Abstract
In socially tolerant settings, naïve individuals may have opportunities to interact jointly with knowledgeable demonstrators and novel tasks. This process is expected to facilitate social learning. Individual experience may also be important for reinforcing and honing socially acquired behaviours. We examined the role of joint interaction and individual experience in the acquisition of a novel foraging task in captive cottontop tamarins. The task involved learning how to locate and access two hidden food rewards from among 10 differently cued forage sites. Tamarins were tested in three different conditions: (1) individually, (2) while interacting with a naïve mate, and (3) while interacting with a mate trained as a knowledgeable demonstrator. For tamarins tested with mates present, we interspersed social input test days with exposure to the task while alone. Tamarins were tested again 17 months after their last exposure to the task, to assess long-term memory. All tamarins tested with knowledgeable demonstrators solved the task. In contrast, tamarins tested alone or with naïve mates had similarly high levels of neophobia and low levels of task acquisition. We conclude that joint interaction occurs in mated pairs of cottontop tamarins and facilitates the spread of novel behaviour. Interspersing test days with a knowledgeable demonstrator present and test days alone with the task helped tamarins to achieve the ultimate goal of the task: obtaining food rewards. Tamarins performed similarly when tested 17 months later, regardless of their initial learning environment. Tamarins had memory deficits for the location of hidden food rewards, but retained memory of the necessary motor actions and solved the task.
Identifying novel foods and finding novel ways to access or process foods are innovative behaviours with clear fitness implications for individuals. There is evidence that animals across a range of taxa use social cues as part of an adaptive strategy to gain new information about where to forage, what to eat and how to access foods (reviewed in Galef & Giraldeau 2001; pigeons, Columba livia: Palameta & Lefebvre 1985; Norway rats, Rattus norvegicus: Laland & Plotkin 1991; guppies, Poecilia reticulata: Laland & Williams 1997; tufted capuchin monkeys, Cebus apella: reviewed in Fragaszy & Visalberghi 2004). In natural settings, the type and degree of social input available to naïve individuals can vary greatly, from indirect cues reflecting a conspecific’s previous activities to direct observation of a conspecific engaged in a foraging behaviour (Coussi-Korbel & Fragaszy 1995).
In social settings characterized by high levels of social tolerance, naïve individuals may have opportunities to observe a knowledgeable demonstrator performing a novel foraging behaviour, while simultaneously interacting with the demonstrator and with features of the novel task (Coussi-Korbel & Fragaszy 1995; Fritz & Kotrschal 1999; Caldwell & Whiten 2003). These situations have been referred to as coaction (Fragaszy & Visalberghi 1989) and joint interaction (Caldwell & Whiten 2003). In this paper, we use the term ‘joint interaction’ to refer to this type of social input. Joint interaction is expected to promote the acquisition of complex behaviour requiring close-range observation and practise during learning (Fragaszy & Visalberghi 1989; Caldwell & Whiten 2003). Caldwell & Whiten (2003) proposed three ways that joint interaction may facilitate learning: (1) social support may reduce neophobia, (2) close social interaction with a knowledgeable demonstrator may aid in learning about specific actions related to the task and (3) opportunities to scrounge food rewards may be important for stimulating interest in the task and promoting individual learning.
In the context of foraging behaviour, spacing patterns and competitive interactions are expected to limit opportunities for joint interaction in many species to only a subset of the most tolerant social relationships (van Schaik et al. 1999; van Schaik 2003). In support of this hypothesis, when trained demonstrators and unrelated naïve group members are tested together with free access to a foraging task in captive settings, dominance interactions can limit opportunities for joint interaction, and naïve individuals do not consistently acquire novel behaviour (e.g. tufted capuchin monkeys: Fragaszy & Visalberghi 1989; hens, Gallus gallus domesticus: Nicol & Pope 1994). In contrast, in experimental settings when the trained demonstrators and naïve individuals are closely related individuals, such as parents and offspring, or siblings, joint interaction typically occurs and naïve individuals show high levels of novel task acquisition after being tested with demonstrators (e.g. tufted capuchin monkeys: Visalberghi & Fragaszy 1990a; black rats, Rattus rattus: Aisner & Terkel 1992; ravens, Corvus corax: Fritz & Kotrschal 1999; canaries, Serinus canaria: Cadieu & Cadieu 2004).
Species characterized by more egalitarian and pacific social interactions are expected to have greater opportunities within social groups for close behavioural coordination in time and space (Coussi-Korbel & Fragaszy 1995). We further predict increased opportunities for joint interaction in socially tolerant species across a wider range of social relationships, including unrelated individuals. Increased opportunities for joint interaction may result in increased evidence for social transmission of complex behaviour.
The family Callitrichidae, including marmosets and tamarins, is characterized by cooperatively breeding family groups with high levels of social tolerance, and is well suited for testing predictions of social tolerance on social learning. Family groups typically consist of a socially monogamous breeding pair and their mature and immature offspring (reviewed in Snowdon 2001). Group members maintain close proximity and low levels of overt aggression during daily activities such as foraging (Caine 1993). There is also evidence for high levels of social communication about the identity, palatability and location of food, especially between adult and immature tamarins (Rapaport 1999; Rapaport & Ruiz-Miranda 2002; Snowdon & Boe 2003; reviewed in Brown et al. 2004).
Joint interaction occurs within family groups of common marmosets, Callithrix jacchus, and facilitates social learning about foraging (Vitale & Queyras 1997; Caldwell & Whiten 2003). Caldwell & Whiten (2003) studied acquisition of a novel foraging task by captive common marmosets tested in different social conditions including: (1) social interaction with naïve individuals and the task, (2) observation of a knowledgeable demonstrator solving the task across a partition and (3) joint interaction with a knowledgeable demonstrator and the task. In the third condition, the novel foraging box contained multiple food rewards and the hinged lid of the box remained open after manipulation by the demonstrator. As a result, naïve tamarins were able to scrounge food rewards without performing any of the actions necessary to open the box. Marmosets in the joint interaction plus scrounging group had enhanced levels of novel task acquisition relative to the other groups.
We studied how social context and individual experience affect the acquisition of a novel foraging task by cottontop tamarins. The task required the use of spatial and/or visual cues to locate a hidden food reward and the use of a novel motor action to access the reward. Tamarins in the wild locate patchily distributed fruits and expose hidden insect prey (Garber 1993) and this task was designed to simulate similar, ecologically relevant foraging behaviour. We tested tamarins in three different contexts: (1) individually, (2) while interacting with a naïve mate and (3) while interacting with a mate trained as a knowledgeable demonstrator. No studies have explicitly tested whether joint interaction occurs and facilitates social learning in mated pairs of callitrichids, despite evidence for high levels of affiliation and cooperation between mated pairs (Savage et al. 1988; Cronin et al. 2005). Furthermore, few studies of social learning have focused on differentiating between the numerous social and individual processes that may mediate novel task acquisition in tolerant social settings, including general social input, close attention to demonstrator cues, opportunities to scrounge food rewards and independent experience. Caldwell & Whiten (2003) found that common marmosets learn a novel foraging task by interacting with a knowledgeable demonstrator while scrounging food rewards, but it is not clear whether scrounging was a necessary condition for learning. In other species, scrounging opportunities can distract individuals from attending to social input by providing alternative strategies through which to receive food rewards (Giraldeau & Lefebvre 1987; Fragaszy & Visalberghi 1989). Our experimental design effectively eliminated scrounging under all test conditions, to examine the influence of social attention and individual experience on task acquisition when alternative strategies of receiving food rewards are not available.
