Abstract
Objective
The impact of a couple’s knowledge about healthy pregnancy habits involving alcohol and substance use was assessed in the context of other factors previously identified to predict prenatal alcohol consumption in a sample of 254 pregnant women and their male partners.
Method
Couples were asked to assess independently a series of statements (true or false) describing the consequences of prenatal substance exposure, while also providing information about their own drinking.
Results
Although the couples demonstrated good knowledge of healthy habits during pregnancy, they did not agree when the element of chance was considered. Median household income was more highly predictive of a pregnant woman’s knowledge score than her partner’s score. In turn, the subject’s knowledge of healthy pregnancy habits as manifested in the assessment score had only a weak relationship with prenatal alcohol consumption. Previous alcohol use by the pregnant woman was the strongest predictor of prenatal alcohol use.
Conclusions
Because previous alcohol consumption use by the pregnant woman was the strongest predictor of prenatal alcohol use, the importance of its accurate identification is emphasized. Although pending further investigation, knowledge about healthy pregnancy behaviors may exert greater impact if it is shared by a pregnant woman and her partner.
ABSTINENCE FROM ALCOHOL IS the recommendation of the United States Surgeon General, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists to pregnant and preconceptional women (Office of the Surgeon General, 2005; Sokol et al., 2003). Yet, estimates for prenatal alcohol use range from 5% to 15% among American women, with higher rates reported in other countries (Flynn et al., 2003; McLeod et al., 2002; Pirie et al., 2000). Results from several large scale surveillance studies, such as the Behavioral Risk Factor Surveillance System and the Pregnancy Risk Assessment Monitoring System, indicate certain consistent demographic predictors of prenatal use, including older age, non-Hispanic background, education exceeding high school, and employment (Floyd et al., 1999; Phares et al., 2004). Other studies have shown that alcohol consumption before pregnancy predicts antenatal consumption as well (Chang et al., 1999b; Day et al., 1993).
In contrast, the role of expectant fathers in pregnancy outcome has received less attention. Their potential importance is appreciated primarily on an intuitive or anecdotal level, because most of the literature and research regarding responsibility for fetal health and well-being has concentrated on maternal characteristics and behavior. However, male behavior may have substantial effects at the time of conception (such as producing damaged sperm because of teratogen exposure) or during gestation (in terms of behavioral, emotional, or even material support; Losco and Shublak, 1994). A partner’s health habits and knowledge may influence specific behaviors during pregnancy. For example, research on breast-feeding has consistently identified fathers as an important source of support in the decision to breast-feed and its implementation (Bar-Yam and Darby, 1997; Wolfberg et al., 2004). Partners’ smoking habits have been one of the strongest predictors of prenatal cigarette use (Olsen, 1993; Waterson et al., 1990). Maternal drinking is highly correlated with paternal drinking (Passaro et al., 1998), because the premarriage alcohol consumption of husbands has been found to be unilaterally influential on their wives’ drinking after marriage (Leonard and Das Eiden, 1999; Leonard and Mudar, 2003). Thus, educational efforts in India, Turkey, and Sweden have focused on including expectant fathers in antenatal education, resulting in positive effects on knowledge, attitudes, and behaviors (Finnbogadottir et al., 2003; Hallgren et al., 1999; Pachuri, 2001; Turan et al., 2001).
The purpose of this study was to assess the impact of a couple’s knowledge about healthy habits during pregnancy in the context of other factors known to affect prenatal alcohol consumption in a sample of 254 pregnant women and their partners, drawn from a larger study (Chang et al., 2005). The pregnant women, who themselves were all alcohol screen-positive, and their male partners, identified as husbands or biological fathers, were asked to assess independently as true or false a series of statements about the use of alcohol, caffeine, and illicit substances during pregnancy. Structural equation modeling was then used to investigate the potential linkages between paternal and maternal knowledge of healthy prenatal practices, demographic factors (such as household income), and previous maternal alcohol use on prenatal alcohol consumption.
