Abstract
Whole-head magnetoencephalography (MEG) was used to spatiotemporally map the brain response underlying episodic retrieval of words studied a single time following a long delay (~40min.) Recognition following a long delay occurs as a strong, sustained, differential response, within bilateral, ventral and lateral prefrontal cortex, anterior temporal and medial parietal regions from ~500ms onward, as well as ventral occipitotemporal regions from ~700ms onward. In comparison with previous tasks using multiple repetitions at short delays, these effects were centered within the same areas (anteroventral temporal and ventral prefrontal), but were shifted to longer latencies (~500ms vs. ~200ms), were less left-lateralized, and appear more in anterolateral prefrontal regions and less in lateral temporal cortex. Furthermore, comparison of correctly classified words with misclassified, novel and repeated words, suggests that these frontotemporal-parietocingulate responses are sensitive to actual as well as perceived repetition. The results also suggest that lateral prefrontal regions may participate more in controlled, effortful retrieval while left ventral frontal and anterior temporal responses may support sustained lexicosemantic processing. Additionally, left ventromedial temporal sites may be relatively more involved in episodic retrieval, while lateral temporal sites may participate more in automatic priming.
Keywords: MEG, episodic, retrieval, memory, words, priming
INTRODUCTION
Evidence from lesion based studies demonstrates that damage to the frontal cortex or temporal lobes often results in lasting memory impairment (Scoville and Milner, 1957; Stuss and Benson, 1984; Corkin et al., 1985; Janowsky et al., 1989). Involvement of these areas has since been confirmed by studies performed on normal subjects utilizing functional neuroimaging techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). Indeed, much of the imaging research to date suggests that a wide range of brain areas may be involved in memory retrieval; these include frontal, temporal, parietal, and cingulate cortices as well as occipital and cerebellar regions (for review see Cabeza and Nyberg, 2000). Although PET/fMRI have good spatial localization, their temporal accuracy is poor (>1sec) in comparison with MEG and electroencephalography (EEG), which provide millisecond temporal resolution, and adequate spatial resolution (Dale and Halgren, 2001). Studies utilizing EEG to study memory retrieval have found that the N400 event related potential (ERP) component decreases with stimulus repetition and P3b increases (for review see Halgren and Smith, 1987). Recent studies with MEG (Dale et al., 2000; Dhond et al., 2001; Marinkovic et al., 2003) suggest that these repetition induced changes are mainly localized to anteroventral temporal and posteroventral prefrontal cortices, a conclusion consistent with intracranial recordings (Halgren et al., 1994b; Halgren et al., 1994a). These studies, however, have generally used multiple repetitions of each stimulus, and tested for recognition after delays of ~1 min. The current study attempts to test the generality of the previous MEG results by testing recognition of words presented a single time, after a delay of ~40 min. Such large delays also increase error rates allowing for additional comparison to be made with unrecognized repeated words as well as determination of the areas engaged by ‘perceived’ repetition in the case of incorrectly classified novel words.
We utilized whole-head (204 channel) MEG and applied an anatomically constrained, distributed inverse solution, normalized by estimated noise values, to produce dynamic statistical parametric maps (Dale et al., 2000; Dhond et al., 2001). Spatiotemporal response maps of language/memory processing made previously using this technique (Dhond et al., 2001; Halgren et al., 2002; Dhond et al., 2003; Marinkovic et al., 2003) are consistent with activation patterns found in similar tasks with fMRI and intracranial EEG (Smith et al., 1986; Halgren et al., 1994b; Halgren et al., 1994a; Dale et al., 2000). Response maps were used to characterize the overall spatiotemporal cortical response pattern during delayed episodic retrieval, as well as help understand the effects related to the ‘perception’ of words as repeated.
METHODS
Subjects and Task
Data was collected from 12 normal, right-handed (Oldfield, 1971), native English-speaking males (18–30 years old). Subjects were rejected if they had a history of mental or physical illness, head injuries, or of drug/alcohol dependence/abuse. Subjects were screened for MEG artifacts due to dental work or excessive eye-blinking.
During a study phase, subjects made abstract/concrete judgments on 480 visually presented words. Stimuli were projected through an opening in the wall of the shielded room (which was covered with transparent plexi-glass) onto a screen located within the room. Using Mac Probe software (Hunt, 1994) word stimuli were presented in Geneva font as white letters on a black background, and presented in the central 5% of visual angle for 700ms. Stimulus onset asynchrony was 2.0 sec. Stimuli were randomly intermixed with 240, 2 sec trials of fixation (<<+>>). Following the initial study phase, subjects were removed from the MEG machine to allow them to rest for ~20–30 minute after which they were again placed within the machine and underwent a test phase. Prior to completion of the study phase, subjects were not aware that they would be tested, thus, this was a test for retrieval of incidentally encoded words. During the test phase, participants were shown 960 words, 480 of which were “Old” words (previously shown during the study phase) and 480 of which were “New” words (not previously presented in the study phase). Subjects responded with their left hand and were first required to indicate whether the presented word was “New” or “Old” by lifting their left, middle or index finger respectively. Subjects were then required to rate their confidence for their response as “High” for high-confidence (recollection of word during study) or “Low” for low-confidence (word is familiar), again they were asked to respond by lifting their left, middle or index finger respectively. During the test phase, participants were asked to refrain from blinking as long as possible, however, if absolutely necessary to do so only between the “new/old” and “high/low” decision period of presented trials. All data shown in this study are from the test phase of the experiment only. Stimuli were presented in the same manner as those in the study phase except that the test phase was self-paced. Thus, advancement to the next test-word trial did not occur until subjects had completed responding. Trials in which subject responses were very rapid (occurred <700ms) or slow (>2200ms) were rejected prior to averaging. This response-time window was chosen so that 85–100% of all trials, for each individual, fell within this window. Furthermore, a self-paced response procedure was utilized to ensure a high number of correct responses and limit guessing as the long delay time between study and test was expected to make retrieval difficult.
In the subsequent text, a two word notation is used to signify the trial type. The first word indicates whether the test-word was “New” or was a previously studied “Old” word. The second word indicates the accuracy of the subjects’ categorization with “Hit” for correct or “Miss” for incorrect. Thus, a previously studied test word, which was correctly identified will be referred to as an “Old-Hit”. A new word that was incorrectly assigned as “old” would be a “New-Miss”. Note, that the “Old-Miss” and “New-Miss” condition averages were made by combining all high and low confidence trials together. The “New-Hit” as well as “Old-Hit” conditions only contain trials that were also chosen with high confidence.
MEG Recording
MEG was recorded using a Neuromag VectorViewTM (Elekta, Stockholm, Sweden) with 204 gradiometer channels covering the entire scalp. MEG recordings took place within a magnetically shielded room (IMEDCO, Hagendorf, Switzerland). Signals were sampled at 601Hz after filtering from 0.1–200 Hz. Data was then low-pass filtered at 20 Hz and separate averages of each condition were constructed for every subject. Trials were rejected from analysis, based on amplitude criteria supplemented by visual inspection, if they were contaminated by artifacts (identified as peak-to-peak amplitude >5000fT/cm in any channel) or eye-blinks (>200 μV in the EOG electrode). Head movement was minimized using a bite bar customized for each subject (Marinkovic et al., in press).
Cortical Surface Reconstruction
A geometrical representation of the cortical surface of each subject was obtained using procedures described previously (Dale et al., 1999; Fischl et al., 1999a). First, high-resolution 3-D T1-weighted structural images were acquired for each subject using a 1.5T Picker Eclipse (Marconi Medical, Cleveland, OH). Then, the cortical white matter was segmented, and the estimated border between gray and white matter was tessellated, providing a topologically correct representation of the surface with about 150,000 vertices per hemisphere. For the inverse computation, the cortical surface was decimated to approximately 3000 dipoles, which is roughly equivalent to 1 dipole every 1 cm2 along the cortical surface. Finally the folded surface tessellation was “inflated”, in order to unfold cortical sulci, thereby providing a convenient format for visualizing cortical response patterns (Dale et al., 1999; Fischl et al., 1999a). For purposes of intersubject averaging, the reconstructed surface for each subject was morphed into an average spherical representation, optimally aligning sulcal and gyral features across subjects while minimizing metric distortions and shear (Fischl et al., 1999a) and MEG response power was mapped onto an average sulcal-gyral pattern. Compared to volumetric morphing into Talairach (Collins et al., 1994) space, this method has been found to provide better alignment across subjects of functional activation in a verbal task (Fischl et al., 1999b) and allows direct localization to regular gyri.
