Abstract
Marek's disease virus (MDV) is a herpesvirus of chickens that induces T lymphomas and tumors within 4 to 5 weeks of infection. Although the ability of MDV to induce tumors was demonstrated many years ago and although a number of viral oncogenic proteins have been identified, the mechanism by which the MDV is implicated in tumorigenesis is still unknown. We report the identification of a virus-encoded RNA telomerase subunit (vTR) within the genome of MDV. This gene is found in the genomic DNA of the oncogenic MDV strains, whereas it is not carried by the nononcogenic MDV strains. The vTR sequence exhibits 88% sequence identity with the chicken gene (cTR). Our functional analysis suggests that this telomerase RNA can reconstitute telomerase activity in a heterologous system (the knockout murine TR−/− cell line) by interacting with the telomerase protein component encoded by the host cell. We have also demonstrated that the vTR promoter region is efficient whatever the species of cell line considered and that vTR is expressed in vivo in peripheral blood leukocytes from chickens infected with the oncogenic MDV-RB1B and the vaccine MDV-Rispens strains. The functionality of the vTR gene and the potential implication of vTR in the oncogenesis induced by MDV is discussed.
Marek's disease is characterized by the development of T-cell lymphomas in chickens and turkeys. The etiological agent of this disease is the Marek's disease alphaherpesvirus (MDV). MDV isolates can be divided into three major serotypes according to their pathogenicity. The oncogenic serotype (serotype I) contains very virulent strains (e.g., MDV-RB1B and MDV-Md5), virulent strains (e.g., MDV-GA and MDV-HPRS16), and the MDV-Rispens vaccine strain. The second (e.g., MDV-HPRS24) and third serotypes (the HVT vaccine strain) cause minor lesions and are consequently considered to be nononcogenic and nonpathogenic serotypes. The mechanism by which the oncogenic MDV strains cause tumor formation is still unknown. MDV is found in all areas of the world, and very virulent forms of this virus frequently cause acute explosive outbreaks, despite the availability of vaccines. These vaccines, which were initially made from the turkey virus (HVT) and then from the attenuated MDV-Rispens strain, protect against tumor formation but do not prevent the multiplication and the diffusion of the virus in the poultry industry. The MDV genome is composed of a 180-kbp double-stranded DNA (27, 39) that is structurally homologous to that of the human simplex viruses, consisting of a long sequence (UL) and a short sequence (US), each flanked by terminal and internal repeat sequences, TRL-IRL and TRS-IRS, respectively (6). The ends of the MDV genome and the internal IRL-IRS junction consist of telomeric sequences (23), which allow the preferential integration of the viral DNA located near to the telomeres of host cellular DNA during the latent phase (10). Otherwise, a recent epidemiological study revealed the presence of specific MDV DNA sequences in human serum samples, without any variations in prevalence according to age, sex, or exposure to chickens (26).
Recently, several studies have revealed a correlation between a number of viral infections and the development of cancers in human and animals. Furthermore, a number of oncogenic viruses, including the human papillomavirus HPV16 (34), human T-cell lymphotropic virus (38), hepatitis C virus and herpesvirus (HHV8 [9], herpesvirus saimiri [17], Epstein-Barr virus [35]), are known to have an effect on cellular telomerase activity. The telomerase is a ribonucleoproteic complex that is involved in maintaining the length of telomeres (15, 42), thus preserving the integrity of chromosomes during the cell cycle (3). The telomerase complex consists of two essential components, which account for its enzyme activity: a protein component (TERT), sharing sequence features with reverse transcriptases (28), and a closely associated RNA component (TR), which acts as a template for TERT (16, 42). Vertebrate RNA telomerase subunits consist of four main structural domains: (i) the pseudoknot domain, which consists of the template sequence (the CR1 domain), the CR2, and the CR3 conserved regions; (ii) the CR4/CR5 domain, essential for functional telomerase activity; (iii) the H/ACA box; and (iv) the CR7 domain, which is required for the 3′-end processing, stability, and nucleolar localization of the RNA telomerase genes within cells (7, 8, 14, 30, 33). Although a very low level of telomerase activity can be detected in human somatic cells, it is expressed in germinal and proliferative cells and is highly implicated in a broad range of human cancers (13, 18). At present, the only evidence of a telomerase component encoded by a virus is the telomerase protein component of the temperate Escherichia coli phage N15, which plays a critical role in the replication of this bacteriophage by a cleaving-joining activity (11, 37). We report here for the first time the identification of an RNA telomerase gene (vTR) encoded by a virus, MDV, that infects eukaryotic cells.
In order to characterize this subunit and to determine whether it is involved in MDV tumorigenesis, we first studied the expression of the vTR gene and, second, we investigated whether this gene is functional by using a heterologous murine system that is genetically deficient for the murine telomerase RNA gene.
MATERIALS AND METHODS
Cell lines.
Two human cell lines were used in the present study: the HeLa cell line derived from cervix carcinoma and the lymphoblastoid cell line, SUP-T1. HeLa cells were grown in Dulbecco modified Eagle medium (BioMedia), and SUP-T1 cells were grown in RPMI 1640 medium (Life Technologies).
The lymphoblastoid cell line, MDCC-PA5 (32), was derived from testis tumors of chickens infected with the virulent MDV-HPRS16 strain and propagated in RPMI 1640 complete medium. The chicken hepatocellular carcinoma cell line, LMH, was established in gelatin-coated petri dishes (2% gelatin in phosphate-buffered saline [PBS] for 15 min at 4°C) in William's medium (BioMedia), as described previously (22).
Immortalized mTR+/+ fibroblasts, derived from wild-type murine embryos, and KO3 p23 mTR−/− cells, which lack telomerase activity by knocking out the murine RNA telomerase gene (5), were cultured in Dulbecco modified Eagle complete medium.
All cell lines were grown in their specific medium supplemented with 10% fetal bovine serum (Sigma) at 37°C in a 5% CO2 atmosphere. The only exception was the MDCC-PA5 cell line, which was cultured at 41°C.
Genomic DNA.
Murine genomic DNA and MDV-HPRS16 viral genomic DNA were extracted from mTR+/+ fibroblasts and the MDCC-PA5 cell line, respectively. Briefly, the cells were incubated with 500 μg of proteinase K/ml, 0.5% sodium dodecyl sulfate, and 10 mM Tris (pH 8) for 2 h at 70°C. Nucleic acids were then extracted with phenol-chloroform, followed by ethanol precipitation. The resulting pellet was suspended in 50 μl of water.
The MDV-Rispens and HVT genomic DNA was extracted with the Nucleobond AXG100 kit (Macherey Nagel) from the vaccine strains purchased from Fort-Dodge Santé Animal (Tours, France).
The MDV-RB1B genomic DNA was extracted from a splenic lymphoma (Nucleobond AXG100; Macherey Nagel).
Plasmids and telomerase RNA genes.
Plasmids encoding chicken (pUC-cTR) and human (pUC-hTR) telomerase RNA genes (AF221938 and AF221907, respectively) were kindly provided by Jiunn-Liang Chen (Department of Molecular Biology and Genetics, Johns Hopkins University School of Medicine) (7). These plasmids carry the cTR and hTR genes, respectively, encompassed by genomic sequences.
pBS-mTR was constructed by cloning a 1.9-kbp fragment of mouse genomic DNA containing the wild-type mTR gene (AF221922) into pBS SK− such that it was under the control of its own promoter sequences (30).