Method
Participants included 254 of 304 couples enrolled in a randomized trial of a brief intervention for reducing alcohol consumption in pregnancy. All provided written informed consent for this study that was reviewed and approved by the institutional Human Research Office. The 304 pregnant women were T-ACE alcohol screen-positive and also satisfied other eligibility criteria, including gestation less than 28 weeks, any alcohol consumption while pregnant, drinking in excess of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) sensible drinking limits before pregnancy (more than seven standard drinks per week or more than two standard drinks per episode [NIAAA, 1995a]), and being able to involve a partner of her choice. The T-ACE is a 4-item questionnaire that assesses pregnant women for risk drinking in a clinical practice setting (Chang et al., 1998; Sokol et al., 1989). This sample was limited to only those 254 couples in which the partner was the father of the child.
The pregnant women were asked to complete the following measures at the time of study enrollment: (1) Health and Habits Survey, which contained the T-ACE and questions about stress, exercise, diet, and history of cigarette use; (2) alcohol Timeline Followback (TLFB; Sobell and Sobell, 1992) to obtain estimates of their daily drinking in the 6 months before study enrollment; and (3) Healthy Pregnancy Facts, a series of seven statements about healthy habits during pregnancy that the respondent was asked to judge as true or false. These statements were developed because they reflected current knowledge on the consequences of prenatal substance exposure and were reviewed for accuracy and readability by the study’s obstetric team. After delivery, the women provided estimates of daily alcohol consumption in the time period after study enrollment using the TLFB.
At study enrollment, the partners completed the following items: (1) Health and Habits Survey (as described previously); (2) NIAAA quantity/frequency questions about their use of beer, wine, whiskey, gin, or other distilled spirits in the previous 30 days (NIAAA, 1995b); and (3) Healthy Pregnancy Facts (as described previously). Household income was based on home zip code and served as a measure of socioeconomic status.
Data analysis
Data were analyzed using SAS 8.2 (SAS Institute, Inc., Cary, NC). Results are reported as percentages and medians. To measure the agreement between women and their male partners on knowledge about pregnancy health habits, the kappa statistic and McNemar’s Index of Bias were used. The kappa statistic is an index of concordance that takes into account agreement resulting from the element of chance. It ranges in value from <0 (poor agreement) to 1 (perfect agreement; Feinstein, 1985). McNemar’s Index of Bias expresses the difference in disagreements as a proportion of the total number of disagreements (Landis and Koch, 1977).
Structural equation modeling (SEM) was used to estimate the relationships among mediating variables and antenatal alcohol consumption (Bollen, 1989). With the exception of error terms, latent variables were not modeled. This type of model was chosen over a series of ordinary least squares (OLS) regression for two major reasons. First, the SEM approach is able to estimate simultaneously the effect of predictors on a number of outcomes, even in situations in which an outcome for one set of predictors serves as a predictor for a subsequent outcome: Because the coefficients are estimated simultaneously, they are considered more accurate and valid than treating each outcome separately. Second, by analyzing all outcomes for subjects simultaneously, the SEM approach is able to accommodate correlations across time and across subjects, wherein correlation across subjects alone could be induced by unmeasured covariates. For example, error terms for alcohol use were assumed to be correlated, because a major source of error might be underreporting. Treating the errors as uncorrelated might have led to substantial bias in coefficients. All SEM submodels were replicated using OLS regression, and although the size and significance of the coefficients varied, the direction was consistent.
Three specific questions were examined: (1) What are the predictors of how much a pregnant woman knows about healthy pregnancy habits (e.g., expectant father’s knowledge)? (2) What is the impact of knowledge about the adverse effects of prenatal alcohol and drug use on subsequent antenatal alcohol consumption? (3) What is the relationship of household income, as a measure of socioeconomic status, and prenatal alcohol use? Potential predictor variables (maternal and paternal age, maternal education, race, history of obstetric problems, lifetime maternal alcohol use, paternal alcohol consumption at enrollment, gestational age at enrollment, cigarette use, and brief intervention or treatment control status) were tested for overall effect on prenatal alcohol use. Only lifetime maternal alcohol use and paternal alcohol consumption were included in the final models.