Forward solution
The boundary element method (BEM) was used for calculating the signal expected at each MEG sensor, for each dipole location (deMunck, 1992; Oostendorp and Van Oosterom, 1992). The computation of the MEG forward solution has been shown to only require the inner skull boundary to achieve an accurate solution (Meijs et al., 1987; Meijs and Peters, 1987; Hamalainen and Sarvas, 1989). The T1-wieghted MRI described above was used for construction of the inner skull surface. The MEG sensor coordinate system was aligned with the MRI coordinate system using four head position (HPI) coils, attached to cardinal locations on the scalp (Hämäläinen et al., 1993). The HPI coils generate weak magnetic signals, allowing them to be directly localized by the MEG sensors before and after the recording session. The positions of the HPI coils with respect to the subject’s head (and thus MRI) are determined by measuring ~80 points (including the HPI coils) around the head using a Polhemus FastTrack 3-D digitizer. These digitized points were later registered with the MRI image. Since the HPI coils were thus localized with respect to both the MEG sensors and the structural MRI, a common coordinate system was established allowing neural response to be estimated for each cortical location.
Inverse solution
To estimate the timecourses of cortical response, we used the noise-normalized, anatomically constrained linear estimation approach described by Dale et al (2000). This approach is similar to the generalized least-squares or weighted minimum norm solution (Hamalainen and Ilmoniemi, 1984), except that the modeled sources were constrained to lie in the cortical surface (as determined above) (Dale and Sereno, 1993), and the estimate was normalized for noise sensitivity so that source signal to noise ratio rather than current dipole moment was mapped (Dale et al., 2000). The noise normalization also has the effect of greatly reducing the variation in the point-spread function between locations (Liu et al., 2002). This approach provides statistical parametric maps of cortical response, similar to the statistical maps typically generated using fMRI, or PET data, but with a temporal resolution of 5ms or less. Note that although the noise normalized values are not identical to the source current estimates, these values are directly proportional to the current power estimated for a given site. This is a consequence of the fact that the noise normalized value is calculated as the ratio between the time variant signal power and the time invariant noise power.
Since in the current study, no a priori assumptions were made about the local dipole orientation, three components were calculated for each location. The noise normalized estimate of the source power (sum of squared source component strengths) at location i is given by
where Gi is the set of (three) dipole component indices for the ith location, and wi denotes the ith row of the inverse operator W (Dale and Sereno, 1993; Liu et al., 1998; Dale et al., 2000). The noise covariance matrix C for all conditions was estimated from the baselines of the raw signals after all filtering is applied. For all comparisons (i.e. New-Hit vs. Old-Hit etc.), the waveforms of the individual conditions were subtracted prior to estimating the differential source activity pattern. Both non-subtracted and subtracted conditions were tested for the null hypothesis that the signal was noise. Note, under the null hypothesis, qi(t) is F-distributed, with three degrees of freedom for the numerator and a very large number for the denominator (about 160,000 time points per sensor were used to estimate the spatial sensor noise covariance). In summary, noise-sensitivity normalized cortical surface constrained minimum norm inverse solutions were calculated every 5 ms for every condition and every individual. The square roots of these values were then averaged on the cortical surface across individuals after aligning their sulcal-gyral patterns. The square root was used in order to de-emphasize outliers and ensure that the result is linearly proportional to the magnitude of the estimated sources (Dale et al., 2000; Dhond et al., 2001). The distribution under the null hypothesis of the estimates averaged estimates was sampled using monte carlo simulations in order to obtain significance thresholds. For all figures the minimum significance values were p<10−7 for threshold, and the full red color indicates a minimum significance of p<10−10. These values were chosen to maximize the dynamic range of the color scale, and the statistical thresholds were chosen to conservatively correct for the number of comparisons being performed.
In all figures, the response maps of the non-subtracted conditions were constructed using an equal number of trials in each condition (trial numbers were matched by randomly removing trials from the condition with the larger number of trials.) This permits the statistical maps provided here to be used directly to assess relative MEG signal power between the non-subtracted conditions. The dynamic statistical parametric maps of the subtracted conditions demonstrate the differences between the averages of all trials in the non-subtracted conditions for which the comparison is being made.
It should also be noted that MEG gradiometer data in this study are unlikely to reveal responses that can be confidently assigned to deep, non-cortical structures. These structures lack the synaptic/dendritic arrangements necessary to generate large MEG signals. Furthermore, they are far from the sensors, and MEG gradiometer signals decline greatly with distance. Although subcortical responses can sometimes be detected by MEG, this usually requires a large number of averages, i.e. >1000 and a lack of response in overlying generators (such as may occur at very short latencies prior to cortical involvement.) In contrast, the current study examines response at relatively long latencies and averaged across relatively fewer trials. For these reasons, deep non-cortical structures such as the basal ganglia and corpus callosum are not included as sources in the spatiotemporal maps of the medial and ventral surfaces.
RESULTS
Behavioral Results
Behavioral response times were averaged across 12 subjects for each condition. The average behavioral response latencies and standard deviations for 12 subjects were: New-Hit words 1273.8 (117.2); Old-Hit words 1103.6 (104); Old-Miss words 1284.1ms (162); and New-Miss words 1278.2 (106). Mean reaction time differed significantly between New-Hit and Old-Hit trials t(11) = 3.69, p < .004; between Old-Miss and Old-Hit trials t(11) = 2.39, p < .036 ; and between New-Miss and Old-Hit trials t(11) = 3.65, p < .004. Furthermore, subjects’ accuracy was good and the average hit (.754) and false-alarm (.353) rates for Old words demonstrated significant differences t(11) = 12.65, p < .001.
When comparing the average number of words remembered vs. those that were forgotten, subject responses were not largely biased based on frequency (Kucera, 1967), number of letters or imagery (Coltheart, 1981). On average there was no difference in frequency for words (frequency ratings used when available) selected as old (45.96 ± 2.0, mean ± std. err.) and those selected as new (41.90 ± 1.9), t(22) = 1.25, p < .16. There was a slight difference in the number of letters in words selected as old (6.28 ± .02) vs. new (6.13 ± .04), t(22) = 3.09, p < .007. Imagery ratings, when available, were found to be slightly higher for words selected as new (511.44 ± 3.1) than those selected as old (489.38 ± 2.2), t(22) = 5.86, p < .001.
Overall brain response pattern during word retrieval
Both novel and repeated trial types demonstrated a highly similar posterior to anterior sequence of brain response consistent with classical models of language processing (Benson, 1979; Geschwind, 1979). This characteristic pattern is exemplified by response to the Old-Hit condition shown in Figure 1.
Figure 1. Statistical parameteric maps of spatiotemporal response patterns during delayed recognition (Old-Hit trials).
