Abstract
Cellular phosphorylation events during viral infection are necessary for effective viral replication. Encephalomyocarditis (EMC) virus has been used for studies on the molecular mechanisms of viral replication, but little is known about the cellular signaling pathways involved. This investigation was initiated to determine whether mitogen-activated protein kinases (MAPKs), which are central components of signal transduction pathways in the regulation of cell proliferation, play a role in the replication of EMC virus. We examined the phosphorylation of MAPKs, including extracellular signal-regulated kinase (ERK1/2), p38 MAPK, and stress-activated protein kinase 1/c-Jun NH2-terminal kinase (SAPK/JNK) in EMC virus-infected L929 cells and found that p38 MAPK and SAPK-JNK, but not ERK1/2, were activated during viral infection. We then examined the effect of these kinases on the replication of EMC virus in L929 cells by using specific inhibitors, including genistein or herbimycin A for tyrosine kinase, SB203580 or SB202190 for p38 MAPK, and PD98059 for ERK1/2. We found that the tyrosine kinase and p38 MAPK inhibitors, but not the ERK1/2 inhibitor, suppressed viral replication and that the inhibitory effect was primarily on viral protein synthesis. Finally, we examined whether p38 MAPK is involved in the translation of EMC viral transcripts by using L929 cells transfected with a gene construct containing the internal ribosomal entry site (IRES) of EMC virus and a luciferase reporter gene. We found that the p38 MAPK inhibitor suppressed the translation of EMC viral RNA. On the basis of these observations, we conclude that p38 MAPK plays a critical role in the replication of EMC virus, probably in the translation of viral RNA.
Encephalomyocarditis (EMC) virus, a member of the Cardiovirus genus of the family Picornaviridae, contains a single copy of a positive-sense RNA genome and causes diseases such as type 1 diabetes, encephalitis, and myocarditis in mice (7, 37, 38). EMC virus has been widely used for studies on the molecular mechanisms of virus replication, including the transcription and translation of viral RNA (3, 12). However, little is known about the cellular signaling pathways involved in the replication of EMC virus. It was reported that coxsackievirus infection induced tyrosine phosphorylation of cellular proteins, and this phosphorylation was mediated by Src kinase (15, 16). Recently, the Src family kinase Lck (p56lck) was found to be required for efficient replication of coxsackievirus B3 both in vitro and in vivo (23), and replication of coxsackievirus B3 is also dependent on the host cell cycle status (10). Inhibition of the tyrosine kinase pathway in coxsackievirus-infected cells resulted in the reduction of virus production, suggesting that cellular phosphorylation events during infection may be necessary for effective viral replication.
The mitogen-activated protein kinases (MAPKs), which consist of extracellular signal-regulated kinases (ERK1/2), p38 MAPK, and stress-activated protein kinase 1/c-Jun NH2-terminal kinase (SAPK/JNK), are central components of signal transduction pathways in the regulation of cell proliferation and differentiation, cytokine production and apoptosis (11). Several viruses can induce the activation of MAPK in infected cells, such as human immunodeficiency virus type 1 (21, 22), herpes simplex virus type 1 (39), human cytomegalovirus (31), echovirus 1 (17), and Sindbis virus (26). In addition, it was recently shown that the mitogenic Ras/MEK/MAPK pathway contributes to the replication of herpes simplex virus type 2 (32) and influenza virus (29). However, the precise role of MAPK in the replication of viruses has not been elucidated.
This investigation was initiated to determine whether MAPKs play a role in the replication of EMC virus. We report that EMC virus induces the activation of MAPKs such as p38 MAPK and SAPK/JNK in L929 cells, and the suppression of p38 MAPK by a specific inhibitor (SB203580) results in the inhibition of EMC viral replication, particularly the translation of viral RNA, indicating that p38 MAPK plays a key role in the replication of EMC virus.
MATERIALS AND METHODS
Cells and viruses.
L929 cells and HeLa cells were maintained in RPMI 1640 containing 5% fetal bovine serum (FBS), 2 mM l-glutamine, 50 U of penicillin per ml, and 50 μg of streptomycin per ml. Monkey kidney (MK) cells were obtained from the American Type Culture Collection (Manassas, Va.) and were maintained in medium 199 containing 1% horse serum, 2 mM l-glutamine, 50 U of penicillin per ml, and 50 μg of streptomycin per ml. The D variant of EMC virus was cultured and purified by CsCl2 gradient centrifugation as described previously (13). Mengo virus and vesicular stomatitis virus (VSV) were amplified in L929 cells. Coxsackievirus B4 (CVB4) was amplified in MK cells (19). The titer of each virus was determined, and each virus was kept at −70°C until use.
Reagents and antibodies.
Herbimycin A, genistein, SB203580, SB202190, and PD98059 were purchased from Calbiochem (La Jolla, Calif.). Anti-p38 MAPK phosphospecific antibody, anti-SAPK/JNK phosphospecific antibody, ERK1/2 phosphospecific antibody, and anti-ERK2 antibody were obtained from Calbiochem (for Fig. 1) and Santa Cruz Biotechnology (Santa Cruz, Calif.) (for Fig. 3). Anti-SAPK/JNK antibody and a c-Jun-glutathione S-transferase (GST) fusion protein were purchased from Santa Cruz Biotechnology. Anti-phosphospecific activating transcription factor 2 (ATF-2) antibody and anti-ATF-2 antibody were obtained from Upstate Biotechnology (Lake Placid, N.Y.). Polyclonal anti-EMC virus antibodies were generated from EMC virus-infected mice.
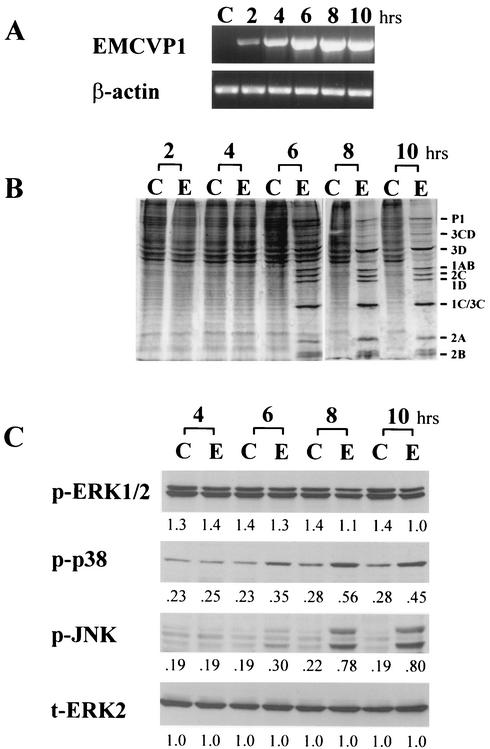
FIG. 1.