Results
The demographic background of the 254 couples is summarized in Table 1. Most couples (86.6%) were formally married. The median household income based on home zip code was $55,700, ranging from $20,354 to $153,918. The average median household income for Massachusetts in the study time period was $50,587 (DeNavas-Walt et al., 2003). The pregnant women were well educated (with at least half of them having a 4-year college degree), were mostly white (82%), had a median age of 31.4 years, and were at 11.5 weeks gestation at the time of study enrollment. The partners’ median age was 32.3 years. Both the women and men reported similar levels of great stress (~10%) and regular exercise (~60%), but more men were smokers (10.3% vs 5.1%) and drank more than the pregnant women before pregnancy, with respect to mean number of drinks consumed per drinking day (2.9 vs 2.0; t = 6.23, p < .001) and the frequency (35.9% vs 20.8%; t = 8.44, p < .001). The women reported a median of 1.7 years of regular, lifetime alcohol consumption.
Table 1.
Sample background: Demographics, health habits, and other characteristics
| Characteristic | % or median (n = 254) |
|---|---|
| Couple characteristics | |
| Married, % | 86.6% |
| Median household income for zip code, in dollars | 55,700.62 |
| Subject characteristics | |
| Median age, in years | 31.4 |
| White, % | 81.9 |
| Median no. of weeks pregnant at study enrollment | 11.5 |
| Median education, in years | 16 |
| Exercises regularly, % | 62.2 |
| Experiences great stress, % | 10.3 |
| Currently smokes cigarettes, % | 5.1 |
| Following a special diet, % | 9.8 |
| First pregnancy, % | 44.1 |
| History of obstetric problems, % | 24.2 |
| Median lifetime alcohol use, in years | 1.7 |
| Median drinks per day prior to pregnancy | 1.8 |
| Median percentage of drinking days prior to pregnancy | 13.7 |
| Median drinks per day during early pregnancy | 1.5 |
| Median percentage of drinking days during early pregnancy | 2.5 |
| Median drinks per day during late pregnancy | 1.0 |
| Median percentage of drinking days during late pregnancy | 0 |
| Median Healthy Pregnancy Facts score | 6 |
| Partner characteristics | |
| Median age, in years | 32.3 |
| Exercises regularly, % | 60.2 |
| Experiences great stress, % | 11.4 |
| Currently smokes cigarettes, % | 10.3 |
| Follows a special diet, % | 7.9 |
| Median no. of drinks consumed per day | 1.5 |
| Median no. of drinks consumed per week | 4.4 |
| Median Healthy Pregnancy Facts score | 6 |
Both pregnant women and their partners had a median score of 6 of 7 correct when asked about healthy habits during pregnancy. They were both most often correct (>85%) when asked about the consequences of prenatal cigarette smoking as a preventable cause of low birth weight and fetal growth retardation (Item 1), the relationship between amount and timing of cocaine use on fetal outcome (Item 4), potential negative effects of prenatal alcohol exposure throughout pregnancy (Item 6), and greater potential harm of heavy episodic drinking (referred to as “binge drinking” in the measure) (Item 7). The couples had more difficulty (<65%, both correct) with questions about prenatal caffeine use (Item 2), marijuana use (Item 3), and the absence of a universally safe drinking limit during pregnancy (Item 5). Based on the McNemar’s test, the women were significantly more often correct concerning caffeine use (Item 2) and the absence of a safe prenatal drinking level (Item 5). When the element of chance was taken into account for their levels of agreement, the couples evinced poor (κ < 0, Item 5) to slight (κ < .20, Items 1–3, 6, and 7) to fair agreement (.21 < κ < .40, Item 4). The specific items, percentages correct, and measures of agreement are listed in Table 2.
Table 2.