The pBMB 8-5 clone was obtained from a MDV-RB1B BamHI genomic library constructed in our laboratory. This clone contains the 3-kbp BamHI L fragment (AF331499) of the MDV-RB1B viral genome, which carries the viral telomerase RNA gene.
PCR amplification.
PCR was carried out in a 100-μl volume. The reaction mixture contained 1× Taq buffer (Promega; supplemented with 1.5 mM MgCl2), an 800 μM concentration of deoxynucleoside triphosphates, 2 pmol of the forward primer, 2 pmol of the reverse primer, 5% dimethyl sulfoxide, 5 U of Taq polymerase (Promega), and 0.5 μg of plasmid DNA or 0.1 μg of genomic DNA/μl. The cycling conditions consisted of 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, followed by a final extension at 72°C for 10 min. PCR products were purified from agarose gels (Ultrafree DA; Millipore) and concentrated with the Microcon 30 kit (Millipore) before being cloned into the T-tailed pGEM-T cloning vector (Promega). They were then subcloned into the vector of interest.
In vivo vTR expression.
Chicken inoculation with the MDV-RB1B or with the vaccine MDV-Rispens or HVT strains, peripheral blood leukocyte (PBL) collection, and semiquantification (by reverse transcription-PCR) of the viral telomerase gene expression were carried out as previously described (12). Briefly, the PBLs were collected from 1 ml of chicken blood and homogenized in 1 ml of RNAble solution (Eurobio Laboratory), and the total RNA was extracted according to the manufacturer's instructions. Reverse transcription was performed with the 790 primer (5′-CTGCAGGCGTGTGGGAGCGACGCCGTCCGC-3′). PCRs were performed in a final volume of 25 μl with 5 μM concentrations of each vTR-specific forward primer TET-882 (5′-TET-GCCCTGGGGTCCTCGCCCGCA-3′) and reverse primer 881 (5′-GCGTGTGGGAGCGACGCCGTCCGC-3′). The cycling conditions consisted of an initial heating step at 94°C for 2 min, followed by 35 cycles at 94°C for 45 s, 80°C for 1 min, and 72°C for 45 s, with a final extension at 72°C for 10 min. A linear range of several dilutions of pBS-vTR and pBS-cTR plasmids was included as positive and negative controls, respectively. β-Actin was amplified as an internal control by 25 cycles at an annealing temperature of 60°C with the specific primers TET-ACTs (5′-TET-CATCACCATTGGCAATGAGAGG-3′) and ACTas (5′-GATTCATCGTACTCCTGCTTGC-3′) in order to standardize the starting cDNA amount of the samples. The PCR products were detected by automated sequencing ABI prism 310 (Perkin-Elmer).
Southern blot hybridization.
Portions (10 μg) of MDV-RB1B, MDV-Rispens, and HVT genomic DNAs were digested overnight at 37°C with the BamHI endonuclease (7 U/μg). Viral DNA BamHI fragments were separated by 1% gel electrophoresis and transferred to nitrocellulose filter. Hybridization was carried out according to the ECL direct nucleic acid labeling and detection system (Amersham Pharmacia Biotech) with a vTR probe obtained by PCR amplification from the pBMB 8-5 clone.
Promoter constructs.
The telomerase RNA promoter sequences from each species (human, mouse, chicken, and MDV) were amplified by PCR. The primer sequences used to amplify these regions were selected on the basis of the published sequences of the hTR promoter region (AF047386), the mTR promoter region (AF047386) (43), the pUC-cTR plasmid, and the MDV-Md5 complete genome (AF243438) (39), respectively. The hTR promoter sequence was amplified from human genomic DNA with the forward primer 787 (5′-CTCGAGGGATCCAGAGAGTGACTCTCACGAGAGCCGC-3′) and the reverse primer788 (5′-CTCGAGGGTGCGCTGCCGGGCGAGTCG-3′), resulting in the pph construct. The mTR promoter construct, ppm, was amplified from murine genomic DNA with the primers 785 (5′-CTCGAGGGATCCAATGGGG AAGAGGGAGCATTTCCGC-3′) and 786 (5′-CTCGAGCCGAGGCCTAGCCGCCCTCGA-3′).
The vTR promoter constructs were obtained from MDV-RB1B genomic DNA with the primer pairs 782 (5′-CTCGAGGGATCCTCCCCGCCGCCAATAGCTAC-3′) and 784 (5′-CTCGAGGCCGGGGGAACCCCGCGTGG-3′), 802 (5′-CTCGAGCCCTAACCCTAACCCCCCAAATTTTCACC-3′) and 784, and 804 (5′-CTCGAGGGATCCGATCCCGCAGACCCCGGC-3′) and 784, yielding the ppv, ppvXL1, and ppvXL3 constructs, respectively.
The ppc and ppcXL3 chicken promoter constructs were generated from pUC-cTR with the primer pairs 783 (5′-CTCGAGGGATCCTCCCCGCGGCCAATAGCGGG-3′)-784 and 706 (5′-CTCGAGGCATCGGACCCCGCGGCCCA-3′)-784, respectively.
These primers all harbor an XhoI restriction site (in boldface), which was used to subclone the PCR product into the XhoI-digested pGL3-Basic luciferase reporter vector (Promega). All constructs were sequenced by using the PGL3 primer (5′-CTAGCAAAATAGGCTGTCCC-3′) to check the orientation and sequence of the inserted fragment.
Telomerase RNA subunit gene constructs.
Since the constructs were transfected into a heterologous system, the human (hTR), murine (mTR), viral (vTR), and chicken (cTR) telomerase RNA genes were individually cloned under the control of the cytomegalovirus (CMV) promoter to ensure gene expression.
All of the RNA telomerase genes, except the cTR gene, were amplified by PCR in the presence of 5% dimethyl sulfoxide. PCR products were purified and cloned into the pGEM-T cloning vector before being subcloned into the EcoRV/NotI-digested pCDNA3 vector, which contains the CMV promoter.
The vTR gene was amplified from the pBMB 8-5 plasmid with the forward primer 791 (5′-ACACGTGGCGGGTGGAAGGCTCCGC-3′) and the reverse primer 790.
The equivalent mTR construct was generated by amplifying the mTR gene from the pBS-mTR plasmid with the primers 792 (5′-CACCTAACCCTGATTTTCATTAGC-3′) and 793 (5′-GGTTGTGAGAACCGAGTTCCGGGTGC-3′).
The hTR gene was amplified from pUC-hTR with the primers 794 (5′-GGGTTGCGGAGGGTGGGCCTGGGA-3′) and 795 (5′-GCATGTGTGAGCCGAGTCCTGGGTGCAC-3′).
Unlike the other telomerase genes, the cTR gene was not obtained in this way since this method resulted in a truncated version of the cTR gene. Consequently, the cTR construct, comprising the complete cTR gene, was obtained by inserting the MluI-NotI fragment of pUC cTR into MluI/NotI-digested pCDNA3 vector.
All halfway and final constructs were checked by sequencing with appropriate primers.
Cloning of the viral telomerase RNA gene from different MDV strains.
The vTR genes from the MDV-HPRS16 and MDV-Rispens strains were PCR amplified from the corresponding genomic DNA by using the 790-791 primer pair as previously described for the MDV-RB1B vTR gene amplification.
Sequence analysis.
Sequencing was carried out by the dye terminator method (Perkin-Elmer).
BLAST (basic local alignment search tool; http://www.ncbi.nlm.nih.gov/) was used to look for sequence homologies. Sequences were aligned by using the Seqman program (DNAstar package; Lasergene).
Promoter assay.
