Abstract
Human studies have shown that iron deficiency and iron deficiency anemia in infants are associated with behavioral impairment, but the periods of brain development most susceptible to iron deficiency have not been established. In the present study, rhesus monkeys were deprived of iron by dietary iron restriction during prenatal (n = 14, 10 μg Fe/g diet) or early postnatal (n = 12, 1.5 mg Fe/L formula) brain development and compared to controls (n = 12, 100 μg Fe/g diet, 12 mg Fe/L formula) in behavioral evaluations conducted during the first four months of life in the nonhuman primate nursery. Iron deficiency anemia was detected in the pregnant dams in the third trimester and compromised iron status was seen in the prenatally iron-deprived infants at birth, but no iron deficiency was seen in either the prenatally or postnatally iron-deprived infants during the period of behavioral evaluation. Neither prenatal nor postnatal iron deprivation led to significant delays in growth, or gross or fine motor development. Prenatally deprived infants demonstrated a 20% reduced spontaneous activity level, lower inhibitory response to novel environments, and more changes from one behavior to another in weekly observation sessions. Postnatally deprived infants demonstrated poorer performance of an object concept task, and greater emotionality relative to controls. This study indicates that different syndromes of behavioral effects are associated with prenatal and postnatal iron deprivation in rhesus monkey infants and that these effects can occur in the absence of concurrent iron deficiency as reflected in hematological measures.
Keywords: Iron deficiency anemia, Monkey, Behavior, Gestation, Lactation, Cognitive, Motor, Emotionality, Activity, Nonhuman primate, Infant formula, Diet
1. Introduction
Iron deficiency (ID) and iron deficiency anemia (IDA), recognized through hematological measures, have been associated with behavioral impairments in infancy and childhood, primarily in the areas of motor development, activity, affect and cognition. Although iron deficiency in infancy is typically associated with other nutritional deficiencies and with socioeconomic deprivation, a causal relationship between iron deficiency and behavioral deficits can be supported in appropriately designed studies with prophylactic iron supplements [45,49]. In addition, diet-induced iron deprivation during development has been demonstrated to influence concurrent and subsequent behavioral performance in rodent models [4,17,37,53,67]. These rodent studies have also been able to begin describing how lack of iron influences brain development to produce the behavioral performance deficits [5,6,12,38].
Recently, focus has turned to evaluation of the effectiveness of iron supplementation for behavioral deficits in infants and children [28,46,59]. This endeavor is made more difficult by a lack of understanding of which periods in brain development are affected by iron deficiency and whether the consequences are reversible or permanent. Resolution of these issues requires the ability to restrict iron deficiency to defined periods of brain development. This in turn requires experimental studies in appropriate animal models.
In the present study, we examined early behavioral development in rhesus monkey infants deprived of iron either prenatally, through feeding of iron-deprived diets to their dams, or postnatally, by feeding a low iron formula to the infants. They were compared to controls fed iron adequate diets throughout prenatal and postnatal maturation. This study was intended to address the behavioral consequences of iron deficiency at two major periods in brain development, the third trimester and early infancy, by using assessments that parallel standard human infant testing in a nonhuman primate model.
Rhesus monkeys appear to offer a good model for the nutritional stress of inadequate iron during pregnancy and infancy. We recently demonstrated third trimester iron deficiency anemia in rhesus monkey dams fed low iron diets beginning in the first trimester [26]. In rhesus monkey colonies, a high prevalence of anemia has been detected late in infancy and the juvenile period, as is the case in humans [1,7,36]. Also, monkey infants can be raised in primate nurseries where the amount and timing of iron in the diet can be precisely controlled through use of infant formulas. Infant monkeys have been used extensively as models for human infants to study absorption, growth and development with formulas of different nutritional content [11,13,35,51,58, 41,42,43,33,40,54,57].
The period of infant evaluation in the present study extended from birth to four months of age, approximately equivalent to birth to 18 months of age in human infants. This estimate is based on an approximate 4:1 human:monkey developmental age equivalence ratio. Development of visual system function including visual recognition memory, follows this 4:1 age ratio [29]. Further, object permanence appears at 8–9 months of age in humans [52] and at 2–3 months of age in rhesus (data from this project). The period studied includes the appearance of basic gross motor abilities, reaching and eye–hand coordination, fine motor skills [31,10,17,20,48,50,60], and early cognitive stages of development, all of which were assessed in the infant monkeys. Emotionality, affect and adrenocortical responsiveness were also assessed at the end of the four-month period through a biobehavioral characterization assay designed for infant monkeys. Finally measures of spontaneous motor activity were obtained during the gross motor observations, during a 24-h biobehavioral characterization, and during a single 48-h session using actimeters at the end of the four-month period.
2. Materials and methods
2.1. Subjects
Newborn infants were transferred on the morning of birth to the primate nursery. These infants were offspring of dams fed iron-regulated purified diets during pregnancy, either 10 μg Fe/g (iron deprived) or 100 μg Fe/g (iron adequate) [27]. Iron status of the dams during pregnancy and of the infants at birth have been previously reported [26]. Briefly, dams fed low iron diets during pregnancy developed IDA in the third trimester and the infants were born with compromised iron and hematological status. Upon transfer to the nursery, infants from the prenatal iron-adequate group were distributed to either an iron-adequate or an iron-deprived formula group based on an initial assignment of their dams at the time of pregnancy detection which balanced dam age, weight and parity across groups. The dietary iron history of each experimental group is shown in Table 1. There were 8 females and 6 males in the prenatally deprived group, 8 females and 4 males in the postnatally deprived group, and 4 females and 8 males in the control group. The infants were born and tested in two cohorts two years apart. The cohort 1:2 ratios were 7/7 for the prenatally deprived group, 5/7 in the postnatally deprived group, and 6/6 in the control group.
Table 1.
Experimental design
| Prenatal deprivation (n = 14) | Control (n = 12) | Postnatal deprivation (n = 12) | |
|---|---|---|---|
| Prenatal diet | 10 μg/g | 100 μg Fe/g | 100 μg/g |
| Postnatal diet | 93 μg/g | 93 μg Fe/g | 12 μg/g |
2.2. Animal housing and care
CNPRC maintains nonhuman primate nurseries housing approximately 200 infants each year using standard protocols that have been found to optimize infant well-being as described previously [27]. Neonates were first housed in heated incubators then transitioned to social housing in small metal cages. Attachment objects (cloth towel and stuffed terrycloth duck) were provided in both incubators and metal cages and the metal cages additionally had toys, swings and perches. Infants were monitored throughout the day by a dedicated nursery staff who recorded any health problems in an individual animal medical record and referred them to the veterinary staff. Body weights were recorded daily for the first two weeks of life and weekly thereafter. Formula intake of individual infants was monitored for individual infants for the first two weeks, and for cages of two infants for an additional two weeks.
2.3. Assurance of compliance with animal codes
All procedures were conducted according to the Guidelines for Use and Care of Laboratory Animals of the National Research Council. CNPRC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Animal husbandry procedures conform to SOPs established by the CNPRC. Experimental protocols were approved prior to implementation by the University of California Davis IAC.
2.4. Infant formulas and feeding
Infants in the postnatally deprived group were fed commercial formula (Similac Advance, Ross Products, Saint-Laurent, QC) containing 12 μg Fe/g powder or 1.5 mg Fe/L. Infants in the control (iron adequate group) and the prenatally deprived group were fed the corresponding commercial formula (Similac Advance with Iron) containing 93 μg Fe/g obtained as a powder or 12 mg Fe/L diluted. The formulas had identical nutritional content except for the iron concentration. The formula was color coded (red, yellow, green) with food dye (McCormick and Co., Inc., Hunt Valley, MD) at the time of dilution to avoid accidental feeding with the wrong formula but maintain blinding. A card of appropriate color on the monkey'scage was used to match the formula. For this experiment, formula was the only source of food for the first four months of life with the exception of food reinforcers used in behavioral testing. All food items used as food reinforcers were screened for low iron content.
Formula feeding followed husbandry SOPs of the primate nursery at CNPRC. All formula feeding was ad libitum. Neonates were hand-held and fed formula from a pet bottle (65 mL/bottle) with nipple at 2 h intervals until judged to be able to nurse from a pet bottle inserted through the side of the cage. Two night feedings (0100 and 0400 h) were given on postnatal days 1 and 2, one night feeding (0100 or 0400) was given on days 3–7 and, after day 7, night feedings were discontinued unless specified by the attending veterinarian. Beginning at 35 days, formula was also introduced in a hanging bottle with a sipper tube and the pet bottle was discontinued at 70 days of age. Infants were transitioned to solid adult food and drinking water over a two-week period beginning at four months of age according to standard CNPRC protocols for nursery-raised infants. The control (iron-fortified) formula was used for all infants during this transition.
2.5. Infant evaluation schedule
The infant evaluation schedule is outlined in Table 2. More detail on timing and duration of individual tests is provided below. Inter and intra-observer reliabilities were obtained for all tests with a criteria of at least 85%(agree/(agree+disagree). Some behavioral tests were conducted for a fixed number of sessions and others until a performance criterion was reached. All testing was conducted in the same test room located near the nursery but in a corridor with no animal housing, with daily sessions, five days a week. Tests were conducted individually (one animal in the room at a time) except for tests utilizing the Wisconsin General Test Apparatus (WGTA), which were conducted two animals at a time in two separate apparatuses positioned out of sight of one another. Data from cerebrospinal fluid (CSF) sampling and auditory brainstem response (ABR) testing will be reported separately.
Table 2.
