Abstract
Ribulose 1,5 bisphosphate carboxylase/oxygenase (RubisCO) catalyzes the biological reduction and assimilation of carbon dioxide gas to organic carbon; it is the key enzyme responsible for the bulk of organic matter found on earth. Until recently it was believed that there are only two forms of RubisCO, form I and form II. However, the recent completion of several genome-sequencing projects uncovered open reading frames resembling RubisCO in the third domain of life, the archaea. Previous work and homology comparisons suggest that these enzymes represent a third form of RubisCO, form III. While earlier work indicated that two structurally distinct recombinant archaeal RubisCO proteins catalyzed bona fide RubisCO reactions, it was not established that the rbcL genes of anaerobic archaea can be transcribed and translated to an active enzyme in the native organisms. In this report, it is shown not only that Methanococcus jannaschii, Archaeoglobus fulgidus, Methanosarcina acetivorans, and Methanosarcina barkeri possess open reading frames with the residues required for catalysis but also that the RubisCO protein from these archaea accumulates in an active form under normal growth conditions. In addition, the form III RubisCO gene (rbcL) from M. acetivorans was shown to complement RubisCO deletion strains of Rhodobacter capsulatus and Rhodobacter sphaeroides under both photoheterotrophic and photoautotrophic growth conditions. These studies thus indicate for the first time that archaeal form III RubisCO functions in a physiologically significant fashion to fix CO2. Furthermore, recombinant M. jannaschii, M. acetivorans, and A. fulgidus RubisCO possess unique properties with respect to quaternary structure, temperature optima, and activity in the presence of molecular oxygen compared to the previously described Thermococcus kodakaraensis and halophile proteins.
In terrestrial and marine environments the major pathway of CO2 fixation is the Calvin-Benson-Bassham (CBB) reductive pentose phosphate pathway (30). Phosphoribulokinase (PRK) and ribulose 1, 5 bisphosphate (RuBP) carboxylase-oxygenase (RubisCO) are the two enzymes unique to this reductive pathway, which is found in plants, algae, and several phototrophic and chemoautotrophic bacteria. For RubisCO, there are classically two structurally distinct forms described: form I, which is composed of both large (catalytic) and small subunits in a hexadecameric structure, and form II, composed exclusively of multiples of large subunits that show only 25 to 30% identity to form I large subunits. Until recently, members of the third recognized kingdom of life, the archaea, were thought not to contain the CBB pathway enzymes. However, RubisCO and PRK activity was detected in crude extracts of extreme halophiles (1, 24, 25) and several experiments indicated that the halophile RubisCO resembled the form I protein found in higher plants (24).
More recently, the completion of several genome projects offered further indications that euryarchaea contain putative RubisCO genes that encode proteins, distinguished from the aforementioned form I and form II enzymes. These proteins are different enough from form I and form II RubisCO that they have been classified as a separate type or form III enzyme (30, 34, 35). The first two archaeal sequencing projects showed RubisCO homologues in the genomes of Methanococcus jannaschii and Archaeoglobus fulgidus (2, 14). M. jannaschii is an obligately autotrophic methanogen, and A. fulgidus is a sulfate-reducing organism (12, 39). Both organisms are obligate anaerobes growing at thermophilic temperatures and are clearly different from typical organisms known to fix carbon dioxide by the CBB pathway. Indeed, there are good indications that the route for primary CO2 fixation in these organisms involves a modified acetyl-coenzyme A pathway, with no indications of CBB-dependent CO2 fixation (28, 39). In addition to these organisms, genomic sequencing projects indicate that Pyrococcus, renamed Thermococcus (6, 13, 16), also contains form III enzymes, with the Thermococcus enzyme forming an interesting and unique quaternary structure (13).
The existence of RubisCO proteins in archaea is not limited to thermophilic organisms. The completed genomes of the Methanosarcina acetivorans (9), Methanosarcina mazei (4), and Methanosarcina barkeri (http://www.jgi.doe.gov/JGI_microbial/html/methanosarcina/methano_homepage.html) mesophilic heterotrophic methanogens were also found to contain genes encoding putative RubisCO proteins. Regardless of growth temperature or nutritional requirements, these archaeal genome projects uncovered for the first time RubisCO genes in organisms that evolved in the absence of molecular oxygen. This discovery could have significant impact on understanding the principles behind CO2/O2 discrimination by RubisCO, which is a fundamental issue in RubisCO enzymology. Certainly, it now appears that examples of RubisCO sequences are widespread in the third domain of life, yet it is also true that Methanobacterium thermoautotrophicum (26) and Methanococcus maripaludis(http://www.genome.washington.edu/UWGC/methanococus/) do not contain recognizable RubisCO sequences.
The expression of archaeal RubisCO genes from extreme thermophiles in Escherichia coli, and the subsequent purification of the resultant recombinant protein, has shown that the archaeal protein catalyzes bona fide RubisCO activity (35). The ability to catalyze the reaction at extreme temperatures is an indication of a unique structural adaptation which could provide insights into general structure-function relationships for all RubisCO molecules. Unlike the reported properties of the Thermococcus kodakaraensis KOD1 enzyme (6, 16), the properties of all of the RubisCO proteins described in this paper include sensitivity to oxygen, suggesting that the archaeal RubisCO form III enzymes represent an extremely diverse group with respect to catalytic function (30). Although the archaeal enzymes have been shown to catalyze the bona fide RubisCO reaction, there is neither convincing biochemical evidence that archaea use the CBB pathway for CO2 fixation nor any evidence from genome sequences, other than the earlier report of PRK activity in extracts of extreme halophiles (1), for the existence of a gene encoding PRK. There has also been no suggestion or evidence to indicate that there are other means to produce RuBP, the substrate for RubisCO. Thus, the physiological role of RubisCO in archaea remains to be determined. As a first step in approaching this conundrum, it was deemed important to demonstrate that the archaeal enzymes are synthesized and active in the native organism. The work reported here shows that extracts from four different archaeal organisms unequivocally catalyze RuBP-dependent CO2 fixation. Western blot analyses showed that the RubisCO gene in M. jannaschii is expressed under normal growth conditions, and radiometric assays demonstrated that the RubisCO molecules were assembled in a catalytically active state in all four organisms. Furthermore, the mesophilic RubisCO gene from M. acetivorans afforded the opportunity to complement RubisCO deletion strains of the photosynthetic bacteria Rhodobacter capsulatus and Rhodobacter sphaeroides. The complementation data, together with the in vitro demonstration of RubisCO activity, provides a strong argument that the form III RubisCO proteins have the ability to catalyze a physiologically significant RuBP-dependent CO2 fixation reaction.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Plasmids used in this study are shown in Table 1. All cloning steps were performed in E. coli strain JM109 (37) prior to transformation into expression strain E. coli BL-21 or BL-21 Codon Plus (Stratagene, La Jolla, Calif.). E. coli cultures were grown in Luria-Bertani (LB) medium containing (per liter of distilled water) 10 g of tryptone-5 g of yeast extract-10 g of NaCl (29). Triparental matings were accomplished using E. coli MM294 containing pRK2013 (8).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strain | ||
| E. coli | ||
| JM109 | 37 | |
| BL-21(DE3) | Expression strain carrying IPTG-inducible T7 RNA polymerase gene | 29 |
| BL-21 Codon Plus | ||
| DE-3RIL | Expression strain carrying IPTG-inducible T7 RNA polymerase gene with additional plasmid containing argU, ileY, and leuW tRNA genes | 29 |
| MM294 | Containing pRK2013 | 8 |
| M. jannaschii | DSM2661 | 2 |
| A. fulgidus | DSM4304 | 14 |
| M. acetivorans | ||
| C2A | Wild type | 9 |
| 4555 | C2A rbcL deletion | W. Metcalf (personal communication) |
| R. sphaeroides | ||
| HR | Wild type; Smr | 36 |
| 16 | cbbLS cbbM | 7 |
| R. capsulatus | ||
| SB1003 | Wild type | 38 |
| SB I-II− | cbbLS cbbM | 22 |
| Plasmid | ||
| pET11A | Apr; E. coli expression vector | 5 |
| pET11AMj | Apr; pET11A with NdeI BamHI Mj 1235 fragment | This study |
| pET11AAf | Apr; pET11A with NdeI BamHI Af 1638 fragment | This study |
| pET11D | Apr; E. coli expression vector | 5 |
| pET11DMa | Apr; pET11D with AflIII BglII Ma 4555 fragment | This study |
| pRPSMCS3 | Tcr | S. Smith (personal communication) |
| pRPSMCS3MA | Tcr; pRPSMCS3 with SpeI XbaI pET11DMa fragment | This study |
| pRK2013 | Knr; conjugative helper plasmid | 8 |
The RubisCO deletion strains SBI-II− of R. capsulatus (22) and 16 of R. sphaeroides (7) were used in the complementation studies. Rhodobacter cultures used in matings were routinely maintained in SOC medium (36). For complementation experiments, photoheterotrophic growth was achieved in Ormerod's minimal medium (20) supplemented with 0.4% dl-malate in bottles that were continually gassed with argon. Photoautotrophic cultures of R. capsulatus were grown in sealed serum vials containing 20% CO2 balanced with hydrogen. The premixed gas was exchanged every 24 h.