All promoter constructs were transfected into murine, human, and avian cell lines in 24-well plates. Each transfection reaction was carried out at least three times in duplicate. pCDNAMLuc, which carries the luciferase gene under the control of the CMV promoter, was also transfected in each plate so that the results for different plates could be compared. Cells were seeded at 105 cells per well, and 2 μg of plasmid DNA was transfected by using the Lipofectin reagent (Life Technologies) according to the manufacturer's instructions. Cells were exposed to the transfection mixture for 6 h. Fresh medium was then added, and the cells were harvested 48 h later. The cells were then washed twice in 1× PBS buffer and suspended in 200 μl of luciferase buffer (25 mM Tris-phosphate [pH 7.4], 8 mM magnesium chloride, 1 mM dithiothreitol, 1 mM EDTA, 1% Triton X-100, 1% bovine serum albumin, 15% glycerol). Cell lysates were clarified (14,000 × g for 2 min) and the luciferase activity of a 100-μl aliquot of the supernatant was measured, as described previously (20), with a luminometer (Autolumat LB 953 Berthold).
The mean pCDNAMLuc luciferase activity was calculated for each cell line. The ratio of pCDNAMLuc activity of each plate to the mean value was calculated. The resulting coefficient was applied to the luciferase activity of each construct for each plate to make it possible to compare plates and experiments. The mean luciferase activity of each construct was calculated, and the final results are expressed as a function of the mean activity of the promoter of interest.
Telomerase assay.
We seeded 106 KO3 p23 mTR−/− cells in 100-mm-diameter dishes. A portion (20 μg) of each of the telomerase RNA gene constructs was transfected by using the Lipofectin reagent (Life Technologies) according to the manufacturer's instructions. Cells were exposed to the transfection mixture for 6 h. Fresh medium was then added, and the cells were harvested 48 h later. Each transfection was carried out three times.
At 48 h posttransfection, cells were washed twice in 1× PBS, and then 106 cells were incubated with 100 μl of ice-cold lysis buffer on ice for 30 min. After centrifugation at 12,000 × g for 30 min, 80 μl of the supernatant was removed and flash frozen before being stored at −80°C. The protein concentration was estimated by the BCA protein assay (Pierce) and adjusted to 1 μg/μl so that 2 μg of protein was used for a 50-μl TRAP assay. Telomerase activity was determined with a semiquantitative fluorescence-based telomere repeat amplification protocol (TRAP) assay as previously described (24). The PCR step was performed with TAMRA-labeled forward TS and CXext as reverse primers (Eurogentec). An internal amplification standard (ITAS), required for the quantitative TRAP assay, was added to the PCR mixture and yielded a 135-bp product with the TS and CXext primers. Samples were analyzed by capillary electrophoresis (ABI Prism 310). The telomerase activity of each sample was quantified by adding the integrated value of four telomerase products (corresponding to four telomerase hexamer repeats beyond the primer dimer peak) and as a function of the integrated value of ITAS.
RESULTS
Comparison of the primary sequence of the viral telomerase RNA subunit gene in the different MDV strains.
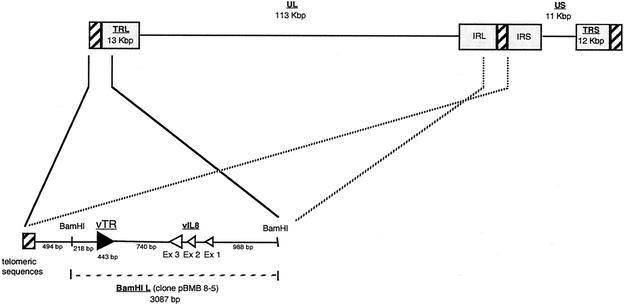
Based on the full sequences of the MDV-GA and MDV-Md5 strains genomes previously published (27, 39) and with the aim of characterizing the ends of the MDV-RB1B strain genome, we screened a MDV-RB1B BamHI genomic library. This led us to select the pBMB 8-5 clone. This clone consists of a 3,087-bp insert, corresponding to the most distal fragment of the genome, the BamHI L fragment (Fig. 1). The sequence of this MDV-RB1B BamHI L fragment is 95.6 and 99.6% identical to that of its MDV-GA and MDV-Md5 orthologs, respectively. According to the genomic organization of MDV, this fragment is mapped both to the terminal long repeat (TRL), 494 bp downstream of the genome end, which consists of telomeric sequences, and to the internal long repeat (IRL). A BLAST search identified a 443-bp sequence located 218 bp downstream of the beginning of the BamHI L fragment. This sequence was identified as being a viral RNA telomerase subunit (vTR) due to its high sequence identity (88%) with the chicken telomerase RNA subunit gene (cTR). This viral telomerase component was found to be located 712 bp downstream of telomeric sequences and 740 bp upstream of the third exon of the viral interleukin-8 gene (vIL8).
FIG. 1.
Genomic organization of MDV and localization of the viral telomerase RNA gene (vTR). UL is the unique long sequence, and US is the unique short sequence of MDV. The telomeric sequences at the end of the genome and at the IRL-IRS junction are represented by hatched boxes. The BamHI L fragment localized in both TRL and IRL regions is enlarged in the bottom panel. The telomerase RNA subunit gene, vTR, is represented by a solid arrow, which also indicates the orientation of the gene, and the three exons of the vIL8 gene are represented by three open arrows.
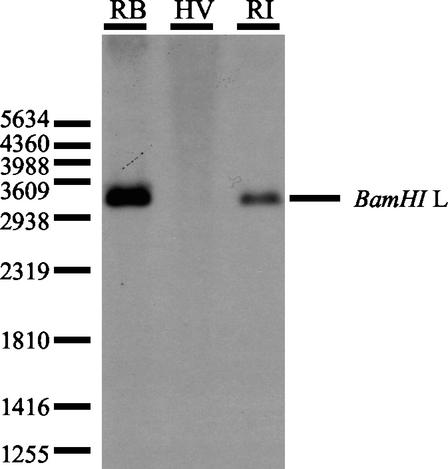
We investigated whether this telomerase subunit was also encoded by the other MDV strains, especially by the vaccine strains. Sequence analysis showed that the MDV oncogenic serotype 1 MDV-GA (AF147806) and the MDV-Md5 (AF243438) strains harbored similar sequences in the BamHI L fragment at positions 247 to 692 and 137984 to 138429 and at positions 1676 to 2117 and 139503 to 139944, respectively, whereas the MDV nononcogenic serotype 2 (MDV-HPRS24 strain [19]) and the HVT vaccine strain (1) did not encode this subunit. By a Southern blotting experiment with the MDV-RB1B vTR gene performed on the genomic DNA of the MDV-RB1B, MDV-Rispens, and HVT strains, we confirmed that the HVT vaccine strain did not encode the vTR gene whereas, like the very virulent MDV-RB1B strain, the MDV-Rispens vaccine strain encoded vTR within the BamHI L fragment (Fig. 2). Thus, we cloned and sequenced the vTR gene from the vaccine MDV-Rispens and the virulent MDV-HPRS16 strains. An alignment of the sequences of the vTR genes from the different MDV strains and their chicken orthologs revealed that the viral genes are all highly similar and are thus all ca. 88% homologous to cTR (Fig. 3). The four main structural domains described in the chicken telomerase RNA subunit sequence are globally conserved in the vTR sequences of all of the viral strains considered. The conserved regions (CR1 to CR8) of the vTR gene from different MDV strains are all highly homologous to the conserved regions of cTR. However, several point mutations and deletion regions were found in the vTR sequence compared to cTR. A one-nucleotide deletion was found in the CR2 domain (position 90) of the vTR gene from all MDV strains, as well as a point mutation (position 348) in the CR5 domain and major mutations in the CR7 domain (positions 439, 441, 443, 446, and 448) of the MDV-HPRS16 vTR gene. The MDV-Rispens vTR sequence also harbors a point mutation (position 401) located in the H box within the CR6 region. Nevertheless, most of mutations and deletions in the vTR sequence are mainly located in junction regions outside of the conserved domains.