Infant evaluation schedule
| Nonbehavioral | Age at evaluation | |
|---|---|---|
| Body weights | 1–28 days, 4–14 weeks | |
| Morphometrics | Birth, 1 and 4 months | |
| Hematology/iron status | Birth, 1 and 4 months | |
| Lymphocyte flow cytometry | 3 to 4 months | |
| Auditory brainstem response | 1 and 4 months | |
| Cerebral spinal fluid sampling | 4 months | |
| Behavioral | Age at evaluation | # Sessions/criterion |
| Motor and postural maturation | 1–14 weeks | 13 sessions |
| Visual novelty preference | 190, 200, 210 | 3 sessions |
| Reach and retrieve/hesitancy | ||
| Home cage | Postnatal day 21 | 5 sessions |
| WGTA; holes, tubes, rod | Postnatal day 28 | Criterion or 14 weeks |
| Fine motor development | Postnatal day 28 | Criterion or 14 weeks |
| Object permanence/A not B | 201 days postconception | Criterion or 14 weeks |
| Activity monitoring | 4 months | One 48 h session |
| Biobehavioral characterization | 3 to 4 months | One 24 h session |
2.6. Hematology and iron status measures
A complete blood count (CBC) and supplemental iron status measures (transferrin receptor (TfR), serum ferritin, serum iron, zinc protoporphyrin (ZPP)) were conducted as described previously [26]. The CBC included RDW (red blood cell distribution width). In addition, quantification of CD4+ and CD8+ lymphocyte subpopulations was performed by flow cytometry at four months of age. For this assay, 50 μL aliquots of whole blood were directly labeled with phycoerythrin (anti-CD4-M-T477; BD Pharmingen), peridinin chlorophyll-alpha protein (anti-CD8-SK1; BD Pharmingen), and fluorescein isothiocyanate (anti-CD3-SP34; BD Pharmingen). A Coulter Q-prep (Coulter Corp., Miami, FL) was used to lyse the red blood cells and fix the sample in paraformaldehyde. Lymphocytes were gated by forward and side light scatter. A FACS Calibur flow cytometer (BD Pharmingen) was used to phenotype the lymphocyte subsets.
2.7. Growth and health
Body weights were measured daily from birth to four weeks of age, and weekly from four to fourteen weeks of age. Morphometric measures (crown–rump length, femur length, foot length, skinfold thickness (back, abdomen, thigh, triceps), and head length, width and circumference were measured at birth, one and four months of age as previously described [28]. Infant health records maintained by colony services were summarized for the incidence of veterinary diagnosis and intervention.
2.8. Motor and postural maturation
Weekly 10-min sessions were conducted in a specialized cage (38 × 38 × 86 cm) with plastic mesh sides that allowed climbing [17,27,21,22,23,19]. Infant behavior initiations were recorded for a 7.5 min session after a 2.5 min acclimation period using a checklist of exhaustive, mutually exclusive categories that included mature and immature forms of motor behaviors (sitting, stand, walk, climb up, climb down, cage manipulation) [17].
2.9. Novelty preference
This test of visual recognition memory is based on the Fagan Infant Intelligence Test [15] for human infants and adapted for use in monkeys [30]. The apparatus and method have been previously described [18]. The seven stimulus pairs used for the monkey infants were the same ones originally developed by Fagan to be equivalent in brightness and in preference and to increase in difficulty. They are black and white heavy line drawings of abstract figures/patterns. Three tests with either two or three sets of familiar/unfamiliar stimuli were conducted at ages based on the date of conception (postconception days 190, 200 and 210 ± 1.) This corresponded to 25, 35 and 45 days postnatal age for a full term (165 days postconception) infant. Briefly the infant was swaddled and held with its head free in front of a vertical board on which two visual stimuli cards could be displayed. There were seven problems (sets of stimuli) across the three day test series increasing in difficulty (similarity between stimuli). An observer sitting behind the display board timed the stimulus familiarization period and operated a video camera focused on the infant's face. The stimulus designated “familiar” was first displayed on both the right and left sides until a total of 20 s of fixation was recorded. Then both familiar and unfamiliar stimuli were presented for two 10-s periods beginning at the first fixation, and the number and total duration of fixations directed at each stimulus card was determined from the videotapes.
2.10. Eye–hand coordination and grasp maturation
A number of tests were used in succession to evaluate the fine motor ability of the infants.
2.10.1. Home cage reach/hesitancy
Five daily sessions were conducted. Three objects (large marshmallow, peanut-shaped marshmallow candy, gummi fish candy) were placed in the infant's home cage. Time to contact an object was recorded by an observer. Subsequent to contact, the maturity of interaction with the object over a 5-min period was scored in three categories:
1=mouths but does not pick up the objects
2=mouths the object and also manipulates it
3=picks up, holds, and mouths the objects.
For the second cohort only, a novel object was used for an additional 5-min reach period after completion of the series with the original three objects.
2.10.2. Reach and retrieve/WGTA
The infant was transferred to the WGTA apparatus for each session. The same objects as used for the home cage reach-and-retrieve evaluations were placed on the WGTA test board, one at a time with three 30-s trials conducted for each object. In this situation the infant had to reach through 6.5 cm diameter circles in the Plexiglas cage front to retrieve the object. Interaction with the object was scored as above. The criterion for passing this test was all nine trials with scores of “3” on two consecutive days. After attaining criterion, a second test of hesitancy (three 30-s trials) was conducted for the second cohort only using a novel object.
2.10.3. Tube reach
This test, based on a method developed to assess motor ability after cortical lesions [39], used Plexiglas tubes, 5 cm in diameter, of two different lengths, 1.5 and 3.0 in long. The tubes were attached to a test board at different distances from the edge (0, 2 and 4 cm for the 1.5 in tube, 0 and 2 cm for the 3.0 in tube. This resulted in a series of five conditions of increasing difficulty. The test required the infant to reach through the tube to retrieve the small food treat at the other end. Three trials were conducted for each tube at each distance. Each trial was scored in one of four categories:
0–no reach attempted
1–hits side of tube when entering
2–hits edges of tube inside or drops the reward when picking up or returning
3–clean entry and retrieval.
Three trials with score of “3” were needed to pass on to the next more difficult condition.
2.10.4. Rod orientation reach
For this test an elongated object (2.5 in piece of licorice or a gummi fish) was held in a horizontal position outside the WGTA test cage. As the infant reached through the circular opening to retrieve the object, the orientation was changed to vertical. Three trials of horizontal to vertical orientation were followed by three trials of vertical to horizontal orientation. Each trial was scored for whether or not the infants reached for the object, whether they were successful in grasping it, whether they used one or both hands, and whether they adjusted the direction of their hand when the object orientation was changed. Criterion for passing this test was use of adjusted hand direction for all six trials on two consecutive sessions. This test is used in human infants [47] and was added for the second cohort to further extend the motor evaluation for comparison to humans.
2.10.5. Fine motor development
On 201 days post-conception, grasp testing was initiated as previously described [21,22,18]. For this test small food items (whole “froot loops” cereal placed on the surface of the board, small marshmallow in a well, crushed cereal on the surface) were placed on a test board in the WGTA in successive 30 s trials. The quality of grasp was scored when the infant retrieved the food item in one of four categories:
sweep
grasp
modified grasp
finger–thumb grasp.
This test continued until a score of “4” was obtained on all three trials for two successive sessions for the crushed cereal and marshmallow in the well, to a maximum of 14 weeks of age.
After five sessions of grasp evaluation, a bimanual coordination task was added. For the first cohort this task consisted of lifting an inverted, transparent beaker with one hand in order to retrieve a food item under it with the other hand. For the second cohort, the task consisted of lifting the lid of a hinged box with one hand to remove a food item inside with the other hand. However, few of the animals attempted to perform either task with both hands, and data are not analyzed or presented here.
2.11. Object permanence/A not B
The WGTA apparatus was used for this test, which is based on procedures for evaluating this early Piagetian cognitive ability [52] in humans and monkeys [21,18,14]. The infant was first given a toy or food object to hold and manipulate for 5 s. Then the object was taken away and placed in a deep well in the test board next to a large plastic block. There were three conditions depending on the position of the plastic block: placed behind the well (uncovered), partially concealing the well (partially covered), or completely concealing the well (fully covered). The infant was allowed a maximum of 30 s to reach for the object. If it was retrieved, the trial was scored “+.” If the infant failed to reach for the object, the trial was scored “−.” The next trial was initiated within 15 s. There were 15 trials (5 per condition) in each session. Sessions continued to a criterion of 8/10 correct trials on two consecutive sessions. Testing was discontinued at 14 weeks of age if criterion was not reached.
Infants received the A-not-B test if they reached the object permanence criterion prior to 14 weeks of age. This protocol, adapted from [14], used two five-session series. A food object was first hidden within view of the infant on one of two (right/left) covered wells, and the infant was allowed to retrieve it. After two consecutive correct retrievals, the object was hidden within view of the infant on the opposite side (reversal). Each session progressed to longer delays (2, 5, 10, 12, 14, 16 s) between hiding the object and allowing access to the test board if the infant reached criterion at any one delay within the maximum 43 trials allowed per delay. Choice of the previously rewarded side is the “A not B” error. Completion of three reversals to a criterion of two consecutive correct responses led to progression to the next longer delay interval.
2.12. Spontaneous activity/diurnal rhythms
Actimeters that use mercury switch closures to record body movement were used to record activity during a 48-h period over the weekend when cage room disruption was limited to regularly scheduled feeding and cleaning. As previously described [27,25,24], actimeters were placed in small pouches attached to a harness so that they were located in the middle of the infant's back where they could not be reached. Infants were individually housed for the activity monitoring.
2.13. Biobehavioral characterization
Infants in the present study participated in a 24 h biobehavioral characterization conducted each year for most three-four month old rhesus infants in the colony at CNPRC. Over 1200 infants have been characterized with this screen since 2001. The biobehavioral characterization screen consisted of removing the infants individually to a larger metal cage (living cage) located in a different room identical in size and appearance to their home cage room. Each infant was relocated into this room along with three to seven other familiar infants for testing during the same 24-h period. The living cage contained a cloth diaper and stuffed terrycloth duck, which had served as attachment objects for the infants in the primate nursery, and also a novel manipulable object (approximately 4×9 cm) with automatic sensors. Appropriate formulas were supplied in hanging bottles identical to those used in the home cage. During the 24-h period, behavior was evaluated under four conditions: undisturbed in the living cage at the beginning and end of the 24-h period, response to novel objects in the living cage, separate test cage), response to monkey videos of aggressive and nonsocial behaviors (separate test cage), response to human intruder (separate test cage). The living cage observations were conducted by a live observer, while videotaping was used for the response to human intruder (the technician conducting the test dressed in protective clothing) and monkey video segments. In addition, adrenocortical response was monitored by serum cortisol (see below). Finally, a list of 26 adjectives used to rate affect quality [63] was completed by the observer at the end of the 24 h period. Details for the biobehavioral characterization are provided in [9].
