaΔ65-infected implants stained with a human polyclonal immune serum (E). Cytopathic effect is indicated by the dark arrows.
Cohen et al. demonstrated that a partial deletion of ORF65 in a vaccine Oka recombinant did not alter infectivity in melanoma cells (5). Using a recombinant cosmid system derived from wild-type P-Oka, we have demonstrated that a full deletion of ORF65 is dispensable for viral replication in vitro, in melanoma and Vero cells, and in vivo, in human skin and T-cell xenografts.
Acknowledgments
We thank Helen Azzam, who made anti-ORF65 antibody reagents in the laboratory of Lynn Enquist, Princeton University. SCID-hu (thy/liv) mice were kindly provided by Cheryl Stoddard (Gladstone Institute, University of California San Francisco).
Our work was supported by grant AI20459 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Arvin, A. M. 2001. Varicella-zoster virus, p. 2731-2767. In B. N. Fields, D. N. Knipe, and P. M. Howley, ed., Virology. Lippincott-Raven Publishers, Philadelphia, Pa.
- 2.Arvin, A. M. 1999. Varicella-zoster virus: virologic and immunologic aspects of persistent infection, p. 183-208. In R. Ahmed and I. Chen (ed.), Persistent viral infections. John Wiley & Sons Ltd., New York, N.Y.
- 3.Brandimarti, R., and B. Roizman. 1997. Us9, a stable lysine-less herpes simplex virus 1 protein, is ubiquitinated before packaging into virions and associates with proteasomes. Proc. Natl. Acad. Sci. USA 94:13973-13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brideau, A. D., B. W. Banfield, and L. W. Enquist. 1998. The Us9 gene product of pseudorabies virus, an alphaherpesvirus, is a phosphorylated, tail-anchored type II membrane protein. J. Virol. 72:4560-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, J. I., H. Sata, S. Srinivas, and K. Lekstom. 2001. Varicella-zoster virus (VZV) ORF65 virion protein is dispensable for replication in cell culture and is phosphorylated by casein kinase II, but not by the VZV protein kinases. Virology 280:62-71. [DOI] [PubMed] [Google Scholar]
- 6.Davison, A. J., and J. E. Scott. 1986. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 67:1759-1816. [DOI] [PubMed] [Google Scholar]
- 7.Dolan, A., F. E. Jamieson, C. Cunningham, B. C. Barnett, and D. J. McGeoch. 1998. The genome sequence of herpes simplex virus type 2. J. Virol. 72:2010-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flowers, C. C., and D. J. O'Callaghan. 1992. The equine herpesvirus type 1 (EHV-1) homolog of herpes simplex virus type 1 US9 and the nature of a major deletion within the unique short segment of the EHV-1 KyA strain genome. Virology 190:307-315. [DOI] [PubMed] [Google Scholar]
- 9.Frame, M. C., D. J. McGeoch, F. J. Rixon, A. C. Orr, and H. S. Marsden. 1986. The 10K virion phosphoprotein encoded by gene Us9 from herpes simplex virus type 1. Virology 150:321-332. [DOI] [PubMed] [Google Scholar]
- 10.Kemble, G., G. Duke, R. Winder, and R. Spaete. 1996. Defined large-scale alterations of the human cytomegalovirus genome constructed by cotransfection of overlapping cosmids. J. Virol. 70:2044-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kemble, G. W., P. Annunziato, O. Lungu, R. E. Winter, T. A. Cha, S. J. Silverstein, and R. R. Spaete. 2000. Open reading frame S/L of varicella-zoster virus encodes a cytoplasmic protein expressed in infected cells. J. Virol. 74:11311-11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung-Tack, P., J. Audonnet, and M. Riviere. 1994. The complete DNA sequence and the genetic organization of the short unique region (Us) of the bovine herpesvirus type 1 (ST strain). Virology 199:409-421. [DOI] [PubMed] [Google Scholar]
- 13.Mallory, S., M. Sommer, and A. M. Arvin. 1997. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J. Virol. 71:8279-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moffat, J. F., M. D. Stein, H. Kaneshima, and A. M. Arvin. 1995. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J. Virol. 69:5236-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moffat, J. F., L. Zerboni, P. R. Kinchington, C. Grose, H. Kaneshima, and A. M. Arvin. 1998. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 72:965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moffat, J. F., L. Zerboni, M. H. Sommer, T. C. Heineman, J. I. Cohen, H. Kaneshima, and A. M. Arvin. 1998. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc. Natl. Acad. Sci. USA 95:11969-11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi, M., T. Otsuka, Y. Okumo, Y. Asano, T. Yazaki, and S. Isomura. 1974. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet ii:1288-1290. [DOI] [PubMed]
- 18.Telford, E. A. R., M. S. Waston, K. McBride, and A. J. Davison. 1992. The DNA sequence of equine herpesvirus-1. Virology 189:304-316. [DOI] [PubMed] [Google Scholar]