Healthy pregnancy knowledge of women and their partners
| % correct
|
Measures of agreement
|
||||||
|---|---|---|---|---|---|---|---|
| Item | Correct answer | Woman | Partner | κa | p | McNemar Indexb | p |
| 1. Prenatal cigarette smoking is a preventable cause of low birth weight and fetal growth and retardation. | T | 94.0 | 94.2 | .187 | .004 | 0.200 | .655 |
| 2. All caffeine use must be eliminated during pregnancy. | F | 84.0 | 61.6 | .149 | .007 | 32.287 | <.001 |
| 3. The impact of prenatal marijuana use on birth outcome is not clearly known. | T | 53.0 | 45.2 | .039 | .539 | 3.139 | .076 |
| 4. The effects of prenatal cocaine exposure are related to amount and timing of use. | T | 88.8 | 94.6 | .390 | <.001 | 5.762 | .016 |
| 5. No universally safe level of prenatal alcohol use has been established. | T | 79.9 | 64.9 | −.070 | .247 | 12.462 | .000 |
| 6. Prenatal alcohol exposure may have negative effects potentially throughout pregnancy. | T | 94.0 | 96.3 | .038 | .539 | 1.636 | .201 |
| 7. Binge drinking (5+ drinks per episode) is less harmful than drinking one drink a day for 5 days. | F | 92.8 | 92.8 | .012 | .856 | 0.926 | .336 |
Notes: T = true; F = false.
The kappa statistic takes into account agreement between the subject and her partner that results from the element of chance;
the McNemar Index expresses the difference in disagreement between the subject and her partner as a proportion of the total number of disagreements.
An SEM was estimated to evaluate the relationship between household income, knowledge, and prenatal alcohol consumption. The exogenous variables—or those constructs influenced only by variables that lie outside the causal model—included household income, white (or not), and lifetime maternal alcohol consumption, estimated as median years of regular drinking. Prenatal alcohol consumption before study enrollment and after study enrollment until delivery was measured in two ways: (1) quantity per drinking day and (2) number of drinking days (or frequency). Household income and the Healthy Pregnancy Facts score were used to predict alcohol consumption at these three time points.
The model is shown in Figure 1, and standardized path coefficients from the models are summarized in Table 3. As shown in the figure, household income was tested as a predictor of drinks per drinking day and percentage of drinking days for each time period as well as partner’s and subject’s Healthy Pregnancy Facts score (single-headed arrows). The standardized coefficients (β) from the predictive model for income with associated probability values are shown in Table 3. Income is a covariate for lifetime alcohol consumption and race (double-headed arrows); thus, no coefficients are listed. Factors e1 to e9 are error terms from the model. Factors e4 to e9 are assumed to be correlated, whereas e1 to e3 are not.
Figure 1.
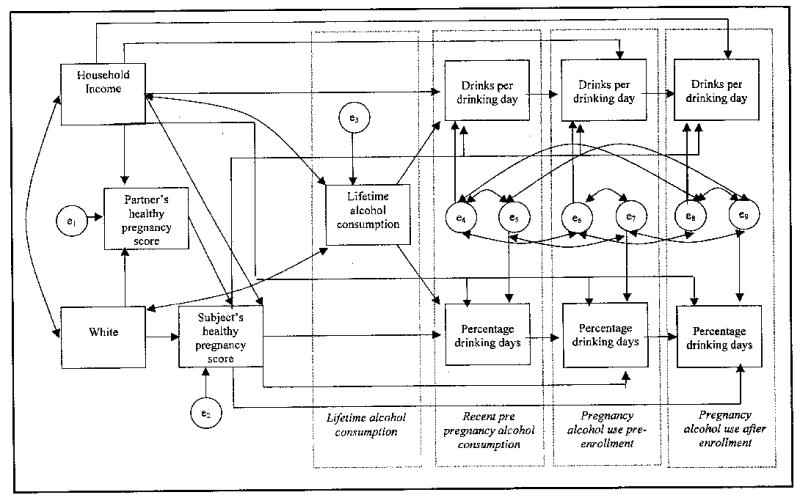
Conceptual model
Table 3.