Based on evidence for high levels of neophobia in Saguinus species (Day et al. 2003), and a feeding ecology that emphasizes visual search strategies over extractive foraging (Garber 1993), we predicted that tamarins tested alone would have low levels of novel task exploration and would be unlikely to attempt novel motor actions in order to obtain hidden food rewards. In several avian and primate species, the presence of naïve groupmates reduces neophobia and enhances exploration of novel objects (Fragaszy & Mason 1978; Visalberghi & Fragaszy 1990b; Coleman & Mellgren 1994; Soma & Hasegawa 2004). In the socially monogamous titi monkey, Callicebus moloch, mates tested together while exposed to novel objects had lower levels of emotional arousal and higher levels of object manipulation than when they were exposed to the same objects individually (Fragaszy & Mason 1978). If tamarins also show reductions in neophobia in the presence of naïve social partners, we would expect shorter latencies to contact the novel apparatus, more overall time spent in contact with the apparatus and possibly more independent task solutions in tamarins tested in naïve pairs relative to tamarins tested individually.
We further predict that a knowledgeable mate provides social cues and social input not available through interactions with naïve mates. In a broad review of social learning across primate taxa, Custance et al. (2002) found that naïve conspecifics were more likely to acquire a novel task in the presence of a skilled demonstrator than when tested with a naïve partner or in naïve social groups. Although the authors did not hypothesize about why this may occur, we propose that skilled demonstrators provide specific cues about the locations, motor actions and object movements related to novel task solutions, and that individuals who have opportunities to attend to these cues will be more likely to acquire novel tasks. If this is the case, then we predict that naïve tamarins tested with knowledgeable mates should have higher levels of exploration and manipulation of specific objects related to task solutions, and they should be more likely to solve the task compared with the other two groups.
We have emphasized that the effectiveness of social learning opportunities may relate to the type of social input available. However, in natural settings, the development of socially learned behaviour is also expected to be reinforced and honed through individual experience (Laland et al. 1993; Galef 1995). In one study, naïve Norway rats were exposed to a novel foraging task while observing a trained demonstrator, then given one trial of individual experience with the task. Subjects performed significantly better on subsequent test days than did rats that had observed the trained demonstrator but that had not received any testing while alone (Laland & Plotkin 1992). We hypothesized that individual experience may be important for honing socially acquired behaviours in cottontop tamarins. To test this prediction, we interspersed test days with either naïve or demonstrator mates present with 2 days of exposure to the task while alone, and we compared task performance between test days with social input and test days with individual experience.
For wild tamarins, detailed knowledge of plant locations is important for coping with seasonal fluctuations in fruit food availability (Garber 1989). Wild moustached tamarins, S. mystax, and saddle-back tamarins, S. fuscicollis, retain memory for the location of particular trees within their home ranges over several months and use this knowledge to make goal-directed foraging decisions (Garber 1989). Based on this evidence, we predicted that cottontop tamarins should retain memory for the location of feeding sites over several months. We retested tamarins approximately 17 months after the initial study to evaluate long-term memory and to determine whether initial mode of learning affected long-term retention. Galef (1995) predicted that long-term retention of behaviour depends not on the context in which the behaviour was acquired, but on the presence and effectiveness of alternative strategies available. However, theoretical work also suggests that socially acquired behaviour may be transmitted relatively intact and faithfully maintained over time, whereas behaviour acquired without social input may be more susceptible to modification over time (Laland et al. 1993). In one of the only studies to address these hypotheses, red-winged blackbirds, Agelaius phoeniceus, that had learned a response to a novel food through individual training only or through social input had similar memory profiles, but memory was tested only over 12 days postacquisition (Mason et al. 1984). This study explores long-term memory over more than a year for socially and individually acquired behaviour in cottontop tamarins.
METHODS
Subjects
We studied 32 mated, but nonbreeding, adult cottontop tamarins from a captive colony at the University of Wisconsin-Madison Psychology Department. We housed all colony animals socially throughout their lives, either in family groups or in mated pairs. The tamarins ranged in age from 1 to 6 years old and had all been paired for at least 6 months prior to the beginning of the study. Tamarin pairs live in cages measuring 160 × 93 × 263 cm, furnished with natural tree branches, ropes, acrylic or polycarbonate sheeted nestboxes and various toys for environmental enrichment. We fed the colony three times daily from food platforms at least 1 m above the floor. Water was available ad libitum. For further details on colony husbandry refer to Ginther et al. (2001). Testing occurred either between 1000 and 1130 hours, before the main feed, or between 1500 and 1600 hours, before a high-protein snack.
Apparatus
We constructed two 70 × 53-cm aluminium frames and attached five removable polyurethane forage sites to each frame, with approximately 42 cm between each site (Fig. 1). The forage sites were cylindrical containers, 7.5 cm deep × 7 cm in diameter, with differently coloured lids: red, green, yellow, white or black. The spatial configuration of the differently coloured lids was the same on the two frames. We attached five 18 × 8-cm wooden perches to each frame, one below each forage site, providing tamarins with easy access to the sites. Each forage site contained a single 2-g slice of an Apple Newton cookie, a preferred food for the tamarins. We placed a cookie slice in each forage site in order to eliminate any differential odour cues. However, only one site on each apparatus (the ‘correct’ site) could be opened, by rotating the lid to the left or the right, to reach the food reward. An internal latch restricted lid movement of the other forage sites, so that the food reward in these sites could not be reached. The colour cue and spatial location of the two correct forage sites were identical on the two foraging frames. Within each test pair, the location and colour of the two correct forage sites remained constant across test days. Between pairs, we varied the location and colour of the correct forage sites to control for possible location or colour biases. We hung the two foraging frames on opposite walls in the pair cages during testing, 1.5 m above the floor, and removed them immediately after a test session.
Figure 1.
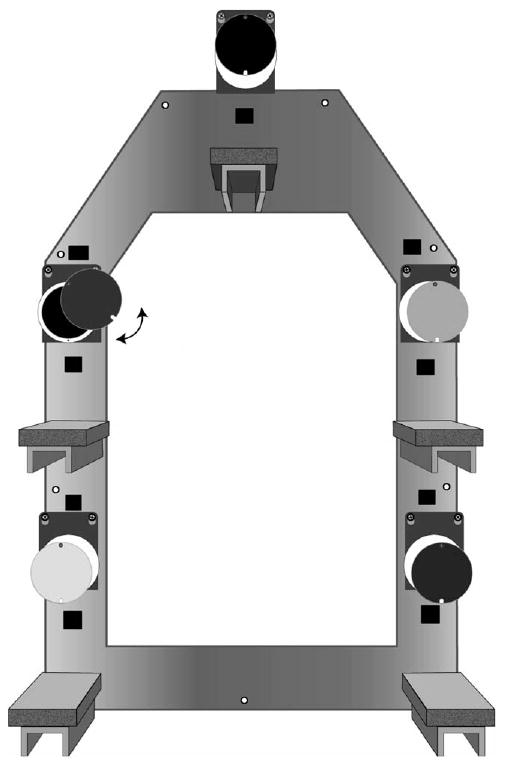
Diagram of one foraging frame, with five forage sites and perches below each site. The lids of the forage sites were coloured (from top, clockwise) black, yellow, red, white and green. Four forage sites had internal latches, restricting lid movement. One forage site could be opened by moving the lid right or left.