Activation of MAPKs in L929 cells infected with EMC virus. (A) RT-PCR analysis of EMC virus RNA. RNA was isolated from uninfected cells (lanes C) or EMC virus-infected cells (MOI = 5) at 2, 4, 6, 8, and 10 h after infection, and EMC viral RNA for the major capsid protein, VP1, was analyzed by RT-PCR (EMCVP1). β-Actin mRNA was amplified from the same cDNA as an internal standard. (B) Metabolic labeling of uninfected (lanes C) and EMC virus-infected (lanes E) L929 cells. The cells were labeled with [35S]methionine for 30 min at 2, 4, 6, 8, and 10 h after infection. The cell lysate was separated by SDS-PAGE and analyzed by autoradiography. The positions of EMC viral proteins are shown on the right side. (C) Western blot analysis of phosphorylated ERK1/2 (p-ERK1/2), phosphorylated p38 MAPK (p-p38), and phosphorylated SAPK/JNK (p-JNK) at 4, 6, 8, and 10 h after EMC virus-infection (lanes E) or from uninfected cells (lanes C). The total ERK2 (t-ERK2) was analyzed as an internal standard. Numbers below bands are density values relative to the density of total ERK2.
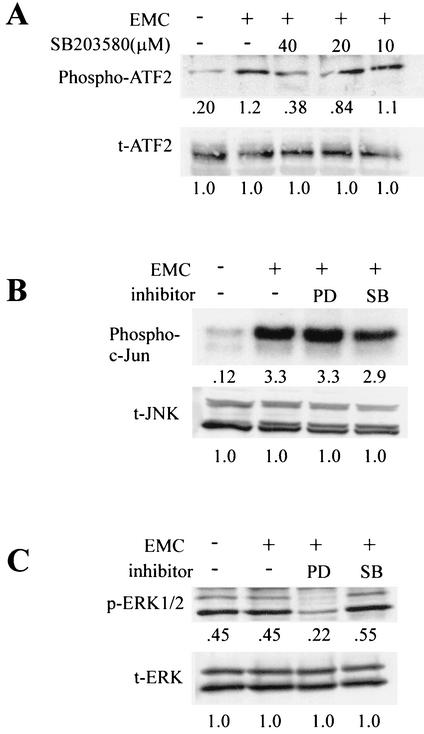
FIG. 3.
Inhibition of MAPK activation in L929 cells infected with EMC virus by SB203580 (SB) or PD98059 (PD). (A) L929 cells were infected with EMC virus (+) in the presence of various concentrations of SB203580 and lysed 10 h after infection. Western blot analysis was performed with anti-phosphospecific ATF-2 antibody (Phospho-ATF2). After stripping, the membrane was reprobed with antibody against total ATF-2 (t-ATF2). The numbers below the bands are density values relative to the density of total ATF-2. (B) In vitro kinase assay of SAPK/JNK (Phospho-c-Jun) performed with c-Jun as a substrate, as described in Materials and Methods, in the presence of PD98059 (PD; 40 μM) or SB203580 (SB; 40 μM). As an internal control, total JNK (t-JNK) was analyzed. Numbers below bands are density values relative to the density of total JNK. (C) Western blot analysis of phosphorylated ERK1/2 (p-ERK1/2) and total ERK2 (t-ERK) in the presence of PD98059 (PD; 40 μM) or SB203580 (SB; 40 μM). Numbers below bands are density values relative to the density of total ERK.
RT-PCR.
The total RNA was extracted from cells with Trizol reagent (Gibco BRL Life Technologies, Inc., Gaithersburg, Md.), and reverse transcriptase (RT)-PCR was performed as described previously (13). The following oligonucleotide sequences were used as primers for PCR: for β-actin, sense, 5′-GTTACCAACTGGGACGACA-3′, and antisense, 5′-TTCGAGCAGGAGATGGCCA-3′; and for EMC virus VP1, sense, 5′-GGAGTTGAGAATGCTGAGAGAGGGGTT-3′, and antisense, 5′-GGAATTCATTCCAGCATAAGGACTCCAGCTCTCTCGG-3′. The PCR products were separated by electrophoresis on a 1.5% agarose gel and detected by ethidium bromide staining.
Measurement of viral replication.
Virus replication was measured as previously described (19, 34-36). Briefly, cells were grown in 24-well culture plates and infected with virus at a multiplicity of infection (MOI) of 5. The viral concentrations of the triplicate culture supernatants were determined by plaque assay with L929 cells for EMC virus, Mengo virus and VSV, and with MK cells for CVB4.
Western blot analysis.
Cells were grown in six-well culture plates with or without EMC virus infection (MOI of 5) for the indicated times and then lysed in lysis buffer (50 mM Tris [pH 7.6], 1% Nonidet P-40, 150 mM NaCl, 50 mM NaF, 1 mM Na3VO4, 5 mM EDTA, 1 mM phenylmethlsulfonyl fluoride, 10 μg of aprotinin per ml) at 4°C for 30 min. Lysates were cleared of debris by centrifugation at 12,000 × g for 20 min. Samples were analyzed by electrophoresis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% polyacrylamide) followed by transfer to nitrocellulose membranes (Amersham Life Sciences, Inc., Oakville, Ontario, Canada). The membrane was blocked with 5% bovine serum albumin in Tris-buffered saline containing 0.1% Tween 20 and then incubated with primary antibody. After washing, the membrane was incubated with peroxidase-conjugated goat anti-mouse immunoglobulin G or anti-rabbit immunoglobulin G antibody, and specific bands were detected with the Amersham ECL enhanced chemiluminescence detection system.
In vivo radiolabeling of EMC virus-infected cells and immunoprecipitation analysis.