Strict anaerobic procedures were used in the preparation of medium for M. jannaschii, A. fulgidus, M. barkeri, and M. acetivorans. All gases were passed through heated copper filings to remove trace amounts of oxygen. A. fulgidus was grown in lactate-sulfate medium with a gas phase of 80% N2-20% CO2 (39). The organism was grown in crimped tubes or 160-ml serum vials that were pressurized to 30 lb/in2. Growth took place in a nonshaking heating oven set to a constant temperature of 85°C. M. jannaschii was grown in serum vials with either the bicarbonate medium (12) or MES (morpholineethanesulfonic acid)-buffered medium (19) in an atmosphere of 80% H2-20% CO2 at 30 lb/in2 at 85 or 65°C. M. acetivorans was grown in HS medium supplemented with either methanol or methanol-acetate in sealed crimped tubes (27) with an atmosphere of 80% H2-20% CO2 at 10 lb/in2 at 37°C without shaking. M. barkeri cells (grown on either methanol or trimethylamines) were a kind gift from J. A. Krzycki.
Cultures were harvested anaerobically in mid to late logarithmic growth by centrifugation. The cells were washed in their respective media to prevent osmotic lysis and centrifuged again at 16,000 × g for 30 min at 4°C. Cell pellets were stored at −70°C in sealed centrifuge bottles.
DNA manipulations and genetic techniques.
To construct an M. jannaschii RubisCO expression plasmid without the previously employed N-terminal His tag (35), primers were designed with an NdeI site at the N terminus (5′GGAATTCCATATGGACTACATAAACTTAAACTACA3′) and a TAA stop codon at the C terminus (5′TTATTTCCAATACTCTAAAGCC3′). Using PFU turbo polymerase (Stratagene) and these primers, the M. jannaschii RubisCO gene (Mj 1235) was amplified from pAMJEV50 (The Institute for Genomic Research, Rockville, Md.). The 1.4-kb PCR fragment was cloned into the NdeI and SmaI sites of pK19. The gene was fully sequenced to ensure that no mutations were introduced during the PCR. The M. jannaschii RubisCO gene was subsequently subcloned into the NdeI and BamHI sites of expression plasmid pET11A (Stratagene).
The A. fulgidus RubisCO gene (Af 1638) was cloned directly from genomic DNA. PFU polymerase (NdeI [5′GGAATTCCATATGGCGGAGTTTGAGATTTACAGA3′] and BamHI [5′ATTTTAGATTGGCGTAACCCTG3′]) and primers designed with an NdeI restriction site at the N terminus and a BamHI site at the C terminus were used to amplify the rbcL-2 gene from A. fulgidus genomic DNA. The gene was ligated into pCR Script (Stratagene) and sequenced for PCR-incorporated mutations. Using the NdeI and BamHI sites in that vector, the gene was subcloned into pET11A.
Using UniPol DNA polymerase (PGC Scientific, Frederick, Md.) with primers that contained an AflIII site at the 5′ end and a BglII site at the 3′ end (AflII primer, 5′ACATGTCCAGGTACTTCCTCCAGCCAG and BglII primer, 5′AGATCTGTGCAGCCGCTCATTATGCAG), the putative RubisCO gene from M. acetivorans (Ma 4555) was amplified from genomic DNA. The amplified gene was ligated into pCR Topo 2.1 and fully sequenced. Using the AflIII and BglII sites, the 1.4-kb fragment was directionally cloned into pET11D (Stratagene). A 200-bp deletion at the N-terminal end of the M. acetivorans rbcL gene was used to inactivate the rbcL gene in its host as described by William Metcalf (personal communication) (18); the resultant double-recombinant knockout strain of M. acetivorans was used to test for any possible growth phenotype.
Using XbaI and SpeI restriction sites for expression in Rhodobacter, the putative rbcL gene from M. acetivorans was further subcloned from pCR Topo 2.1 (Invitrogen, Carlsbad, Calif.) into pRPSMCS3, a broad-host-range expression vector (S. A. Smith and F. R. Tabita, unpublished studies). Using helper plasmid pRK2013 for complementation of mutant strains, the pRPSMCS3-rbcL construct (pRPSMCS3MA) was conjugated into R. capsulatus strain SBI-II and R. sphaeroides strain 16 by triparental matings as previously described (8).
Cell lysis procedures for preparing crude extracts from archaeal cells.
Cells were normally lysed in the anaerobic chamber by a combination of osmotic shock and disruption using glass beads, except for M. barkeri cells, which were lysed solely by osmotic shock. Cell pellets were resuspended in oxygen-free 50 mM Tris-Cl (pH 7.2)-1 mM EDTA-10 mM MgCl2-10 mM sodium bicarbonate-10 mM β-mercaptoethanol (TEMMB buffer) with DNase and acid-washed 100-micron glass beads and lysed for approximately 2 min in a Mini-Bead Beater (Biospec Products, Bartlesville, Okla.) set at 4,800 rpm. The cell lysate was centrifuged at 13,500 × g inside the chamber for 20 min. The supernatant was removed, and the contents were sealed inside a 2-ml serum vial under argon.
Cell extracts were desalted using 2.5-ml HiTrap desalting columns (Amersham Pharmacia, Piscataway, N.J.). Column fractions of 1 ml were taken, and protein was detected by continuous flow measurements at an absorbance of 280 nm. Fractions containing protein were pooled and aliquoted into 1-ml serum vials under argon and either immediately used or frozen in an ethanol dry-ice bath and stored at −70°C.