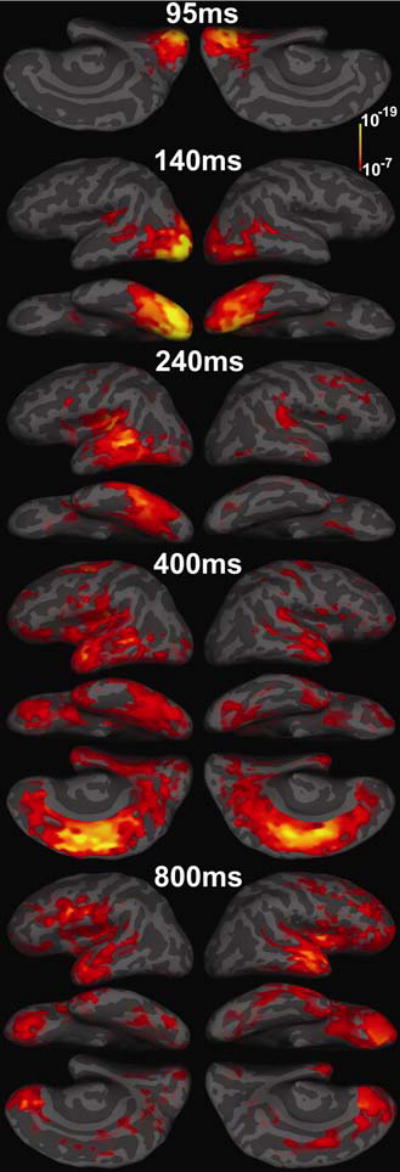
All conditions demonstrated a similar overall pattern of brain response beginning with the bilateral visual response at roughly ~95ms centered on the calcarine sulcus and occipital pole. By ~140ms post-stimulus, response was strongest within occipitotemporal regions (left >right). Subsequently, at ~240ms, response peaked within a band of cortex extending from the posterior insula and posterior superior temporal plane, through the posterior halves of the superior, middle and inferior temporal, fusiform and parahippocampal gyri. At ~400ms response peaked anterolateral temporal, medial temporal, and ventral prefrontal regions (left > right) similar to that seen for the N400m (Halgren et al., 2002). There was also strong bilateral response within anterior and posterior cingulate cortex as well as precuneus and retrosplenial areas. At ~800ms fronto-temporal recruitment was highly extended to dorsal prefrontal regions. Response continued for ~100ms and then began to decrease. For these and all other maps the minimum significance values are p<10−7 for threshold and p<10−10 for full red.
The initial visual response peaks bilaterally at ~95ms post-stimulus in the occipital pole and calcarine sulcus (primary visual cortex). Response then spread rapidly to anteroventral occipital cortex corresponding approximately to Brodmann area 19 (~BA 19) where by ~140ms it was strongest within the language dominant, left hemisphere. This early response extended into left posterior fusiform and slightly more anterior occipitotemporal areas (~BA 37/19) previously implicated in the encoding of letter-strings and other word-like stimuli (Nobre et al., 1994). Response also occurred within the left posterior cingulate area (including precuneus and retrosplenial cortices) at this time. By ~240ms response continues within ventral temporal regions and is maximal in posterior language areas, including Wernicke’s area (~BA 22) and adjacent temporo-parietal cortex both implicated in lexico-phonemic/phonological processing (Rumsey et al., 1997; Paulesu et al., 2000; Price, 2000) and occipitotemporal (ventral ~BA 37/19) cortex implicated in the processing of visual form attributes of words (Tarkiainen et al., 1999; Dhond et al., 2001; Tarkiainen et al., 2002; Marinkovic et al., 2003). At this time, response engaged the entire posterior half of the temporal lobe and also began within bilateral anterior cingulate cortex and medial aspects of the post-central gyrus. Response also began within anterior temporal and prefrontal regions but is not maximal until longer latencies.
The dominant focus of cortical response continued to move forward, and by ~340ms encompassed anterotemporal regions (~BA 38, and anterior 20/21) associated with lexico-semantic processing (Hodges et al., 1992; Damasio et al., 1996; Mummery et al., 2000). Prefrontal regions were also active at this time, and their response increased over the next 100ms, peaking at ~400ms and strongest within the left hemisphere (including ~BA 11, 47, 45, 44, 10). The cortical distribution and timing of these responses were similar to those of the N400m as measured by MEG (Dhond et al., 2001; Halgren et al., 2002; Marinkovic et al., 2003) and intracranial EEG (Smith et al., 1986; Halgren et al., 1994b; Halgren et al., 1994a; Guillem et al., 1995; McCarthy et al., 1995). Other MEG studies utilizing single or a few dipoles have found context sensitive N400-like components at similar latencies localizing within temporal and/or frontal regions (Simos et al., 1997; Helenius et al., 1998, 1999; Penney et al., 2003; Puregger et al., 2003; Pylkkanen and Marantz, 2003). At this time, there was also strong bilateral recruitment of cingulate cortex, which may play a role in attention and working memory as well as response choice (Bush et al., 2000; Braver and Barch, 2002; Cabeza et al., 2003; Wang et al., in press). Response also occurred within precuneus and retrospenial areas. Fronto-temporal response continued until the end of the epoch, with lateral prefrontal areas showing their greatest response at longer latencies (~800ms onward). These areas included classical Broca’s area (BA 44) (Benson, 1979) and neighboring regions (~BA 9/46/45), especially of the left, as well as anterior/dorsal (~BA 10 and 9) and ventral prefrontal cortices (~BA 47/11) on the right, often activated during memory retrieval tasks (Cabeza and Nyberg, 2000).
New-Hit vs. Old-Hit: Repetition with recognition (the New/Old effect) (Fig 2)
Figure 2. Significant cortical responses for successful recognition following a long delay (New-Hits vs. Old-Hits).
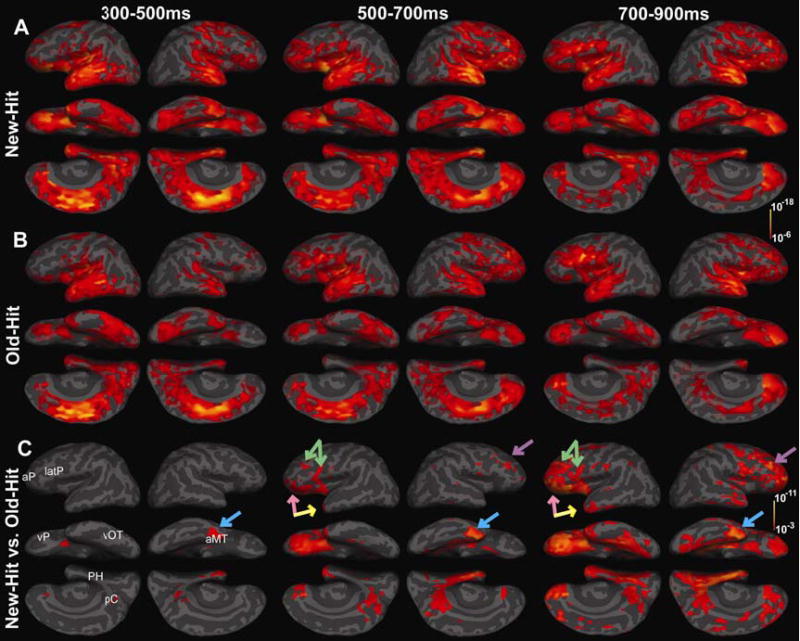
At ~300–500ms post-stimulus, differential response began in right anteromedial temporal (light blue arrow, aMT), and then left ventral prefrontal cortex (pink arrow, vP), and bilateral posterior cingulate cortices (pC). From ~500–700ms, these responses become more significant, and additional differences were observed in lateral prefrontal regions (green and purple arrows, latP), as well as right parahippocampal gyrus (PH) and left anteromedial temporal cortex (yellow arrow). From 700–900ms there was involvement of ventral occipitotemporal (vOT) areas particularly within the left. All responses were greater for New-Hits except within Broca’s Area where it was greater to repeated words. Compared to previous MEG and EEG studies of word recognition which used multiple repetitions and a shorter delay, these differential responses occurred at longer in latencies and involved additional response within more lateral prefrontal and anterior prefrontal (aP) regions. Thus, as repetition delay (and retrieval difficulty) increase, sustained response within a frontotemporal-parietocingulate network supporting controlled/directed retrieval of semantic associations is associated with item recognition.
In order to assess the effect of item repetition during successful recognition, we contrasted New-Hits with Old-Hits (Figure 2).