Standardized path coefficients and fit statistics
| Predictors
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject’s demographics
|
Healthy Pregnancy Facts score
|
|||||||||
| White
|
Household income
|
Subject
|
Partner
|
Lagged dependent variablea |
||||||
| Variable | β | r | β | r | β | r | β | r | β | r |
| Standardized path coefficients | ||||||||||
| Health Pregnancy Facts score | ||||||||||
| Subject | .091 | .1611* | .1338* | .1944* | .1281* | .1703* | ||||
| Partner | .1469* | .1837* | .0846 | .1501* | ||||||
| Lifetime alcohol use | .0746 | .1018 | .0349 | .0894 | ||||||
| Drinking, prior to pregnancy | ||||||||||
| Drinks per day | .0315 | .1389* | −.0399 | −.0449 | .3415‡ | .4753‡ | ||||
| Percentage drinking days | .1161 | −.2226† | −.0701 | −.0148 | .3412‡ | .0689 | ||||
| Prenatal drinking, pre-enrollmentDrinks per day | .0204 | .0513 | .1151* | .1092 | .5124‡ | .5411‡ | ||||
| Percentage drinking days | −.0481 | −.0563 | .0897 | −.0976 | .5316‡ | .4183‡ | ||||
| Prenatal drinking, after enrollment | ||||||||||
| Drinks per day | .1790 | .1109 | .1414* | .0397 | .4826‡ | |||||
| Percentage drinking days | .1295† | .1027 | −.1314 | .1776† | .2054* | |||||
| Fit statistics | ||||||||||
| Independence model chi-square (55 df) | 1454.10 | |||||||||
| Model chi-square (20 df) | 66.97‡ | |||||||||
| Goodness of Fit Index | .9567 | |||||||||
| RMSEA (95% CI) | .0930 (.0685–.1185) | |||||||||
| Bentler and Bonett’s NFI | .9539 | |||||||||
Notes: RMSEA = root mean square error of approximation; CI = confidence interval; NFI = normed fit index.
The lagged dependent variable is drinking in the prior period.
p < .05;
p < .01;
p < .001.
Similarly, the subject’s Healthy Pregnancy Facts score was tested as a predictor for prenatal drinking, measured as drinks per drinking day and percentage of drinking days, at three points (single-headed arrows, Figure 1). The standardized coefficients (β) p values are listed in Table 3. The subject’s score on the Healthy Pregnancy Facts assessment showed a relationship with two other factors: (1) the partner’s healthy pregnancy knowledge (β = .1281, p < .05) and (2) household income ((β = .1338, p < .05).
Factors that influenced the subject’s prenatal alcohol consumption were identified. The single strongest predictor of prenatal alcohol use at most time points was the lagged dependent variable, or alcohol use at the prior time point. Lifetime maternal alcohol use predicted drinks per drinking day before pregnancy (β = .3415, p < .001) and percentage of drinking days before pregnancy (β = .3412, p < .001). Alcohol consumption just before pregnancy predicted drinks per drinking day during early pregnancy before study enrollment (β = .5124, p < .001) and percentage of drinking days during early pregnancy (β = .5316, p < .001). Alcohol consumption in early pregnancy predicted subsequent percentage of drinking days during late pregnancy (β = .1776, p < .001) but not drinks per drinking day in late pregnancy (β = .0397, p > .05).
Other predictors of prenatal alcohol use were examined. The subject’s knowledge of healthy pregnancy habits as manifest in the assessment score had only one statistically significant relationship to prenatal alcohol consumption that was positive for quantity of prenatal alcohol use before enrollment (β = .1151, p < .05). Subjects with higher income drank more frequently while pregnant after enrollment (β = .1295, p < .01).
Discussion
The main finding of this study of 254 couples is that knowledge about pregnancy risks from the use of substances and alcohol was not as influential in prenatal drinking as the women’s prepregnancy drinking. Although the couples demonstrated good knowledge overall, they agreed little on their responses when the element of chance was taken into account. Perhaps most important, only 56% of the couples agreed that there is no universally safe level of prenatal alcohol use and that the women were significantly more knowledgeable about this specific issue than their partners. Both median household income and the partner’s score were predictive of the pregnant woman’s overall knowledge score in the SEM. Knowledge alone, however, is not enough to change norms and actual behavior, and this may be especially true when the importance of prenatal abstinence is not shared by a couple (Hankin, 2002).