Synthesis and purification of recombinant RubisCO.
The A. fulgidus rbcL2 (isolated by J.-P. Yu; unpublished results of this laboratory) and M. acetivorans rbcL genes were expressed in E. coli BL-21 (Stratagene), whereas recombinant M. jannaschii RubisCO was obtained from strain BL-21 Codon Plus. E. coli cultures expressing the A. fulgidus and M. acetivorans genes were grown in 2-liter flasks containing 1 liter of LB medium, with continuous shaking at 120 rpm at 37°C. When the cells reached an optical density at 660 nm of 0.5 to 0.6, the cells were shifted to a temperature of 42°C for 30 min. This artificial heat shock induces the E. coli chaperone system, which increases the amount of soluble, active recombinant protein. The culture was then shifted to 25°C, and isopropyl β-d-galactopyranoside (IPTG) was added to a final concentration of 0.1 mM. Induction was continued overnight (15 h) at 25°C.
M. jannaschii recombinant RubisCO protein was produced in six 10-liter batch cultures of LB medium (Ohio State University Fermentation Facility). The airflow into the fermenter was 2.0 liters of air/min, with constant stirring of 120 rpm. Oxygen probe measurements proved that this airflow was adequate to maintain an oxygen-limited environment in the fermenter. Cultures were grown at 37°C until an optical density at 660 nm of 0.5 to 0.6 was reached and then were shifted to 42°C for 30 min. After cooling to 25°C, the culture was induced overnight as previously described. Following the addition of IPTG, the stirring rate was reduced to 80 rpm overnight. Cells were harvested aerobically by centrifugation and stored at −70°C.
The purification protocols prior to column chromatography were nearly identical for the two recombinant thermophilic RubisCO proteins from A. fulgidus and M. jannaschii. All manipulations were performed with buffers sparged thoroughly with argon gas. The cell pellets were resuspended in an anaerobic chamber in TEMMB buffer supplemented with DNase. The cells were disrupted in a French pressure cell (102,000 kPa), with the crude extract flowing into a sealed vial flushed with argon gas. The extracts were decanted into centrifuge tubes inside an anaerobic chamber. All centrifuge bottles contained a screw cap with an O-ring seal to assure proper sealing outside of the chamber. The cells were centrifuged at 16,000 × g and 4°C for 10 min. The supernatant was decanted inside an anaerobic chamber into new serum vials, which were subsequently pressurized to 10 lb/in2 with argon gas. The vials were placed into an 85°C water bath for 20 min with gentle shaking. After 20 min the extract was immediately placed on ice for 60 min. The extract was taken back into the chamber, decanted into anaerobic equilibrated centrifuge bottles, and centrifuged at 30,000 × g and 4°C for 30 min. Extracts were decanted into clean serum vials and frozen in an ethanol dry-ice bath and stored overnight at −70°C in preparation for column chromatography.
For purification of the M. acetivorans recombinant protein, the procedure was the same except that the heat step was altered for this mesophilic protein prior to column chromatography. After the first centrifugation, the MgCl2 concentration was increased to 50 mM with an anaerobic stock of 1.0 M MgCl2; the extract was heated to 50°C for 10 min and then transferred to ice for 1 h. Precipitated protein was removed by centrifugation.
Column chromatography was performed inside a chamber with a Bio-Rad BioLogic Workstation purification apparatus. Column fractions were collected in 1.0-ml volumes and assayed using the standard anaerobic RubisCO assay. The purification of the M. jannaschii RubisCO employed G-25 Superfine, DEAE FF, QSHP, and Superose 12 column chromatography. Purification of the recombinant A. fulgidus enzyme was similar to that of M. jannaschii RubisCO except that the G-25 Superfine and the DEAE FF columns were not used. The M. acetivorans RubisCO protein purification followed the same column order as the M. jannaschii enzyme, with the omission of G25 Superfine chromatography. Gel filtration standards were purchased from Bio-Rad (Hercules, Calif.) (catalogue number 151-1901). These standards were used to calibrate the 110-ml Superfine Gel Filtration column used in the above purification protocols. Purified enzymes (Fig. 1) were used to generate antibodies; the purified proteins were also utilized in some of the reported enzyme assays.
FIG. 1.
Purity of protein fractions eluted from Superose 12 gel filtration columns of recombinant RubisCO preparations from M. jannaschii (A), A. fulgidus (B), and M. acetivorans (C). Protein standards are shown in the leftmost lanes of panels A and B. SDS-PAGE was performed as described in Materials and Methods. Purified proteins were used to prepare antibodies of each enzyme as described in Materials and Methods.
Radiometric RubisCO assay.
The radiometric RubisCO assay was modified from the standard assay (31) to create the anaerobic conditions necessary for full catalytic activity. Buffers and substrates were bubbled with argon gas prior to all reactions. The assay buffer for A. fulgidus and M. jannaschii RubisCO molecules contained 80 mM N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES)-KOH, pH 7.2; for the M. acetivorans RubisCO molecules, the assay buffer contained 80 mM N,N-bis(2-hydroxyethyl)glycine (Bicine)-KOH, pH 7.5. Sealed serum vials pressurized to 10 lb/in2 were incubated in a dry heating block (Reacti-Therm IIITM heating/stirring module; Pierce, Rockford, Ill.) placed inside a chemical fume hood. Using gas-tight Hamilton syringes with beveled needles, samples were injected into the assay. Assays for A. fulgidus and M. jannaschii RubisCO were performed at 85°C. The assay temperature for the M. acetivorans RubisCO was 37°C. All assays contained a final concentration of 2 μCi H14CO3−. Assays were stopped by the injection of aerobic propionic acid. Vials were opened to the aerobic environment and allowed to dry overnight at 85°C. Samples were resuspended in 200 μl of 1.0 N HCl and placed into scintillation cocktail. Radiolabeled 14C was detected using a Beckman scintillation counter. Aerobic controls were conducted in an identical manner except that the serum vials were never sealed, allowing ambient air to freely exchange in the reaction during the assay. The transition state analog 2-carboxyarabinitol-1,5-bisphosphate (CABP) was used to verify the authenticity of all RuBP-dependent CO2 fixation reactions with crude extracts, as previously described (35).
Using BSA as a standard, protein concentrations were determined by the Lowry method (17).
Western immunoblot analysis using polyclonal antibodies to archaeal RubisCO proteins.
The purity of each respective RubisCO protein was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (15) (Fig. 1). Antiserum directed against purified M. jannaschii RubisCO was prepared in rabbits by Cocalico Biologicals, Inc. (Reamstown, Pa.), and Western immunoblotting was used to test the specificity of the antiserum. Using a Transblot semidry transfer cell (Bio-Rad), proteins resolved by SDS-PAGE (15) were transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, Mass.) according to directions supplied by the manufacturer. Using antibodies directed against the archaeal RubisCO used, washes and incubations with antibodies were carried out at a dilution of 1:1,000 as described previously (32). Immunoblots were developed with Attophos detection reagent according to the manufacturer's instructions (Amersham, Buckinghamshire, England) and visualized with a Storm 840 imaging system (Molecular Dynamics, Sunnyvale, Calif.).