Confluent monolayers of L929 cells grown in six-well plates were infected with EMC virus at an MOI of 5. At each time point, the cells were washed in methionine-free Dulbecco's modified Eagle's medium (DMEM) and replaced with methionine-free DMEM containing 2% dialyzed FBS and 0.1 mCi of [35S]methionine per ml. After a 30-min incubation at 37°C, the cells were washed in phosphate-buffered saline and lysed in lysis buffer as described above. Lysates were cleared of cell debris by centrifugation at 12,000 × g for 5 min. Immunoprecipitation of 35S-labeled cell lysates with polyclonal anti-EMC virus serum or anti-β-actin antibody was performed as described elsewhere (33). Immunoprecipitates were analyzed by SDS-PAGE (10% polyacrylamide).
In vitro kinase assay.
Whole-cell lysates were incubated with 1 μg of anti-SAPK/JNK antibody for 1 h at 4°C. Immune complexes were precipitated with protein A-Sepharose (Pharmacia Biotech, Uppsala, Sweden) overnight at 4°C, washed twice with lysis buffer, and suspended in 30 μl of kinase assay buffer (25 mM HEPES [pH 7.6], 20 mM MgCl2, 100 μM Na3VO4, 20 mM β-glycerolphosphate, 20 mM p-nitrophenyl phosphate, 2 mM dithiothreitol [DTT], 50 μM ATP, 5 μCi of [γ-32P]ATP) with 0.5 μg of c-Jun-GST fusion protein as a substrate. After incubation for 20 min at 30°C, the reaction was stopped by adding sample buffer, and the products were resolved by SDS-PAGE.
Preparation of probe for RNase protection assay (RPA).
The EMC-VP1 fragment (181 bp), encompassing nucleotides 2964 to 3145, was amplified with primers containing the restriction enzyme site BamHI in the sense primer and KpnI in the antisense primer (sense, 5′-CCCGGATCCCTAACGGAGATTTGG G-3′; antisense, 5′-TGGGAAGCTTAGTTGGGCACCACCTAAC-3′). The fragment was digested with BamHI and KpnI and cloned into Bluescript vector (Stratagene, La Jolla, Calif.). A labeled probe was generated with the MAXIscript in vitro transcription kit (Ambion, Austin, Tex.) according to the manufacturer's instructions. Briefly, 1 μg of subcloned EMC-VP1 (virus specific) or p-TRI-β-actin as an internal control was incubated in reaction mixture containing 2 μl of 10× transcription buffer (Ambion); 1 μl each of 10 mM ATP, CTP, and GTP; 5 μl of [α-32P]UTP; and 2 μl of RNA polymerase for 20 min at 37°C. One microliter of 0.5 M EDTA was added, followed by an equal volume of Gel Loading Buffer II (95% formamide, 0.025% xylene cyanol, 0.025% bromophenol blue, 18 mM EDTA, 0.025% SDS; Ambion). The mixture was heated for 3 min at 95°C. The resulting labeled probe fragments were then gel purified by PAGE. The labeled probe was then precipitated with ethanol, lyophilized, and reconstituted in 100 μl of RNase-free water. A 2-μl aliquot was taken for quantitation by scintillation counting.
RPA.
L929 cells were grown in six-well culture plates and infected with EMC virus at an MOI of 5. At 8 h postinfection, cells were treated with a p38MAPK inhibitor (SB203580). RNA was isolated at 1, 3, and 5 h posttreatment with Trizol reagent (Gibco BRL). RNA was then digested with DNase I (Roche, Laval, PQ) for 1 h at 37°C and reisolated with the RNeasy Cleanup Protocol (Qiagen, Mississauga, Ontario, Canada). The RPA was carried out with the HybSpeed RPA kit (Ambion) according to the manufacturer's instructions. Briefly, a precipitation mixture was prepared with 1 μg of RNA, 150,000 cpm each of EMC virus-specific probe and β-actin control probe, 10 μl of 5-mg/ml yeast tRNA, and 10 μl of 5 M NH4Ac in a final volume of 100 μl. After precipitation with ethanol, the pellet was resuspended in 10 μl of hybridization buffer (Ambion) for 5 min at 95°C. After a 10-min incubation at 68°C, the resuspended RNA was treated with RNase for 50 min at 37°C. RNA was precipitated with ethanol and resuspended in 15 μl of loading dye for 4 min at 95°C. Digested RNA was then separated by PAGE and vacuum dried before exposure to Biomax film (Kodak, Toronto, Ontario, Canada).
Construction of pEMCV-Luc.
EMCV-Luc-PV plasmid DNA (18) was digested with SacI and BglII to delete the poliovirus replicon (from poliovirus internal ribosomal entry site [IRES] to region 3c), treated with T4 DNA polymerase, and self-ligated (pEMCV-Luc).
Transfection and luciferase assay.
L929 monolayers were grown to 60% confluence in six-well plates. Cells were washed with prewarmed serum-free RPMI 1640 medium (Gibco BRL) and transfected with a 1-ml transfection solution of 37°C serum-free RPMI 1640 containing 4 μg of EMC-Luc RNA, which was prepared by in vitro transcription with plasmid DNA, pEMCV-Luc, and 10 μl of DMRIE-C transfection reagent (Gibco BRL). Cells were incubated in a CO2 incubator at 37°C for 30 min. The transfection solution was aspirated and replaced with 2 ml of 5% RPMI 1640 with or without SB203580. The cells were incubated, and samples were prepared for a luciferase assay by using a commercial luciferase assay system (Promega, Madison, Wis.). Briefly, the cells were lysed in lysis buffer as described above, and the lysates were centrifuged for 15 s. The supernatants were removed and stored at −70°C until use. Lysates were quick thawed immediately in a heating block at 25°C, and 20 μl of lysate was added to 100 μl of reconstituted luciferase assay reagent. The resulting luminescence was immediately measured with a Turner TD-20E luminometer (Turner Designs, Inc., Sunnyvale, Calif.).
Statistical analysis.
Statistical analyses were conducted with Student's t test.
RESULTS
Activation of p38 MAPK and SAPK/JNK in L929 cells infected with EMC virus.