FIG. 2.
Detection of the viral telomerase RNA gene in very virulent (MDV-RB1B) and vaccine (MDV-Rispens and HVT) strains of MDV. Viral genomic DNAs of the MDV-RB1B (RB), MDV-Rispens (RI), and HVT (HV) strains were digested by the BamHI endonuclease, electrophoresed through a 1% agarose gel, and blot hybridized to the vTR gene. The position of the molecular weight marker and the BamHI L fragment from pBMB 8-5 clone are shown at the left and the right, respectively.
FIG. 3.
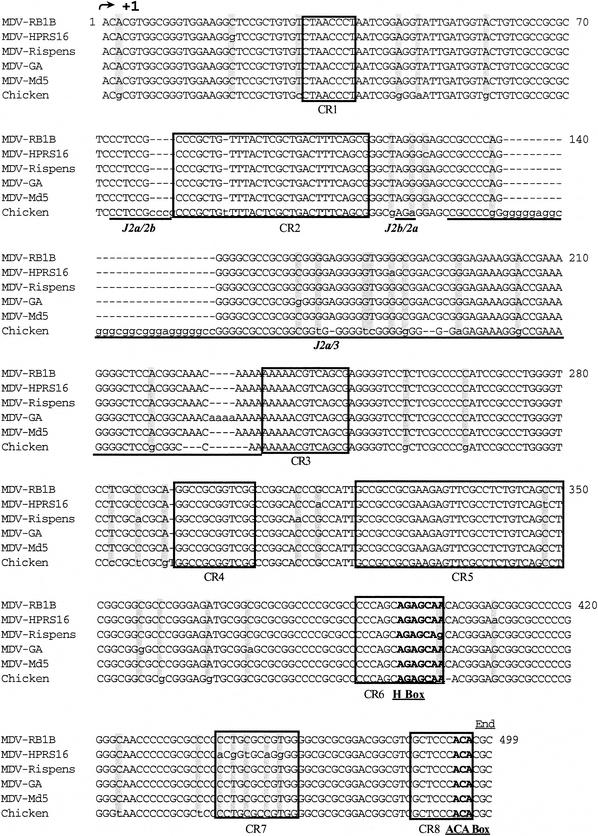
Alignment of the vTR sequences of different MDV strains and the chicken telomerase RNA subunit. The primary sequences of vTR and cTR were aligned by using conserved sequences as anchors. Each line consists of 70 characters. Identical nucleotides are shown in uppercase, point mutations are in lowercase and gray boxes, and deletions are indicated by dashes. The conserved domains of the cTR and vTR genes (CR) are boxed in black, functional boxes are underlined and shown in boldface, and junction regions (J) are underlined.
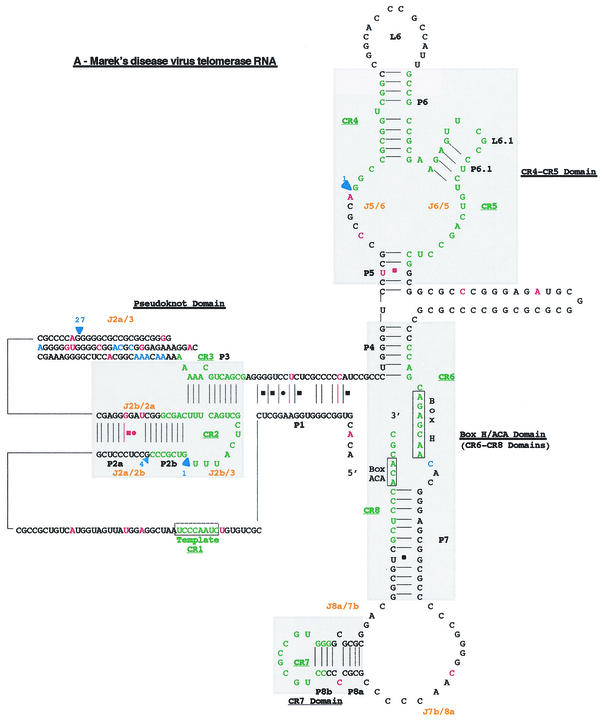
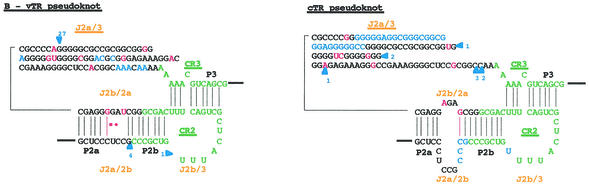
The high degree of similarity between the primary sequences of vTR and cTR genes was also reflected in their overall secondary structures (Fig. 4A). However, most of the mutations and deletions scattered along the vTR sequence affected preferentially the pseudoknot domain (Fig. 4B). The J2a/3 loop of the vTR pseudoknot carries the largest deletion region of 27 bp (positions 132 to 158). Moreover, the four-unpaired-nucleotide deletion (positions 79 to 82) in the J2a/2b junction region, bordering the CR2 domain, led to the disappearance of the loop found in the cTR pseudoknot. A one-nucleotide mutation (positions 118) within the J2b/2a junction, which did not alter the size of the loop, made conventional base pairing possible. Likewise, two mutations (positions 257 and 266) within the P1 helix of vTR allowed conventional base pairing.
FIG. 4.
Proposed secondary structure of the MDV. Comparison of the overall proposed secondary structure of MDV and cTR (A) and their pseudoknot domains structures (B) are based on the vertebrate TR structure published by Chen et al. (9). Paired regions (P) are numbered from 5′ to 3′ as P1 to P8. Junction regions (J) between two paired regions are named with reference to the flanking paired regions and are given in orange type. The four universal structural domains conserved in all vertebrate TRs are shaded in gray and labeled. The template region, box H, and box ACA motifs are labeled, and their conserved nucleotides are boxed. Universal base pairing (according to the Watson-Crick scheme) is represented by dashes, whereas G-U pairs and noncanonical A-C pairs are indicated by squares and circles, respectively. Mutations in the vTR sequence are in red, as is additional base pairing in the vTR structure. Additional nucleotides in the vTR sequence are shown in blue, and deletion regions are indicated by blue arrows showing the location and the nucleotide length of the deletion.
Efficiency of the vTR promoter region in vitro and in vivo.