RESULTS
Activity in crude extracts.
Although the overall homology between representative enzymes from the different groups of RubisCO is low (Table 2), key residues known to be important in catalysis (3) are conserved between typical form I and form II proteins and the four (form III) archaeal proteins used in this study (Fig. 2). The demonstrable activity of recombinant archaeal RubisCO supports the notion that these are bona fide RubisCO enzymes (30). It was thus of interest to determine whether the form III RubisCO gene is actually translated and assembled into an active protein in archaea; in addition, it would be useful to determine whether there are growth conditions that favor maximum accumulation of the enzyme. Radiometric RubisCO assays of crude extracts prepared from M. jannaschii, M. acetivorans, M. barkeri, and A. fulgidus grown under optimized conditions demonstrated that there was catalytically active RubisCO present in all cases (Table 3). For comparison, RubisCO activity from R. capsulatus (grown chemoheterotrophically) was included to illustrate that the activity levels obtained for M. jannaschii and A. fulgidus extracts approximated what one typically obtains when synthesis is repressed in nonsulfur purple bacteria (22). There was no significant activity in the presence of the specific inhibitor CABP, a known transition state analogue for RubisCO catalysis (23, 35) (data not shown).
TABLE 2.
Percent identity/percent similarity for several representative I, II, and III RubisCO proteins
| Protein form and source strain | % Identity/% similarity for proteins of form (source strain):
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IA (R. capsulatus) | IB (Synechococcus sp. strain PCC6301) | IB (Spinacia oleracea) | IC (R. sphaeroides) | ID (Cylindrotheca sp. strain NI) | II (Rhodospirillum rubrum) | II (R. sphaeroides) | III (M. jannaschii) | III (A. fulgidus II) | III (M. acetivorans) | III (M. barkeri) | III (T. kodakaraensis) | |
| IA | ||||||||||||
| R. capsulatus | 67/80 | 66/78 | 53/67 | 50/66 | 32/45 | 30/45 | 41/57 | 33/49 | 35/49 | 34/49 | 34/49 | |
| IB | ||||||||||||
| Synechococcus sp. strain PCC6301 | 79/88 | 55/67 | 54/67 | 34/46 | 30/45 | 39/54 | 37/52 | 38/53 | 38/54 | 38/51 | ||
| Spinacia oleracea | 56/68 | 52/67 | 31/43 | 30/44 | 38/52 | 35/50 | 35/50 | 34/48 | 35/49 | |||
| IC | ||||||||||||
| R. sphaeroides | 67/79 | 33/46 | 28/42 | 36/52 | 36/52 | 36/50 | 36/51 | 35/51 | ||||
| ID | ||||||||||||
| Cylindrotheca sp. strain NI | 31/46 | 29/42 | 34/52 | 34/52 | 33/49 | 33/50 | 34/51 | |||||
| II | ||||||||||||
| Rhodospirillum rubrum | 76/85 | 31/47 | 37/52 | 38/53 | 38/54 | 31/46 | ||||||
| R. sphaeroides | 32/48 | 31/47 | 30/47 | 30/46 | 33/48 | |||||||
| III | ||||||||||||
| M. jannaschii | 44/65 | 48/67 | 49/67 | 44/64 | ||||||||
| A. fulgidus II | 46/65 | 44/66 | 72/82 | |||||||||
| M. acetivorans | 86/94 | 47/64 | ||||||||||
| M. barkeri | 44/63 | |||||||||||
| T. kodakaraensis | ||||||||||||
FIG. 2.
Sequence alignment showing that the key catalytic residues important for RuBP-dependent CO2 fixation are maintained in the archaeal RubisCO proteins used in this study. The numbers represents the amino acid residues corresponding to the Synechococcus PCC 6301 form I protein. Residues known to be important for either catalysis (C) or RuBP binding (R) are indicated. I-Syn 6301, Synechococcus PCC 6301 form I; II-Rr, R. rubrum form II; III-Mj, Methanocaldococcus (Methanococcus) jannaschii form III; III-Af RbcL-2, A. fulgidus RbcL-2 form III; III-Ma, M. acetivorans form III; III-Mb, M. barkeri form III; III-Mm, M. mazei form III; III-Ph, Pyrococcus horikoshii form III; III-Tk, T. (Pyrococcus) kodakaraensis form III.
TABLE 3.
RubisCO levels of crude extracts prepared from archaeaa
| Organism | Medium/bufferb | Specific activity (nmol/min/mg) | Specific activity of purified protein (nmol/min/mg)c |
|---|---|---|---|
| M. jannaschii | Defined/bicarbonate | 10 | 6,500 |
| M. jannaschii | Undefined/bicarbonate (yeast extract added) | 12 | 6,500 |
| A. fulgidus | Defined/bicarbonate | 15 | 26,000d |
| M. acetivorans | HS medium with methanol or methanol-acetate/phosphate | 0.2 | 400 |
| M. barkeri | Trimethylamine or methanol/phosphate | 0.1 | NDf |
| R. capsulatus | (Aerobic) malate/phosphate | 10 | 4,000e |
Assays of extracts from the thermophiles were performed at 85°C; all the other extracts were assayed at 30°C. All crude extracts, substrates, and buffers were kept anaerobic in sealed vials under a headspace of argon.
Growth media (defined and undefined, described in Materials and Methods) were buffered as indicated.
Values represent results for purified recombinant protein synthesized in E. coli.
Unpublished results (J.-D. Sears and F. R. Tabita).
Horken and Tabita (11).
ND, not determined.
It is not clear why M. acetivorans or M. barkeri RubisCO activity is so low in crude extracts, but further optimization of the assay may be in order, as the purified M. acetivorans recombinant protein also had much lower activity than its counterpart from M. jannaschii and A. fulgidus (Table 3). The specific activities for the mesophilic archaea are low but significant, because no significant radiolabel incorporation took place when assays were performed in the presence of CABP. Another potential explanation for the possible low activity of these mesophilic form III RubisCO proteins is the conserved change (of threonine to serine at position 62) that is specific to these proteins alone among all of the bonafide RubisCO proteins, including all known form I and form II proteins (Fig. 2). Threonine 62 was previously shown to be essential for RuBP binding of form I and II RubisCO (3).
The presence of RubisCO in archaeal extracts was further investigated using antibodies directed against recombinant M. jannaschii RubisCO. The Western immunoblotting results (Fig. 3) clearly indicated that significant levels of M. jannaschii RubisCO protein were produced under normal growth conditions and optimal growth temperatures. It is interesting that the original whole-cell 14CO2 label incorporation studies performed with M. jannaschii did not indicate that [14C]phosphoglyceric acid was formed (indicative of RubisCO activity); however, these studies were performed at 65°C (28). Thus, growth experiments with M. jannaschii were repeated at the optimal growth temperature of 83°C and at 65°C, the same temperature at which the original label-incorporation studies had been performed. The Western blotting results (Fig. 3) demonstrated that significant amounts of RubisCO protein were produced by M. jannaschii at both temperatures.
FIG. 3.
Western immunoblot showing RubisCO protein accumulation in M. jannaschii with respect to growth temperature. The antiserum used in Western immunoblotting was generated against M. jannaschii recombinant RubisCO protein purified from E. coli. (A) Purified recombinant M. jannaschii RubisCO (3 μg) from E. coli grown at 37°C; (B) crude extract (49 μg) prepared from M. jannaschii grown at 65°C; (C) crude extract (49 μg) prepared from M. jannaschii grown at 85°C.