Differential response between New-Hit and Old-Hit conditions first occurred within the right, anteromedial temporal region, corresponding to entorhinal and perirhinal cortices at ~310ms followed by left anterior insular cortex at ~425ms. There were also differences within the posterior cingulate area including the precuneus and retrosplenial cortex. By ~455ms, response spread anteriorly to left ventral prefrontal cortex (~BA 11, 47). Differences began at ~490ms, in more lateral prefrontal regions (~BA 45, 46) including Broca’s area (~BA 44) and also within left anterior/dorsal prefrontal cortex. However, these responses only became strong and sustained at longer latencies (>600ms). All responses (between 300–500ms) were greater for correctly classified novel items, and all continued on through longer latencies (compare Figure 2, New-Hit vs. Old-Hit). In the right hemisphere, differences within similar lateral and ventral prefrontal as well as dorsal prefrontal regions were significant from ~600ms onward. Figure 2, New-Hit vs. Old-Hit, shows that the greatest average difference between 500–700ms occurred within the right anteromedial temporal cortex (entorhinal/perirhinal and parahippocampal areas), and left ventral prefrontal cortex. Starting at ~660ms, but most significant from ~700–900ms, the differential response in the left hemisphere shifted posteriorly to include occipitotemporal regions encompassing the fusiform and parahippocampal gyri. There were also small differences within lateral parietal regions at longer latencies (~600–900ms). All responses were greater for New-Hit words with the exception of the response within Broca’s area, which was greater to repeated words. Responses within all of the above regions gradually peaked at ~815ms post-stimulus, and then continued until the end of epoch (1000ms.)
Overall these results suggest that successful recognition depends critically on the semantic circuits in the anterior temporal (~BA 38/20) and ventral prefrontal (~BA 47/11) cortices. Furthermore, at these longer latencies, the differences increasingly involved bilateral anterior/dorsal (~BA 9,10) and lateral prefrontal (~BA 44/45) perhaps supporting directive retrieval processes (Buckner and Koutstaal, 1998; Wheeler and Buckner, 2003).
New-Hit vs. Old-Miss: Repetition in the absence of recognition
To determine if the responses to delayed word repetition noted above are specific to successful retrieval New-Hits were contrasted with Old-Miss trials (Figure 3A). In both conditions, the words were classified as new, but in the Old-Miss trials they were in fact repeated words.
Figure 3. Effects of Actual vs. Perceived Repetition/Novelty: A: New-Hit vs. Old-Miss.
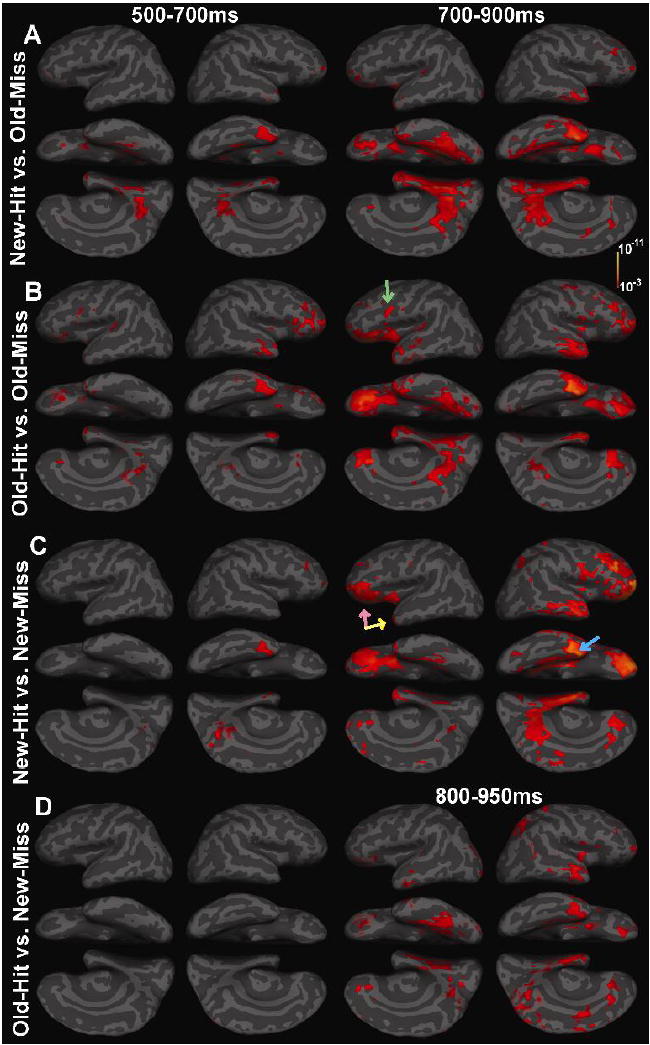
The actual repetition of words in the absenFce of recognition produced differential response that was similar to that seen when repetition was correctly recognized in anteromedial temporal, ventral prefrontal and posterior cingulate areas (see Figure 2, lower panel C). However, unlike correct recognition, little lateral prefrontal (~BA 44/45) difference was observed, implicating this area in aspects of conscious recognition. B. Old-Hit vs. Old-Miss: Correctly recognized words differed from those that were forgotten in bilateral prefrontal, anterior and ventral temporal as well as posterior cingulate regions suggesting that sustained cooperative response within these areas is required for recognition to occur. Greater response modulation within lateral prefrontal sites (green arrow) was seen for recognized words. C. New-Hit vs. New-Miss: The incorrect perception of novel items as repeated also produced frontotemporal differences that were similar to those seen for actual repetition (pink arrows), however, the lack of left lateral prefrontal differences again suggests that response within this region may be associated with correctly perceived repetition. Furthermore, increased right anteromedial temporal response (light blue arrow) for words judged “new” suggests that response modulation within this area is related to the conscious “perception” of a word as novel even if it is not. D. Old-Hit vs. New-Miss: Unperceived repetition resulted in response modulation within bilateral ventral prefrontal and temporal areas, similar but later and weaker than those seen for correctly perceived repetition in Figure 2C. The presence of bilateral ventral prefrontal, anteromedial temporal and posterior cingulate response differences suggests that these areas are part of a general network sensitive to repetition, whereas the lack of strong lateral prefrontal response modulation suggests that these regions require that the repetition be perceived.
The differences began slightly later (at ~530ms post-stimulus) than those described above where Old words were correctly recognized. The most significant effects between 500 and 700ms were located within the right anteromedial temporal and bilateral posterior cingulate areas, with additional responses in bilateral posterior parahippocampal and fusiform gyri activity. These differential responses increased and continued at longer latencies (700–900ms) when there was also response within ventral prefrontal regions bilaterally but more significant in the left hemisphere.
These results contrast with the findings above showing that, when Old words were not correctly recognized, they produced less response in many of the same areas as when they were correctly recognized, including bilateral anteromedial temporal, ventral prefrontal, and posterior cingulate areas. However, these decreases began later and were less significant. Furthermore, repetition in the absence of recognition did not alter response within left lateral prefrontal cortex (~BA 44/45) suggesting that this area may participate in accurate explicit recognition. Overall these data suggest that beginning at an early latency, sustained differential response within bilateral prefrontal regions may be necessary for successful retrieval.
Old-Hit vs. Old-Miss: Repetition without Recognition (Figure 3B)
A similar response pattern was observed when contrasting activity evoked by repeated words that were recognized vs. those that were not. Again, the main areas responding differentially were ventromedial temporal, ventral and lateral prefrontal and posterior cingulate areas. Of these, the first significant differences occurred within the right anterior middle prefrontal cortex at ~450ms post-stimulus. Differential response then spread to the right frontal pole and inferior prefrontal regions by ~510ms and began at ~540ms within the right anteromedial temporal cortex continuing through longer latencies (~800ms). At ~ 650ms small differences occurred within the left inferior prefrontal cortex (~BA 47/11, 44) and temporal pole, then within bilateral posterior cingulate cortex at ~600ms (left > right) and left posterior fusiform and parahippocampal gyri at ~740ms post-stimulus.
Thus, correctly recognized repeated words (Old-Hit) demonstrate increased response within Broca’s area, when they were contrasted with words perceived as new, regardless of whether the words are actually new (Figure 2) or had actually been seen before (the current contrast). This again suggests that increased response within Broca’s area may to some extent be associated with successful retrieval of words or explicit recognition. Similarly, increased response within the right anteromedial temporal cortex may signal the eventual categorization of an item as novel (even if it is not).