Videotapes were scored using commercial software (The Observer, Noldus Information Technology, Wageningen, Netherlands) by experienced technicians blind to the purpose of the experiment or the treatment groups of individual animals. Behavior was coded in nine states (frequency and duration) (sleep, lie, sit, stand, crouch, active (locomotion), rock/sway, and motor stereotypy) and 22 events (frequency only; includes vocalizations, self-directed behaviors (e.g. scratch), social behaviors (e.g. fear grimace) and other discrete actions). Additional categories for individual tests included: looking at the video monitor (monkey videos), location in the cage relative to the monitor or the human intruder (monkey videos and human intruder). Instances of the 22 individual events were low during the observation period; an index of distress events was constructed for purposes of this analysis as the sum of cage shake, convulsive jerk, self-clasp, crouch, motor stereotypy, self bite, scratch, toothgrind, and screech vocalization.
2.14. Blood sampling for plasma cortisol
Blood samples (0.5 or 1.0 mL) were obtained by femoral venipuncture at four timepoints during the biobehavioral characterization:
“response to relocation,” ∼1030 h, 1–2.5 h after relocation to the new environment,
“adaptation to relocation,” ∼1600 h, 5.5 h after the first sample and immediately after the human intruder test,
“response to dexamethasone” ∼0830 h the next day, 16.5 h after an injection of 500 μg/kg dexamethasone i.m., which occurred immediately after the second blood sample,
“response to ACTH,” 30 min after injection of 2.5 IU ACTH i.m. which occurred immediately after the third blood sample.
Samples were transferred to EDTA tubes, centrifuged and frozen (−80 °C) until assayed for cortisol by RIA (Diagnostic Products Corp., Los Angeles, CA).
2.15. Statistical analysis
Statistical analysis was conducted using JMP software (SAS Institute, Cary, NC). Linear modeling was used and each endpoint was screened prior to analysis for potential covariates, including cohort, sex, gestation age at birth, birth weight, and delivery mode (vaginal, c-section). ANOVAs used linear modeling with post hoc contrasts of least-square means (control vs. prenatal, control vs. postnatal). Some variables were analyzed with nonparametric statistics or transformed with square root transformation if distributions were not normal or adequate fit was not obtained with the linear model. Graphs present descriptive statistics from the raw data file, rather than least square means and are not adjusted for covariates.
3. Results
3.1. Growth and health
There were no diet group effects on weights obtained daily from one to four weeks of age, or monthly at 1, 2, and 4 months of age (Fig. 1). Gestation length was a covariate at <one month of age, while cohort was a covariate throughout the period. Sex was not a covariate at these ages. The prenatally deprived group appeared to fall behind in weight gain beginning in the second week of life, but month-to-month weight gain was comparable in all groups. Notably, weight gain in the postnatally deprived group was nearly identical to the control group that was fed iron supplemented formula. No illnesses were seen in the infants during the first four months of life.
Fig. 1.
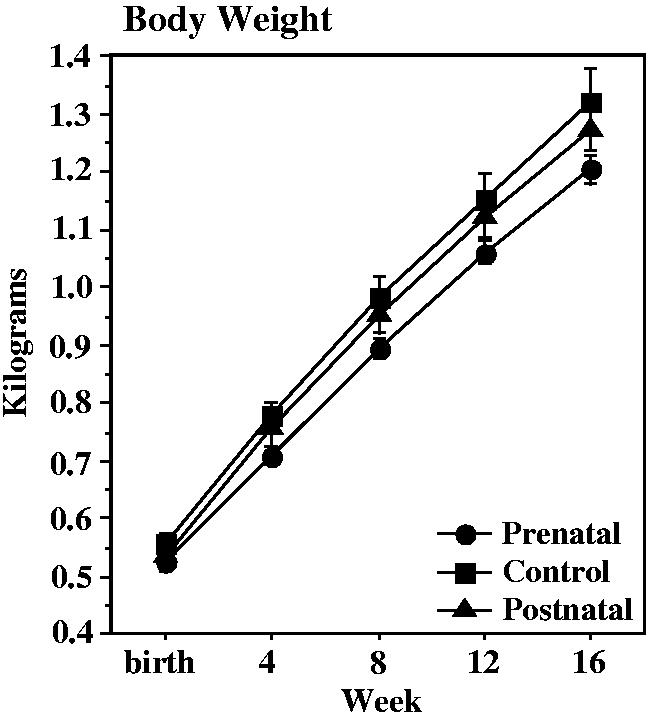
Body weight of control (n = 12), prenatally iron-deprived (n = 14), and postnatally iron-deprived (n = 12) rhesus monkey infants during the first four months of life. There were no group differences in body weight or body weight gain.
Other measures of growth and body composition were similar across groups with the exception of head measurements. Diet group effects, first observed at birth [26], were also seen at one month (F = 3.60, p = .04) and four months (F = 3.83, p = .03). Prenatally deprived group had smaller head width than controls (see Table 3 for individual group comparisons). There were no covariates for head width. The head length and head circumference measures did not differ by group. Sex and gestation length were significant covariates for head length and circumference.
Table 3.
Developmental head measurements in control and iron-deprived infants
| Prenatal (n = 14) | Control (n = 12) | Postnatal (n = 12) | |
|---|---|---|---|
| Head width birth | 51.47 ± 0.32a | 53.2 ± 0.42 | 52.2 ± 0.53 |
| 1 month | 54.40 ± 0.52a | 56.53 ± 0.54 | 55.10 ± 0.66 |
| 4 months | 59.10 ± 0.41a | 61.07 ± 0.33 | 59.59 ± 0.76 |
| Head length birth | 68.66 ± 0.57 | 68.75 ± .62 | 68.75 ± .61 |
| 1 month | 73.87 ± 0.75 | 74.75 ± 0.65 | 74.02 ± 0.61 |
| 4 months | 78.10 ± 0.98 | 78.9 ± 0.91 | 79.55 ± 0.31 |
| Head circumference birth | 193 ± 17 | 198 ± 22 | 193 ± 23 |
| 1 month | 209 ± 16 | 212 ± 15 | 212 ± 16 |
| 4 months | 226 ± 14 | 230 ± 15 | 227 ± 16 |
p<=.01 vs. control.
3.2. Hematology and iron status measures
Results from the complete blood count and from additional measures of iron status (serum Fe, TfR, ZPP, and ferritin) are shown in Table 4. No significant differences between dietary iron groups were seen at any timepoint. In addition to the one and four month timepoints, a CBC and flow cytometry for CD4 and CD8 lymphocyte subsets were performed at the time of the biobehavioral characterization. No significant differences were found in the CBC parameters. CD4+/CD8+ ratios were lower in the prenatal deprivation group than controls (F = 3.65, p = .037, prenatal vs. control p = .01); however all values were within a clinically normal age-appropriate range of 1 to 5.
Table 4.
Hematological and iron status parameters
| 1 month of age |
4 months of age |
|||||
|---|---|---|---|---|---|---|
| Prenatal | Control | Postnatal | Prenatal | Control | Postnatal | |
| RBC (× 106/μL) | 4.8 ± 0.1a | 4.8 ± 0.1 | 4.8 ± 0.1 | 5.0 ± 0.1 | 5.1 ± 0.1 | 5.0 ± 0.1 |
| Hemoglobin (g/dL) | 12.2 ± 0.2 | 12.7 ± 0.2 | 12.6 ± 0.4 | 11.9 ± 0.1 | 12.3 ± 0.1 | 11.9 ± 0.2 |
| Hematocrit (%) | 37 ± 1 | 38 ± 1 | 38 ± 1.2 | 36 ± 0.6 | 37 ± 0.7 | 36 ± 0.8 |
| MCV (fL) | 78 ± 0.9 | 79 ± 0.7 | 80 ± 1 | 72 ± 0.6 | 72 ± 0.5 | 73 ± 0.8 |
| MCH (pg) | 25.7 ± 0.3 | 26.4 ± 0.3 | 26.3 ± 0.4 | 23.6 ± 0.3 | 24.1 ± 0.3 | 24.1 ± 0.5 |
| MCHC (pg/fL) | 32.9 ± 0.3 | 33.3 ± 0.5 | 32.7 ± 0.5 | 32.9 ± 0.3 | 33.6 ± 0.4 | 33.2 ± 0.5 |
| RDW (%) | 13.1 ± 0.2 | 13.0 ± 0.3 | 12.9 ± 0.2 | 13.4 ± 0.2 | 13.7 ± 0.2 | 13.3 ± 0.2 |
| ZPP (μmol/mol heme) | 51 ± 5 | 57 ± 6 | 74 ± 17 | 34 ± 6 | 34 ± 6 | 44 ± 15 |
| TfR (μg/mL) | 2.6 ± 0.3 | 2.8 ± 0.4 | 2.2 ± 0.3 | 1.7 ± 0.2 | 2.0 ± 0.3 | 1.5 ± 0.2 |
| Serum Fe (μg/dL) | 123 ± 9 | 132 ± 12 | 159 ± 13 | 139 ± 12 | 117 ± 10 | 128 ± 13 |
| CD4+/CD8+ | 2.8 ± 0.2b | 3.8 ± 0.5 | 3.1 ± 0.2 | |||
Mean ± SEM.
p<.01 vs. control.
Serum ferritin values at four months of age indicated an effect of the postnatal iron deprivation on iron storage pools (Fig. 2). The ANOVA at this age was significant, but pairwise contrasts with controls did not reach statistical significance. However, variability in the prenatally deprived group was strongly influenced by one high value (32.0), which may have been due to an inflammatory event. If the postnatally deprived group was directly compared to controls with a two-group ANOVA, the values were significantly different (F = 9.20, p = .007).
Fig. 2.
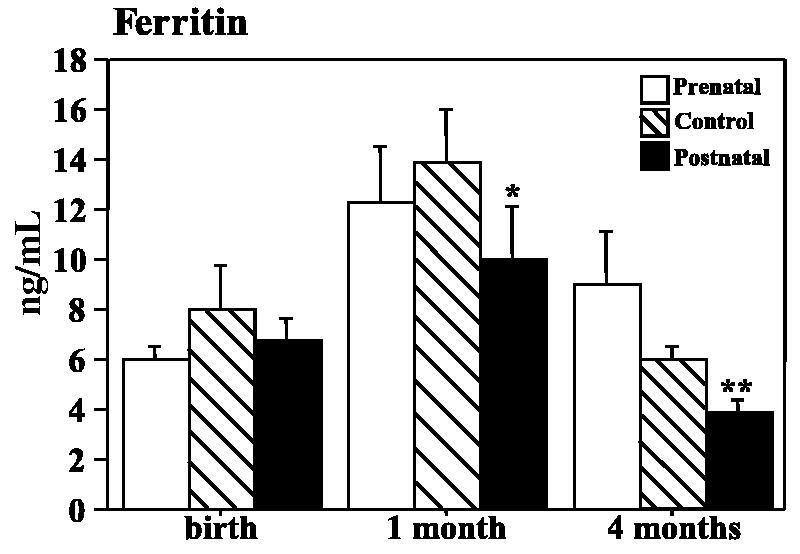
Serum ferritin concentrations at three time points during the first four months of life in control, prenatally iron-deprived and postnatally iron-deprived rhesus monkey infants. The ferritin assay uses a human antibody that detects approximately 10% of monkey ferritin. *p = .055, **p<.01, t-test vs. control.