Gel filtration of crude extracts indicated that the enzyme had a native molecular mass of 95 kDa, suggesting the presence of a homodimer structure (data not shown). Additional gel filtration studies performed on calibrated Superose 12 columns indicated that purified recombinant M. jannaschii, A. fulgidus, and M. acetivorans RubisCO proteins were dimers (data not shown).
Antibodies to recombinant M. acetivorans RubisCO verified that the mutant strain did not synthesize the protein (data not shown).
Recombinant archaeal RubisCO.
The rbcL genes of M. jannaschii, A. fulgidus, and M. acetivorans were each expressed in E. coli, and recombinant proteins were purified to homogeneity (Fig. 1). In all three cases, the proteins were found to be homodimers of large subunits, in keeping with the nature of the enzyme synthesized by M. jannaschii itself and in agreement with previous results with the M. jannaschii recombinant protein (35). The activity of purified recombinant M. jannaschii and A. fulgidus RubisCO was examined with respect to assay temperature. Recombinant M. jannaschii RubisCO did not exhibit detectable activity at room temperature, and incorporation of 14CO2 into acid-stable labeled product was barely detectable at 65°C compared to that seen at the optimal temperature of 85°C (Fig. 4A). In addition, there was a notable lag time of about 5 min before significant activity ensued and became linear with time. The narrow temperature range for catalytic activity for the M. jannaschii enzyme contrasted to that seen with A. fulgidus RubisCO (Fig. 4B), as this protein catalyzed activity over a very broad temperature range (12). It was also apparent that the A. fulgidus enzyme did not exhibit a time-dependent lag in activity.
FIG. 4.
Activity time course for purified recombinant M. jannaschii RubisCO assayed at 65°C (♦) and 85°C (▪) (A) and A. fulgidus RubisCO assayed at 90°C (▴), 65°C (▪), and 37°C (♦) (B). Each assay contained 5 μg of protein and was performed under anaerobic conditions; no activity was detected at 37°C for the M. jannaschii enzyme.
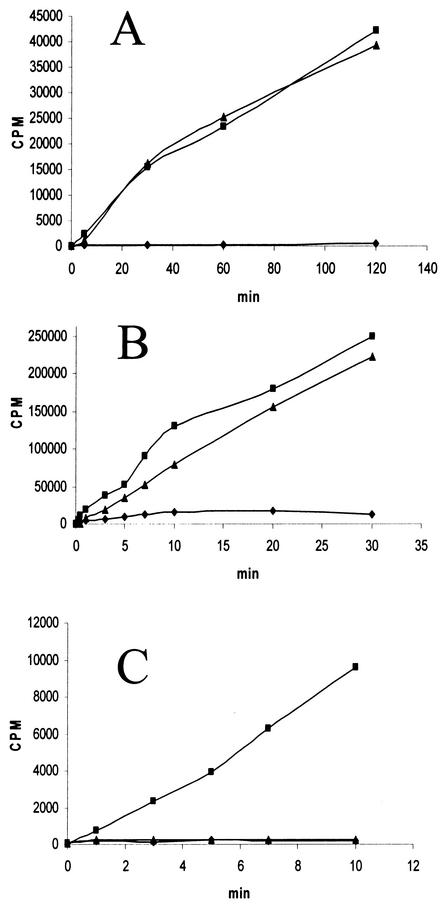
RubisCO from M. jannaschii, A. fulgidus, and M. acetivorans showed interesting responses to the presence of molecular oxygen. Reversible inhibition of RubisCO activity was observed when the M. jannaschii and A. fulgidus proteins were exposed to oxygen (Fig. 5A and B); surprisingly, the activity of the M. acetivorans enzyme did not recover after 45 min of exposure (Fig. 5C). The A. fulgidus protein did catalyze significant RuBP-dependent CO2 fixation in an aerobic environment, albeit at a lower rate than under anaerobic conditions (i.e., about 15% of the anaerobic rate).
FIG. 5.
Exposure of recombinant M. jannaschii, A. fulgidus, and M. acetivorans RubisCO to oxygen (2.5 μg of protein in all cases). M. jannaschii (A) and A. fulgidus (B) RubisCO were assayed at 83°C, while M. acetivorans RubisCO (C) was assayed at 37°C. The aerobic assay (♦) was performed in vials in the presence of ambient air, whereas the anaerobic assay (▪) was carried out under an argon atmosphere. The recovered assay (▴) represents an assay in which a continuous stream of oxygen was allowed to permeate the assay vial headspace for 45 min; the oxygen-exposed enzyme was then injected into an anaerobic vial and assayed for the indicated times.
Despite sharing a similar quaternary structure with the form II protein from Rhodospirillum rubrum (16), the form III proteins employed in this investigation, which possess low primary structure homology, exhibit vastly different catalytic properties. Furthermore, the form III RubisCO proteins from the obligate anaerobes M. jannaschii, A. fulgidus, and M. acetivorans show different degrees of oxygen sensitivity (Fig. 5), unlike the form III Thermococcus RubisCO, which can apparently fully catalyze the reaction in the presence of molecular oxygen (16). The present results (Fig. 5) indicate that it would be fruitful to further investigate the active site of form III RubisCO proteins.
Complementation of bacterial RubisCO deletion mutants with the M. acetivorans rbcL gene.
RubisCO deletion mutants of R. capsulatus and R sphaeroides cannot grow under photoheterotrophic or photoautotrophic conditions. Initial attempts to complement the RubisCO deletion mutants of R. capsulatus (strain SBI-II−) and R. sphaeroides (strain 16) with the M. jannaschii rbcL gene failed (data not shown). Lack of complementation was most likely due to the fact that while R. sphaeroides and R. capsulatus grow at 30°C, the M. jannaschii RubisCO shows little or no activity at temperatures at or below 65°C (Fig. 4). However, RubisCO from the mesophile M. acetivorans was shown to be active at 25 to 40°C (results not shown). Thus, expression of the M. acetivorans rbcL gene in Rhodobacter RubisCO knockout strains afforded an opportunity to test whether the archaeal enzyme can function in a physiological background where it would be required to support growth. This was deemed the ultimate test of the functionality of archaeal RubisCO. It was shown that expression of the M. acetivorans rbcL gene in R. capsulatus strain SBI-II− complemented growth under anaerobic photoheterotrophic conditions with cultures bubbled with argon gas (Fig. 6A). No special precautions were taken to eliminate or scrub vestiges of oxygen from the gas atmosphere in these growth experiments, and it appeared that photoheterotrophic cultures of complemented R. capsulatus strain SBI-II− took some time to even approach the growth rate of wild-type R. sphaeroides under these conditions.
FIG. 6.