We first examined the amplification of EMC viral RNA in EMC virus-infected L929 cells at different times after viral infection by RT-PCR analysis with oligonucleotide primers specific for the major capsid protein, VP1, of the EMC virus. We found that EMC viral RNA was detected within 2 h postinfection and increased to a peak level at 8 h (Fig. 1A). When we examined the synthesis of EMC viral protein in L929 cells by metabolic labeling with [35S]methionine at 2 to 10 h after infection, we found that the synthesis of EMC viral protein was detected from 6 h after infection and increased thereafter (Fig. 1B). The synthesis of EMC viral proteins resulted in the shutoff of host protein synthesis from 8 h after infection (Fig. 1B). To determine whether MAPKs are activated during EMC virus infection, we examined the phosphorylation of MAPKs including ERK1/2, p38 MAPK, and SAPK/JNK in EMC virus-infected L929 cells at 4 to 10 h after infection by immuoblotting the cell lysate with antibodies against phosphospecific ERK1/2, p38 MAPK, or SAPK/JNK, respectively. We found that the phosphorylation of p38 MAPK and SAPK/JNK increased from 6 to 10 h after infection, while the phosphorylation level of ERK1/2 did not increase during infection (Fig. 1C), indicating that p38MAPK and SAPK/JNK are activated during EMC viral infection.
Inhibition of EMC virus replication in L929 cells by blocking tyrosine kinase or p38 MAPK.
To determine whether the activation of MAPKs plays any role in the replication of EMC virus, we examined the effect of these kinases on progeny virus production in EMC virus-infected L929 cells by blocking tyrosine kinase or MAPKs. We infected L929 cells with EMC virus in the presence of the tyrosine kinase inhibitors genistein and herbimycin A at various concentrations and determined the viral concentration in the cell culture supernatant by plaque assay 24 h after infection. We found that genistein inhibited viral replication to 0.5% of the control level (infected cells without tyrosine kinase inhibitor) at a concentration of 50 μM, and herbimycin A inhibited viral replication to 10% of that of the control at a concentration of 40 μM (Fig. 2A and B). Reduction of progeny virus production by genistein was also observed in EMC virus-infected HeLa cells (Fig. 2C).
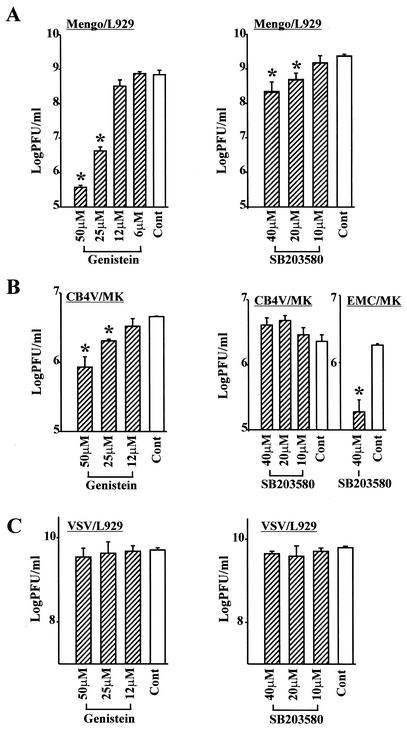
FIG. 2.
Effects of tyrosine kinase inhibitors and MAPK inhibitors on replication of EMC virus. L929 cells (A, B, and D to F) or HeLa cells (C) were infected with EMC virus (MOI = 5) in the presence of the indicated concentrations of genistein (A), herbimycin A (B), genistein (C), SB203580 (D), SB202190 (E), or PD98059 (F) or in the absence of inhibitor (control [Cont]). At 24 h after infection, progeny virus was determined by plaque assay (measurement of PFU per milliliter) from triplicate samples. Bars represent standard deviations. *, P < 0.01 versus the control.
We then determined the effects of various concentrations of p38 MAPK inhibitors (SB203580 and SB202190) or an ERK1/2 inhibitor (PD98059) on progeny virus production (8, 9, 24). We found that SB203580 and SB202190 inhibited the production of EMC progeny virus to 12% (at 40 μM) and 5% (at 30 μM), respectively (Fig. 2D and 2E), whereas PD98059 showed no effect on the production of progeny virus (Fig. 2F). We examined whether these inhibitors had toxic effects on the treated cells by examining cell morphology by light microscopy and by testing for cell viability by trypan blue exclusion staining. We found that the inhibitors used, including tyrosine kinase inhibitors, p38 MAPK inhibitors, and ERK1/2 inhibitor, did not show toxic effects on the treated L929 cells (data not shown). These results suggest that p38 MAPK plays an important role in the replication of EMC virus in L929 cells.
To confirm that the p38 MAPK inhibitors that we used truly inhibit the signal pathways, we measured the activities of their downstream elements, such as the phosphorylation of ATF-2, in the presence of these inhibitors. We found that the phosphorylation of ATF-2, which is phosphorylated by the p38 MAPK pathway, was blocked by a p38 MAPK-specific inhibitor (SB203580) in a dose-dependent manner, while the activation of ATF-2 was substantially increased after EMC virus-infection (Fig. 3A). In addition, the activation of SAPK/JNK was slightly inhibited by treatment with SB203580 (Fig. 3B), perhaps resulting from the p38 MAPK inhibitor-induced suppression of EMC virus replication. When we examined the phosphorylation of ERK1/2 after treatment with the MEK1 inhibitor, PD98059, we found that ERK1/2 phosphorylation was significantly inhibited by PD98059, but not by SB203580 (Fig. 3C).
Differential effects of tyrosine kinase and p38 MAPK inhibitors on the replication of Mengo virus, CVB4, and VSV.
To examine whether p38 MAPK plays a role in the replication of other picornaviruses or nonpicornaviruses, such as Mengo virus (Picornaviridae, Cardiovirus), CVB4 (Picornaviridae, Enterovirus) and VSV (Rhabdoviridae) in addition to EMC virus, we infected L929 cells with Mengo virus or VSV and MK cells with CVB4 at an MOI of 5 in the presence of the tyrosine kinase inhibitor genistein or the p38 MAPK inhibitor SB203580 and examined the production of progeny virus in the culture supernatant. We found that both genistein and SB203580 inhibited the replication of Mengo virus, while genistein, but not SB203580, inhibited the replication of CVB4 (Fig. 4A and B). Progeny virus production of EMC virus-infected MK cells was decreased by the treatment of SB203580 (Fig. 4B). Neither genistein nor SB203580 inhibited the production of progeny virus in VSV-infected L929 cells (Fig. 4C).