Several aspects of our test design restricted tamarins from obtaining a food reward without opening a correct forage site. Each forage site was relatively small, allowing only one animal to reach into the forage site at a time, and the lids of the correct forage sites returned to a closed position after each manipulation. The presence of two correct forage sites reduced any possible competition for food rewards by providing opportunities for both tamarins to receive food rewards through independent task solutions when tested together. Most importantly, there was only one, discrete cookie slice available in each forage site and it could be quickly consumed by an individual tamarin. As a result of these combined factors, scrounging was effectively eliminated and a tamarin had to open a correct forage site independently to receive a food reward.
There were several phases of task acquisition. These included: (1) overcoming neophobia, (2) learning the location of the correct forage sites using novel colour and/or spatial cues and (3) using a novel motor action to open correct forage sites. The lack of scrounging opportunities meant that, on days when pairs were tested together, tamarins had to open a correct forage site before their mate in order to receive a food reward.
Procedure
Habituation
We began habituation 2 weeks before testing for each pair. We attached a portable transport cage (66 × 64 × 190 cm) to the pair cage via a removable tunnel 15 cm in diameter, made from ventilation ducting. On either end of the tunnel, movable flaps could be closed, restricting monkeys to either their home cage or the transport cage. We have used transport cages previously for research and environmental enrichment, so many of the tamarins were already comfortable with moving between the two cages. We attached sheets to the mesh walls of the transport cage, so that individuals inside were visually isolated from their mates in the home cage. Each tamarin had six to eight habituation trials, to familiarize them to separation from their mate for short intervals. During a habituation trial, the flap to the transport cage was opened until one monkey entered the cage. After the tamarin entered, we closed the flap and rewarded the monkey with two slices of Apple Newton cookie. We kept the tamarin in the transport cage for 10 min, then allowed it to return to the home cage. We repeated the procedure with the other tamarin.
Experiment 1: The Role of Social Context and Individual Experience in Novel Task Acquisition
Individual learning
We attempted to randomly assign equal numbers of males and females to the individual learner test group. However, we switched the sex assignment in one pair, because of difficulties in habituating one individual to enter a transport cage. As a result, we had five pairs with male individual learners and three pairs with female individual learners. Individual learners received one 10-min trial per day for eight consecutive days. We tested these monkeys with the novel foraging task in the home cage while their mate was isolated in the transport cage. We recorded focal animal data on the number and duration of bouts of contact with each foraging frame, defined as touching or sniffing any part of the frame, and the number and order of visits and revisits to each forage site, defined as sniffing or touching the lid of the forage site. We distinguished visits that resulted in obtaining food rewards from unrewarded visits. We considered three main measures of task acquisition: opening correct forage sites, receiving food rewards and visiting both correct forage sites before investigating any incorrect forage sites, referred to in subsequent analyses as an ‘accurate’ trial. At the completion of each trial, we removed the apparatus from the room and then allowed the mate in the transport cage to return to the pair cage. Individual learners provided a baseline measure of performance on the task without social input.
The initial individual learners were trained to be knowledgeable demonstrators for the joint-interaction test. Before proceeding to the joint-interaction test, each individual learner had to open the correct forage sites, receive food rewards and complete an accurate trial on three consecutive test days. Most individual learners were not proficient on all three measures of performance by the end of the individual learning test. These tamarins received further training from human demonstrators during 10-min trials in the home cage while their mates were visually isolated in the transport cage. During the first three days of training, we propped open the lids of the correct forage sites so that the two food rewards were visible and tamarins were able to reach the rewards without manipulating the lids. We incrementally lowered the lids over days until, on the fourth day, the lids were closed as in the individual learning test and monkeys had to manipulate the lids to receive food. Once the tamarins began to open lids, we continued training until each tamarin demonstrated success on all three measures of task performance on three consecutive days without human assistance. This procedure was meant to ensure that all individual learners became equally effective demonstrators.
Joint interaction with a knowledgeable demonstrator
The joint-interaction test began on the day immediately following the completion of demonstrator training. The knowledgeable demonstrator performed the task with his/her naïve mate present. Testing followed the same general format as individual learning (i.e. 10-min trial with the foraging task in the home cage for eight consecutive days). On days 1–3 and 5–7, we tested both tamarins together in their home cage with the foraging task. On days 4 and 8, we tested the naïve tamarin alone in the home cage with the foraging task, while its demonstrator was isolated in the transport cage. During joint-interaction testing, two experimenters collected focal animal data, one on each tamarin. Focal data recorded were the same as in individual learning. We also used these data to calculate a measure of joint interaction. We measured the number of times that a naïve tamarin followed a demonstrator to a forage site and visited that site immediately after the demonstrator visited it, and before either monkey visited another forage site. This behaviour is referred to as ‘follows’ in subsequent analyses.
To minimize observer bias, experimenters switched test subjects across test days. To determine interobserver reliability, experimenters recorded focal animal data on the same tamarin during individual learning test days and during days 4 and 8 of the joint-interaction test. The mean ± SE index of concordance for these focal data was 96 ± 0.02%.
Social interaction with a naïve mate
We tested eight additional tamarin pairs in the ‘social interaction with naïve mate’ condition. We used the same testing procedure as in joint interaction with a knowledgeable mate. On days 1–3 and 5–7, we tested the pairs together in the home cage with the foraging task. On days 4 and 8, we tested each tamarin individually in the home cage with the task. We recorded the same measures as during joint interaction with a knowledgeable mate.
Experiment 2: Memory Test
The one-trial memory test occurred after a mean ± SE of 17.14 ± 1.96 months since the last exposure to the task. Subjects were 12 tamarins who had previously learned the task, either through individual learning plus training or through joint interaction with their knowledgeable mate. These monkeys received a single exposure to the task together with their mate in their home cage. We tested mated pairs together on the memory test to determine how the social dynamics between the original joint-interaction tamarins and their original demonstrators would affect performance. We recorded the same measures of performance as during joint-interaction and social-interaction testing.
Data Analysis
Small sample sizes and violations of normality required us to use nonparametric tests for data analysis. All analyses were performed with SPSS 11.0 software (Chicago, Illinois, U.S.A.). We measured differences in performance and behaviour between test groups with the Fisher’s exact test and the Mann–Whitney U test. We examined relationships between performance of joint-interaction tamarins and their interactions with knowledgeable mates using Spearman rank correlations. We measured differences in performance within groups on different test days using the sign test and the Wilcoxon signed-ranks test. All tests were two tailed. When making multiple comparisons between groups, we used the Bonferroni correction to control for familywise error rate. We designate these tests by α* = α/c, where α equals 0.05 and c equals the number of comparisons performed. For all other tests, alpha was set at 0.05. Unless otherwise noted, results are presented as median and ranges.