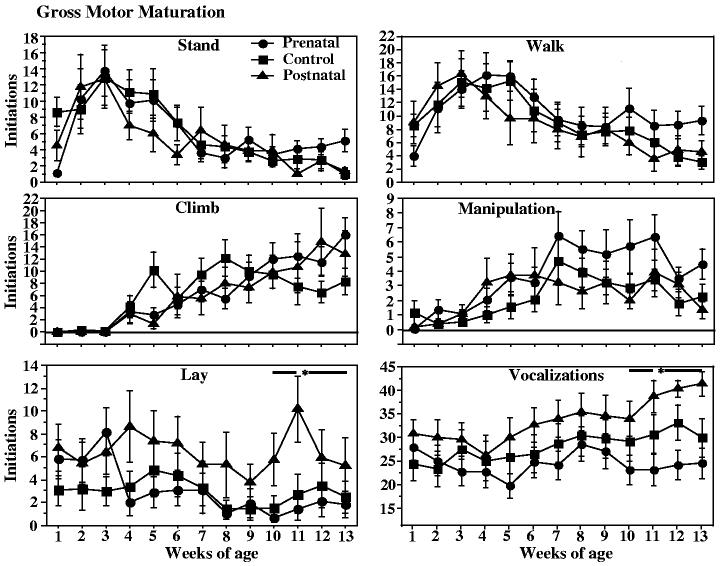
3.3. Gross motor observations
There was no indication of a lag in maturation of gross motor function. The iron deprived groups began sitting, walking, climbing, and manipulating objects at similar ages to controls (Fig. 3).
Fig. 3.
Behavior initiations during weekly 7.5-min observation sessions from one to fourteen weeks of age in control, prenatally iron-deprived and postnatally iron-deprived rhesus monkey infants. The top and middle graphs show maturation of major gross motor behaviors (stand, walk, climb, manipulation). The bottom graphs show observations related to emotionality (lying down, a withdrawal behavior, and vocalizations). *p < .05 postnatal deprivation vs. control, post hoc contrast of least square means, sum of last four observation sessions.
Some measures of emotionality were available from the “lying down” and “vocalization” measures taken during the gross motor observation periods (Fig. 3). Lyingdownis typically a withdrawal behavior after the immediate neonatal period, and vocalization rate can reflect distress. Iron deprivation influenced the incidence of lying down behaviors summed across all observation sessions (F = 5.38, p = .009); the postnatal deprivation group differed from controls (p = .002). In addition there was a significant iron deprivation effect on vocalizations during the last month of observation (F =6.50, p = .004). Postnatally deprived infants had higher vocalization frequencies than controls (p < .05), while prenatally deprived infants had a lower incidence of vocalizations than controls (not significant).
Also, the weekly observation period allowed some qualitative evaluation of regulation of behavior. Although durations of individual behaviors were not recorded, the number of behavior initiations (changes from one behavior to another) in each session provided a measure of persistence in individual behaviors. Iron deprivation effects were seen in the last month of the four month period (F = 3.41, p = .04). The prenatally deprived group differed from controls (Fig. 4).
Fig. 4.
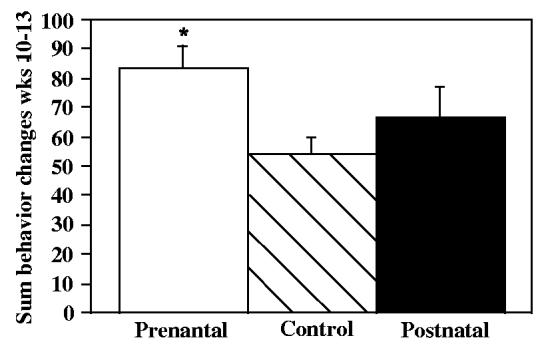
Sum of all behavior initiations during the last four weeks of the weekly observations sessions in control, prenatally iron-deprived and postnatally iron-deprived rhesus monkey infants. A larger number of behavior initiations reflects a shorter duration of each behavior. *p = .01 relative to controls, post hoc contrast of least square means.
3.4. Reach/fine motor tests
There were no group differences in performance of the reach and fine motor tests (Table 5). Pre- and postnatally iron-deprived infants were able to use smooth reaching trajectories and finger–thumb opposition as well as controls. Similar numbers of infants in each group failed to reach the preset performance criteria. There were no significant iron deprivation effects on the hesitancy measures taken during reach testing.
Table 5.
Reach and fine motor tests
| # Reaching criterion |
Sessions to criteriona |
|||||
|---|---|---|---|---|---|---|
| Pre-natal | Control | Post-natal | Pre-natal | Control | Post-natal | |
| Reach and retrieve | 14/14 | 12/12 | 14/14 | 8 ± 1 | 11 ± 3 | 11 ± 2 |
| Tube reach | 14/14 | 11/12 | 12/12 | 12 ± 1 | 12 ± 1 | 12 ± 1 |
| Rod orientation reachb | 7/7 | 5/6 | 5/7 | 8 ± 2 | 6 ± 1 | 8 ± 2 |
| Grasp maturity | 8/14 | 8/12 | 5/12 | 29 ± 2 | 32 ± 3 | 33 ± 1 |
Mean ± SEM of infants who reached criterion.
Cohort 2 only.
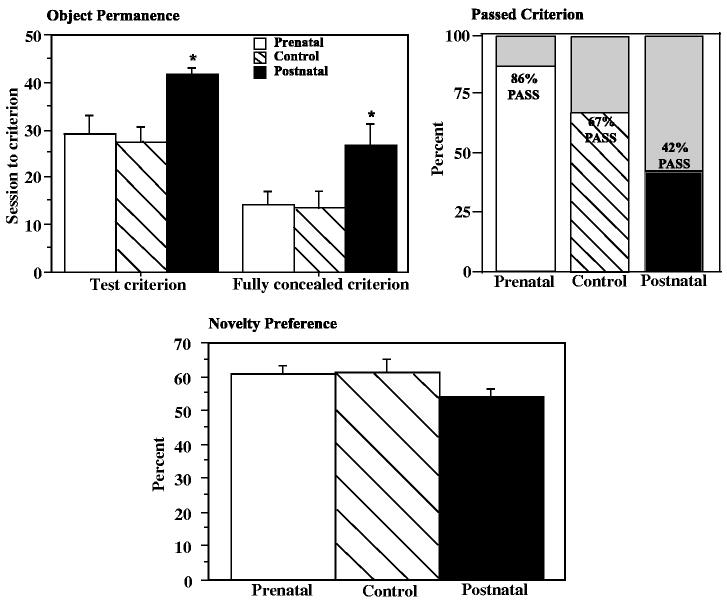
3.5. Cognitive testing: novelty preference and object permanence
The novelty preference and object permanence tests are intended to tap cognitive abilities of infants, although performance can be affected by motor, motivational and emotional factors.
Novelty preference scores are shown in Fig. 5 as the average of the three sets of problems presented at three ages. Sex and cohort were significant covariates on some problems. The majority of the infants preferred the novel stimulus on all problems except the last one. The proportion of infants with overall novelty preference was greater than chance for the control group (p = .02, binomial test, one-tailed), and the prenatally deprived group (p = .0008), but not the postnatally deprived group (p = .054). There were no group differences in average percent novelty preference over all seven problems. Examination of the data showed that very low preference scores in individual problems sometimes occurred in postnatally deprived infants. Across individual infant scores on individual problems (266 total), there were three preference scores less than 10% (1% of all scores), all recorded in the postnatally deprived infants, and 12 preference scores less than 20% (4% of all scores) of which seven were recorded in postnatally deprived infants.
Fig. 5.
Cognitive assessments in control, prenatally iron-deprived and postnatally iron-deprived rhesus monkey infants. Object permanence (top graphs): number of sessions required to meet criterion on all conditions (not hidden, partially hidden, fully hidden), and the number of sessions required to meet a less stringent criterion for the fully hidden condition (2/5 correct in one session) (left graph); the percent of infants reaching a criteria of 8/10 correct on all conditions (right graph). Novelty preference (bottom graph). Mean preference score (time looking at novel stimulus/time looking at both novel and familiar stimuli) averaged across seven problems at three testing ages. *p < .05 relative to controls, post hoc contrast of least square means.
Fewer postnatally deprived infants reached criterion (8 of 10 correct in two consecutive sessions) on the object permanence task (Fig. 5)(χ2 = 5.76, p = .056). More trials were required to reach criterion (F = 5.37, p = .009, control vs. postnatal p = .020) (infants not reaching criteria were given a score of 50 sessions). Further analysis was conducted to determine whether infants were slower in acquiring the concept of retrieving the fully covered food item, or in reaching a stable performance level. Groups were compared on the number of sessions before the fully covered food treat was retrieved at least two times in a session, thus demonstrating acquisition of the concept. Analysis indicated that the postnatally deprived group was delayed relative to controls (F = 4.23, p = .02, postnatal vs. control p = .02). There were no apparent group differences in performance of the A-not-B task in the 25 infants that reached criterion on object permanence. However group sizes were small in the control and postnatally deprived groups (n = 8, 5).
3.6. Emotionality and affect: biobehavioral characterization
This assessment was done in the context of introduction to a novel environment and to additional challenging components introduced individually into that environment (novel object, human intruder, monkey videotapes). Thus, the measures taken are considered to reflect the emotional or affective domain of behavior.