FIG. 4.
Effects of genistein and SB203580 on the replication of various viruses: Mengo virus in L929 cells (A), CVB4 in MK cells and EMC virus in MK cells (B), and VSV in L929 cells (C). Cells were infected with virus (MOI = 5) in the presence of the indicated concentrations of genistein or SB203580 or in the absence of inhibitor (control [Cont]). At 24 h after infection, progeny virus was determined by plaque assay (PFU per milliliter) from triplicate samples. Bars represent standard deviations. *, P < 0.01 versus controls.
Effects of p38 MAPK inhibitor on viral RNA transcription and viral protein synthesis.
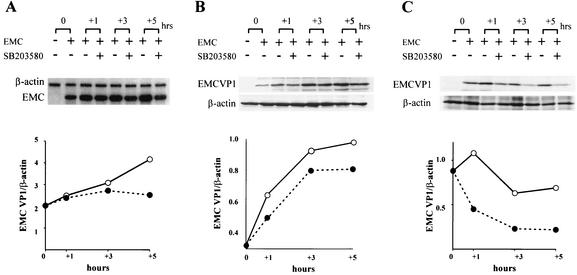
Because SB203580 inhibited EMC viral replication in L929 cells, we determined which phase of the replication cycle, viral transcription or translation, is affected. To examine the effect of SB203580 on the synthesis of viral RNA, we infected L929 cells with EMC virus at an MOI of 5, incubated the cells for 8 h, and then replaced the medium with medium containing SB203580. The amount of accumulated EMC viral RNA was measured by RPA 0 to 5 h after addition of SB203580. Significant inhibition of viral RNA synthesis was not found with up to 3 h of treatment with p38 MAPK inhibitor, but was found at 5 h of treatment (Fig. 5A). To examine the effect of SB203580 on EMC viral protein synthesis, we performed Western blot analysis to measure the accumulated amount of viral protein in lysates of EMC virus-infected L929 cells after treatment with SB203580. After 1 h of SB203580 treatment, we found that accumulated protein synthesis in EMC virus-infected cells was reduced by 25% compared to that in untreated cells (Fig. 5B). To measure ongoing protein synthesis, we immunoprecipitated [35S]methionine-labeled cell lysates with anti-EMC viral antibody. After 1 h of SB203580 treatment, we found that ongoing protein synthesis in EMC virus-infected cells was reduced by 59% compared to that of untreated cells (Fig. 5C). These results indicate that viral translation may be the first step of EMC viral replication that is affected by the suppression of p38 MAPK.
FIG. 5.
Effects of SB203580 on EMC viral transcription and protein synthesis. L929 cells were infected with EMC virus (MOI = 5), and fresh medium with (solid circles) or without (open circles) SB203580 (40 μM) was added 8 h later. (A) Total RNA was isolated after 0, 1, 3, and 5 h of SB203580 treatment, and the RPA was performed with an EMC virus-specific probe. β-Actin mRNA was measured as an internal standard (upper panel). Relative EMC viral RNA synthesis was measured as a ratio of β-actin mRNA (lower panel). (B) Cell lysates were prepared after 0, 1, 3, and 5 h of SB203580 treatment, and Western blotting was performed with polyclonal anti-EMC virus antibodies for VP1 (EMCVP1) or anti-β-actin antibodies (upper panel). (C) Cells were labeled with [35S]methionine for 30 min after 0, 1, 3, and 5 h of SB203580 treatment, and cell lysates were immunoprecipitated with polyclonal anti-EMC virus antibodies for VP1 (EMC VP1) or anti-β-actin antibodies. The samples were separated by SDS-PAGE and analyzed by autoradiography (upper panel). Relative EMC protein synthesis was measured as ratio of β-actin protein (B and C, lower panel). Nonsaturated film exposures were selected, scanned, and imported into the UTHSCASA Image Tool for quantification.
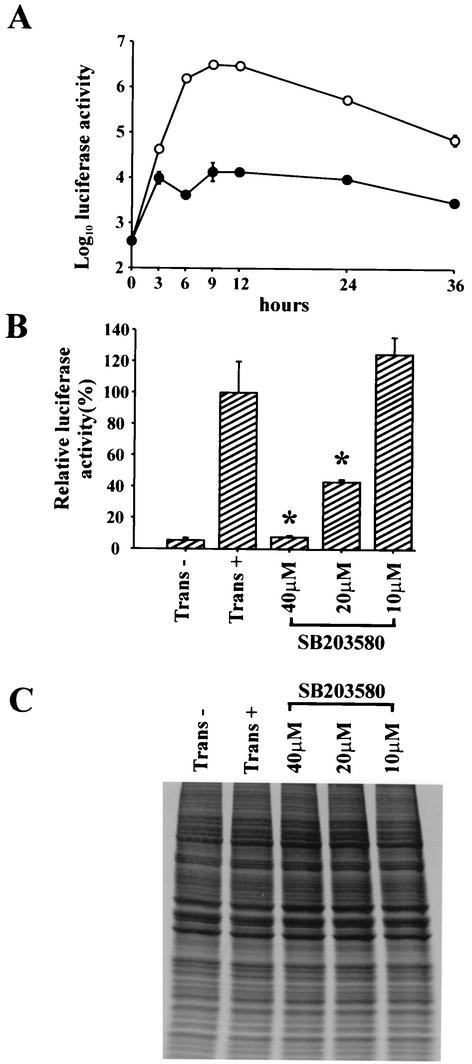
To further determine whether p38 MAPK is involved in the translation of EMC viral transcripts, we transfected L929 cells with RNA from a construct containing the IRES of EMC virus and a luciferase reporter gene (EMC-Luc) and examined luciferase activity in the presence or absence of SB203580. The expression of luciferase from EMC-Luc RNA increased rapidly from 3 to 6 h and reached a maximal level at 9 and 12 h after transfection (Fig. 6A). The expression of luciferase was significantly inhibited in the presence of SB203580 during the experimental period. Moreover, luciferase activity was reduced in a dose-dependent manner in transfected cells treated with SB203580 and was almost equivalent to that of the untransfected control at 40 μM SB203580 (Fig. 6B).
FIG. 6.