Previous alcohol use was the strongest predictor of prenatal alcohol use at nearly every point in time. Household income was a weaker predictor of prenatal consumption. Those with higher incomes drank more frequently in later pregnancy after enrollment than those with lower incomes. All women drank less in early pregnancy. The overall spontaneous remission rate in prenatal alcohol consumption has been estimated to about 50% in a sample of high-risk pregnant drinkers (Smith et al., 1987).
Partner’s alcohol use was not predictive of prenatal alcohol consumption. This was not necessarily expected. Although speculative, it may be possible that partners underreported their own consumption, or simply reflected the reduction in alcohol drinking that is reported with the transition to marriage and imminent parenthood (Mudar et al., 2002). The pregnant women were all alcohol screen-positive, and their prenatal drinking may have been more reflective of their own patterns of use rather than those of their partners. Finally, this is one of the few studies that has prospectively evaluated the role of the male partner in prenatal alcohol use; thus, further investigation is needed.
Potential limitations to the generalizability of study findings include the characteristics of the study sample, the knowledge assessment, and reliance on self-reports of alcohol consumption. Study participants were generally well educated, with higher than average median incomes and involvement in relationships stable enough to include their partners in the study. Higher education and employment, in addition to being non-Hispanic, are demographic characteristics associated with increased risk of prenatal alcohol use (Floyd et al., 1999; Phares et al., 2004). The knowledge assessment was developed by the study team to ascertain level of knowledge about substance use during pregnancy, but was necessarily brief and has not been tested in other settings. These findings do suggest that there is a gap between what expectant mothers and fathers know. Although some may question the accuracy of self-reported alcohol use, antenatal alcohol interviews have been found to be valid and to exceed collateral reports (Chang et al., 1999a; Jacobson et al., 2002).
Thus, several recommendations might be made on the basis of study findings. First, all pregnant women should be screened for previous alcohol use, because prior use is most predictive of subsequent antenatal use. Second, it should not be assumed that familiarity with the recommendations about prenatal alcohol use will necessarily be translated into actual behaviors, as demonstrated in other studies (Kesmodel and Kesmodel, 2002). Thus, physicians and clinicians should educate all pregnant women about the recommendations of abstinence from alcohol during pregnancy, even those who seem to enjoy the apparent advantages of higher education and income. Third, if social support during pregnancy improves fetal outcomes, then expectant fathers might also be included in prenatal education that not only focuses on abstinence from alcohol, but also on ways to be supportive during an important time in their relationship.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism grants R01 AA12548 and K24 AA 00289 to Grace Chang.
References
- Bar-Yam NB, Darby L. Fathers and breastfeeding: A review of the literature. J Human Lactat. 1997;13:45–50. doi: 10.1177/089033449701300116. [DOI] [PubMed] [Google Scholar]
- Bollen, K.A. Structural Equations with Latent Variables, New York: John Wiley & Sons, 1989.