RESULTS
Experiment 1
Comparison of novel task acquisition between test groups
All eight tamarins tested with trained demonstrators (joint-interaction group) opened correct forage sites and received food rewards within the 8-day test period. In contrast, none of the tamarins tested with naïve mates (social-interaction group) and only two of the tamarins tested alone (individual learners) opened correct forage sites and received food rewards (2 × 3 Fisher’s exact tests: Ps < 0.001). Post hoc analyses indicated that significantly more tamarins in the joint-interaction group opened forage sites and received food rewards compared with social-interaction tamarins (2 × 2 Fisher’s exact tests: Ps < 0.001, α* = 0.017), and compared with individual learners (Ps = 0.007, α* = 0.017; Fig. 2). Seven joint-interaction tamarins completed an accurate trial during testing, whereas only one social-interaction tamarin and two individual learners completed an accurate trial during testing (2 × 3 Fisher’s exact test: P = 0.001). Significantly more joint-interaction tamarins completed an accurate trial than did social-interaction tamarins (P = 0.001, α* = 0.017), but there were no significant differences between joint-interaction tamarins and individual learners (P = 0.041, α* = 0.017). There were no significant differences between the social-interaction group and the individual learners in the number of tamarins to open forage sites, receive food rewards or make accurate trials (Ps = 0.10–0.25).
Figure 2.
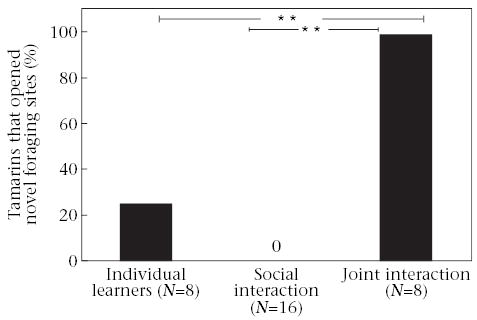
Percentage of tamarins in each test group to open novel forage sites. Individual learners were tested alone with the novel task. Social-interaction tamarins were tested with naïve mates present. Joint-interaction tamarins were tested with knowledgeable mates present. **P < 0.01.
We did not detect any significant influences of sex on duration of time spent on apparatus or percentage of visits to correct forage sites within individual learners (Mann–Whitney U tests: Us = 4–6.5, N1 = 5, N2 = 3, Ps = 0.30–0.76), within the social-interaction group (Us = 25.5–26, N1 = N2 = 8, Ps = 0.49–0.53) or within the joint-interaction group (Us = 6, N1 = 3, N2 = 5, Ps = 0.655). There were also no detectable sex differences within the joint-interaction tamarins in number of days taken to first open a forage site or to first receive food reward (Us = 6.5–7.5, N1 = 3, N2 = 5, Ps = 0.76–1.0). Based on the lack of significant sex differences in any of the groups, we combined sexes within each test group for subsequent analyses.
Differences in neophobia between test groups
Across test days, median latency to contact the apparatus was significantly shorter for joint-interaction tamarins (14 s, range 10–30 s) than for social-interaction tamarins (62 s, range 14–109 s; U = 15, N1 = 8, N2 = 16, P = 0.003, α* = 0.017). Latencies of joint-interaction tamarins did not differ significantly from latencies of individual learners (28 s, range 7–100 s; U = 25, N1 = N2 = 8, P = 0.46). Individual learners also had significantly shorter latencies to contact the apparatus than did social-interaction tamarins (U = 15, N1 = 8, N2 = 16, P = 0.003, α* = 0.017).
Joint-interaction tamarins spent significantly more time on the apparatus than did individual learners (U = 8, N1 = N2= 8, P = 0.012, α* = 0.017) and social-interaction tamarins (U = 6, N1 = 8, N2 = 16, P < 0.001, α* = 0.017). There were no significant differences in time spent on the apparatus between individual learners and social-interaction tamarins (U = 58, N1 = 8, N2 = 16, P = 0.71; Fig. 3).
Figure 3.
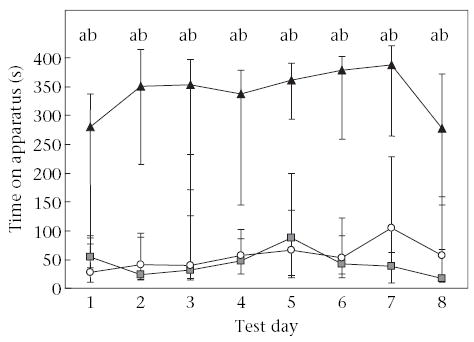
Exploration of a novel task across test days. Points represent group medians and whiskers represent the interquartile range containing 50% of the values. Joint-interaction tamarins (▴) were tested with knowledgeable mates present on days 1–3 and 5–7, and were tested alone with the task on days 4 and 8. Social-interaction tamarins (○) were tested with naïve mates present on days 1–3 and 5–7, and were tested alone with the task on days 4 and 8. Individual learners (◼) were tested alone with the task on all test days. Letters denote significant differences between groups: joint-interaction tamarins versus social-interaction tamarins (a = P < 0.01); joint-interaction tamarins versus individual learners (b = P < 0.05).
Knowledge of correct forage site locations
The total number of visits to correct forage sites measured knowledge of the locations of correct forage sites and motivation to solve the task. Joint-interaction tamarins made significantly more median visits to correct forage sites than did individual learners (U = 8, N1 = N2 = 8, P = 0.012, α* = 0.017) and social-interaction tamarins (U = 0, N1 = 8, N2 = 16, P < 0.001, α* = 0.017). There were no significant differences in median visits to correct forage sites between individual learners and social-interaction tamarins (U = 55.5, N1 = 8, N2 = 16, P = 0.60; Fig. 4).
Figure 4.
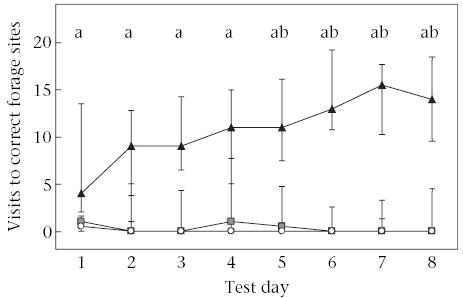
Visits to correct forage sites across test days. Points represent group medians and whiskers represent the interquartile range containing 50% of the values.▴: joint-interaction tamarins;○: social-interaction tamarins;◼: individual learners. Letters denote significant differences between groups: joint-interaction tamarins versus social-interaction tamarins (a = P < 0.05); joint-interaction tamarins versus individual learners (b = P < 0.05).