3.6.1. Living cage observations
Observations taken in the home cage during a 5-min period near the beginning and end of the 24 h novel environment experience were summarized as the duration of five activity states (Fig. 6) and a disturbance index (based on the frequency of nine distress-related behaviors). In addition, the frequency of environmental exploration was analyzed. Prenatally deprived infants had greater duration of motor activity (F = 3.32, p = .048, prenatal vs. control p < .05), a lower duration of lying down, and a higher incidence of exploratory activity (F = 4.04, p = .026, prenatal vs. control p < .05) than controls during the 5-min period which occurred within the first hour after the infants were first placed in the novel environment. The majority of infants (20/38) did not demonstrate any disturbance-related behaviors during the first 5 min; the major distress related behavior was withdrawal, manifested as sleeping or lying down in the bottom of the cage.
Fig. 6.
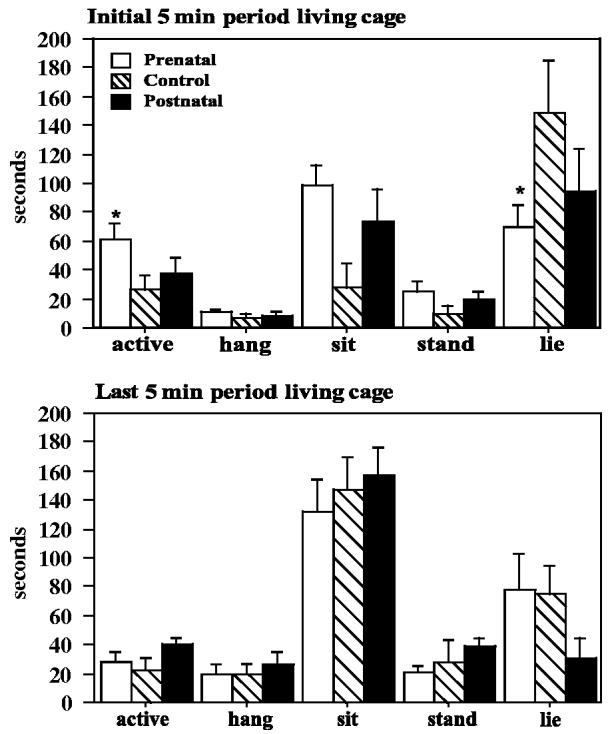
Durations of major behavior states during 5-min periods in the living cage near the beginning and the end of the 24-h biobehavioral characterization. Lying in the bottom of the cage, a withdrawal behavior, was more prominent shortly after introduction to the living cage. Prenatally iron-deprived infants demonstrated less lying behavior and more locomotor activity during the initial 5-min period, 15 min after the infants were relocated to the living cage. *p<.05 relative to controls, post hoc contrast least square means.
No iron deprivation effects were identified for the five activity states or for the composite distress index during the 5-min period observed near the end of the 24 h session.
3.6.2. Novel objects
Movement of novel objects in the living cage as detected by the automatic sensor did not differ between groups except during the initial period after the infants entered the cage. Data on the first novel object were available for a 45-min period beginning 30 min after the infants were transferred to the living cage when all aspects of the environment were novel. The sensors counts during this time varied from 0 to 20, with a median of 3, in a single 5-min period. Repeated measure analysis demonstrated a significant interaction between time and group (F = 1.9, p = .042); the prenatally deprived group had generally higher counts and the difference from control became larger later in the time period (Fig. 7). An individual group comparison was significant only at 30 min (F = 1.9, p = .05, prenatal vs. control p = .046). The major difference between groups was in the occurrence of 5 min periods with high sensor counts. The number of infants with >10 sensor counts in at least one 5 min period was 8/14 prenatal deprivation 3/12 control and 5/12 postnatal deprivation (not significant). Sensor counts from the first novel object later in the day, between challenge tests, and from the second novel object overnight were similar across the three groups.
Fig. 7.
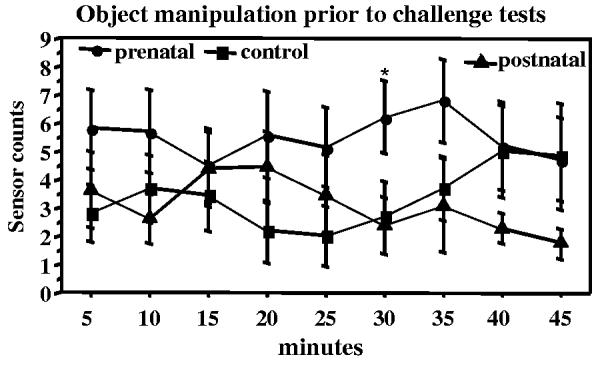
Manipulation of a novel object in the living cage during the biobehavioral characterization. Data shown is from a 45-min period after introduction into the living cage and before the human intruder test. Manipulation of the object was recorded by sensors (mercury switch) in the object. Prenatally iron-deprived infants showed more sensor counts than controls at some timepoints. *p<.05 relative to controls, post hoc contrast least square means.
3.6.3. Human intruder
Observations during the human intruder test demonstrated that the prenatal deprivation group was generally less active (locomotor activity) under all four conditions (Fig. 8). The difference in duration of time spent active was significant for the least challenging condition (profile far) F = 5.75, p = .01, prenatal vs. control p < .05), and also across all conditions (RMANOVA F(2, 35) = 4.8, p = .015, prenatal vs. control p = .05). Frequencies of locomotor activity were also lower in the prenatally deprived group than controls under the profile condition (profile far, F = 5.75, p < .01 prenatal vs. control p < .05; profile near F = 4.26, p = .02, prenatal vs. control p = .01). Activity decreased across the conditions in the control group, so that control and prenatal deprivation groups were similar under the near-stare condition. Corresponding to reduced locomotor activity was increased duration of sitting in the prenatally deprived group (RMANOVA across all conditions (F = 8.8, p < .001, prenatal vs. control p = .002). The groups did not differ in the amount of time spent in the cage nearer or farther from the intruder (data not shown). There was significant effect of condition on the distress index (F = 4.1, p = .01) with the frequency of distress-related behaviors increasing from the least to the most challenging condition. Analysis by group demonstrated a significant increase in the control group only from the profile far to the profile near condition (F=3.24, p=.051) and a significant increase in the prenatally deprived group only between the profile near and the stare far conditions (F=3.57, p=.03). The disturbance index of the postnatally deprived group was on the average higher than that of the other two groups and did not increase across conditions.
Fig. 8.
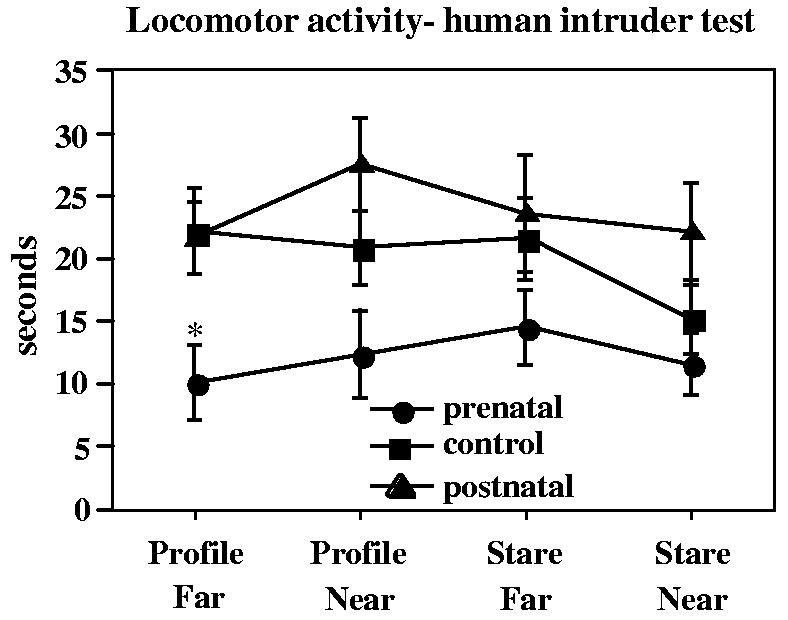
Duration of locomotor activity during the human intruder test of the biobehavioral characterization. There were four conditions in the human intruder test, each lasting 1 min, as shown on the x-axis. *p<.05 relative to controls, post hoc contrast least square means.
3.6.4. Monkey videotapes
Observations taken during the playing of videotapes of monkeys showed a similar pattern to the human intruder test in that the prenatally deprived group were less active, although no statistical group differences were demonstrated. The disturbance index was significantly greater for the aggressive than nonsocial video segments (paired t-test, t=4.41, p<.0001), but there was no group effect on the disturbance index during any video segment. The prenatally deprived group showed less cage roof clinging during most of the videotape segments, but group differences were significant for only for the second nonsocial segment (F=5.5, p=.01, prenatal vs. control p=.03). Cage roof clinging can be interpreted as part of a fleeing response in which infants move as far away as possible from the video monitor.
3.6.5. Affect rating
At the completion of the biobehavioral characterization, each infant was scored with a checklist of 20 adjectives using a 7-point scale. The adjective that best distinguished the prenatal group from controls was “fearful”—the prenatal group was significantly less fearful than controls (F=5.50, p=.01, prenatal vs. control p=.004) (Fig. 9). The postnatal group was best distinguished from controls as more “tense” (F=4.04, p=.03, control vs. postnatal p=.03). Cohort was a covariate in both analyses. The adjective descriptor for “fear” was “Fear grimace; retreats readily from others or from outside disturbances.” The adjective descriptor for “tense” was “Shows restraint in posture and movement; carries body stiffly, which suggests a shrinking tendency, as if trying to pull back and be less conspicuous.”
Fig. 9.
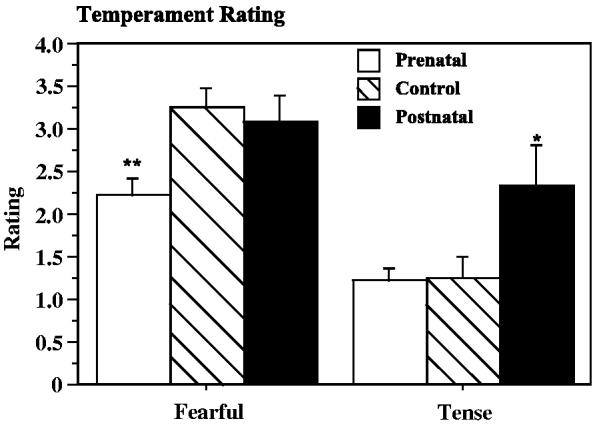
Affect quality rating categories (of 20 categories) showing differences between control, prenatally iron-deprived and postnatally iron-deprived rhesus monkey infants. *p<.05, **p<.01 post hoc contrast least square means vs. controls.