New-Hit vs. New-Miss: Perceived Repetition in the absence of Actual Repetition
To gain more insight into the brain areas that were differentially affected by perceived ‘oldness’, novel words misperceived as ‘old’ (New-Miss), were subtracted from correctly classified novel trials (New-Hit) as seen in Figure 3C. Response was greater to trials correctly classified as new (New-Hit trials) from ~395ms onward in the right anteromedial temporal cortex. As seen earlier, larger response power within this area also occurred for words categorized as “new” (even if they were not); thus, sustained response within this area may indicate the perception of a stimulus as novel, though the strongest response occurs when judgments are accurate. By ~500ms there was also differential response within the right posterior cingulate area, and right dorsal prefrontal regions by ~600ms. Differences began within the ventral prefrontal in the right at ~625ms and left from ~670ms onward. The prefrontal differences peaked (bilaterally) between 800–850ms. There were also small differences within left medial and polar temporal cortex, as well as strong responses in the right anterior, medial and lateral temporal lobe from ~700ms onward.
The overall network seen in this contrast is highly similar to that observed in the previous contrasts. Relatively little differential response was seen within ventral occipitotemporal or left lateral prefrontal regions suggesting that these areas may be slightly more sensitive to actual repetition or its recognition. Conversely, relatively more significant differences occurred in right prefrontal and anteromedial temporal areas, suggesting that the perception of repetition may be sufficient to modulate response within these areas.
Old-Hit vs. New-Miss: Actual Novelty in the absence of Perceived Novelty
Further analysis of the effects of actual repetition were assessed by considering the effects of words incorrectly vs. correctly perceived as ‘old’ (Figure 3D.) Significant differences occurred mainly at longer latencies >700ms, and were smaller than those of the preceding contrasts. Response peaked ~915ms within left parahippocampal and ventral occipitotemporal regions as well as right anteromedial temporal cortex. Differential response began in right ventral and polar prefrontal regions at ~800ms. Differences were strongest within the left ventral prefrontal cortex from ~900ms onward. All of these responses were greater for the New-Miss condition. Again, the overall network observed was similar to that found for the other contrasts, but was late and weak. Preserved differential response in the right anteroventral temporal, and left ventral occipitotemporal cortices suggest that these areas may weakly respond to repetition even when it is not sufficient to guide behavior.
Comparisons of High and Low confidence Trials
The comparison of high and low confidence trials for novel words demonstrated increased response to high-confidence trials within regions of the right anteromedial temporal and parahippocampal cortices from ~300–900ms post-stimulus (Figure 4). This association of confidence in novelty judgments with right medial temporal response is consistent with the notion that novelty detection is based on a signal from that area (Tulving et al., 1996). For repeated words the comparison of “high” confidence judgments with “low” confidence trials yielded no sustained significant differences.
Figure 4. Comparison of novel high vs. low confidence trials.
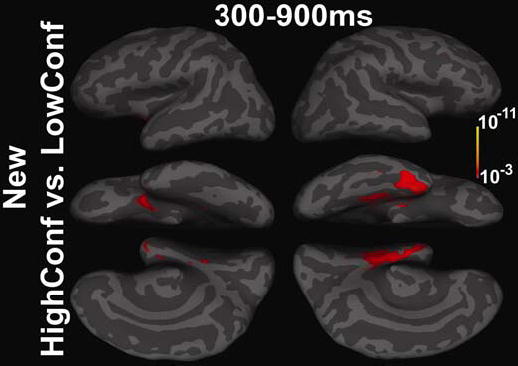
Differential effects were significant within the right anteromedial temporal cortex suggesting that response within these areas may partially support the experience of novelty.
DISCUSSION
Overall pattern of response during delayed recognition
While a posterior to anterior sequential pattern of response was observed at early latencies, after ~250ms, simultaneous response occurred within bilateral frontal, temporal and medial cortices. The location of response is consistent with most hemodynamic observations (for review see Cabeza and Nyberg, 2000) as well as intracranial EEG recordings during word recognition tasks (Smith et al., 1986; Halgren et al., 1994b; Halgren et al., 1994a). Furthermore, the present spatiotemporal response pattern strongly resembles those seen with MEG during semantic judgment tasks (Dale et al., 2000; Marinkovic et al., 2003), verb past-tense inflection (Dhond et al., 2003), sentence reading (Halgren et al., 2002) and word-stem completion (Dhond et al., 2001). The concentration of MEG response within areas involved in verbal processing demonstrates that visuo-verbal episodic retrieval is supported by the same networks, and lends support for the existence of a general, distributed, intracortical network underlying all higher order language functions (Halgren, 1990).
Brain Responses to Word Repetition
Numerous studies with scalp and intracranial EEG have demonstrated that word repetition results in prominent N400 decreases from ~300–500ms, however, these studies utilized repetition intervals of <2min (for review see Halgren and Smith, 1987). Furthermore, anatomically constrained MEG as well as intracranial EEG localize these effects to multiple overlapping frontotemporal sites (Halgren et al., 1994b; Halgren et al., 1994a; Dale et al., 2000; Dhond et al., 2001; Marinkovic et al., 2003). The present investigation tests the generality of these findings by observing stimulus repetition at longer latencies ~40 min.
Timing of Responses to Word Repetition
MEG responses to delayed word repetition occurred predominantly as a late (>500ms), sustained, bilateral, response modulation of prefrontal and ventral temporal sites (Figure 5). In contrast, previous MEG studies utilizing numerous repetitions and shorter delays demonstrated that the greatest differences began much earlier (~210ms) and were strongest within left, ventral prefrontal and lateral temporal sites (Dhond et al., 2001; Marinkovic et al., 2003). The data are consistent with EEG studies demonstrating that as repetition delay is increased repetition related differences occur primarily at longer latencies, >500ms (Nagy and Rugg, 1989; Rugg and Nagy, 1989; Karayanidis et al., 1991; Van Petten et al., 1991). When words are repeated many times at relatively short intervals, automatic priming effects are likely to be more significant than in the current study where controlled effortful recognition may be required. Thus, the present results suggest that similar frontotemporal networks are involved in these different recollective tasks, but that left lateral temporal regions are relatively more involved in automatic processing, and bilateral prefrontal regions in controlled processing. Furthermore, previous MEG studies utilizing repetition priming at shorter delays found repetition effects that were much more laterally distributed in the temporal lobe. Thus, the present findings are also consistent with the general idea that more medially situated temporal lobe structures are involved in controlled episodic memory processes, whereas lateral temporal areas are more specialized for automatic priming (Brown and Aggleton, 2001).
Figure 5. Sensor waveforms demonstrating late differences between New-Hit and Old-Hit words during the delayed recognition task.
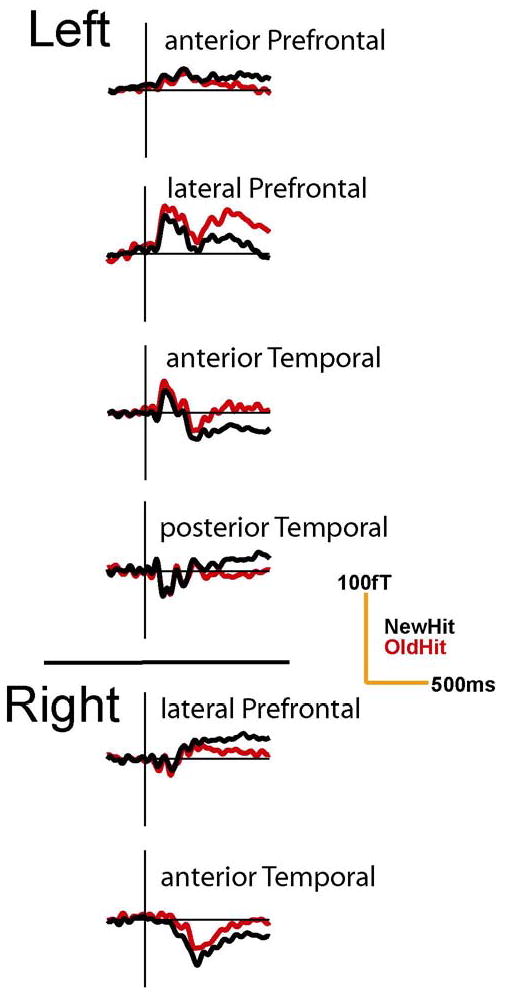
Sensor waveforms from a single subject are shown. Differences between New-Hit and Old-Hit words were most significant at longer latencies (>500ms) though some smaller differences were also seen earlier within right anterior temporal sites. Left lateral prefrontal sites demonstrated larger responses for correctly recognized repeats while the other sites demonstrated larger responses for novel words.