Inhibition of luciferase gene expression in EMC-Luc RNA-transfected L929 cells by SB203580. L929 cells were transfected with EMC-Luc RNA and then incubated for 30 min. (A) The cells were washed and incubated for the indicated times with (solid circles) or without (open circles) 40 μM SB203580, and luciferase gene expression was analyzed. Bars represent standard deviations. (B) Luciferase gene expression was analyzed at 24 h after treatment with the indicated concentrations of SB203580. Untransfected (trans−) and transfected cells without SB203580 treatment (trans+) were used as controls. The expression of luciferase generated from EMC-Luc RNA in each group was calculated as a percentage of the average expression of luciferase in trans+ cells. All experiments were performed in triplicate. Bars represent standard deviation. *, P < 0.01 as compared with transfected cells without SB203580. (C) Cells were labeled with [35S]methionine for 30 min after treatment with the indicated concentrations of SB203580 for 24 h, and proteins were analyzed by SDS-PAGE and subsequent autoradiography. Untransfected (trans−) and transfected cells without SB203580 treatment (trans+) were used as controls.
To examine whether there is any effect of SB203580 treatment on host cellular protein synthesis, we transfected L929 cells with EMC-Luc RNA, labeled them in vivo with [35S]methionine in the presence of SB203580 at 24 h after transfection, and examined protein synthesis by SDS-PAGE analysis. We found that treatment of the cells with SB203580 at the concentrations used in this experiment (10 to 40 μM) did not affect host protein synthesis (Fig. 6C).
DISCUSSION
Viral infections of cells are known to result in activation of intracellular signaling molecules that can affect cellular function and viral replication. Many viruses have been shown to induce MAPK signaling pathways in infected host cells (4, 5, 29-31). Recently, we showed that infection of macrophages with EMC virus results in activation of Src kinase, particularly p59/p56 Hck, which results in the production of the inflammatory molecules tumor necrosis factor alpha and nitric oxide, which contribute to the destruction of pancreatic β cells (6, 14). Blocking this signaling pathway by treatment with the Src kinase inhibitor PP2 suppressed production of these inflammatory molecules and protected β cells from destruction, resulting in significant prevention of EMC virus-induced diabetes in mice (6). In this study, we investigated the role of MAPK signaling pathways in the replication of EMC virus.
First, we examined whether MAPKs, including ERK1/2, p38 MAPK, and SAPK/JNK, are activated during the course of EMC viral infection in L929 cells and found that p38 MAPK and SAPK/JNK, but not ERK1/2, were clearly activated. We then asked whether the activation of p38 MAPK in the infected cells is due to binding of the EMC virus to a cellular receptor or replication of the virus within the cells. When we treated L929 cells with cycloheximide to inhibit protein synthesis, p38 MAPK was not activated in the infected cells. In addition, p38 MAPK was not activated in cells exposed to UV-irradiated EMC virus (data not shown). These results indicate that activation of p38 MAPK is not due to binding of the virus to the cells, but to actual replication of the virus within the cells. However, it is not known how p38 MAPK is activated in EMC virus-infected cells. There is some evidence for cross talk between viral proteins and cellular signaling in picornaviruses. Poliovirus 3Dpol interacts with the cellular adapter protein, Sam68, which is a target for Src-like tyrosine kinase during mitosis (25). Coxsackievirus B3 was shown to induce cleavage of p21ras GTPase-activating protein in infected cells, resulting in the activation of its downstream Ras pathways, including ERK1/2 (16). Thus, it may be possible that cross talk between viral RNA and cellular proteins and/or viral proteins and cellular proteins in L929 cells during EMC viral replication may induce the activation of the p38 MAPK and SAPK/JNK pathways. Alternatively, activation of p38 MAPK and SAPK/JNK may result from stress caused by inhibition of host protein synthesis by the replication of EMC virus in infected cells. Whether either of these or other mechanisms are involved in the activation of p38 MAPK in EMC virus-infected cells remains to be determined.
Second, we determined whether EMC virus-induced MAPKs are involved in viral replication by examining the effects of various tyrosine kinase and MAPK inhibitors on the production of EMC progeny virus. We found that the tyrosine kinase inhibitors genistein and herbimycin A inhibited virus production in L929 cells. EMC virus production was also inhibited by the p38 MAPK inhibitors SB203580 and SB202190, but not by the ERK inhibitor PD98059. In contrast, it has been reported that ERK activation plays an important role in the replication of visna virus and influenza virus. The inhibition of visna virus-induced ERK1/2 activation by treatment with PD98059 reduced the expression of gag and env mRNAs, resulting in the inhibition of virus production (2). Inhibition of the Ras/MEK/ERK signaling pathway by treatment with U0126, a MEK inhibitor, in influenza virus-infected cells interfered with the nuclear export of viral ribonucleoprotein complexes (RNPs), resulting in the concomitant inhibition of virus production (29). It is apparent that there is virus specificity in the action of MAPK inhibitors, because the ERK inhibitor did not show any inhibitory effect on the production of EMC virus, but did show a clear inhibitory effect on the production of visna virus and influenza virus. In addition, the effect of the MEK inhibitor PD98059 on the replication of visna virus was different from that of the MEK inhibitor U0126 on the replication of influenza virus. The mechanism involved in this virus specificity is not known, although it is probably due to differences in the signaling pathways involved in the replication of each virus. Nevertheless, it is clear that MAPKs are truly involved in the replication of EMC virus in L929 cells.
Third, we examined the effect of the p38 MAPK inhibitor SB203580 on the replication of other picornaviruses, such as Mengo virus (a cardiovirus) in L929 cells and CVB4 (an enterovirus) in MK cells. We found that SB203580 inhibited the replication of Mengo virus, but not the replication of CVB4, suggesting p38 MAPK is involved in the replication of cardioviruses such as EMC and Mengo virus, but not all picornaviruses. We also examined the effect of SB203580 on a nonpicornavirus, VSV (a rhabdovirus), in L929 cells and found that the p38 MAPK inhibitor had no inhibitory effect on VSV replication. Taken together, the effect of p38 MAPK on viral replication appears to be virus specific, rather than cell specific. However, further studies of this specificity involving several different virus families and different cell types are needed prior to making any definite conclusion.