3.6.6. Adrenocortical response
Plasma cortisol decreased between the first sample, taken at the time of introduction to the novel environment, and the second sample taken five h later, decreased in response to dexamethasone and increased in response to ACTH (RMANOVA, F=23.29, p<.0001) (Table 6). The three experimental groups did not differ in adrenocortical response at any of the timepoints or overall. Covariates did not affect cortisol measures. Profile analysis indicated an interaction between group and change in cortisol at transfer to the living cage and 5 h after transfer; cortisol values of controls decreased during this interval (F=7.13, p=.02) while those of the other two groups did not change significantly.
Table 6.
Adrenocortical response during the biobehavioral characterization
| 2 h after relocation | 7 h after relocation | 16 h after dexamethasone | 30 min after ACTH | |
|---|---|---|---|---|
| Prenatal | 47 ± 6a | 42 ± 5 | 22 ± 4 | 64 ± 5 |
| Control | 63 ± 6 | 48 ± 5 | 26 ± 4 | 63 ± 3 |
| Postnatal | 65 ± 9 | 51 ± 6 | 25 ± 2 | 66 ± 3 |
Mean ± SEM, μg/mL.
3.7. Activity monitoring
Activity was measured as whole body movements that triggered the mercury switch of the actimeter. Mean activity averaged across the 48-h period (counts per 2-min period) was about 20% lower in the prenatal group than controls (F=5.14, p=.01, prenatal vs. control, p=.04) and somewhat higher in the postnatal group, although not significant in pairwise comparisons (Fig. 10). The same pattern was seen for daytime activity as for overall activity. There were no group differences in the duration of wake and sleep periods. However, this analysis may have been complicated by a very large cohort effect; some infants in the first cohort had much earlier “bedtimes” than those in the second cohort. We attributed this to a different age range of infants together in the nursery in the two cohorts. The groups did not differ in length of the longest period of inactivity during the day or night. However, closer examination of individual animal data suggested prolonged periods of inactivity and very low average activity counts in several of the prenatally deprived monkeys in the home cage.
Fig. 10.
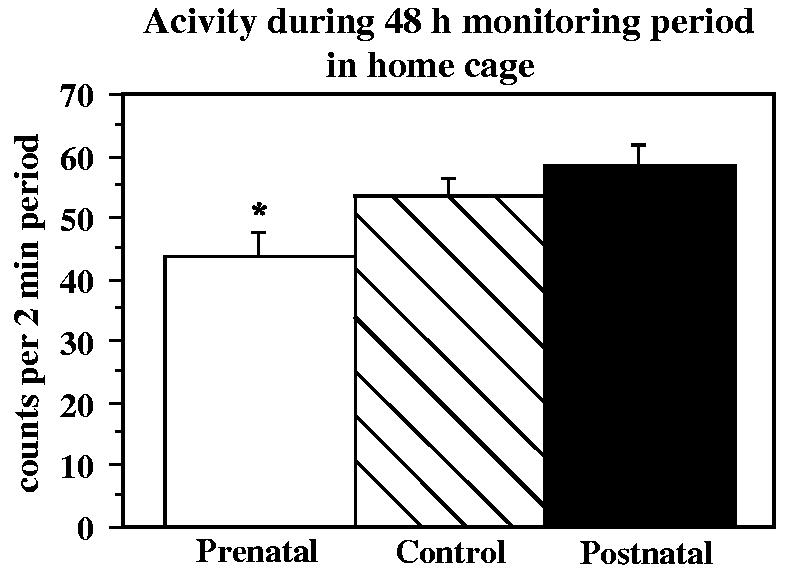
Actimeter counts during a 48-h period in the home cage at four months of age in control, prenatally iron-deprived and postnatally iron-deprived rhesus monkey infants. *p<.05 relative to controls, post hoc contrast least square means.
Because of consistent group differences in activity across the biobehavioral characterization observations and the actimeter monitoring, both of which were conducted at 3–4 months of age, a regression analysis was conducted using hematological measures and activity measures at four months. Activity measures were highly correlated within conditions (human intruder, monkey videotapes, actimeter monitoring) and showed modest associations across conditions, but were not significantly associated with any of the hematological measures (hemoglobin, hematocrit, MCV, MCH, MCHC, ferritin).
A summary of the findings by functional domain is presented in Table 7.
Table 7.
Summary of findings concerning time-dependent effects of developmental iron deprivation on behavior by functional domain
| Prenatal iron deprivation | Postnatal iron deprivation | |
|---|---|---|
| Motor: gross | No effects | No effects |
| Motor: fine | No effects | No effects |
| Cognition | No effects | Delayed object concept |
| Emotionality | Less inhibition (novel environment) more changes between behaviors | Heightened emotionality (familiar environment) |
| Affect rating | Less fearful | More tense |
| Activity | Lower (familiar environment) | Trend toward higher activity |
4. Discussion
No effects of iron deprivation on growth were detected with fairly extensive morphometric exams. Growth retardation is not a common finding unless IDA is severe; retarded growth linked to altered metabolism has been demonstrated in rodent models [38,3] and stunting is found in connection with anemia in generally malnourished populations [34]. A smaller head width was detected in the prenatally deprived group, but head circumferences did not differ, suggesting a conformational rather than growth related source of the head width difference. Head circumference is one of the measures associated with growth retardation in newborns of mothers with moderate or severe anemia [8,62].
An important characteristic of this study was that no group differences in hematology or iron status were observed in the monkey infants at one or four months of age. Lower hemoglobin and red cell parameters seen at birth in the prenatally deprived group [26] had apparently recovered by one month of age. Hematological parameters showed maturation-related changes as seen in human infants including a decrease in ferritin near the end of the nursing period, which was somewhat greater in the postnatally deprived group than in controls. Criteria are not available for determining anemia in infant monkeys; one prenatally deprived infant had hemoglobin <11 g/dL at one month of age, and one prenatally deprived and one postnatally deprived infant had hemoglobin <11 g/dL at four months of age. All three of these values were >10 g/dL.
Lack of effect of the low iron formula (postnatal iron deprivation) on hematology, clinical condition and growth is in agreement with clinical trials in healthy well-nourished human infants fed iron supplemented or non-supplemented (low iron) formulas [49,32,61,65]. In contrast, several behavioral measures were influenced by postnatal iron deprivations relative to controls, as has been suggested by studies of high risk populations with iron supplemented and non-supplemented formulas [49]. (It should be recognized that breast feeding, rather than commercial formula, is the most appropriate normal or control condition for infant nutrition of both human and nonhuman primates.)
The behavioral test battery attempted to assess three domains of behavior, motor, cognitive and emotional, that have been implicated by studies of iron deficiency in human infants using a test battery constructed to parallel an ongoing human study of iron deficiency.
No effect on motor development was seen in this study, although both gross and fine motor delays are a characteristic finding in studies of anemic human infants and motor deficits often respond to iron supplementation in infancy. Differences in design and evaluation methods may be relevant. For instance, human motor testing usually involves verbal instruction and encouragement by the tester or observation by the caretaker rather than sequential, time sampled observations as used in our experiment. Also, most gross motor abilities were acquired by the infant monkeys in the first two months of life while postnatal iron depletion was likely to appear only toward the end of the formula feeding period. But the most important difference from human and rodent studies may be the fact that our monkey infants were not anemic at the time of evaluation. An interpretation consistent with both monkey and human studies on infant motor effects of IDA is that motor development effects require concurrent iron deficiency anemia.
A potential effect of postnatal iron deprivation on cognitive development was seen in the object permanence test. Fewer infants in the postnatally deprived group reached criterion on this test after extensive testing, and the infants that did reach criterion required more sessions, suggesting a later appearance of this cognitive ability. Group effects were not seen for visual novelty preference, a test of recognition memory that is predictive of later cognitive ability in humans [56]. Novelty preference does not have a discrete age-related appearance during maturation. The Bayley Scales are commonly used to test cognitive ability of ID/IDA infants; one experiment that used novelty preference and object permanence did not find effects of a combination energy/micronutrient supplement [55].
No effects of prenatal iron deprivation were seen in the novelty preference and object permanence tests. Cognitive deficits associated with prenatal iron deprivation are little studied in human infants. Tamura et al. [64] studied the association between previously obtained cord blood ferritin and scores of five-year olds on motor and cognitive tests, including the Wechsler IQ test. Children with cord blood ferritin in the lowest quartile had significantly lower full scale IQs and about twice as many fell below 70 points.
The most striking effects of the iron deprived diets in this study appeared on measures of emotion/reactivity. These group differences were seen during testing conducted at the end of the four month evaluation period. Heightened emotionality, as reflected by withdrawal (lying down) and vocalizations was seen in the postnatally deprived group in the weekly observations. Postnatally deprived infants were also rated as more “tense” than controls during biobehavioral characterization. On the other hand, prenatally deprived infants appeared to react less strongly to relocation during the biobehavioral characterization test. Prenatally deprived monkeys were judged less “fearful” and also tended to explore their environment and manipulate the novel object in their living cage more during biobehavioral characterization.
Prenatally deprived monkey infants were less active over the 48-h actimeter monitoring period in the home cage and during the latter part of the 24-h relocation period during the biobehavioral characterization. This agrees with findings in rats that limited periods of dietary iron deprivation during brain development can lead to long-lasting changes in spontaneous activity levels [53], and also findings of lower activity in anemic children after treatment with iron [2]. Experiments with reduced activity and concurrent iron deficiency in rats have demonstrated associations with brain dopamine systems (reduced iron content and dopamine D1 receptor density in midbrain) [4]. Postnatally deprived monkey infants did not have reduced activity levels. Although reduced activity has been shown in anemic rats [53,16] and humans during development, it is important to note that our postnatally deprived monkey infants were not anemic.
Differences from controls in activity level in the prenatally deprived infants were situation specific. Prenatally deprived infants were more active during the initial period after relocation to a new environment, but were generally found to be less active later in the relocation period, and also in their home cages during 48-h actimeter monitoring. Activity durations were not recorded in the familiar weekly observation situation. However, the number of initiations of different behaviors was greater in the prenatally deprived infants toward the end of the observation period. Different characteristics of the infants can be reflected in activity measures in different situations, depending on whether they are novel or familiar and simple or complex. The general finding of reduced activity in familiar, nonthreatening situations in the prenatally deprived group suggests that lower activity in IDA infants may be a result of earlier rather than concurrent iron deficiency. Postnatally deprived infants had higher average activity scores than controls under familiar nonthreatening environmental conditions. However, post hoc comparisons to controls were not significant.