Localization of New/Old Effects
Prefrontal Responses
In the present study, prefrontal differences were centered within anterior, lateral and ventral areas. In general, left prefrontal areas are recruited during semantic retrieval/manipulation (Kapur et al., 1994; Buckner et al., 1995; Demb et al., 1995; Vandenberghe et al., 1996; Poldrack et al., 1999) and the generation of the N400 cognitive component indicating lexico-semantic integration (Halgren et al., 1994b; Dale et al., 2000; Halgren et al., 2002; Marinkovic et al., 2003). Anterior regions of prefrontal cortex (~BA 10, 9) have also been associated with directed/effortful retrieval (Schacter et al., 1996; Buckner et al., 1998a; Nolde et al., 1998), though more recently greater new/old differences in ~BA 44 have also been linked to increased “controlled processing” occurring for retrieval following a single repetition as opposed to many (20x) repetitions (Wheeler and Buckner, 2003). In the present study, late sustained response within left ventral prefrontal cortex (which is also modulated at shorter repetition delays) may represent “re-access” to lexico-semantic associations activated during study, providing a basis for the recognition of words through ‘controlled’ or directed retrieval mechanisms possibly supported by more anterior and lateral prefrontal regions. It should be noted, however, that the strongest frontotemporal activations occurred ~500–900ms, which is subsequent to the peak latencies of the N400m (~300–500ms) supporting semantic integration (Halgren et al., 2002). Thus, ventral frontotemporal responses may to some extent represent ‘sustained/prolonged’ access to semantic associates while aspects of controlled retrieval and presumably ‘conscious’ recognition, thought to occur at longer latencies (>500ms) (Smith, 1993; Smith and Guster, 1993), may be supported by lateral prefrontal sites (which demonstrated the strongest responses for words correctly perceived as repeats).
Previous data have also linked areas within the right prefrontal cortex to retrieval monitoring (Buckner et al., 1998b; MacLeod et al., 1998), retrieval attempt (Kapur et al., 1995; Nyberg et al., 1995) as well as post-retrieval evaluation (Rugg et al., 1999; Trott et al., 1999; Wilding, 1999). In the present data, however, response within right prefrontal regions was not specific to retrieval success and occurred within all conditions suggesting that it may be associated with general effortful retrieval processes.
Temporal Responses
The present results also demonstrated repetition related decreases within bilateral anterior temporal cortex. Similar areas within the left hemisphere, may in coordination with left ventral prefrontal cortex, constitute a supramodal semantic association network (Marinkovic et al., 2003). In the present data, right anteromedial temporal regions had the strongest responses for words that were perceived as novel (regardless of whether they actually were) and demonstrated more significant responses for words perceived as novel with high (vs. low) confidence from~300ms onward). Previous investigations have implicated anteromedial temporal regions in the judgment of familiarity (Henson et al., 2003) as well as novelty/encoding (Tulving et al., 1996). However, the present results do not permit a clear functional differentiation. It is possible that significant right temporal modulation in the present study is related to the number of times a stimulus was previously presented, with the left MTL benefiting most from multiple repetitions, while the right is affected by a single exposure (Heckers et al., 2002).
From ~700–900ms there were strong differences within the left ventral occipitotemporal cortex (including middle and posterior aspects of the fusiform gyrus). The specific nature of potential functional divisions within the ventral temporal cortex is debatable (Cohen et al., 2000; Dehaene et al., 2002; Price et al., 2003). Recent MEG data suggest that early (~200ms) modulation of response within similar areas may support automatic access to perceptual attributes of words (Tarkiainen et al., 1999; Dhond et al., 2001; Tarkiainen et al., 2002; Marinkovic et al., 2003). In the present study, however, differential response occurred primarily at very long latencies (~700–900ms). One possibility is that late simultaneous response modulation within the occipitotemporal/fusiform area, and within anteroventral frontotemporal networks, may be related to continued activation of visuoperceptual word attributes (in posterior 37/19), in attempts to reconstruct prior lexico-semantic representations made during the initial study session.
Medial Parietal and Cingulate Responses
Delayed recognition also resulted in sustained differential response within bilateral posterior cingulate area including the precuneus, and retrosplenial cortex. The precuneus has been implicated in imagery and general retrieval processes (Grasby et al., 1993; Shallice et al., 1994; Buckner et al., 1996; Krause et al., 1999; Schmidt et al., 2002); while the retrosplenial cortex in primates has extensive reciprocal connections with adjacent parietal, lateral prefrontal and superior temporal regions as well as the parahippocampal gyrus (Morris et al., 1999b; Morris et al., 1999a). These areas responded differentially to both repetition and subject perception. However, word repetition that was accompanied with correct new/old judgments produced the strongest and longest sustained responses within these areas. Thus, overall the present data suggest that recognition is supported by sustained response within a distributed frontotemporal-parietocingulate network. Parietal differences have been shown in numerous hemodynamic studies of memory retrieval (Henson et al., 1999; Konishi et al., 2000; McDermott et al., 2000), however, in the present study the dominant effects occurred medially rather than laterally. Strong parietal activation is also noted in many EEG studies however, it is very well possible that such signals are generated by medial parietal, medial temporal or even orbital sources such as those seen here.
Conclusion
Previous data has clearly demonstrated that stimulus repetition can result in behavioral modifications without conscious recollection of the stimulus or event (Squire and Schacter, 2002); this ‘priming’ is presumably supported by brain regions that demonstrate activity regardless of whether the subject explicitly recognizes the stimulus. In contrast, brain activity that supports explicit memory for a stimulus would presumably be correlated with correct behavioral recognition. In the present study, this contrast was possible because of the relatively large number of forgotten items. Overall, the areas affected by repetition (regardless of recognition) and recognition (regardless of repetition) overlapped substantially. However, some mild dissociations were found. Specifically, the ventral occipitotemporal cortex, tended to be associated with repetition more than behavioral recognition. In contrast, other areas, such as the left lateral prefrontal region showed the converse association, being associated more with behavioral recognition that with actual repetition. Thus, both word repetition and behavioral recognition modulated similar frontotemporal-parieto-cingulate networks. The distinguishing feature when these influences were combined (i.e., when repeated words were correctly recognized), as compared to either alone, was not in the locations engaged but in their temporal dynamics. Specifically, the differential response to correctly recognized repeated words began earlier, at ~500ms, in contrast with unrecognized repeats where the differences did not begin until ~700–900ms. In conclusion, successful recognition of word repetition seems to depend not only a specific cortical network, but also requires that the network be modulated at an earlier latency.
Table 1.
Results of Item Repetition of on the MEG Spatiotemporal Response Pattern
| Type of Task | ~1 min. delay, 10 repetitions | ~40 minute delay, 1 repetition |
| Initial Onset Latency | ~210 ms | ~430 ms |
| Origin | Temporal | Prefrontal |
| Laterality | left >> right | left ~ right |
| Temporal Sites | lateral + medial | Anteromedial |
| Frontal Sites | ventrolateral | anterior, ventral, lateral |
Acknowledgments
This work was funded by USPHS (NIH grants R01NS18741, R01NS44623 and P41RR14075), and the Mental Illness and Neuroscience Discovery (MIND) Institute. We thank Anthony Wagner, Ksenija Marinkovic, Dave Post, Sharelle Baldwin, Jeremy Jordin, Bruce Fischl, Arthur Liu, Jeffrey Lewine, Kim Paulson and Bruce Rosen.
References
- Benson DF (1979) Aphasia, Alexia, and Agraphia. New York: Churchill Livingstone.