- Chang G, Goetz MA, Wilkins-Haug L, Berman S. Prenatal alcohol consumption: Self versus collateral report. J Subst Abuse Treat. 1999a;17:85–89. doi: 10.1016/s0740-5472(98)00053-1. [DOI] [PubMed] [Google Scholar]
- Chang G, McNamara TK, Orav EJ, Koby D, Lavigne A, Ludman B, Vincitorio NA, Wilkins-Haug L. Brief intervention for prenatal alcohol use: A randomized trial. Obstet Gynecol. 2005;105:991–998. doi: 10.1097/01.AOG.0000157109.05453.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G, Wilkins-Haug L, Berman S, Goetz MA. Brief intervention for alcohol use in pregnancy: A randomized trial. Addiction. 1999b;94:1499–1508. doi: 10.1046/j.1360-0443.1999.941014996.x. [DOI] [PubMed] [Google Scholar]
- Chang G, Wilkins-Haug L, Berman S, Goetz MA, Behr H, Hiley A. Alcohol use and pregnancy: Improving identification. Obstet Gynecol. 1998;91:892–898. doi: 10.1016/s0029-7844(98)00088-x. [DOI] [PubMed] [Google Scholar]
- Day NL, Cottreau CM, Richardson GA. The epidemiology of alcohol, marijuana, and cocaine use among women of childbearing age and pregnant women. Clin Obstet Gynecol. 1993;36:237–245. doi: 10.1097/00003081-199306000-00005. [DOI] [PubMed] [Google Scholar]
- DeNavas-Walt C, Cleveland RW, Webster BH., Jr Washington: Government Printing Office; Income in the United States: 2002. Bureau of the Census, Current Population Reports, P60–221. 2003 available at http://www.census.gov/prod/2003pubs/p60–221.pdf.
- Feinstein, A.R. Clinical Epidemiology: The Architecture of Clinical Research, Philadelphia, PA: W.B. Saunders, 1985, pp. 182–186.
- Finnbogadottir H, Crang Svalenius E, Persson EK. Expectant first-time fathers’ experiences of pregnancy. Midwifery. 2003;19:96–105. doi: 10.1016/s0266-6138(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Floyd RL, Decoufle P, Hungerford DW. Alcohol use prior to pregnancy recognition. Amer J Prev Med. 1999;17:101–107. doi: 10.1016/s0749-3797(99)00059-8. [DOI] [PubMed] [Google Scholar]
- Flynn HA, Marcus SM, Barry KL, Blow FC. Rates and correlates of alcohol use among pregnant women in obstetrics clinics. Alcsm Clin Exp Res. 2003;27:81–87. doi: 10.1097/01.ALC.0000046595.47491.37. [DOI] [PubMed] [Google Scholar]
- Hallgren A, Kihlgren M, Forslin L, Norberg A. Swedish fathers’ involvement in and experiences of childbirth preparation and childbirth. Midwifery. 1999;15:6–15. doi: 10.1016/s0266-6138(99)90032-3. [DOI] [PubMed] [Google Scholar]
- Hankin JR. Fetal alcohol syndrome prevention research. Alcsm Res Hlth. 2002;26:58–65. [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Kesmodel U, Kesmodel PS. Drinking during pregnancy: Attitudes and knowledge among pregnant Danish women, 1998. Alcsm Clin Exp Res. 2002;26:1553–1560. doi: 10.1097/01.ALC.0000034702.14322.25. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Leonard KE, Das Eiden R. Husband’s and wife’s drinking: Unilateral or bilateral influences among newlyweds in a general population sample. J Stud Alcohol. 1999;(Supplement No 13):130–138. doi: 10.15288/jsas.1999.s13.130. [DOI] [PubMed] [Google Scholar]
- Leonard KE, Mudar P. Peer and partner drinking and the transition to marriage: A longitudinal examination of selection and influence processes. Psychol Addict Behav. 2003;17:115–125. doi: 10.1037/0893-164x.17.2.115. [DOI] [PubMed] [Google Scholar]
- Losco J, Shublak M. Paternal-fetal conflict: An examination of paternal responsibilities to the fetus. Politics Life Sci. 1994;13:63–75. doi: 10.1017/s073093840002222x. [DOI] [PubMed] [Google Scholar]
- McLeod D, Pullon S, Cookson T, Cornford E. Factors influencing alcohol consumption during pregnancy and after giving birth. New Zeal Med J. 2002;115:U29. [PubMed] [Google Scholar]
- Mudar P, Kearns JN, Leonard KE. The transition to marriage and changes in alcohol involvement among black couples and white couples. J Stud Alcohol. 2002;63:568–576. doi: 10.15288/jsa.2002.63.568. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Bethesda, MD: Department of Health and Human Services; The Physicians’ Guide to Helping Patients with Alcohol Problems, NIH Publication No. 95–3769. 1995a
- National Institute on Alcohol Abuse and Alcoholism. Quantity-Frequency—Questions. In: Allen, J.P. and Columbus M. (Eds.) Assessing Alcohol Problems: A Guide for Clinicians and Researchers. National Institute on Alcohol Abuse and Alcoholism Treatment Handbook Series No. 4, NIH Publication No. 95–3745, Washington: Government Printing Office, 1995b, pp. 460–461.