The percentage of visits to correct forage sites relative to total visits to any forage site measured tamarin precision when investigating forage sites. The percentage of visits to correct forage sites was significantly greater for joint-interaction tamarins (70%, range 46–78%), than for social-interaction tamarins (13%, range 0–44%; U = 2, N1 = 8, N2 = 16, P < 0.001, α* = 0.017). Joint-interaction tamarins showed a nonsignificant tendency towards a greater percentage of visits to correct forage sites than individual learners (18%, range 0–84%; U = 11, N1 = N2 = 8, P = 0.027, α* = 0.017). There were no differences in percentage of visits to correct forage sites between individual learners and social-interaction tamarins (U = 52, N1 = 8, N2 = 16, P = 0.46).
Comparing social input from knowledgeable demonstrators and naïve mates
To evaluate whether knowledgeable mates provided quantitatively different social input from naïve mates, we compared performance of demonstrators during joint-interaction testing with performance of naïve tamarins during social interaction. Median duration on the apparatus was significantly greater for demonstrators (266 s, range 91–370 s) than for social-interaction tamarins (54 s, range 3–335 s; U = 17, N1 = 8, N2 = 16, P = 0.004). Median visits to correct forage sites was significantly greater for demonstrators (11 visits, range 6–16 visits) than for social-interaction tamarins (0.2 visits, range 0–2 visits; U = 0, N1 = 8, N2 = 16, P < 0.001). Percentage of visits to correct forage sites was also significantly higher for demonstrators (89%, range 64–100%) than for social-interaction tamarins (13%, range 0–37%; U = 0, N1 = 8, N2 = 16, P < 0.001).
Relationship between social attention to a demonstrator and rates of novel task acquisition
Joint-interaction tamarins took a median of 2 days (range 1–7 days) to first open a forage site, 3 days (range 2–6 days) to first complete an accurate trial, and 4 days (range 3–8 days) to first receive a food reward. The number of days taken for joint-interaction tamarins to first open a forage site, complete an accurate trial and receive a food reward were not correlated with their demonstrator’s median duration on the apparatus (Spearman rank correlation: rS = −0.06 to 0.23, P = 0.58–0.89), median latency to open a forage site (rS = −0.04 to 0.24, P = 0.58–0.93) or median percentage of visits to correct forage sites (rS = 0.36–0.63, P = 0.10–0.97). All demonstrators had similarly high levels of task performance because of the training procedure.
However, the performance of joint-interaction tamarins was influenced by their number of follows of demonstrators’ visits to forage sites. Across the 6 test days with demonstrators present, joint-interaction tamarins made a median of 3.2 follows (range 1.2–4.5 follows) of demonstrators’ visits to forage sites per day. Tamarins who made more follows during the first 3 test days took significantly fewer days to first open a forage site (rS = −0.76, N = 8, P = 0.029) and to first complete an accurate trial (rS = −0.82, N = 7, P = 0.023). There was also a tendency for tamarins who made more follows during the first 3 days of testing to take fewer days to first receive a food reward (rS = −0.70, N = 8, P = 0.054). However, only the follows made during the first 3 days of testing were correlated with faster task solutions. The number of follows during the last 3 days of testing with demonstrators present (days 5–7) was not correlated with time taken to first open a forage site, first complete an accurate trial or first receive a food reward (rS = −0.41 to −0.56, N = 8, P = 0.15–0.36).
Influence of experience alone with the task on performance
For the joint-interaction and social-interaction test groups, day 4 and day 8 were the only test days when tamarins were able to interact with the novel task while alone. Individual experience with the task did not appear to influence the behaviour of the social-interaction tamarins, since tamarins performed poorly across test days. However, in the joint-interaction group, individual experience test days did influence some aspects of performance. All joint-interaction tamarins first opened a forage site on test days with their demonstrator present, and 75% (N = 6) first completed an accurate trial on test days with their demonstrator present; however, only 25% (N = 2) first received a food reward on test days with their demonstrator present. The majority of joint-interaction tamarins first received a food reward either on day 4 or day 8, when they were tested alone. This helps to explain why joint-interaction tamarins took significantly more days to first receive a food reward than to first open a forage site (Wilcoxon signed-ranks test: T = 8, N = 8, P = 0.01) or to first complete an accurate trial (T = 5, N = 7, P = 0.038; Fig. 5). Once joint-interaction tamarins had received their first food reward, they continued to receive food rewards on 96% (25 of 26) of subsequent test days, regardless of whether the demonstrator was present. When tested with the demonstrator present, joint-interaction tamarins were able to visit a forage site before their mate, and received food rewards on these test days ( ± SE = 1 ± 0.09). Thus, being tested alone appeared to be important for the first experience receiving food rewards, but not for subsequent success with receiving food rewards.
Figure 5.
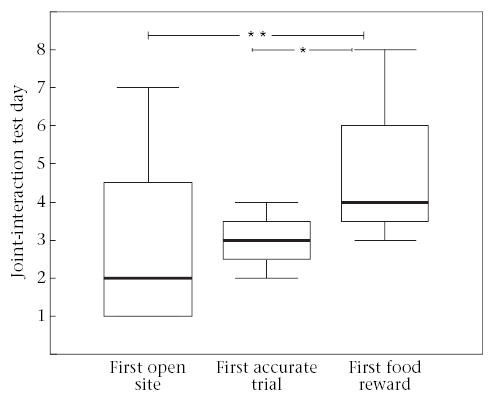
Number of days taken for joint-interaction tamarins (N = 8) to first achieve different phases of the task. Boxes represent the inter-quartile range containing 50% of the values. The line across the box represents the median value. The whiskers extend to the smallest and largest values (excluding outliers). *P < 0.05; **P < 0.01.
Experiment 2: Memory Test
During the one-trial memory test, all 12 tamarins opened correct forage sites. In three pairs, both tamarins received one food reward and in the other three pairs, either the original individual learners or the original joint-interaction tamarins visited both correct forage sites before their mates and received both food rewards. Five tamarins (47%) visited a correct forage site before visiting any incorrect sites. Other tamarins tried to open one incorrect forage site several times before opening the correct forage site. As a group, tamarins made a median of 1.5 visits (range 0–13 visits) to incorrect forage sites before visiting a correct forage site. We detected no significant differences between original individual learners and original joint-interaction tamarins on latency to contact the apparatus, total time on the apparatus, proportion of visits to correct forage sites or number of incorrect visits before visiting a correct forage site (Mann–Whitney U test: U = 9–17.50, N1 = N2 = 6, P = 0.15–0.93). We also detected no sex differences on these variables with our sample sizes (U = 10–18, N1 = N2 = 6, P = 0.20–1.0).
As a result of no detectable group or sex differences, we grouped data from all tamarins and compared their performance on the memory test with their performance when they were last tested together in pairs, on day 7 of the joint-interaction test. There were no differences between joint-interaction day 7 and the memory test in the time that tamarins spent on the apparatus (Wilcoxon signed-ranks test: T = 5, N = 12, P = 0.58). However, compared with their performance on joint-interaction day 7, tamarins took significantly longer to first make contact with the apparatus (T = 0, N = 12, P = 0.002) and to first open a correct forage site (T = 0, N = 12, P = 0.002; Fig. 6a). Tamarins also made more visits to incorrect sites before visiting a correct forage site than they had on joint-interaction day 7 (T = 0, N = 12, P = 0.017; Fig. 6b) and memory-test tamarins had a lower percentage of visits to correct forage sites than they had on joint-interaction day 7 (T = 11, N = 12, P = 0.003). Overall, tamarins in the memory test did not perform as well as they had at the end of joint-interaction testing.