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev. 2002;26:809–817. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W. Functional neuroimaging studies of encoding, priming, and explicit memory retrieval. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:891–898. doi: 10.1073/pnas.95.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Petersen SE. Dissociation of human prefrontal cortical areas across different speech production tasks and gender groups. J Neurophysiol. 1995;74:2163–2173. doi: 10.1152/jn.1995.74.5.2163. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Miezin FM, Petersen SE. Functional anatomic studies of memory retrieval for auditory words and visual pictures. J Neurosci. 1996;16:6219–6235. doi: 10.1523/JNEUROSCI.16-19-06219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Wagner AD, Rosen BR. Functional-anatomic study of episodic retrieval using fMRI. I. Retrieval effort versus retrieval success. Neuroimage. 1998a;7:151–162. doi: 10.1006/nimg.1998.0327. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Dale AM, Rotte M, Rosen BR (1998b) Functional-anatomic study of episodic retrieval. II. Selective averaging of event-related fMRI trials to test the retrieval success hypothesis. NeuroImage in press. [DOI] [PubMed]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Prince SE, Rice HJ, Weissman DH, Nyberg L. Attention-related activity during episodic memory retrieval: a cross-function fMRI study. Neuropsychologia. 2003;41:390–399. doi: 10.1016/s0028-3932(02)00170-7. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients [In Process Citation] Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Data in Standardized Talairach Space. Journal of Computer Assisted Tomography. 1994;18:292–205. [PubMed] [Google Scholar]
- Coltheart M. The MRC psycho-linguistic database. Quarterly Journal of Experimemtal Psychology. 1981;33A:497–505. [Google Scholar]
- Corkin S, Cohen NJ, Sullivan EV, Clegg RA, Rosen TJ, Ackerman RH. Analyses of global memory impairments of different etiologies. Ann N Y Acad Sci. 1985;444:10–40. doi: 10.1111/j.1749-6632.1985.tb37577.x. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale AM, Halgren E. Spatiotemporal mapping of brain activity by integration of multiple imaging modalities. Curr Opin Neurobiol. 2001;11:202–208. doi: 10.1016/s0959-4388(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis I: Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Dehaene SGLCHJBP, Le Bihan D, Cohen L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport. 2002;15:321–325. doi: 10.1097/00001756-200203040-00015. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JDE. Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. Journal of Neuroscience. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deMunck JC. A linear dicretization of the volume conductor boundary integral equation using analytically integrated elements. IEEE Transactions on Biomedical Engineering. 1992;39:986–990. doi: 10.1109/10.256433. [DOI] [PubMed] [Google Scholar]
- Dhond RP, Buckner RL, Dale AM, Marinkovic K, Halgren E. Spatiotemporal maps of brain activity underlying word generation and their modification during repetition priming. J Neurosci. 2001;21:3564–3571. doi: 10.1523/JNEUROSCI.21-10-03564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhond RP, Marinkovic K, Dale AM, Witzel T, Halgren E. Spatiotemporal maps of past-tense verb inflection. Neuroimage. 2003;19:91–100. doi: 10.1016/s1053-8119(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis II: Inflation, flattening, a surface-based coordinate system. NeuroImage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Specializations of the human brain. Sci Am. 1979;241:180–199. doi: 10.1038/scientificamerican0979-180. [DOI] [PubMed] [Google Scholar]
- Grasby PM, Frith CD, Friston K, Frackowiak RSJ, Dolan RJ (1993) Activation of the human hippocampal formation during auditory-verbal long-term memory function. [DOI] [PubMed]
- Guillem F, N'Kaoua B, Rougier A, Claverie B. Intracranial topography of event-related potentials (N400/P600) elicited during a continuous recognition memory task. Psychophysiology. 1995;32:382–392. doi: 10.1111/j.1469-8986.1995.tb01221.x. [DOI] [PubMed] [Google Scholar]
- Halgren E (1990) Insights from evoked potentials into the neuropsychological mechanisms of reading. In: Neurobiology of Cognition (Scheibel A, Weschsler A, eds), pp 103–150. New York: Guilford.
- Halgren E, Smith ME. Cognitive evoked potentials as modulatory processes in human memory formation and retrieval. Human Neurobiology. 1987;6:129–139. [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Clarke M. Spatio-temporal stages in face and word processing. 1. Depth-recorded potentials in the human occipital, temporal and parietal lobes. Journal of Physiology (Paris) 1994a;88:1–50. doi: 10.1016/0928-4257(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Chauvel P, Clarke M. Spatio-temporal stages in face and word processing. 2. Depth-recorded potentials in the human frontal and Rolandic cortices. Journal of Physiology (Paris) 1994b;88:51–80. doi: 10.1016/0928-4257(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Halgren E, Dhond RP, Christensen N, Van Petten C, Marinkovic K, Lewine JD, Dale AM. N400-like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. Neuroimage. 2002;17:1101–1116. doi: 10.1006/nimg.2002.1268. [DOI] [PubMed] [Google Scholar]
- Hamalainen MS, Ilmoniemi RJ (1984) Interpreting measured magnetic fields of the brain: Estimates of current distribution. Helsinki: University of Technology, Dept. of Technical Physics Report TKK-F-A559.
- Hamalainen MS, Sarvas J. Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans Biomed Eng. 1989;36:165–171. doi: 10.1109/10.16463. [DOI] [PubMed] [Google Scholar]
- Hämäläinen MS, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV. Magnetoencephalography - theory, instrumentation, and applications to noninvasive studies of the working human brain. Reviews of Modern Physics. 1993;65:413–497. [Google Scholar]
- Heckers S, Weiss AP, Alpert NM, Schacter DL. Hippocampal and brain stem activation during word retrieval after repeated and semantic encoding. Cereb Cortex. 2002;12:900–907. doi: 10.1093/cercor/12.9.900. [DOI] [PubMed] [Google Scholar]
- Helenius P, Salmelin R, Service E, Connolly JF. Distinct time courses of word and context comprehension in the left temporal cortex. Brain. 1998;121:1133–1142. doi: 10.1093/brain/121.6.1133. [DOI] [PubMed] [Google Scholar]
- Helenius P, Salmelin R, Service E, Connolly JF. Semantic cortical activation in dyslexic readers. J Cogn Neurosci. 1999;11:535–550. doi: 10.1162/089892999563599. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Cansino S, Herron JE, Robb WG, Rugg MD. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13:301–304. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic Dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hunt SMJ. MacProbe: A Macintosh-based experimenter's workstation for the cognitive sciences. Behavioral Research Methods,Instrumentation and Computing. 1994;26:345–351. [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989;27:1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Tulving E, Wilson AA, Houle S, Brown GM. Neuroanatomical correlates of encoding in episodic memory: levels of processing effect [see comments] Proc Natl Acad Sci U S A. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Jones C, Brown GM, Houle S, Tulving E. Functional role of the prefrontal cortex in retrieval of memories: a PET study. Neuroreport. 1995;6:1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- Karayanidis F, Andrews S, Ward PB, McConaghy N. Effects of inter-item lag on word repetition: an event-related potential study. Psychophysiology. 1991;28:307–318. doi: 10.1111/j.1469-8986.1991.tb02200.x. [DOI] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. Neuroimage. 2000;12:276–286. doi: 10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- Krause BJ, Schmidt D, Mottaghy FM, Taylor J, Halsband U, Herzog H, Tellmann L, Muller-Gartner HW. Episodic retrieval activates the precuneus irrespective of the imagery content of word pair associates. A PET study. Brain. 1999;122 ( Pt 2):255–263. doi: 10.1093/brain/122.2.255. [DOI] [PubMed] [Google Scholar]
- Kucera H, & Francis, W. N. (1967). .Providence, RI: Brown University Press. (1967) Computational analysis of present-day American English. Providence, RI: Brown University Press.