Effects on affect and emotionality are consistently found in human infant studies of iron deficiency. One study coded affect from videotapes made during a 15-min play session and administration of the Bayley scales to 12–23 month old IDA infants [44]. They recorded less participation in motor subscales of the Bayley, less activity and exploration during the play session and greater proximity to caregivers during both testing and play. The Bayley Infant Behavior Record also characterized the infants as showing markedly less endurance and also less responsiveness to the investigator. Differences between IDA and non-IDA infants were reported to be present after three months of iron supplementation, which corrected the IDA. These test groups can be contrasted to our sample of monkey infants, none of whom met the criteria for IDA when they were being evaluated.
Another study [66] correlated temperament scores (standardized 8 item, 4 to 6 point scale) of neonates (first week of life) observed at home and in the laboratory before discharge from the hospital to cord blood hemoglobin, serum iron and ferritin. They found a situation dependent (home, laboratory) pattern of results and concluded that low hemoglobin and iron were associated with “higher levels of emotionality and lower levels of alertness and soothability.”
In summary, several findings noted in human infants with ID and IDA, namely delayed gross and fine motor development and apathy/lethargy, were not seen in this study. This may be due to the fact that the monkey infants did not have concurrent iron deficiency (ID or IDA at the time of evaluation) as reflected in hematological indices, although their dietary iron intake was inadequate. However, other characteristics of ID infants such as reduced activity, heightened emotionality, and cognitive deficits were seen. These three characteristics were associated with different periods of iron deprivation during brain development.
A new and potentially valuable finding relevant to human populations is the ability of the prenatal iron deprivation, repleted at birth, to influence infant behavior. Few human studies have approached this issue. One study found an effect of prenatal iron deficiency, as reflected in quartiles of ferritin in cord blood at birth, on IQ, fine motor, language skills and conduct (tractability) [64]. However, postnatal iron deprivation may have covaried with prenatal deprivation and was not statistically controlled for in this study. While pre and postnatal iron deprivation are unlikely to be dissociated in generally malnourished populations, the extensive use of subsidized iron-fortified formulas in some developed countries could lead to this situation.
Finally, some new effects of developmental-stage specific iron deprivation on emotionality were identified. Prenatally deprived infants were less fearful and inhibited than controls in novel environments, and postnatally deprived infants demonstrated more distress behavior in familiar environments. These emotionality/affect characteristics, if they persist, could be antecedents of later behavioral dysfunction related to school performance and conduct disorders in populations with iron deprived diets in pregnancy and infancy.
Acknowledgments
Dr. Golub designed the experiment, analyzed the data and wrote the manuscript. Ms. Hogrefe and Ms. Germann designed and wrote the experimental protocols and were responsible for day-to-day conduct of the study. The authors acknowledge the contribution of Laura DelRosso, who conducted the biobehavioral characterization, Abigail Spinner who supervises the CNPRC clinical laboratory that performed the CBCs, Kelly Weaver who supervises the CNPRC nonhuman primate nurseries, and program project investigators who have provided information and feedback throughout the project. Supported by PO1 HD39386, Betsy Lozoff, Principal Investigator, and RR00169.
References
- 1.Anderson J, Keen C, Lonnerdal B. Iron deficiency in outdoor corral housed juvenile rhesus monkeys. Lab. Anim. Sci. 1983;33:494. [Google Scholar]
- 2.Angulo-Kinzler RM, Peirano P, Lin E, Garrido M, Lozoff B. Spontaneous motor activity in human infants with iron-deficiency anemia. Early Hum. Dev. 2002;66:67–79. doi: 10.1016/s0378-3782(01)00238-9. [DOI] [PubMed] [Google Scholar]
- 3.Beard JL, Zhan CS, Brigham DE. Growth in iron-deficient rats. Proc. Soc. Exp. Biol. Med. 1995;209:65–72. doi: 10.3181/00379727-209-43879. [DOI] [PubMed] [Google Scholar]
- 4.Beard JL, Erikson KM, Jones BC. Neurobehavioral analysis of developmental iron deficiency in rats. Behav. Brain Res. 2002;134:517–524. doi: 10.1016/s0166-4328(02)00092-x. [DOI] [PubMed] [Google Scholar]
- 5.Beard J, Erikson KM, Jones BC. Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J. Nutr. 2003;133:1174–1179. doi: 10.1093/jn/133.4.1174. [DOI] [PubMed] [Google Scholar]
- 6.Beard JL, Wiesinger JA, Connor JR. Pre- and postweaning iron deficiency alters myelination in Sprague – Dawley rats. Dev. Neurosci. 2003;25:308–315. doi: 10.1159/000073507. [DOI] [PubMed] [Google Scholar]
- 7.Bicknese EM, George JW, Hird DW, Anderson JH. Prevalence and risk factors for iron deficiency anemia in weanling rhesus macaques. Lab. Anim. Sci. 1993;43:434–438. [PubMed] [Google Scholar]
- 8.Camp BW, Broman SH, Nichols PL, Leff M. Maternal and neonatal risk factors for mental retardation: defining the ‘at-risk’ child. Early Hum. Dev. 1998;50:159–173. doi: 10.1016/s0378-3732(97)00034-9. [DOI] [PubMed] [Google Scholar]
- 9.Capitanio JP, Mason WA, Mendoza SP, DelRosso L, Roberts JA. Nursery rearing and biobehavioral organization. In: Sackett GP, Ruppenthal G, editors. Nursery Rearing of Non-human Primates in the 21st century. Kluwer/Plenum; in press. [Google Scholar]
- 10.Champoux M, Suomi S. Behavioral development of nursery-reared rhesus macaque (Macaca mulatta) neonates. Infant Behav. Dev. 1988;11:363–367. [Google Scholar]
- 11.Chao AC, Ziadeh BI, Diau GY, Wijendran V, Sarkadi-Nagy E, Hsieh AT, Nathanielsz PW, Brenna JT. Influence of dietary long-chain PUFA on premature baboon lung FA and dipalmitoyl PC composition. Lipids. 2003;38:425–429. doi: 10.1007/s11745-003-1079-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen Q, Connor JR, Beard JL. Brain iron, transferrin and ferritin concentrations are altered in developing iron-deficient rats. J. Nutr. 1995;125:1529–1535. doi: 10.1093/jn/125.6.1529. [DOI] [PubMed] [Google Scholar]
- 13.Davidson LA, Litov RE, Lonnerdal B. Iron retention from lactoferrin-supplemented formulas in infant rhesus monkeys. Pediatr. Res. 1990;27:176–180. doi: 10.1203/00006450-199002000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Diamond A, Goldman-Rakic P. Comparison of human infants and rhesus monkeys on Piaget's A-not-B task: evidence for dependence on dorsolateral prefrontal cortex. Exp. Brain Res. 1989;74:24–40. doi: 10.1007/BF00248277. [DOI] [PubMed] [Google Scholar]
- 15.Fagan JF, Singer LT. Infant recognition memory as a measure of intelligence. In: Lipsitt LP, editor. Advances in Infancy Research. Ablex; Norwood, NJ: 1983. pp. 31–78. [Google Scholar]
- 16.Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J. Nutr. 1996;126:693–701. doi: 10.1093/jn/126.3.693. [DOI] [PubMed] [Google Scholar]
- 17.Golub MS. Use of monkey neonatal neurobehavioral test batteries in safety testing protocols. Neurotoxicol. Teratol. 1990;12:537–541. doi: 10.1016/0892-0362(90)90019-9. [DOI] [PubMed] [Google Scholar]
- 18.Golub MS, Donald JM. Effect of intrapartum meperidine on behavior of 3- to 12- month-old infant rhesus monkeys. Biol. Neonate. 1995;67:140–148. doi: 10.1159/000244155. [DOI] [PubMed] [Google Scholar]
- 19.Golub MS, Germann SL. Perinatal bupivacaine and infant behavior in rhesus monkeys. Neurotoxicol. Teratol. 1998;20:29–41. doi: 10.1016/s0892-0362(97)00068-8. [DOI] [PubMed] [Google Scholar]
- 20.Golub MS, Gershwin ME. Standardized neonatal assessment in the rhesus monkey. In: Nathanielsz JTPPW, editor. Research in Perinatal Medicine. Plenum Press; NY: 1984. pp. 56–86. [Google Scholar]
- 21.Golub MS, Eisele JH, Jr., Donald JM. Obstetric analgesia and infant outcome in monkeys: infant development after intrapartum exposure to meperidine or alfentanil. Am. J. Obstet. Gynecol. 1988;159:1280–1286. doi: 10.1016/0002-9378(88)90464-4. [DOI] [PubMed] [Google Scholar]
- 22.Golub MS, Eisele JH, Jr., Donald JM. Effect of intrapartum meperidine on the behavioral consequences of neonatal oxygen deprivation in rhesus monkey infants. Dev. Pharmacol. Ther. 1991;16:231–240. [PubMed] [Google Scholar]
- 23.Golub MS, Galliher NJ, Working PK, Greenspan A. Twelve month evaluation of rhesus monkey dams and infants after hRlx-2 infusion during late pregnancy. Reprod. Toxicol. 1996;10:29–36. doi: 10.1016/0890-6238(95)02015-2. [DOI] [PubMed] [Google Scholar]
- 24.Golub MS, Takeuchi PT, Keen CL, Hendrickx AG, Gershwin ME. Activity and attention in zinc-deprived adolescent monkeys. Am. J. Clin. Nutr. 1996;64:908–915. doi: 10.1093/ajcn/64.6.908. [DOI] [PubMed] [Google Scholar]
- 25.Golub MS, Keen CL, Gershwin ME. Behavioral and hematologic consequences of marginal iron – zinc nutrition in adolescent monkeys and the effect of a powdered beef supplement. Am. J. Clin. Nutr. 1999;70:1059–1068. doi: 10.1093/ajcn/70.6.1059. [DOI] [PubMed] [Google Scholar]