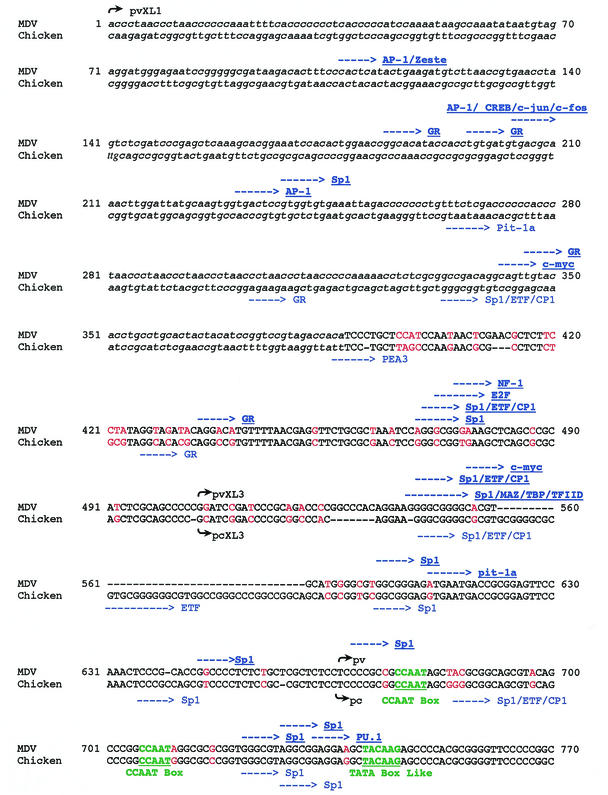
Given that vTR is located at the very end of the MDV genome, we defined its promoter region between the MDV telomeric sequences and the cTR transcription start site. The alignment of the vTR and cTR promoter sequences (Fig. 5) revealed a 379-bp region that was 72.8% homologous to the chicken and the viral sequences (positions 388 to 770). The sequences upstream of this region were not comparable. The vTR promoter region contains the two CCAAT boxes (positions 676 and 706) and the TATA-like box (position 741) that have been described in the cTR promoter region. Transcription factor recognition site analysis revealed four sites of conserved sequence and localization in the cTR and vTR promoter regions: the Sp1/ETF/CP1 (positions 470 to 475) and the SP1-binding sites (positions 603 to 608, 722 to 727, and 728 to 733). However, several point mutations and deletion regions (positions 526 to 532 in cTR and positions 552 to 591 in vTR) were found along this homologous sequence, altering the positions of the transcription factors binding sites between the cTR and vTR promoter regions. Potential transcription factor recognition sites were identified in the vTR promoter region but not in the cTR sequence (the NF-1, E2F, Sp1, ETF, and CP1 recognition sites [positions 469 to 480]; c-myc and Sp1/MAZ/TBP/TFIID [positions 536 to 551]; Pit-1a [positions 611 to 616]; and PU.1 [positions 734 to 739]). Conversely, the cTR promoter region was found to contain Pit-1a (positions 264 to 270), PEA3 (positions 386 to 392), ETF (positions 561 to 571), and SP1/ETF/CP1 (positions 675 to 680) recognition sites, unlike vTR. Some binding sites were present in both sequences, but these were located a few base pairs further downstream in vTR. For example, the Sp1 site is located at positions 645 to 650 in the vTR promoter sequence and at positions 635 to 640 in the cTR promoter sequence, and the glucocorticoid-binding site is located at positions 435 to 440 in MDV and at positions 426 to 431 in the chicken sequence. The 5′ nonhomologous flanking sequence of the vTR promoter region also exhibits some potential binding sites, including three AP-1 sites (positions 107, 204, and 231) and CREB/c-jun/c-fos sites (positions 204 to 210).
FIG. 5.
Alignment of the 5′ flanking region of the viral and chicken telomerase RNA genes. The primary sequences of the promoter regions of the vTR and cTR genes were aligned according to conserved sequences. Each line consists of 70 characters. Homologous sequences are shown in uppercase letters, and nonhomologous sequences are given in lowercase letters. Mutations are in red, and deletions are indicated by dashes. Putative regulatory elements are indicated by bold blue arrows in the viral sequence (top) and by blue arrows in the chicken sequence (bottom). The start sites of each of the defined promoter regions are identified by black arrows, followed by the name of the concerned region.
To determine whether the 5′ flanking region of vTR exhibits promoter activity, we tested the efficiency of nested promoter sequences from this 770-bp promoter region by using the luciferase gene as a reporter system. We truncated the sequence upstream of vTR into three potential promoter regions extending 103 to 770 bp upstream of the transcription start site of vTR. The 103-bp ppv construct, defined in relation to the previously described chicken promoter sequence, contained both CCAAT boxes and the TATA-like box. The ppvXL3 construct, located within the defined homologous region between vTR and cTR, extended over 265 bp (from position 504). Finally, the longest construct (ppvXL1) covered the entire 5′ flanking region of vTR, starting immediately upstream of the telomeric MDV repeat sequences.
To compare the activity of the vTR promoter sequence, we built constructs containing the orthologous promoter regions of the cTR gene (ppc and ppcXL3), defined according to the equivalent nucleotide lengths of the viral promoter regions pv, pvXL3 and pvXL1, respectively. We also built constructs containing the murine and human promoter constructs (ppm and pph), which are equivalent to the ppv and ppc constructs. First, we compared the relative activities in vitro of the vTR promoter constructs with those of the cTR constructs in a homologous system consisting of LMH avian cells and MDV-infected PA-5 avian cell lines (Fig. 6A and B). We then compared the viral promoter constructs to the chicken, murine, and human promoter constructs in three heterologous systems: a murine embryo fibroblast cell line (MEF) and two human cell lines (HeLa and SupT1) (Fig. 6C and D).
FIG. 6.
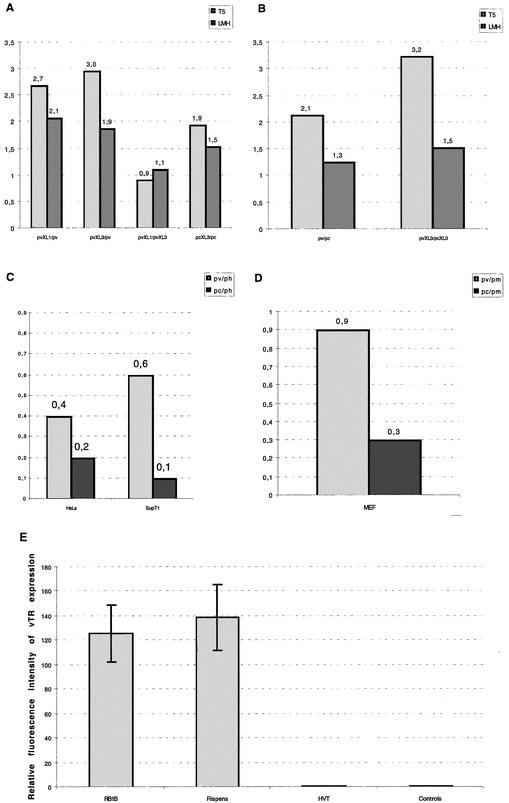
Efficiency of the vTR promoter sequences and the endogenous TR promoter sequences in homologous and heterologous systems and in vivo vTR expression. The promoter activity resulting from the transfection of the different viral, avian, murine, and human promoter constructs into avian MDV-infected (PA-5) and noninfected (LMH) cells (A and B), human (HeLa and SupT1), or murine (MEF) cell lines (C and D, respectively) was measured. The results are given as the ratio between the mean luciferase activity of the two promoter constructs considered (mentioned on the x axis or in the legend). (E) The levels of in vivo vTR expression were determined in PBLs collected from three chickens inoculated with the MDV-RB1B oncogenic strain and three chickens inoculated with the vaccine strains (HVT and MDV-Rispens) compared to three noninoculated control chickens. The results are presented as histograms, and the standard deviations are represented by bars.