Finally, we determined which step of EMC viral replication is affected by p38 MAPK by using its specific inhibitor, SB203580. As shown in Fig. 5, we found that p38 MAPK inhibitor clearly blocked both EMC viral protein synthesis and transcription. It was not surprising that treatment of EMC virus-infected cells with p38 MAPK inhibitor reduced viral transcripts as well as proteins, because viral transcription and translation are closely related in the replication of picornaviruses. Therefore, the role of p38 MAPK in EMC viral translation could not be clearly demonstrated with a model of natural infection. Since the inhibition of EMC viral protein synthesis was observed earlier than the inhibition of EMC viral transcription, we suggest that inhibition of viral RNA synthesis in p38 MAPK inhibitor-treated cells might be a secondary effect resulting from the inhibition of viral protein synthesis. However, our study does not exclude the possibility of direct effects of the inhibitor on EMC viral RNA transcription.
Translational initiation of EMC viral RNA is known to be mediated by a cap-independent mechanism, which requires the IRES in the 5′ untranslated region (12). Therefore, to determine whether p38 MAPK inhibitor has a direct effect on the translation of EMC viral RNA, we examined the translation activity of IRES in L929 cells transfected with RNA from a reporter gene construct containing the IRES of EMC virus and the luciferase gene after treatment with SB203580. We found that p38 MAPK signaling is directly involved in the translation of EMC viral RNA through the IRES of the EMC virus. In several picornaviruses, including EMC virus, the internal entry process of ribosomes requires the cellular polypyrimidine tract-binding protein (PTB) (12, 28), eukaryotic initiation factors (eIFs) (27), and IRES-specific cellular transacting factors (ITAFs) (20, 28). Therefore, the activation of p38 MAPK by EMC virus infection may phosphorylate these cellular factors and consequently affect the translation of EMC viral proteins. This hypothesis is supported by the recent observation that the phosphorylation of eIF4E in mouse hepatitis virus-infected cells was p38 MAPK dependent (1). However, whether cellular factors are truly modulated by p38 MAPK signaling pathways in EMC virus-infected cells remains to be determined.
In conclusion, EMC virus induces the activation of a central component of a signal transduction pathway, p38 MAPK, in L929 cells and suppression of p38 MAPK activity by a specific inhibitor results in inhibition of EMC viral replication. p38 MAPK appears to play a critical role in translation of the EMC viral transcript, because blocking the p38MAPK pathway inhibits the translation of EMC viral RNA, and the p38 MAPK signaling pathway may be associated with modulation of cellular proteins that bind to the IRES.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (MOP-13224), the Canadian Diabetes Association, and the Alberta Heritage Foundation for Medical Research to J.-W.Y. J.-W.Y. is a Canada Research Chair in Diabetes.
We thank C. D. Morrow for providing EMCV-Luc-PV plasmid and A. L. Kyle for editorial assistance.
REFERENCES
- 1.Banerjee, S., K. Narayanan, T. Mizutani, and S. Makino. 2002. Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells. J. Virol. 76:5937-5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber, S. A., L. Bruett, B. R. Douglass, D. S. Herbst, M. C. Zink, and J. E. Clements. 2002. Visna virus-induced activation of MAPK is required for virus replication and correlates with virus-induced neuropathology. J. Virol. 76:817-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belsham, G. J., and N. Sonenberg. 1996. RNA-protein interactions in regulation of picornavirus RNA translation. Microbiol. Rev. 60:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benn, J., F. Su, M. Doria, and R. J. Schneider. 1996. Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-regulated and c-Jun N-terminal mitogen-activated protein kinases. J. Virol. 70:4978-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruder, J. T., and I. Kovesdi. 1997. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J. Virol. 71:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi, K. S., H. S. Jun, H. N. Kim, H. J. Park, Y. W. Eom, H. L. Noh, H. Kwon, H. M. Kim, and J. W. Yoon. 2001. Role of Hck in the pathogenesis of encephalomyocarditis virus-induced diabetes in mice. J. Virol. 75:1949-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craighead, J. E., and M. F. McLane. 1968. Diabetes mellitus: induction in mice by encephalomyocarditis virus. Science 162:913-914. [DOI] [PubMed] [Google Scholar]
- 8.Cuenda, A., J. Rouse, Y. N. Doza, R. Meier, P. Cohen, T. H. Gallagher, P. R. Young, and J. C. Lee. 1995. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 364:229-233. [DOI] [PubMed] [Google Scholar]
- 9.Dudley, D. T., L. Pang, S. J. Decker, A. J. Bridges, and A. R. Saltiel. 1995. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 92:7686-7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feuer, R., I. Mena, R. Pagarigan, M. K. Slifka, and J. L. Whitton. 2002. Cell cycle status affects coxsackievirus replication, persistence, and reactivation in vitro. J. Virol. 76:4430-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrington, T. P., and G. L. Johnson. 1999. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 11:211-218. [DOI] [PubMed] [Google Scholar]
- 12.Hellen, C. U., and E. Wimmer. 1995. Translation of encephalomyocarditis virus RNA by internal ribosomal entry. Curr. Top. Microbiol. Immunol. 203:31-63. [DOI] [PubMed] [Google Scholar]