Figure 6.
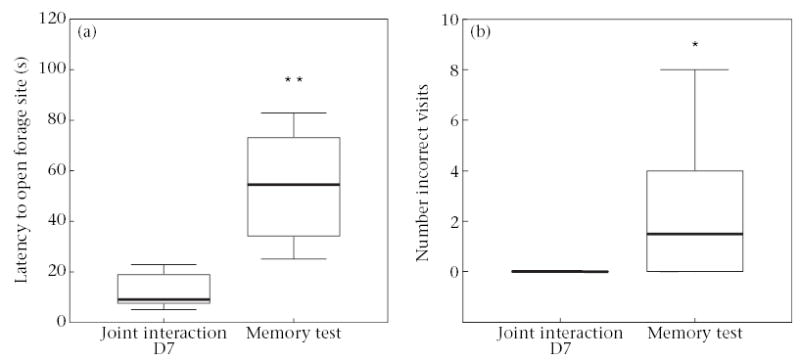
Performance of tamarins on a long-term memory test compared with joint-interaction testing. Boxes represent the interquartile range containing 50% of the values. The line across the box represents the median value. The whiskers extend to the smallest and largest values (excluding outliers). (a) Latency to open a correct forage site. (b) Number of incorrect visits before locating a correct forage site. *P < 0.05; **P < 0.01.
To examine whether previously experienced tamarins performed better on the memory test relative to naïve tamarins without any previous exposure to the task, we compared the performance of the original individual learners on their memory test with their first day of exposure to the task during individual learning. We did not consider the original joint-interaction tamarins, because their performance on their first day of exposure to the task was influenced by the presence of trained demonstrators. Compared with their performance as individual learners, memory-test tamarins spent significantly more time on the apparatus (Wilcoxon signed-ranks test: T = 0, N = 6, P = 0.043), but their latency to contact the apparatus and percentage of visits to correct forage sites did not differ between the two test conditions (T = 2–3, N = 6, P = 0.14–0.40). Importantly, all six former individual learners opened forage sites and five received food rewards on the memory test, although none of the tamarins performed either of these phases of the task on their first day of individual learning. Significantly more of the tamarins opened a forage site on the memory test than on their first day of exposure to the task (sign test: P = 0.031) and there was a nonsignificant tendency for more of the tamarins to obtain food rewards on the memory test than on their first day of exposure to the task (sign test: P = 0.063).
We also compared the performance of the six pairs of memory-test tamarins with the performance of the eight pairs of social-interaction tamarins on their first day of exposure to the task. Compared with naïve social-interaction tamarins, memory-test tamarins spent significantly more time on the apparatus (Mann–Whitney U test: U = 5, N1 = 16, N2 = 12, P < 0.001) and made a significantly greater percentage of visits to correct forage sites (U = 45, N1 = 16, N2 = 12, P = 0.017). There were no significant differences in latency to contact the apparatus between memory-test tamarins and social-interaction tamarins (U = 91.5, N1 = 16, N2 = 12, P = 0.83). Although all 12 memory-test tamarins opened forage sites and nine received food rewards, none of the social-interaction tamarins achieved either of these phases of the task (2 × 2 Fisher’s exact tests: Ps < 0.001).
DISCUSSION
This study extends current evidence of tolerant social learning environments within callitrichid family groups (Vitale & Queyras 1997; Caldwell & Whiten 2003; Snowdon & Boe 2003) to include joint interaction between mated adult cottontop tamarins. We found no evidence for aggression, displacement or monopolization of the foraging task by skilled demonstrators or naïve individuals when tested together. This negative result contrasts with evidence in other species that skilled demonstrators can aggressively displace naïve group members from novel foraging tasks, thereby limiting opportunities for joint interaction (tufted capuchin monkeys: Fragaszy & Visalberghi 1989; hens: Nicol & Pope 1994). Joint-interaction tamarins tested with knowledgeable demonstrators were socially attentive, as evident in their follows of demonstrators’ visits to forage sites. Joint-interaction tamarins made more investigations of the salient features of the task than did naïve individuals in other test groups, and they were able to rapidly learn the novel foraging task.
When tamarins were tested individually, high levels of neophobia interfered with novel task exploration and acquisition. Individual learners had a tendency to make rapid but brief bouts of contact with the apparatus at the beginning of a trial, which may explain why latencies to contact the apparatus did not differ significantly between individual learners and joint-interaction tamarins. However, individual learners had low levels of overall contact with the foraging apparatus across test days and showed several behavioural signs of neophobia, including piloerection and alarm calling.
We predicted that having naïve mates present may enhance general activity and interest in novel objects, leading to improved performance over tamarins tested individually. Naïve mates showed high levels of social tolerance and low levels of aggression during the social-interaction test, but there was no evidence that this helped to reduce neophobia and enhance novel task exploration. In contrast, social-interaction tamarins had longer latencies to contact the apparatus and lower levels of task exploration than did individual learners, and although two individual learners independently solved the task, none of the social-interaction tamarins did so. This result contrasts with evidence in other species that the presence of naïve social partners can increase exploration of novel objects and promote novel task acquisition (Fragaszy & Mason 1978; Visalberghi & Fragaszy 1990b; Coleman & Mellgren 1994; Soma & Hasegawa 2004). However, our results correspond with Day et al. (2003), who found that Saguinus exposed to novel foraging tasks in naïve groups had high levels of neophobia and low levels of novel task manipulations. In more neophilic species, a social partner’s interest in novel objects may provide a form of local enhancement that aids in novel task acquisition (Visalberghi & Fragaszy 1990b). However, in more neophobic species like cottontop tamarins, the presence of a naïve social partner may not provide sufficient social input to enhance novel object exploration or support novel task acquisition.
Knowledgeable demonstrators had higher levels of activity on the apparatus and made a greater percentage of visits to correct forage sites than did naïve mates. Thus, knowledgeable demonstrators provided forms of local enhancement through their increased activity with the novel foraging task. When solving the task, demonstrators also provided more specific cues about the motor actions needed to open forage sites, the movements of the forage site lids and the presence of food rewards within the forage sites. None of these cues were consistently available to tamarins in the other test groups.
Caldwell & Whiten (2003) proposed that, when common marmosets were tested in joint interaction with trained demonstrators opening novel foraging boxes, the close visual contact between the naïve individual and the demonstrator may have aided learning. In reviewing that study and other examples of primate social learning, Fragaszy & Visalberghi (2004) suggested that demonstrators may provide important cues about the altered positions of objects. They pointed out that the actions that place objects in new positions are often brief, but objects may stay in new positions for a longer interval, making object movements more readily perceived and remembered by naïve monkeys who are able to attend to demonstrators. Evidence from our study supports this hypothesis. When demonstrators opened a correct forage site, the swinging lid of the forage site often remained in motion after the demonstrator had left the area. Close follows of demonstrators’ visits to forage sites may have led many joint-interaction tamarins to investigate and manipulate a lid while it was still in motion from the demonstrator’s previous visit. As a result, joint-interaction tamarins received extended visual and tactile input from the moving lid. Joint-interaction tamarins who made more follows of demonstrators’ visits to forage sites during the first half of testing took fewer days to begin to independently open forage sites. We also note anecdotally that the only two tamarins in the individual learning condition to spontaneously solve the task were able to do so after they noticed slight movements of correct forage site lids caused by their high levels of undirected activity on the foraging apparatus. Taken together, this evidence suggests that observation of the movements of forage site lids, and possibly tactile input from moving lids, was critical for task acquisition. Joint interaction may have been important mainly in drawing attention to altered positions of forage site lids, and by providing opportunities for naïve tamarins to touch moving lids.