Complementation and growth of R. capsulatus cbbLS/cbbM knockout strain (form I [cbbLS] and II [cbbM] RubisCO genes deleted) SBI-II− (22) at the expense of the M. acetivorans RubisCO (rbcL) gene in plasmid pRPSMCS3MA. (A) Using malate as the carbon source, photoheterotrophic cultures were grown in glass bottles continuously bubbled with argon (without scrubbing the gas through a heated-copper-filings system) to remove trace amounts of oxygen. ▪, R. capsulatus strain SBI-II−; ♦, R. capsulatus strain SBI-II− containing plasmid pRPSMCS3MA; ▴, wild-type R. sphaeroides strain HR containing plasmid pRPSMCS3MA. (B) Photoautotrophic growth of R. capsulatus knockout strain SBI-II− (cbbLS/cbbM) (▪) along with wild-type R. sphaeroides HR (♦) and R. sphaeroides knockout strain 16 (cbbLS/cbbM) (▴), all containing plasmid pRPSMCS3MA. The cells were grown in sealed and crimped tubes pressurized with oxygen-free 20% CO2 balanced with H2. The gas was exchanged every 24 h.
Since RubisCO knockout strains of Rhodobacter do eventually develop adaptive mutations over long time intervals that enable photoheterotrophic growth in the absence of the CBB pathway (33), we determined whether M. acetivorans rbcL expression allows photoautotrophic growth of Rhodobacter RubisCO knockout strains. The CBB pathway and functional RubisCO are absolutely required when these organisms are grown photoautotrophically in an H2/CO2 environment (21, 30). It was shown that complementation to photoautotrophic growth depended on strict anaerobiosis, and sealed and crimped tubes were used in these experiments. When sealed vessels and a gas phase of 20% CO2 balanced with hydrogen were used, M. acetivorans RubisCO supported growth of both R. capsulatus and R. sphaeroides RubisCO knockout strains under photoautotrophic conditions only under conditions of rigorous anaerobiosis (Fig. 6B); by contrast, there was little or no photoautotrophic growth when complemented strains were continuously bubbled with unscrubbed mixtures of H2 and CO2, as is typically done for wild-type Rhodobacter cultures (22). This is analogous to the difficulty of achieving optimum photoheterotrophic growth by complemented strains in the presence of unscrubbed argon (Fig. 6A). After photoautotrophic growth of the complemented strains, plasmid pRPSMCS3 (containing the M. acetivorans rbcL gene) was back mated into E. coli and sequenced to determine whether there were any point mutations that were selected under these growth conditions. No mutations were detected. Subsequent reintroduction of the plasmid into a new SBI-II− background again resulted in photoautotrophic growth, eliminating the possibility that mutations in strain SBI-II− somehow allowed complementation. Furthermore, assays performed on crude extracts from autotrophically grown cells indicated that RubisCO activity was strictly dependent on anaerobiosis (data not shown), in keeping with the observed properties of the M. acetivorans enzyme (Fig. 5C). While R. capsulatus or R. sphaeroides knockout strains grow under aerobic chemoautotrophic conditions in an H2/CO2/O2 atmosphere when any number of form I or form II RubisCO genes are used, the M. acetivorans rbcL gene did not complement these strains to aerobic chemoautotrophic growth.
Finally, Western immunoblot experiments provided further evidence that the M. acetivorans gene was expressed in the R. capsulatus and R. sphaeroides RubisCO deletion strains under anaerobic phototrophic growth conditions. Levels of protein produced were consistent with known regulatory features of the cbb promoter of the vector employed under the indicated growth conditions (Fig. 7). Under photoheterotrophic growth conditions and, to a lesser extent, under photoautotrophic growth conditions, extracts from complemented R. sphaeroides strain 16 appeared to contain low-molecular-mass cross-reactive protein species that were perhaps indicative of proteolysis (Fig. 7, lanes F and G). This result was not so apparent for complemented R. capsulatus (Fig. 7, lanes D and E).
FIG. 7.
Western immunoblot of extracts of R. capsulatus SBI-II− and R. sphaeroides strain 16 complemented with plasmid pRPSMCS3MA (containing M. acetivorans rbcL). The immunoblot was tested using antibodies directed against purified recombinant M. acetivorans RubisCO. All lanes contained soluble crude extract prepared from stationary phase cultures from the following strains: photoheterotrophically grown wild-type R. sphaeroides strain HR (lane A); photoautotrophically grown wild-type R. sphaeroides strain HR (lane B); purified recombinant M. acetivorans RubisCO (lane C); photoheterotrophically grown R. capsulatus SBI-II− complemented with plasmid pRPSMCS3MA (containing M. acetivorans rbcL) (lane D); photoautotrophically grown R. capsulatus SBI-II− complemented with plasmid pRPSMCS3MA (lane E); photoautotrophically grown R. sphaeroides strain 16 complemented with plasmid pRPSMCS3MA (lane F); photoheterotrophically grown R. sphaeroides strain 16 complemented with plasmid pRPSMCS3MA (lane G); photoheterotrophically grown wild-type R. capsulatus strain SB1003 (lane H); and purified recombinant M. acetivorans RubisCO (lane I). Each lane received approximately 15 μg of protein.
DISCUSSION
Although the level of amino acid identity for forms I, II, and III of RubisCO is low, residues shown to be essential for catalysis are present and conserved in each of the archaeal enzymes used in this study. Until this study, there had been no experimental information relative to the functional properties and expression of active nonhalophilic archaeal RubisCO in its native host. Previous work using Northern blotting and Western immunoblotting by Ezaki et al. (6) demonstrated that T. kodakaraensis KOD1 transcribed and translated the RubisCO gene; however, the ability of this organism to catalyze RuBP-dependent CO2 fixation in crude extracts was never reported by these authors. Western immunoblot analysis and RubisCO assays of crude extracts from four different organisms employed in this study clearly demonstrated that significant levels of catalytically active RubisCO were produced by M. jannaschii and A. fulgidus and that M. acetivorans and M. barkeri exhibited low but measurable activity. It is possible that further assay optimization can raise the levels of RubisCO-dependent CO2 fixation in crude extracts from the mesophilic methanogens used in this study. Alternatively, the unique substitution of a serine residue for the catalytically significant threonine residue at position 62 in the mesophilic proteins might relate to the poor inherent activity of these enzymes.
With respect to M. jannaschii, the original labeling experiments might have missed potential whole-cell evidence for RubisCO activity because the cells were grown at 65°C (28), a temperature at which this enzyme shows poor activity in vitro. Thus, it might be useful to consider performing similar label incorporation studies at optimum temperatures for both growth and enzyme activity (83 to 85°C). Interesting yet puzzling is the fact that RubisCO protein accumulation appeared to be independent of whether M. jannaschii was grown at 65 or 83°C. The two thermophilic form III RubisCO proteins, from A. fulgidus and M. jannaschii, also showed great differences in the range of temperature-dependent activity; they certainly differed from the mesophilic enzyme from M. acetivorans in this respect. That the A. fulgidus enzyme retained activity at the same temperature as the M. acetivorans RubisCO might lead to potential future structure-function studies related to defining structural requirements for thermostability.
Other interesting properties relate to the differential effect of oxygen exposure. Both the M. jannaschii (35) and A. fulgidus RubisCO were reversibly inhibited by oxygen. For RubisCO, oxygen is classically known to be a competitive inhibitor with respect to carbon dioxide (30); however, in no instance have air levels of oxygen been shown to inhibit form I or form II RubisCO at the saturating levels of CO2 employed in normal assays. Previous work had shown that the M. jannaschii RubisCO can use oxygen as a gaseous substrate and form phosphoglycolate (35); however, the enzyme is rapidly inactivated under these conditions, as shown in this study as well. While not directly addressed, it may be that the archaeal enzymes considered here have extremely high affinities for oxygen or there may be some special way in which oxygen interacts with the M. jannaschii and A. fulgidus proteins. Whatever the mechanism for reversible oxygen inhibition, clearly the mesophilic M. acetivorans enzyme behaved differently, as this enzyme did not recover from oxygen inactivation under the experimental conditions reported.