- 13.Hirasawa, K., H. S. Jun, K. Maeda, Y. Kawaguchi, S. Itagaki, T. Mikami, H. S. Baek, K. Doi, and J. W. Yoon. 1997. Possible role of macrophage-derived soluble mediators in the pathogenesis of encephalomyocarditis virus-induced diabetes in mice. J. Virol. 71:4024-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirasawa, K., H. S. Jun, H. S. Han, M. L. Zhang, M. D. Hollenberg, and J. W. Yoon. 1999. Prevention of encephalomyocarditis virus-induced diabetes in mice by inhibition of the tyrosine kinase signalling pathway and subsequent suppression of nitric oxide production in macrophages. J. Virol. 73:8541-8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber, M., H.-C. Selinka, and R. Kandolf. 1997. Tyrosine phosphorylation events during coxsackievirus B3 replication. J. Virol. 71:595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huber, M., K. A. Watson, H.-C. Selinka, C. M. Carthy, K. Klingel, B. M. McManus, and R. Kandolf. 1999. Cleavage of RasGAP and phosphorylation of mitogen-activated protein kinase in the course of coxsackievirus B3 replication. J. Virol. 73:3587-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huttunen, P., T. Hyypia, P. Vihinen, L. Nissinen, and J. Heino. 1998. Echovirus 1 infection induces both stress- and growth-activated mitogen-activated protein kinase pathways and regulates the transcription of cellular immediate-early genes. Virology 250:85-93. [DOI] [PubMed] [Google Scholar]
- 18.Johansen, L. K., and C. D. Morrow. 2000. Inherent instability of poliovirus genomes containing two internal ribosome entry site (IRES) elements supports a role for the IRES in encapsidation. J. Virol. 74:8335-8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang, Y., N. K. Chatterjee, M. J. Nodwell, and J. W. Yoon. 1994. Complete nucleotide sequence of a strain of coxsackie B4 virus of human origin that induces diabetes in mice and its comparison with nondiabetogenic coxsackie B4 JBV strain. J. Med. Virol. 44:353-361. [DOI] [PubMed] [Google Scholar]
- 20.Kolupaeva, V. G., C. U. Hellen, and I. N. Shatsky. 1996. Structural analysis of the interaction of the pyrimidine tract-binding protein with the internal ribosomal entry site of encephalomyocarditis virus and foot-and-mouth disease virus RNAs. RNA 2:1199-1212. [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar, A., S. K. Manna, S. Dhawan, and B. B. Aggarwal. 1998. HIV-Tat protein activates c-Jun N-terminal kinase and activator protein-1. J. Immunol. 161:776-781. [PubMed] [Google Scholar]
- 22.Li, C. J., Y. Ueda, B. Shi, L. Borodyansky, L. Huang, Y. Z. Li, and A. B. Pardee. 1997. Tat protein induces self-perpetuating permissivity for productive HIV-1 infection. Proc. Natl. Acad. Sci. USA 94:8116-8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, P., K. Aitken, Y. Y. Kong, M. A. Opavsky, T. Martino, F. Dawood, W. H. Wen, I. Kozieradzki, K. Bachmaier, D. Straus, T. W. Mak, and J. M. Penninger. 2000. The tyrosine kinase p56lck is essential in coxsackievirus B3-mediated heart disease. Nat. Med. 6:429-434. [DOI] [PubMed] [Google Scholar]
- 24.Manthey, C. L., S. W. Wang, S. D. Kinney, and Z. Yao. 1998. SB202190, a selective inhibitor of p38 mitogen-activated protein kinase, is a powerful regulator of LPS-induced mRNAs in monocytes. J. Leukoc. Biol. 64:409-417. [DOI] [PubMed] [Google Scholar]
- 25.McBride, A. E., A. Schlegel, and K. Kirkegaard. 1996. Human protein Sam68 relocalization and interaction with poliovirus RNA polymerase in infected cells. Proc. Natl. Acad. Sci. USA 93:2296-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakatsue, T., I. Katoh, S. Nakamura, Y. Takahashi, Y. Ikawa, and Y. Yoshinaka. 1998. Acute infection of Sindbis virus induces phosphorylation and intracellular translocation of small heat shock protein HSP27 and activation of p38 MAP kinase signaling pathway. Biochem. Biophys. Res. Commun. 253:59-64. [DOI] [PubMed] [Google Scholar]
- 27.Pestova, T. V., C. U. T. Hellen, and I. N. Shatsky. 1996. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Mol. Cell. Biol. 16:6859-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pilipenko, E. V., T. V. Pestova, V. G. Kolupaeva, E. V. Khitrina, A. N. Poperechnaya, V. I. Agol, and C. U. Hellen. 2000. A cell cycle-dependent protein serves as a template-specific translation initiation factor. Genes Dev. 14:2028-2045. [PMC free article] [PubMed] [Google Scholar]
- 29.Pleschka, S., T. Wolff, C. Ehrhaardt, G. Hobom, O. Planz, U. R. Rapp, and S. Ludwig. 2001. Influenza virus propagation is impaired by inhibition of the Ras/MEK/ERK signaling cascade. Nat. Cell Biol. 3:301-305. [DOI] [PubMed] [Google Scholar]
- 30.Popik, W., and P. M. Pitha. 1998. Early activation of mitogen-activated protein kinase kinase, extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and c-Jun N-terminal kinase in response to binding of simian immunodeficiency virus to Jurkat T cells expressing CCR5 receptor. Virology 252:210-217. [DOI] [PubMed] [Google Scholar]
- 31.Rodems, S. M., and D. H. Spector. 1998. Extracellular signal-regulated kinase activity is sustained early during human cytomegalovirus infection. J. Virol. 72:9173-9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, C. C., J. Nelson, L. Aurelian, M. Gober, and B. B. Goswami. 2000. Ras-GAP binding and phosphorylation by herpes simplex virus type 2 RR1 PK (ICP10) and activation of the Ras/MEK/MAPK mitogenic pathway are required for timely onset of virus growth. J. Virol. 74:10417-10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strong, J. E., M. C. Coffey, D. Tang, P. Sabinin, and P. W. Lee. 1998. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 17:3351-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whelan, S. P., L. A. Ball, J. N. Barr, and G. T. Wertz. 1995. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc. Natl. Acad. Sci. USA 92:8388-8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon, J.-W., A. K. C. Wong, Y.-S. Bae, and H.-M. Eun. 1988. An apparent deletion of an oligonucleotide detected by RNA fingerprint in the nondiabetogenic B variant of encephalomyocarditis virus is caused by a point mutation. J. Virol. 62:637-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon, J. W., M A. Lesniak, R. Fussganger, and A. L. Notkins. 1976. Genetic differences in susceptibility of pancreatic beta cells to virus-induced diabetes mellitus. Nature 264:178-180. [DOI] [PubMed] [Google Scholar]
- 37.Yoon, J. W., P. R. McClintock, T. Onodera, and A. L. Notkins. 1980. Virus-induced diabetes mellitus. XVIII. Inhibition by a nondiabetogenic variant of encephalomyocarditis virus. J. Exp. Med. 152:878-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon, J. W., M. M. Rodrigues, C. Currier, and A. L. Notkins. 1982. Long-term complications of virus-induced diabetes mellitus in mice. Nature 296:566-569. [DOI] [PubMed] [Google Scholar]
- 39.Zachos, G., B. Clements, and J. Conner. 1999. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J. Biol. Chem. 274:5097-5103. [DOI] [PubMed] [Google Scholar]