To our knowledge, few studies have attempted to divide a broader social learning activity into separate components, apart from defining the motor components involved (e.g. Whiten 1998; Fritz & Kotrschal 1999). Our novel task involved four clear phases of acquisition: (1) overcoming neophobia (2) learning the location of the correct forage sites using spatial and/or colour cues (3) learning the novel motor patterns necessary to open correct forage sites and (4) opening one of the two correct forage sites before the mate, in order to obtain a food reward. Most tamarins tested alone, or tested with naïve mates, were unable to overcome neophobia in order to reach subsequent phases of the task. Joint interaction with a knowledgeable mate facilitated the first three phases of task acquisition. However, joint interaction may have interfered with the last phase of the task, the need to diverge from the demonstrators’ pattern of investigation in order to receive food rewards.
Six of the eight joint-interaction tamarins opened correct forage sites and completed an accurate trial within the first 3 days of testing, suggesting that they had knowledge of the motor actions necessary to solve the task and the locations of the correct forage sites. Despite this result, joint-interaction tamarins generally followed their demonstrator’s pattern of visits to forage sites and opened each correct forage site after the demonstrator had taken the food reward. The majority of tamarins first received food rewards on test day 4 or 8, when they were tested alone and had opportunities to initiate independent investigation of forage sites. While follows of demonstrators’ visits in the initial days of testing may have aided in learning about features of the task, high levels of social attentiveness to demonstrators on later test days may have delayed or reduced the independent interactions with a novel task that were ultimately necessary to receive food rewards. Thus, even in tolerant social contexts, some individual experience with a novel task separate from social input may be important for reinforcing independent task solutions.
On the one-trial memory test, we found no evidence for differences in task performance between original joint-interaction tamarins and original individual learners. Although based on small sample sizes, our results offer tentative support for Galef’s (1995) hypothesis that long-term task retention is independent of mode of task acquisition, either individually or through social learning. On further reflection, it is noteworthy that original social learners performed as well as original individual learners on the memory test, given that the individual learners had at least twice as much total exposure to the task as did original joint-interaction tamarins, and that most joint-interaction tamarins did not receive food rewards during the initial days of joint-interaction testing.
Based on evidence from field studies of foraging behaviour in Saguinus (Garber 1989), we predicted that cottontop tamarins would show long-term memory for the location of discrete feeding sites. Memory-test tamarins performed poorly relative to their high levels of performance at the end of joint-interaction testing. In our study, the interval between original testing and memory testing was probably longer than the typical interval between revisiting feeding sites in the wild. Also, the close spatial configuration of different forage sites on each frame may have made it difficult for tamarins to remember relevant location cues. These factors may have interfered with memory for the location of correct forage sites.
However, comparisons with naïve tamarins suggest that memory-test tamarins did show long-term memory of the foraging task. Memory-test tamarins spent more time on the apparatus than they had as individual learners and than did social-interaction tamarins, suggesting lower levels of neophobia during the memory test. Memory-test tamarins also made a greater percentage of visits to correct forage sites than did social-interaction tamarins, suggesting some memory for the locations of correct forage sites. Most importantly, all tamarins opened correct forage sites on the one-trial memory test and 75% (N = 9) received food rewards, whereas none of the individual learners or social-interaction tamarins were able to open forage sites or receive food rewards on their first day of exposure to the task. Considering that memory-test tamarins as a group took only 1 min longer to receive food rewards than they had at the peak of their performance during joint-interaction testing, we suggest that differences in performance on the memory test may not be ecologically significant.
Fragaszy & Visalberghi (2004) suggested that models of social learning should consider how individual propensities and social context can interact and influence the ability of individuals to acquire novel behaviour. With our experimental design, we examined how both aspects affect novel task acquisition in cottontop tamarins. We found that in test conditions without knowledgeable tamarins present, high levels of neophobia prevented all but a few more neophilic individuals from acquiring a novel task. In contrast, when knowledgeable demonstrators were present, the tolerant social context facilitated joint interaction and promoted transmission of novel behaviour from knowledgeable to naïve individuals. Once joint-interaction tamarins had observed demonstrations of task solutions, individual experience became useful in promoting independent task solutions. Our results suggest that naïve cottontop tamarins may be unlikely to acquire novel behaviour through individual experience alone. However, novel behaviour may be effectively transmitted within cottontop tamarin groups through close social attention and joint interaction with more knowledgeable group members.
Acknowledgments
We thank Aimee V. Kurian for helping with the majority of data collection. We also thank Adria Heymsfield, Tatyana Humle and Sofia K. Zahed for additional assistance with data collection. Andrew Mulder constructed the apparatus. Nicholas Keuler provided important statistical help. Robert A. Becker designed the figure of the apparatus for this paper. We thank Rosamunde E. Almond, Matthew W. Campbell, Katherine A. Cronin, Tatyana Humle and two anonymous referees for providing critical feedback on initial drafts. The research was supported by United States Public Health Service Grant MH29775 and an American Psychological Association Forest Honaker Scholarship for Masters Research.
References
- Aisner R, Terkel J. Cultural transmission of pine cone opening behaviour in the black rat, Rattus rattus. Animal Behaviour. 1992;44:327–36. [Google Scholar]
- Brown GR, Almond REA, van Bergen Y. Begging, stealing and offering: food transfer in nonhuman primates. Advances in the Study of Behavior. 2004;34:265–296. [Google Scholar]
- Cadieu N, Cadieu JC. The influence of free interactions and partner familiarity on social transmission in the young canary. Animal Behaviour. 2004;67:1051–1057. [Google Scholar]
- Caine, N. 1993. Flexibility and co-operation as unifying themes in Saguinus social organization and behaviour: the role of predation pressures. In: Marmosets and Tamarins (Ed. by A. B. Rylands), pp. 200–219. Oxford: Oxford University Press.