- Liu AK, Belliveau JW, Dale AM. Spatiotemporal imaging of human brain activity using fMRI constrained MEG data: Monte Carlo simulations. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8945–8950. doi: 10.1073/pnas.95.15.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AK, Dale AM, Belliveau JW. Monte Carlo simulation studies of EEG and MEG localization accuracy. Human Brain Mapping. 2002;16:47–62. doi: 10.1002/hbm.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod AK, Buckner RL, Miezin FM, Petersen SE, Raichle ME. Right anterior prefrontal cortex activation during semantic monitoring and working memory. Neuroimage. 1998;7:41–48. doi: 10.1006/nimg.1997.0308. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Cox B, Reid K, Halgren E (in press) Head position in the MEG helmet affects the sensitivity to anterior sources. Neurology and Clinical Neurophysiology. [PMC free article] [PubMed]
- Marinkovic K, Dhond RP, Dale AM, Glessner M, Carr V, Halgren E. Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron. 2003;38:487–497. doi: 10.1016/s0896-6273(03)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Nobre AC, Bentin S, Spencer DD. Language-related field potentials in the anterior-medial temporal lobe: I. Intracranial distribution and neural generators. Journal of Neuroscience. 1995;15:1080–1089. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Jones TC, Petersen SE, Lageman SK, Roediger HL., 3rd Retrieval success is accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: an event-related fMRI study. J Cogn Neurosci. 2000;12:965–976. doi: 10.1162/08989290051137503. [DOI] [PubMed] [Google Scholar]
- Meijs JWH, Peters MJ. The EEG and MEG, Using a model of eccentric spheres to describe the head. IEEE Transactions on Biomedical Engineering. 1987;34:913–920. doi: 10.1109/tbme.1987.325929. [DOI] [PubMed] [Google Scholar]
- Meijs JWH, Bosch FGC, Peters MJ, Lopes da Silva FH. On the magnetic field distribution generated by a dipolar current source situated in a realistically shaped compartment model of the head. Electroencephalography and Clinical Neurophysiology. 1987;66:286–298. doi: 10.1016/0013-4694(87)90078-2. [DOI] [PubMed] [Google Scholar]
- Morris R, Petrides M, Pandya DN. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta) Eur J Neurosci. 1999a;11:2506–2518. doi: 10.1046/j.1460-9568.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Morris R, Pandya DN, Petrides M. Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. J Comp Neurol. 1999b;407:183–192. doi: 10.1002/(sici)1096-9861(19990503)407:2<183::aid-cne3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47:36–45. [PubMed] [Google Scholar]
- Nagy ME, Rugg MD. Modulation of event-related potentials by word repetition: the effects of inter-item lag. Psychophysiology. 1989;26:431–436. doi: 10.1111/j.1469-8986.1989.tb01946.x. [DOI] [PubMed] [Google Scholar]
- Nobre A, Allison T, McCarthy H. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–263. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- Nolde SF, Johnson MK, D'Esposito M. Left prefrontal activation during episodic remembering: an event- related fMRI study. Neuroreport. 1998;9:3509–3514. doi: 10.1097/00001756-199810260-00032. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Tulving E, Habib R, Nilsson LG, Kapur S, Houle S, Cabeza R, McIntosh AR. Functional brain maps of retrieval mode and recovery of episodic information [see comments] Neuroreport. 1995;7:249–252. [PubMed] [Google Scholar]
- Oldfield The assesment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oostendorp TF, Van Oosterom A (1992) Source parameter estimation using realistic geometry in bioelectricity and biomagnetism. In: Biomagnetic Localization and 3D Modeling (Nenonen J, Rajala HM, Katila T, eds). Helsinki: Helsinky Univ. of Technology, Rport TKK-F-A689.
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, Cotelli M, Cossu G, Corte F, Lorusso M, Pesenti S, Gallagher A, Perani D, Price C, Frith CD, Frith U. A cultural effect on brain function. Nat Neurosci. 2000;3:91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- Penney TB, Maess B, Busch N, Derrfuss J, Mecklinger A. Cortical activity reduction with stimulus repetition: a whole-head MEG analysis. Brain Res Cogn Brain Res. 2003;16:226–231. doi: 10.1016/s0926-6410(02)00277-x. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. Journal of Anatomy. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Winterburn D, Giraud AL, Moore CJ, Noppeney U. Cortical localisation of the visual and auditory word form areas: A reconsideration of the evidence. Brain Lang. 2003;86:272–286. doi: 10.1016/s0093-934x(02)00544-8. [DOI] [PubMed] [Google Scholar]
- Puregger E, Walla P, Deecke L, Dal-Bianco P. Magnetoencephalographic--features related to mild cognitive impairment. Neuroimage. 2003;20:2235–2244. doi: 10.1016/j.neuroimage.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Pylkkanen L, Marantz A. Tracking the time course of word recognition with MEG. Trends Cogn Sci. 2003;7:187–189. doi: 10.1016/s1364-6613(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Nagy ME. Event-related potentials and recognition memory for words. Electroencephalogr Clin Neurophysiol. 1989;72:395–406. doi: 10.1016/0013-4694(89)90045-x. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Chua PM, Dolan RJ. The role of the prefrontal cortex in recognition memory and memory for source: an fMRI study. Neuroimage. 1999;10:520–529. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B, Donohue BC, Nace K, Maisog JM, Andreason P. Phonological and orthographic components of word recognition. A PET- rCBF study. Brain. 1997;120:739–759. doi: 10.1093/brain/120.5.739. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS. Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proc Natl Acad Sci U S A. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Krause BJ, Mottaghy FM, Halsband U, Herzog H, Tellmann L, Muller-Gartner HW. Brain systems engaged in encoding and retrieval of word-pair associates independent of their imagery content or presentation modalities. Neuropsychologia. 2002;40:457–470. doi: 10.1016/s0028-3932(01)00102-6. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology,Neurosurgery and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- Simos PG, Basile LF, Papanicolaou AC. Source localization of the N400 response in a sentence-reading paradigm using evoked magnetic fields and magnetic resonance imaging. Brain Res. 1997;762:29–39. doi: 10.1016/s0006-8993(97)00349-1. [DOI] [PubMed] [Google Scholar]
- Smith ME. Neurophysiological manifestations of recollective experience during recognition memory judgments. Journal of Cognitive Neuroscience. 1993;5:1–13. doi: 10.1162/jocn.1993.5.1.1. [DOI] [PubMed] [Google Scholar]
- Smith ME, Guster K. Decomposition of recognition memory event-related potentials yields target, repetition, and retrieval effects. Electroencephalography and Clinical Neurophysiology. 1993;86:335–343. doi: 10.1016/0013-4694(93)90046-x. [DOI] [PubMed] [Google Scholar]
- Smith ME, Stapleton JM, Halgren E. Human medial temporal lobe potentials evoked in memory and language tasks. Electroencephalogr Clin Neurophysiol. 1986;63:145–159. doi: 10.1016/0013-4694(86)90008-8. [DOI] [PubMed] [Google Scholar]
- Squire LR, Schacter DL (2002) The Neuropsychology of Memory, 3rd Edition. New York: Guilford Publications, Inc.
- Stuss DT, Benson DF. Neuropsychological studies of the frontal lobes. Psychological Bulletin. 1984;95:3–28. [PubMed] [Google Scholar]
- Tarkiainen A, Cornelissen PL, Salmelin R. Dynamics of visual feature analysis and object-level processing in face versus letter-string perception. Brain. 2002;125:1125–1136. doi: 10.1093/brain/awf112. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Helenius P, Hansen PC, Cornelissen PL, Salmelin R. Dynamics of letter string perception in the human occipitotemporal cortex. Brain. 1999;122 ( Pt 11):2119–2132. doi: 10.1093/brain/122.11.2119. [DOI] [PubMed] [Google Scholar]
- Trott CT, Friedman D, Ritter W, Fabiani M, Snodgrass JG. Episodic priming and memory for temporal source: event-related potentials reveal age-related differences in prefrontal functioning. Psychol Aging. 1999;14:390–413. doi: 10.1037//0882-7974.14.3.390. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Craik FE, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Kutas M, Kluender R, Mitchiner M, McIsaac H. Fractionating the word repetition effect with event-related potentials. Journal of Cognitive Neuroscience. 1991;3:131–150. doi: 10.1162/jocn.1991.3.2.131. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RS. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E (in press) Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci. [DOI] [PMC free article] [PubMed]
- Wheeler ME, Buckner RL. Functional dissociation among components of remembering: control, perceived oldness, and content. J Neurosci. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding EL. Separating retrieval strategies from retrieval success: an event- related potential study of source memory. Neuropsychologia. 1999;37:441–454. doi: 10.1016/s0028-3932(98)00100-6. [DOI] [PubMed] [Google Scholar]