- Office of the Surgeon General. Bethesda, MD: Department of Health and Human Services; Press release, February 21, 2005: U.S. Surgeon General Releases Advisory on Alcohol Use in Pregnancy. available at www.hhs.gov/surgeongeneral/pressreleases/sg02222005.html.
- Olsen J. Predictors of smoking cessation in pregnancy. Scand J Social Med. 1993;21:197–202. doi: 10.1177/140349489302100309. [DOI] [PubMed] [Google Scholar]
- Pachauri S. Male involvement in reproductive health care. J Indian Med Assoc. 2001;99:138–141. [PubMed] [Google Scholar]
- Passaro KT, Little RE, Savitz DA, Noss J ALSPAC Study Team. Effect of paternal alcohol consumption before conception on infant birth weight. Teratology. 1998;57:294–301. doi: 10.1002/(SICI)1096-9926(199806)57:6<294::AID-TERA2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Phares TM, Morrow B, Lansky A, Barfield WD, Prince CB, Marchi KS, Braveman PA, Williams LM, Kinniburgh B. Surveillance for Disparities in Maternal Health-Related Behaviors—Selected States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2000–2001. MMWR. 2004;53 (SS4):1–13. [PubMed] [Google Scholar]
- Pirie PL, Lando H, Curry SJ, McBride CM, Grothaus LC. Tobacco, alcohol, and caffeine use and cessation in early pregnancy. Amer J Prev Med. 2000;18:54–61. doi: 10.1016/s0749-3797(99)00088-4. [DOI] [PubMed] [Google Scholar]
- Smith IE, Lancaster JS, Moss-Wells S, Coles CD, Falek A. Identifying high-risk pregnant drinkers: Biological and behavioral correlates of continuous heavy drinking during pregnancy. J Stud Alcohol. 1987;48:304–309. doi: 10.15288/jsa.1987.48.304. [DOI] [PubMed] [Google Scholar]
- Sobell, L.C. and Sobell, M.B. Timeline followback: A technique for assessing self-reported alcohol consumption. In: Litten, R.Z. and Allen, J.P. (Eds.) Measuring Alcohol Consumption: Psychosocial and Biological Methods, Totowa, NJ: Humana Press, 1992, pp. 41–72.
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Martier SS, Ager JW. The T-ACE questions: Practical prenatal detection of risk-drinking. Amer J Obstet Gynecol. 1989;60:863–870. doi: 10.1016/0002-9378(89)90302-5. [DOI] [PubMed] [Google Scholar]
- Turan JM, Nalbant H, Bulut A, Shaip Y. Including expectant fathers in antenatal education programmes in Istanbul, Turkey. Reproduct Hlth Matters. 2001;9:114–125. doi: 10.1016/s0968-8080(01)90098-9. [DOI] [PubMed] [Google Scholar]
- Waterson EJ, Evans C, Murray-Lyon IM. Is pregnancy a tune for changing drinking and smoking patterns for fathers as well as mothers? An initial investigation Brit J Addict. 1990;85:389–396. doi: 10.1111/j.1360-0443.1990.tb00655.x. [DOI] [PubMed] [Google Scholar]
- Wolfberg AJ, Michels KB, Shields W, O’Campo P, Bronner Y, Bienstock J. Dads as breastfeeding advocates: Results from a randomized controlled trial of an educational intervention. Amer J Obstet Gyncol. 2004;191:708–712. doi: 10.1016/j.ajog.2004.05.019. [DOI] [PubMed] [Google Scholar]