- Caldwell CA, Whiten A. Scrounging facilitates social learning in common marmosets, Callithrix jacchus. Animal Behaviour. 2003;65:1085–1092. [Google Scholar]
- Coleman SL, Mellgren RL. Neophobia when feeding alone or in flocks in zebra finches, Taeniopygia guttata. Animal Behaviour. 1994;48:903–907. [Google Scholar]
- Coussi-Korbel S, Fragaszy DM. On the relation between social dynamics and social learning. Animal Behaviour. 1995;50:1441–1453. [Google Scholar]
- Cronin KA, Kurian AV, Snowdon CT. Cooperative problem solving in a cooperatively breeding primate (Saguinus oedipus) Animal Behaviour. 2005;69:133–142. doi: 10.1016/j.anbehav.2004.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custance DM, Whiten A, Fredman T. Social learning and primate reintroduction. International Journal of Primatology. 2002;23:479–499. [Google Scholar]
- Day RL, Coe RL, Kendal JR, Laland KN. Neophilia, innovation and social learning: a study of intergeneric differences in callitrichid monkeys. Animal Behaviour. 2003;65:559–571. [Google Scholar]
- Fragaszy DM, Mason WA. Response to novelty in Saimiri and Callicebus: influence of social context. Primates. 1978;19:311–331. [Google Scholar]
- Fragaszy DM, Visalberghi E. Social influences on the acquisition of tool-using behaviors in tufted capuchin monkeys (Cebus apella) Journal of Comparative Psychology. 1989;103:159–170. doi: 10.1037/0735-7036.103.2.159. [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Visalberghi E. Socially biased learning in monkeys. Learning & Behaviour. 2004;32:24–35. doi: 10.3758/bf03196004. [DOI] [PubMed] [Google Scholar]
- Fritz J, Kotrschal K. Social learning in common ravens, Corvus corax. Animal Behaviour. 1999;57:785–793. doi: 10.1006/anbe.1998.1035. [DOI] [PubMed] [Google Scholar]
- Galef BG. Why behaviour patterns that animals learn socially are locally adaptive. Animal Behaviour. 1995;49:1325–1334. [Google Scholar]
- Galef BG, Giraldeau L. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Animal Behaviour. 2001;61:3–15. doi: 10.1006/anbe.2000.1557. [DOI] [PubMed] [Google Scholar]
- Garber PA. Role of spatial memory in primate foraging patterns: Saguinus mystax and Saguinus fuscicollis. American Journal of Primatology. 1989;19:203–216. doi: 10.1002/ajp.1350190403. [DOI] [PubMed] [Google Scholar]
- Garber, P. A. 1993. Feeding ecology and behaviour of the genus Saguinus In: Marmosets and Tamarins: Systematics, Behaviour and Ecology (Ed. by A. B. Rylands), pp. 273–295. Oxford: Oxford University Press.
- Ginther AJ, Ziegler TE, Snowdon CT. Reproductive biology of captive male cottontop tamarin monkeys as a function of social environment. Animal Behaviour. 2001;61:65–78. doi: 10.1006/anbe.2000.1587. [DOI] [PubMed] [Google Scholar]
- Giraldeau L, Lefebvre L. Scrounging prevents cultural transmission of food-finding behaviour in pigeons. Animal Behaviour. 1987;35:387–394. [Google Scholar]
- Laland KN, Plotkin HC. Excretory deposits surrounding food sites facilitate social learning of food preferences in Norway rats. Animal Behaviour. 1991;41:997–1005. [Google Scholar]
- Laland KN, Plotkin HC. Further experimental analysis of the social learning and transmission of foraging information amongst Norway rats. Behavioural Processes. 1992;27:53–64. doi: 10.1016/0376-6357(92)90040-K. [DOI] [PubMed] [Google Scholar]
- Laland KN, Williams K. Shoaling generates social learning of foraging information in guppies. Animal Behaviour. 1997;56:181–190. doi: 10.1006/anbe.1996.0318. [DOI] [PubMed] [Google Scholar]
- Laland, K. N., Richerson, P. J. & Boyd, R. 1993. Animal social learning: toward a new theoretical approach. In: Perspectives in Ethology. Vol. 10: Behaviour and Evolution (Ed. by P. P. G. Bateson, P. H. Klopfer & N. S. Thompson), pp. 249–277. New York: Plenum.
- Mason JR, Arzt AH, Reidinger RF. Comparative assessment of food preferences and aversions acquired by blackbirds via observational learning. Auk. 1984;101:796–803. [Google Scholar]
- Nicol CJ, Pope SJ. Social learning in small flocks of laying hens. Animal Behaviour. 1994;47:1289–1296. doi: 10.1006/anbe.1998.0920. [DOI] [PubMed] [Google Scholar]
- Palameta B, Lefebvre L. The social transmission of a food-finding technique in pigeons: what is learned? Animal Behaviour. 1985;33:892–896. [Google Scholar]
- Rapaport LG. Provisioning of young in golden lion tamarins (Callitrichidae, Leontopithecus rosalia): a test of the information hypothesis. Ethology. 1999;105:619–636. [Google Scholar]
- Rapaport LG, Ruiz-Miranda CR. Tutoring in wild golden lion tamarins. International Journal of Primatology. 2002;23:1063–1070. [Google Scholar]
- Savage A, Ziegler TE, Snowdon CT. Sociosexual development, pair bond formation, and mechanisms of fertility suppression in female cotton-top tamarins (Saguinus oedipus oedipus) American Journal of Primatology. 1988;14:345–359. doi: 10.1002/ajp.1350140404. [DOI] [PubMed] [Google Scholar]
- van Schaik, C. P. 2003. Local traditions in orangutans and chimpanzees: social learning and social tolerance. In: The Biology of Traditions: Models and Evidence (Ed. by D. M. Fragaszy & S. Perry), pp. 297–328. Cambridge: Cambridge University Press.
- van Schaik CP, Deaner RO, Merrill MY. The conditions for tool use in primates: implications for the evolution of material culture. Journal of Human Evolution. 1999;36:719–741. doi: 10.1006/jhev.1999.0304. [DOI] [PubMed] [Google Scholar]
- Snowdon CT. Social processes in communication and cognition in callitrichid monkeys: a review. Animal Cognition. 2001;4:247–257. doi: 10.1007/s100710100094. [DOI] [PubMed] [Google Scholar]
- Snowdon CT, Boe CY. Social communication about unpalatable foods in tamarins (Saguinus oedipus) Journal of Comparative Psychology. 2003;117:142–148. doi: 10.1037/0735-7036.117.2.142. [DOI] [PubMed] [Google Scholar]
- Soma M, Hasegawa T. The effect of social facilitation and social dominance on foraging success of budgerigars in an unfamiliar environment. Behaviour. 2004;141:1121–1134. [Google Scholar]
- Visalberghi, E. & Fragaszy, D. M. 1990a. Do monkeys ape? In: Language and Intelligence in Monkeys and Apes: Comparative Developmental Perspectives (Ed. by S. T. Parker & K. R. Gibson), pp. 247–273. New York: Cambridge University Press.
- Visalberghi E, Fragaszy DM. Food-washing behaviour in tufted capuchin monkeys (Cebus apella) and crab eating macaques (Macaca fascicularis) Animal Behaviour. 1990b;40:829–836. [Google Scholar]
- Vitale A, Queyras A. The response to novel foods in common marmosets (Callithrix jacchus): the effects of different social contexts. Ethology. 1997;103:395–403. [Google Scholar]
- Whiten A. Imitation of the sequential structure of actions by chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1998;112:270–281. doi: 10.1037/0735-7036.112.3.270. [DOI] [PubMed] [Google Scholar]