In the homologous systems, the longest viral promoter constructs (ppvXL3 and ppvXL1) exhibited similar levels of promoter activity. These two constructs were about three- and twofold more efficient than the shortest promoter construct (ppv) in the PA-5 and LMH cell lines, respectively. Although the longest avian promoter construct, ppcXL3, was slightly more efficient than the ppc construct, it exhibited a similar level of activity to its viral ortholog (ppvXL3) in LMH cells but was about threefold weaker than ppvXL3 in the MDV-infected PA-5 cell line. Nevertheless, the viral ppv construct did not seem to be significantly more efficient than the ppc promoter construct in any of the homologous cell lines considered. In the heterologous systems, the viral ppv construct was slightly less efficient than the human or murine endogene promoter but was about two- to sixfold more efficient than the chicken promoter sequence, pc.
Moreover, we confirmed the efficient transcription of the vTR gene in PBLs extracted from chickens at 30 days postinoculation with MDV serotype 1 strains (MDV-RB1B and MDV-Rispens), whereas vTR was not detected in noninfected PBLs and in the PBLs of HVT-infected chickens (Fig. 6E).
Functional study of the vTR gene in a heterologous system.
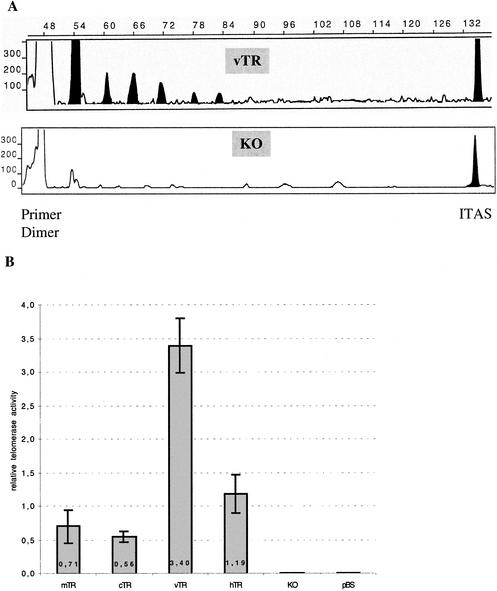
Although the sequence and structure of the vTR gene were highly similar to those of the cTR gene, some point mutations and deletions occurred along the vTR sequence. These differences may affect the functionality of vTR. We used telomerase activity reconstitution assays to test whether the viral telomerase RNA subunit is functional. These assays were carried out in the heterologous system that is usually used to test the efficiency of telomerase RNA genes, which consists of expressing the RNA telomerase subunit of interest in a murine cell line lacking its endogenous mTR gene (KO3 p23 mTR−/− cells). This involved the transfection of constructs consisting of the viral (vTR), the murine (mTR), avian (cTR), and human (hTR) telomerase RNA genes under the control of the CMV promoter. We used a modified TRAP assay based on fluorescence to measure the resulting telomerase activity generated by each construct at 48 h posttransfection. The vTR gene was functional, as shown by the 6-bp profile obtained (Fig. 7A), and seems to be at least as efficient as its murine, chicken, or human telomerase RNA genes orthologs (Fig. 7B), whereas, as expected, the murine invalidated mTR−/− cells, as well as cells transfected with the empty vector pBS, do not present any telomerase activity.
FIG. 7.
In vivo reconstitution of telomerase activity in telomerase-deficient KO3 p23 mTR−/− cells. The different constructs consisting of the mTR, cTR, vTR, or hTR genes under the control of the CMV promoter region and the pBS empty vector as negative control were transfected into murine cells lacking telomerase activity (KO3 p23 mTR−/−). Each transfection was carried out three times, and the resulting telomerase activity was measured by a modified TRAP assay based on fluorescence. (A) Classic profiles of electrophoregrams showing the six-base TRAP product for vTR (upper panel) and the KO3 p23 mTR−/− (lower panel) cells. The positions of the primer dimer and ITAS (internal control) are indicated. Filled-in areas identify the 6-bp profile of the telomerase product. (B) The relative telomerase activity reconstitution of each construct is presented as a histogram, and the standard deviations are represented by bars.
DISCUSSION
We identified the coding sequence of a telomerase RNA subunit in a virus genome. We showed that the viral TR is encoded by the sequence of MDV strains from serotype 1 (27, 39) but not by any of the nononcogenic MDV strains from serotype 2 (1, 19) and serotype 3. Furthermore, we tested the functionality of this gene in a heterologous system consisting of a murine cell line knocked out for its endogenous RNA telomerase subunit. This assay is usually used to test the functionality of RNA telomerase genes due to the ability of TERT to bind with heterologous telomerase RNA, as has been demonstrated for the human RNA component and mTERT (31), as well as for the rabbit telomerase RNA component and the human protein subunit, hTERT (41). Our results demonstrate that the vTR gene can also take part to this heterologous telomerase complex, which in turn generates telomerase activity. Furthermore, it should be stressed that the viral gene was at least as efficient as the endogenous mTR subunit and the heterologous human or the chicken TRs. Consequently, since the vTR gene can be functional outside of its natural host, it would be conceivable that the introduction and the overexpression of such a viral subunit might increase a risk factor in a number of species, particularly in those in which telomerase activity is regulated by the expression of the RNA telomerase subunit (e.g., the murine model in which mTR is the limiting factor [4]).
The functionality of vTR confirms that it was correctly folded, allowing it to associate with the telomerase protein subunit. In addition to the conservation of the overall structure, the sequences of all of the main functional domains were identical to those of the chicken and other species (7). A recent report concluded that both CR4/CR5 domains and the pseudoknot domain are required and sufficient to reconstitute telomerase activity in vitro (2, 30). Our results show that the sequences of the CR4/CR5 domains that are essential for telomerase activity are conserved between vTR and cTR. Furthermore, their secondary structures are similar to that of mTR (8), consisting of the P6.1 stem and the L6.1 loop required for interaction with the telomerase protein subunit and essential for efficient telomerase activity. Likewise, the domains responsible for the 3′-end processing, the maintenance, and the stability of TRs within cells are conserved between vTR and other vertebrates. However, despite a high degree of sequence homology (88%) between vTR and its chicken counterpart, the vTR seems to restore more telomerase activity in our detection system than cTR. Since all functional domains are conserved between cTR and vTR, the differences in telomerase activity may be explained by differences scattered along the vTR and cTR sequences, especially in the pseudoknot domain. The main difference in this region was the largest deletion region in the J2a/3 loop of the vTR pseudoknot sequence. However, this deletion region has been described to vary considerably in length between species (7). Apart from this deletion region, the vTR pseudoknot contains a four-unpaired-nucleotide deletion and a mutation in the J2a-2b and J2b-2a junction regions compared to the cTR pseudoknot that cause the loop to disappear. This is particularly interesting because this four-nucleotide deletion corresponds to an mTR-depleted mutant that was recently shown to restore telomerase activity more efficiently than the wild-type mTR subunit in the same experimental system (30). Consequently, vTR can be considered to be a natural positive cTR mutant in which the 4-bp deletion in the pseudoknot structure might stabilize the P2 stem, thus enhancing telomerase activity. The two mutations involving conventional base pairing within the vTR P3 helix are consistent with this hypothesis, as shown by stabilization of the P3 helix, which is highly implicated in the specific telomerase activity, as reported for the hTR pseudoknot (2). We will use mutagenesis assays to check these assumptions and to determine whether there is a relationship between the pseudoknot structure and the functionality of vTR.