- 26.Golub MS, Hogrefe CE, Tarantal AF, Germann SL, Beard JL, Calatroni A. Diet-induced iron deficiency anemia and pregnancy outcome in the rhesus monkey. Am. J. Clin. Nutr. doi: 10.1093/ajcn.83.3.647. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golub MS, Hogrefe CE, Germann SL, Tran TL, Beard JL, Crinella FM, Lonnerdal B. Neurobehavioral evaluation of rhesus monkey infants fed cow's milk formula, soy formula, or soy formula with added manganese. Neurotoxicol. Teratol. 2005;27:615–627. doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131:649S–666S. doi: 10.1093/jn/131.2.649S. discussion 666S – 668S. [DOI] [PubMed] [Google Scholar]
- 29.Gunderson VM, Sackett GP. Development of pattern recognition in infant pig-tailed macaques (Macaca nemestrina) Dev. Psychol. 1984;20:418–426. [Google Scholar]
- 30.Gunderson VM, Grant KS, Burbacher TM. The effect of low-level prenatal methylmercury exposure on visual recognition memory in infant crab-eating macaques. Child Dev. 1986;57:1076–1083. [PubMed] [Google Scholar]
- 31.Hansen EW. The developmental of maternal and infant behavior in the rhesus monkey. Behaviour. 1966;27:107–149. doi: 10.1163/156853966x00128. [DOI] [PubMed] [Google Scholar]
- 32.Haschke F, Vanura H, Male C, Owen G, Pietschnig B, Schuster E, Krobath E, Huemer C. Iron nutrition and growth of breast- and formula-fed infants during the first 9 months of life. J. Pediatr. Gastroenterol. Nutr. 1993;16:151–156. doi: 10.1097/00005176-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Imaki H, Neuringer M, Sturman J. Long-term effects on retina of rhesus monkeys fed taurine-free human infant formula. Adv. Exp. Med. Biol. 1996;403:351–360. doi: 10.1007/978-1-4899-0182-8_37. [DOI] [PubMed] [Google Scholar]
- 34.Kariger PK, Stoltzfus RJ, Olney D, Sazawal S, Black R, Tielsch JM, Frongillo EA, Khalfan SS, Pollitt E. Iron deficiency and physical growth predict attainment of walking but not crawling in poorly nourished Zanzibari infants. J. Nutr. 2005;135:814–819. doi: 10.1093/jn/135.4.814. [DOI] [PubMed] [Google Scholar]
- 35.Kelleher SL, Casas I, Carbajal N, Lonnerdal B. Supplementation of infant formula with the probiotic Lactobacillus reuteri and zinc: impact on enteric infection and nutrition in infant rhesus monkeys. J. Pediatr. Gastroenterol. Nutr. 2002;35:162–168. doi: 10.1097/00005176-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Kriete MF, Champoux M, Suomi SJ. Development of iron deficiency anemia in infant rhesus macaques. Lab. Anim. Sci. 1995;45:15–21. [PubMed] [Google Scholar]
- 37.Kwik-Uribe CL, Gietzen D, German JB, Golub MS, Keen CL. Chronic marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition. J. Nutr. 2000;130:2821–2830. doi: 10.1093/jn/130.11.2821. [DOI] [PubMed] [Google Scholar]
- 38.Kwik-Uribe CL, Golub MS, Keen CL. Chronic marginal iron intakes during early development in mice alter brain iron concentrations and behavior despite postnatal iron supplementation. J. Nutr. 2000;130:2040–2048. doi: 10.1093/jn/130.8.2040. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence D. The development of motor control in the rhesus monkey: evidence concerning the role of corticomotoneuronal connections. Brain. 1976;99:235–254. doi: 10.1093/brain/99.2.235. [DOI] [PubMed] [Google Scholar]
- 40.Lewis DS, McMahan CA, Mott GE. Breast feeding and formula feeding affect differently plasma thyroid hormone concentrations in infant baboons. Biol. Neonate. 1993;63:327–335. doi: 10.1159/000243949. [DOI] [PubMed] [Google Scholar]
- 41.Lonnerdal B, Bell JG, Hendrickx AG, Burns RA, Keen CL. Effect of phytate removal on zinc absorption from soy formula. Am. J. Clin. Nutr. 1988;48:1301–1306. doi: 10.1093/ajcn/48.5.1301. [DOI] [PubMed] [Google Scholar]
- 42.Lonnerdal B, Jayawickrama L, Lien EL. Effect of reducing the phytate content and of partially hydrolyzing the protein in soy formula on zinc and copper absorption and status in infant rhesus monkeys and rat pups. Am. J. Clin. Nutr. 1999;69:490–496. doi: 10.1093/ajcn/69.3.490. [DOI] [PubMed] [Google Scholar]
- 43.Lonnerdal B, Kelleher SL, Lien EL. Extent of thermal processing of infant formula affects copper status in infant rhesus monkeys. Am. J. Clin. Nutr. 2001;73:914–919. doi: 10.1093/ajcn/73.5.914. [DOI] [PubMed] [Google Scholar]
- 44.Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. Behavior of infants with iron-deficiency anemia. Child. Dev. 1998;69:24–36. [PubMed] [Google Scholar]
- 45.Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112:846–854. [PubMed] [Google Scholar]
- 46.Martin S, Logan S, Gilbert R. Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency (Review) In: TC, editor. Collaboration. Vol. 4. John Wiley; New York: 2004. [DOI] [PubMed] [Google Scholar]
- 47.McCarty ME, Clifton RK, Ashmead DH, Lee P, Goubet N. How infants use vision for grasping objects. Child Dev. 2001;72:973–987. doi: 10.1111/1467-8624.00329. [DOI] [PubMed] [Google Scholar]
- 48.Mendelson M. Clinical examination of visual and social responses in infant monkeys. Dev. Psychobiol. 1982;18:658–664. [Google Scholar]
- 49.Moffatt ME, Longstaffe S, Besant J, Dureski C. Prevention of iron deficiency and psychomotor decline in high-risk infants through use of iron-fortified infant formula: a randomized clinical trial. J. Pediatr. 1994;125:527–534. doi: 10.1016/s0022-3476(94)70003-6. [DOI] [PubMed] [Google Scholar]
- 50.Mowbray J, Caddell T. Early behavior patterns of rhesus monkeys. J. Com. Physiol. Psychol. 1962;55:350–357. doi: 10.1037/h0041420. [DOI] [PubMed] [Google Scholar]
- 51.Neuringer M, Sturman J. Visual acuity loss in rhesus monkey infants fed a taurine-free human infant formula. J. Neurosci. Res. 1987;18:597–601. doi: 10.1002/jnr.490180413. [DOI] [PubMed] [Google Scholar]
- 52.Piaget J. The Construction of Reality in the Child. Basic Books; New York: 1954. [Google Scholar]
- 53.Pinero D, Jones B, Beard J. Variations in dietary iron alter behavior in developing rats. J. Nutr. 2001;131:311–318. doi: 10.1093/jn/131.2.311. [DOI] [PubMed] [Google Scholar]
- 54.Polberger S, Fletcher MP, Graham TW, Vruwink K, Gershwin ME, Lonnerdal B. Effect of infant formula zinc and iron level on zinc absorption, zinc status, and immune function in infant rhesus monkeys. J. Pediatr. Gastroenterol. Nutr. 1996;22:134–143. doi: 10.1097/00005176-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Pollitt E, Saco-Pollitt C, Jahari A, Husaini MA, Huang J. Effects of an energy and micronutrient supplement on mental development and behavior under natural conditions in undernourished children in Indonesia. Eur. J. Clin. Nutr. 2000;54(Suppl 2):S80–S90. doi: 10.1038/sj.ejcn.1601009. [DOI] [PubMed] [Google Scholar]
- 56.Rose S, Feldman J. Prediction of IQ and specific cognitive abilities at 11 years from infancy measures. Dev. Psychol. 1995;31:685–696. [Google Scholar]
- 57.Rudloff S, Lonnerdal B. Calcium retention from milk-based infant formulas, whey – hydrolysate formula, and human milk in weanling rhesus monkeys. Am. J. Dis. Child. 1990;144:360–363. doi: 10.1001/archpedi.1990.02150270110037. [DOI] [PubMed] [Google Scholar]
- 58.Rudloff S, Lonnerdal B. Calcium and zinc retention from protein hydrolysate formulas in suckling rhesus monkeys. Am. J. Dis. Child. 1992;146:588–591. doi: 10.1001/archpedi.1992.02160170068017. [DOI] [PubMed] [Google Scholar]
- 59.Sachdev H, Gera T, Nestel P. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr. 2005;8:117–132. doi: 10.1079/phn2004677. [DOI] [PubMed] [Google Scholar]
- 60.Schneider M, Suomi S. Neurobehavioral assessment in rhesus monkey neonates (Macaca mulatta): developmental changes, behavioral stability, and early experience. Infant Behav. Dev. 1992;15:155–177. [Google Scholar]
- 61.Singhal A, Morley R, Abbott R, Fairweather-Tait S, Stephenson T, Lucas A. Clinical safety of iron-fortified formulas. Pediatrics. 2000;105:E38. doi: 10.1542/peds.105.3.e38. [DOI] [PubMed] [Google Scholar]
- 62.Singla PN, Tyagi M, Kumar A, Dash D, Shankar R. Fetal growth in maternal anaemia. J. Trop. Pediatr. 1997;43:89–92. doi: 10.1093/tropej/43.2.89. [DOI] [PubMed] [Google Scholar]
- 63.Stevenson-Hinde J, Stillwell-Barnes R, Zunz M. Subjective assessment of rhesus monkeys over four successive years. Primates. 1980;21:66–82. [Google Scholar]
- 64.Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J. Pediatr. 2002;140:165–170. doi: 10.1067/mpd.2002.120688. [DOI] [PubMed] [Google Scholar]
- 65.Tuthill DP, Cosgrove M, Dunstan F, Stuart ML, Wells JC, Davies DP. Randomized double-blind controlled trial on the effects on iron status in the first year between a no added iron and standard infant formula received for three months. Acta Paediatr. 2002;91:119–124. doi: 10.1080/080352502317285072. [DOI] [PubMed] [Google Scholar]
- 66.Wachs TD, Pollitt E, Cueto S, Jacoby E, Creed-Kanashiro H. Relation of neonatal iron status to individual variability in neonatal temperament. Dev. Psychobiol. 2005;46:141–153. doi: 10.1002/dev.20049. [DOI] [PubMed] [Google Scholar]
- 67.Weinberg J, Levine S, Dallman PR. Long-term consequences of early iron deficiency in the rat. Pharmacol. Biochem. Behav. 1979;11:631–638. doi: 10.1016/0091-3057(79)90254-5. [DOI] [PubMed] [Google Scholar]