Future work should address the differential effects of oxygen on these proteins, since the molecular basis for RubisCO's interaction with oxygen is fundamental to elucidating the mechanism of CO2/O2 specificity (10). It should also be noted that, unlike the three RubisCO enzymes studied here, the recombinant T. kodakaraensis KOD1 RubisCO does not appear to be sensitive to oxygen (6); however, it is not clear how the T. kodakaraensis enzyme was assayed in the cited study. Furthermore, based on room temperature gel filtration studies, each of the enzymes of this study is a homodimer and thus quite different from the T. kodakaraensis enzyme, which shows an unusual and very interesting pentameric structure (13, 16). These previous studies, along with the present investigation, indicate that form III RubisCO proteins comprise a highly divergent group with respect to catalytic and structural properties. This is probably reflective of the rather low primary structural identity of proteins from this class (Table 2).
Complementation of RubisCO knockout strains of R. capsulatus and R. sphaeroides with the mesophilic archaeal rbcL gene from M. acetivorans provided excellent evidence that this RubisCO was functional in an environment where all other required CBB intermediates are known to be present. This result, plus the fact that enzymatic activity and immunological evidence for the archaeal enzymes were demonstrable in crude extracts from the native organisms, strongly suggested that these organisms produce active RubisCO. The absence of any known purpose for archaeal RubisCO in primary CO2 fixation could mean that this enzyme has some other function in these organisms. Unfortunately, the phenotype of the rbcL strain of M. acetivorans has yet to provide any clue as to the function of RubisCO in these organisms. The M. acetivorans rbcL knockout strain (strain 4555) prepared by W. Metcalf (18) was fully capable of using its preferred growth substrates, namely, acetate, methanol, or trimethylamine, with no noticeable difference in growth rate compared to the wild type, yet RubisCO activity and immunoreactive protein were clearly absent in this strain (data not shown).
As a result of the findings of this study and an earlier investigation (35), it is apparent that oxygen inhibited archaeal RubisCO activity; yet it is also apparent that O2 can serve as a substrate, albeit weakly (35). From these results, it can be suggested that the archaeal enzyme functions in some oxygen-scavenging capacity in vivo. However, this seemed unlikely in light of the oxygen sensitivity of the three archaeal enzymes studied here as well as the strict anaerobic requirement for photoautotrophic growth of RubisCO knockout strains of Rhodobacter complemented with M. acetivorans rbcL. In addition, the biphasic growth of argon-bubbled photoheterotrophic cultures of complemented strain R. capsulatus SBI-II− appears to further discount the oxygen-scavenging theory. Complemented photoheterotrophic-grown cultures were not strictly anaerobic, as the argon gas used to bubble cultures was not scrubbed to remove vestiges of oxygen. Normally, this is not a problem with typical form I or form II proteins, because these enzymes are not overly sensitive to oxygen. Thus, the achievement of nearly exponential photoheterotrophic growth by the complemented culture only after 7 to 10 days may reflect the fact that this facultative anaerobe removed inhibitory levels of oxygen only after the culture density reached some defined level.
Speculations as to the function of RubisCO in anaerobic archaea must also contend with the means by which these organisms synthesize the substrate RuBP. This is particularly relevant, as there is no other known potential CO2 acceptor for RubisCO beyond RuBP. We entertained the possibility that the archaeal enzyme might be unusual in this regard and might use other potential and structurally similar sugar phosphates or perhaps other acceptor molecules. No substrate other than RuBP served as a substrate for the archaeal enzyme. In addition, attempts to demonstrate the presence of PRK activity in extracts of these organisms or to extract RuBP from these cells have also consistently failed (M. W. Finn and F. R. Tabita, unpublished observations). Of course, as discussed in the introduction, there is no homologous PRK sequence that can be found in any available archaeal genome. It may be that we are unable to recognize a potentially unusual type of PRK, since sequences of this enzyme have not been studied or compared to the extent that those of RubisCO have been. In addition, the various methods used thus far to extract RuBP from M. jannaschii may have failed because levels are not high enough for detection in these organisms or because the extraction methods do not work in archaea. It is also possible that the failure to detect PRK by homology searches or enzyme assay means that alternative pathways leading to the synthesis of RuBP exist in anaerobic archaea. Current work is thus focused at elucidating potential alternative routes for RuBP synthesis. Resolution of these very interesting and perplexing questions would also undoubtedly invite intriguing evolutionary speculations, especially if anaerobic archaea are shown to fix CO2 by the use of RuBP as the CO2 acceptor while gaining energy and assimilating most of the CO2 required for growth by established methanogenesis pathways.
Acknowledgments
We are grateful to William Metcalf for providing us with the rbcL knockout strain of M. acetivorans and Jon-David Sears for the purified recombinant A. fulgidus RubisCO. In addition, we thank Janet Gibson for comments on the manuscript and for assistance with the complementation studies.
This study was supported by National Institutes of Health grant GM24497.