The functionality of vTR is directly associated with its expression in cells; thus, we tested the promoter region of vTR in homologous and heterologous systems. We did not restrict our study to the minimal promoter sequence that had previously been defined for mammalian TR (7). Instead, we included the whole 5′ flanking region of vTR from the upstream telomeric sequences. We demonstrated that the longest vTR promoter regions were slightly more efficient than the shorter one, which might be due to particular regulatory elements scattered along this region. A number of potential transcription factor-binding sites are present in this region, including two c-myc consensus sequences that might be implicated in the transcriptional activation of vTR as described previously for TERT subunits (36, 40). Furthermore, because of the presence of several Sp1-binding sites, the transcription of vTR could be at least more active due to the cooperation of Sp1 and the c-myc factors, which are major determinants of hTERT expression (25, 36). It is noteworthy that these c-myc-binding sites may also be implicated in the expression of vTR in a heterologous system, given that the viral promoter sequence exhibits promoter activity in nonavian cell lines. Unlike the cTR promoter region, the 5′-flanking region of vTR also contains the TATA box-binding complex from the TBP consensus site (TFIID and TBP), which may lead to the active transcription of vTR when associated with the MAZ transcription factor. Otherwise, it should also be stressed that the pvXL1 promoter sequence contains a potential CREB/c-jun/c-fos-binding site that is not present in the other promoter constructs tested, including the longest avian promoter sequence. Furthermore, the MDV encodes a viral protein, the Meq protein, which may transactivate both viral and host genes by dimerizing with the cellular Jun and Fos proteins (29). However, in our homologous system, we did not observe significant differences in efficiency whatever viral or avian promoters were considered. This may be due to the fact that the two c-myc sites might regulate the expression as much as it could hide the c-jun/c-fos regulation. Alternatively, this may be because we currently lack information concerning the Meq expression status in the PA-5 cell line and the c-jun/c-fos expression status in the LMH avian cell line. Consequently, we are now carrying out mutagenesis and point deletion studies to test the potential effects of such transactivators on viral promoter sequences. Aside from this potential transactivation of the vTR transcription, we have shown that, whatever cell line is used to test the activity of the chicken and viral promoter sequences, the viral promoter is always more efficient than its avian ortholog. A comparison of the sequences of these constructs revealed mutations and deletions scattered along the viral sequence, which induced the appearance of the PU.1 consensus-binding site in the viral promoter sequence. Given that little is known about the functions of the PU.1 transcription factor, we cannot exclude the possibility that this protein enhances the transcription of vTR besides that of cTR.
Given that vTR and the vIL8 gene are colocalized near the end of the viral genome, we suggest that these two genes were acquired from the chicken genome by recombination in the course of viral reactivation. Although the nucleotide sequence of the vIL8 gene is considerably different from that of the chicken interleukin-8 gene, the amino acid sequence of vIL8 is at least 20% identical with its chicken ortholog (21). Conversely, the comparison of the primary sequences of the vTR and cTR genes revealed that these two genes are 88% homologous and that their 5′ and 3′ encompassed regions are 72.8 and 78.8% identical, respectively. This suggests (i) that the viral TR may be acquired after the vIL8 gene and in an independent manner because of the high level of sequence identity between the cTR and vTR genes and their flanking regions and (ii) that MDV could take advantage of the viral TR holding within its genome during its infectious cycle because of the high conservation of the vTR gene sequence added to its functional conservation and because of the increased efficiency of the promoter region.
To conclude, we demonstrated that vTR is functional in vitro as a template for TERT and is efficiently expressed in PBLs extracted from chickens infected with the MDV-RB1B (oncogenic) and the MDV-Rispens (nononcogenic) serotype 1 strains. Since the vTR sequence is highly homologous (99,4% identity) between those two strains, vTR could be involved in the MDV-induced tumorigenesis, but not as a major determinant. However, it would be interesting to estimate its potential implication in the viral replication and the cell immortalization induced by MDV.
Acknowledgments
We thank Carol W. Greider and Jiunn-Liang Chen for kindly providing the chicken and human telomerase RNA genes and Luis Martin-Rivera for generating the murine telomerase RNA gene constructs. We are also grateful to Evelyne Esnault for invaluable assistance in building the different constructs used in the present study, Aouatef Djeraba Ait-Lounis for the in vivo experiments, and Sylvie Laurent for helpful comments on the manuscript.
REFERENCES
- 1.Afonso, C. L., E. R. Tulman, Z. Lu, L. Zsak, D. L. Rock, and G. F. Kutish. 2001. The genome of turkey herpesvirus. J. Virol. 75:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachand, F., and C. Autexier. 2001. Functional regions of human telomerase reverse transcriptase and human telomerase RNA required for telomerase activity and RNA-protein interactions. Mol. Cell. Biol. 21:1888-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackburn, E. H. 1991. Telomeres. Trends Biochem. Sci. 16:378-381. [DOI] [PubMed] [Google Scholar]
- 4.Blasco, M. A., W. Funk, B. Villeponteau, and C. W. Greider. 1995. Functional characterization and developmental regulation of mouse telomerase RNA. Science 269:1267-1270. [DOI] [PubMed] [Google Scholar]
- 5.Blasco, M. A., H. W. Lee, M. P. Hande, E. Samper, P. M. Lansdorp, R. A. DePinho, and C. W. Greider. 1997. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91:25-34. [DOI] [PubMed] [Google Scholar]
- 6.Cebrian, J., C. Kaschka-Dierich, N. Berthelot, and P. Sheldrick. 1982. Inverted repeat nucleotide sequences in the genomes of Marek disease virus and the herpesvirus of the turkey. Proc. Natl. Acad. Sci. USA 79:555-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, J. L., M. A. Blasco, and C. W. Greider. 2000. Secondary structure of vertebrate telomerase RNA. Cell 100:503-514. [DOI] [PubMed] [Google Scholar]
- 8.Chen, J. L., K. K. Opperman, and C. W. Greider. 2002. A critical stem-loop structure in the CR4-CR5 domain of mammalian telomerase RNA. Nucleic Acids Res. 30:592-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, Z., K. J. Smith, H. G. Skelton III, T. L. Barrett, H. T. Greenway, Jr., and S. C. Lo. 2001. Telomerase activity in Kaposi's sarcoma, squamous cell carcinoma, and basal cell carcinoma. Exp. Biol. Med. 226:753-757. [DOI] [PubMed] [Google Scholar]
- 10.Delecluse, H. J., and W. Hammerschmidt. 1993. Status of Marek's disease virus in established lymphoma cell lines: herpesvirus integration is common. J. Virol. 67:82-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deneke, J., G. Ziegelin, R. Lurz, and E. Lanka. 2002. Phage N15 telomere resolution: target requirements for recognition and processing by the protelomerase. J. Biol. Chem. 277:10410-10419. [DOI] [PubMed] [Google Scholar]
- 12.Djeraba, A., E. Musset, N. Bernardet, Y. Le Vern, and P. Quere. 2002. Similar pattern of iNOS expression, NO production and cytokine response in genetic and vaccination-acquired resistance to Marek's disease. Vet. Immunol. Immunopathol. 85:63-75. [DOI] [PubMed] [Google Scholar]
- 13.Doyle, L. A., and W. E. Highsmith. 2002. Telomerase as a diagnostic and therapeutic target for cancer. Expert Rev. Anticancer Ther. 2:217-225. [DOI] [PubMed] [Google Scholar]
- 14.Gilley, D., and E. H. Blackburn. 1999. The telomerase RNA pseudoknot is critical for the stable assembly of a catalytically active ribonucleoprotein. Proc. Natl. Acad. Sci. USA 96:6621-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greider, C. W., and E. H. Blackburn. 1985. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43:405-413. [DOI] [PubMed] [Google Scholar]
- 16.Greider, C. W., and E. H. Blackburn. 1989. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 337:331-337. [DOI] [PubMed] [Google Scholar]
- 17.Harnack, U., C. Lehmann, E. Matthes, and G. Pecher. 2001. Up-regulation of telomerase activity in herpesvirus saimiri immortalized human T-lymphocytes. Anticancer Res. 21:3969-3972. [PubMed] [Google Scholar]
- 18.Hiyama, E., and K. Hiyama. 2002. Clinical utility of telomerase in cancer. Oncogene 21:643-649. [DOI] [PubMed] [Google Scholar]
- 19.Izumiya, Y., H. K. Jang, M. Ono, and T. Mikami. 2001. A complete genomic DNA sequence of Marek's disease virus type 2, strain HPRS24. Curr. Top. Microbiol. Immunol. 255:191-221. [DOI] [PubMed] [Google Scholar]
- 20.Joubert, P., C. Pautigny, M. F. Madelaine, and D. Rasschaert. 2000. Identification of a new cleavage site of the 3C-like protease of rabbit haemorrhagic disease virus. J. Gen. Virol. 81:481-488. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser, P., S. Hughes, and N. Bumstead. 1999. The chicken 9E3/CEF4 CXC chemokine is the avian orthologue of IL8 and maps to chicken chromosome 4 syntenic with genes flanking the mammalian chemokine cluster. Immunogenetics 49:673-684. [DOI] [PubMed] [Google Scholar]
- 22.Kawaguchi, T., K. Nomura, Y. Hirayama, and T. Kitagawa. 1987. Establishment and characterization of a chicken hepatocellular carcinoma cell line, LMH. Cancer Res. 47:4460-4464. [PubMed] [Google Scholar]
- 23.Kishi, M., G. Bradley, J. Jessip, A. Tanaka, and M. Nonoyama. 1991. Inverted repeat regions of Marek's disease virus DNA possess a structure similar to that of the a sequence of herpes simplex virus DNA and contain host cell telomere sequences. J. Virol. 65:2791-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klapper, W., K. K. Singh, K. Heidorn, R. Parwaresch, and G. Krupp. 1998. Regulation of telomerase activity in quiescent immortalized human cells. Biochim. Biophys. Acta 1442:120-126. [DOI] [PubMed] [Google Scholar]
- 25.Kyo, S., M. Takakura, T. Taira, T. Kanaya, H. Itoh, M. Yutsudo, H. Ariga, and M. Inoue. 2000. Sp1 cooperates with c-Myc to activate transcription of the human telomerase reverse transcriptase gene (hTERT). Nucleic Acids Res. 28:669-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurent, S., E. Esnault, G. Dambrine, A. Goudeau, D. Choudat, and D. Rasschaert. 2001. Detection of avian oncogenic Marek's disease herpesvirus DNA in human sera. J. Gen. Virol. 82:233-240. [DOI] [PubMed] [Google Scholar]
- 27.Lee, L. F., P. Wu, D. Sui, D. Ren, J. Kamil, H. J. Kung, and R. L. Witter. 2000. The complete unique long sequence and the overall genomic organization of the GA strain of Marek's disease virus. Proc. Natl. Acad. Sci. USA 97:6091-6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lingner, J., T. R. Hughes, A. Shevchenko, M. Mann, V. Lundblad, and T. R. Cech. 1997. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science 276:561-567. [DOI] [PubMed] [Google Scholar]
- 29.Liu, J. L., S. F. Lin, L. Xia, P. Brunovskis, D. Li, I. Davidson, L. F. Lee, and H. J. Kung. 1999. MEQ and V-IL8: cellular genes in disguise? Acta Virol. 43:94-101. [PubMed] [Google Scholar]
- 30.Martin-Rivera, L., and M. A. Blasco. 2001. Identification of functional domains and dominant negative mutations in vertebrate telomerase RNA using an in vivo reconstitution system. J. Biol. Chem. 276:5856-5865. [DOI] [PubMed] [Google Scholar]
- 31.Martin-Rivera, L., E. Herrera, J. P. Albar, and M. A. Blasco. 1998. Expression of mouse telomerase catalytic subunit in embryos and adult tissues. Proc. Natl. Acad. Sci. USA 95:10471-10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazzella, O., L. Cauchy, F. Coudert, and J. Richard. 1986. Chicken thymocyte-specific antigens identified by monoclonal antibodies: characterization and distribution in normal tissues and in tumoral tissues from Marek's disease chicken. Hybridoma 5:319-328. [DOI] [PubMed] [Google Scholar]
- 33.Narayanan, A., A. Lukowiak, B. E. Jady, F. Dragon, T. Kiss, R. M. Terns, and M. P. Terns. 1999. Nucleolar localization signals of box H/ACA small nucleolar RNAs. EMBO J. 18:5120-5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh, S. T., S. Kyo, and L. A. Laimins. 2001. Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J. Virol. 75:5559-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okubo, M., Y. Tsurukubo, T. Higaki, T. Kawabe, M. Goto, T. Murase, T. Ide, Y. Furuichi, and M. Sugimoto. 2001. Clonal chromosomal aberrations accompanied by strong telomerase activity in immortalization of human B-lymphoblastoid cell lines transformed by Epstein-Barr virus. Cancer Genet. Cytogenet. 129:30-34. [DOI] [PubMed] [Google Scholar]
- 36.Park, N. H., W. Guo, H. R. Kim, and M. K. Kang. 2001. c-Myc and Sp1/3 are required for transactivation of hamster telomerase catalytic subunit gene promoter. Int. J. Oncol. 19:755-761. [DOI] [PubMed] [Google Scholar]
- 37.Ravin, N. V., T. S. Strakhova, and V. V. Kuprianov. 2001. The protelomerase of the phage-plasmid N15 is responsible for its maintenance in linear form. J. Mol. Biol. 312:899-906. [DOI] [PubMed] [Google Scholar]
- 38.Tsumuki, H., M. Nakazawa, T. Hasunuma, T. Kobata, T. Kato, A. Uchida, and K. Nishioka. 2001. Infection of synoviocytes with HTLV-I induces telomerase activity. Rheumatol. Int. 20:175-179. [DOI] [PubMed] [Google Scholar]
- 39.Tulman, E. R., C. L. Afonso, Z. Lu, L. Zsak, D. L. Rock, and G. F. Kutish. 2000. The genome of a very virulent Marek's disease virus. J. Virol. 74:7980-7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu, K. J., C. Grandori, M. Amacker, N. Simon-Vermot, A. Polack, J. Lingner, and R. Dalla-Favera. 1999. Direct activation of TERT transcription by c-MYC. Nat. Genet. 21:220-224. [DOI] [PubMed] [Google Scholar]
- 41.Xiang, H., J. Wang, Y. W. Mao, and D. W. Li. 2000. hTERT can function with rabbit telomerase RNA: regulation of gene expression and attenuation of apoptosis. Biochem. Biophys. Res. Commun. 278:503-510. [DOI] [PubMed] [Google Scholar]
- 42.Yu, G. L., J. D. Bradley, L. D. Attardi, and E. H. Blackburn. 1990. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature 344:126-132. [DOI] [PubMed] [Google Scholar]
- 43.Zhao, J. Q., S. F. Hoare, R. McFarlane, S. Muir, E. K. Parkinson, D. M. Black, and W. N. Keith. 1998. Cloning and characterization of human and mouse telomerase RNA gene promoter sequences. Oncogene 16:1345-1350. [DOI] [PubMed] [Google Scholar]