REFERENCES
- 1.Altekar, W., and R. Rajagopalan. 1990. Ribulose bisphosphate carboxylase activity in halophilic archaebacteria. Arch. Microbiol. 153:169-174. [Google Scholar]
- 2.Bult, C. J., O. White, G. J. Olsen, et al. 1996. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science 273:1058-1073. [DOI] [PubMed] [Google Scholar]
- 3.Cleland, W. W., T. J. Andrews, S. Gutteridge, F. C. Hartman, and G. H. Lorimer. 1998. Mechanism of rubisco: the carbamate as general base. Chem. Rev. 98:549-562. [DOI] [PubMed] [Google Scholar]
- 4.Deppenmeier, U., A. Johann, T. Hartsch, et al. 2002. The genome of Methanosarcina mazei: evidence for lateral gene transfer between bacteria and archaea. J. Mol. Microbiol. Biotechnol. 4:453-461. [PubMed] [Google Scholar]
- 5.Dubendorff, J. W., and F. W. Studier. 1991. Controlling basal expression in an inducible T7 expression system by blocking the target T7 promoter with lac repressor. J. Mol. Biol. 219:45-59. [DOI] [PubMed] [Google Scholar]
- 6.Ezaki, S., N. Maeda, T. Kishimoto, H. Atomi, and T. Imanaka. 1999. Presence of a structurally novel type ribulose-bisphophate caroboxylase/oxygenase in hyperthermophilic archaeon, Pyrococcus kodakaraensis KOD1. J. Biol. Chem. 274:5078-5082. [DOI] [PubMed] [Google Scholar]
- 7.Falcone, D. L., and F. R. Tabita. 1991. Expression of endogenous and foreign ribulose 1,5-bisphosphate carboxylase-oxygenase (RubisCO) genes in a RubisCO deletion mutant of Rhodobacter sphaeroides. J. Bacteriol. 173:2099-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figurski, D. H., and D. R. Helinsky. 1979. Replication of an origin-containing derivative of the plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galagan, J. E., C. Nusbaum, A. Roy, M. G. Endrizzi, et al. 2002. The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res. 12:532-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horken, K. M., and F. R. Tabita. 1999. Closely related form I ribulose bisphosphate carboxylase/oxygenase molecules that possess different CO2/O2 substrate specificities. Arch. Biochem. Biophys. 361:183-194. [DOI] [PubMed] [Google Scholar]
- 11.Horken, K. M., and F. R. Tabita. 1999. The “green” form I ribulose 1,5-bisphosphate carboxylase/oxygenase from the nonsulfur purple bacterium Rhodobacter capsulatus. J. Bacteriol. 181:3935-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones, W. J., J. A. Leigh, F. Mayer, C. R. Woese, and R. S. Wolfe. 1983. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch. Microbiol. 136:254-261. [Google Scholar]
- 13.Kitano, K., N. Maeda, T. Fukui, H. Atomi, T. Imanaka, and K. Miki. 2001. Crystal structure of a novel-type archaeal rubisco with pentagonal symmetry. Structure 9:473-481. [DOI] [PubMed] [Google Scholar]
- 14.Klenk, H. P., R. A. Clayton, J. F. Tomb, et al. 1997. The complete sequence of the hyperthermophilic sulfate-reducing archaeon Archaeoglobus fulgidus. Nature 390:364-370. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleaving of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Maeda, N., K. Kitano, T. Fukui, S. Ezaki, H. Atomi, K. Miki, and T. Imanaka. 1999. Ribulose bisphosphate carboxylase/oxygenase from the hyperthermophilic archaeon Pyrococcus kodakaraensis KOD1 is composed solely of large subunits and forms a pentagonal structure. J. Mol. Biol. 293:57-66. [DOI] [PubMed] [Google Scholar]
- 17.Markwell, M. K., S. M. Hass, L. L. Bieber, and N. E. Tolbert. 1978. A modification of the Lowry method to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87:206-210. [DOI] [PubMed] [Google Scholar]
- 18.Metcalf, W. W., J. K. Zhang, E. Apolinario, K. R. Sowers, and R. S. Wolfe. 1997. A genetic system for archaea of the genus Methanosarcina: liposome-mediated transformation and construction of shuttle vectors. Proc. Natl. Acad. Sci. USA 94:2626-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukhopadhyay, B., E. F. Johnson, and R. S. Wolfe. 1999. Reactor-scale cultivation of the hyperthermophilic methanarchaeon Methanococcus jannaschii to high cell densities. Appl. Environ. Microbiol. 65:5059-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ormerod, J. G., K. S. Ormerod, and H. Gest. 1961. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by the photosynthetic bacteria; relationships with nitrogen metabolism. Arch. Biochem. Biophys. 94:449-463. [DOI] [PubMed] [Google Scholar]
- 21.Paoli, G. C., N. S. Morgan, F. R. Tabita, and J. M. Shively. 1995. Expression of the structural cbbLcbbS and cbbM genes and distinct organization of the cbb Calvin cycle structural genes of Rhodobacter capsulatus. Arch. Microbiol. 164:396-405. [PubMed] [Google Scholar]
- 22.Paoli, G. C., P. Vichivanives, and F. R. Tabita. 1998. Physiological control and regulation of the Rhodobacter capsulatus cbb operons. J. Bacteriol. 180:4258-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierce, J., N. E. Tolbert, and R. E. Barker. 1980. Interaction of ribulose-bisphosphate carboxylase/oxygenase with transition-state analogues. Biochemistry 19:934-942. [DOI] [PubMed] [Google Scholar]
- 24.Rajagopalan, R., and W. Altekar. 1994. Characterization and purification of ribulose-bisphosphate carboxylase from heterotrophically grown halophilic archaebacterium, Haloferax mediterranei. Eur. J. Biochem. 221:863-869. [DOI] [PubMed] [Google Scholar]
- 25.Rawal, N., S. M. Kelkar, and W. Altekar. 1988. Ribulose 1,5-bisphosphate dependent CO2 fixation in the halophilic archaebacterium Halobacterium mediterranei. Biochem. Biophys. Res. Commun. 156:451-456. [DOI] [PubMed] [Google Scholar]
- 26.Smith, D. R., L. A. Doucette-Stamm, C. Deloughery, H. Lee, J. Dubois, T. Aldredge, R. Bashirzadeh, D. Blakely, R. Cook, K. Gilbert, D. Harrison, L. Hoang, P. Keagle, W. Lumm, B. Pothier, D. Qiu, R. Spadafora, R. Vicaire, Y. Wang, J. Wierzbowski, R. Gibson, N. Jiwani, A. Caruso, D. Bush, and J. N. Reeve. 1997. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genomics. J. Bacteriol. 179:7135-7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sowers, K. R., J. E. Boone, and R. P. Gunsalus. 1993. Disaggregation of Methanosarcina spp. and growth as single cells at elevated osmolarity. Appl. Environ. Microbiol. 59:3832-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprott, G. D., I. Ekiel, and G. Patel. 1993. Metabolic pathways in Methanococcus jannaschii and other methanogenic bacteria. Appl. Environ. Micrbiol. 59:1092-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 30.Tabita, F. R. 1999. Microbial ribulose 1,5-bisphosphate carboxylase/oxygenase: a different perspective. Photosynth. Res. 60:1-28. [Google Scholar]
- 31.Tabita, F. R., P. Caruso, and W. Whitman. 1978. Facile assay of enzyme unique to the Calvin cycle in intact cells, with special reference to ribulose 1,5 bisphosphate carboxylase. Anal. Biochem. 84:462-472. [DOI] [PubMed] [Google Scholar]
- 32.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, X., D. L. Falcone, and F. R. Tabita. 1993. Reductive pentose phosphate-independent CO2 fixation in Rhodobacter sphaeroides and evidence that ribulose bisphosphate carboxylase/oxygenase activity serves to maintain the redox balance of the cell. J. Bacteriol. 175:3372-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson, G. M. F., and F. R. Tabita. 1997. Microbial ribulose 1,5-bisphosphate carboxylase/oxygenase: a molecule for phylogenetic and enzymological investigation. FEMS. Lett. 146:13-22. [DOI] [PubMed] [Google Scholar]
- 35.Watson, G. M. F., J. P. Yu, and F. R. Tabita. 1999. Unusual ribulose 1,5-bisphosphate carboxylase/oxygenase of anoxic archaea. J. Bacteriol. 181:1569-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weaver, K. E., and F. R. Tabita. 1983. Isolation of partial characterization of Rhodobacter sphaeroides mutants defective in regulation of ribulose bisphosphate carboxylase/oxygenase. J. Bacteriol. 156:507-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M14mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 38.Yen, H. C., and B. Mars. 1976. Map of genes for carotenoid and bacteriochlorophyll biosynthesis in Rhodobacter capsulatus. J. Bacteriol. 126:619-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zellner, B., E. Stackebrandt, H. Kneifel, P. Messner, W. E. B. Sleytr, E. C. De Marcario, H. P. Zabel, K. O. Stetter, and J. Winter. 1989. Isolation and characterization of thermophilic, sulfate reducing archaebacterium. Archaeoglobus fulgidus strain Z. Syst. Appl. Microbiol. 11:151-160. [Google Scholar]