Abstract
Staphylococcus aureus is a common pathogen associated with nosocomial infections. It can persist in clinical settings and gain increased resistance to antimicrobial agents through biofilm formation. We have found that alpha-toxin, a secreted, multimeric, hemolytic toxin encoded by the hla gene, plays an integral role in biofilm formation. The hla mutant was unable to fully colonize plastic surfaces under both static and flow conditions. Based on microscopy studies, we propose that alpha-hemolysin is required for cell-to-cell interactions during biofilm formation.
Biofilms are surface-associated, sessile bacterial communities. A mature biofilm is formed when planktonic cells initially colonize a surface, aggregate and/or grow into multicellular colonies, and embed themselves in an exopolysaccharide matrix. Staphylococcus aureus is capable of biofilm formation, which increases its persistence and boosts its levels of antimicrobial resistance (5), and biofilms of this organism have been observed on surfaces ranging from intravascular catheters to pacemaker leads (17, 18). Genetic analyses of staphylococci have shown that the progression of biofilm development consists of two steps: initial cell-to-surface interactions followed by cell-to-cell interactions (9). Recent reports have shown autolysin (10), teichoic acids (8), and surface proteins such as Bap to be integral to the initial stages of colonization (4). The ica locus, which is required for the synthesis of the polysaccharide intracellular adhesin (PIA), plays a role in subsequent cell-to-cell interactions (3, 14).
The accessory gene regulator (agr) is a two-component regulatory system in S. aureus that has been implicated in biofilm formation—an agr mutant is a hyper-biofilm-forming strain (22). To study biofilms of S. aureus, we took the approach of examining known downstream targets regulated by the agr system and determining their impact on biofilm formation. We show that one of these targets, alpha-hemolysin, a 34-kDa protein that causes host cell lysis by heptamerizing upon insertion into eukaryotic cell membranes, plays a role in biofilm formation (21, 23). Mutants defective in alpha-hemolysin production failed to form biofilms under both static and flow conditions, and strains lacking alpha-hemolysin have an apparent defect in cell-to-cell interactions.
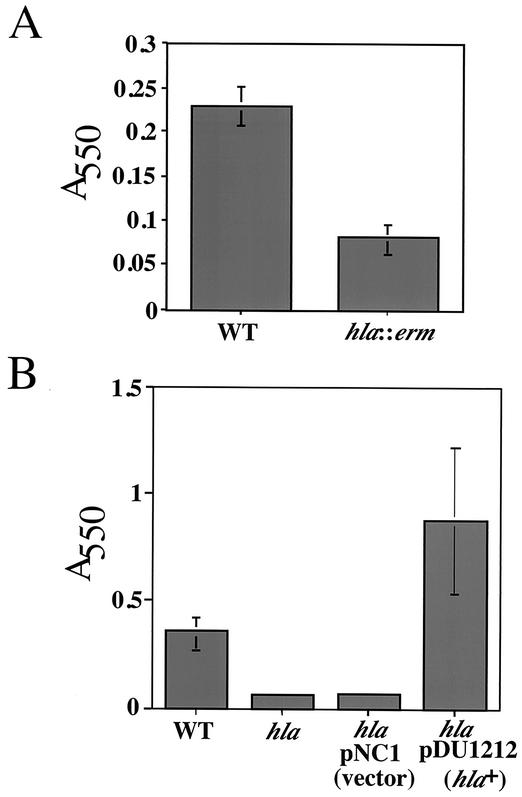
Figure 1A shows the results of a biofilm assay wherein bacteria were grown at 37°C in tryptic soy broth (TSB) and 0.2% glucose for 8 h, as described by Heilmann et al. (9, 11). The level of bacterial adhesion, as quantified by crystal violet staining, is ∼3-fold lower for the hla mutant than for the wild type (strains are described in Table 1). The alpha-hemolysin-deficient strain was also defective for biofilm formation when compared to the wild type at 16 h (data not shown). Plasmid pDU1212 contains a wild-type copy of the hla gene, and when this plasmid is introduced into the hla::erm strain, biofilm formation is induced to a level above that of even the wild-type strain (Fig. 1B), whereas the vector control pNC1 has no effect on biofilm formation. It has been shown previously that the supernatant of S. aureus DU1090/pDU1212 (hla+) contains 2.5- and 110-fold more hemolytic activity (in hemolytic units per milliliter) than wild-type and hla::erm strains, respectively (1). Thus, the level of alpha-hemolysin may correlate with the level of biofilm formation.
FIG. 1.
The hla mutant is defective in biofilm formation. (A) Biofilm formation phenotypes. Biofilm formation by the wild type (WT; S. aureus 8325-4) and a hla::erm mutant (S. aureus DU1090) was quantitated. Crystal violet was used to stain cells adhering to polystyrene after 8 h of growth at 37°C. (B) Complementation of the hla::erm mutant. Biofilms formed on polystyrene (8 h at 37°C) were analyzed for an hla::erm mutant harboring a vector control plasmid (pNC1) or a plasmid providing a wild-type copy of the hla gene (pDU1212 [hla+]).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Description | Reference |
|---|---|---|
| S. aureus strains | ||
| 8325-4 | 19 | |
| DU1090 | Strain 8325-4 with hla::erm | 20 |
| DU1090/pDU1212 | Strain 8325-4 with hla::erm, harboring pDU1212 (Apr Cmr/hla+) | 6 |
| DU1090/pNC1 | Strain 8325-4 with hla::erm, harboring pNC1 (Apr Cmr) | This study |
| 113 | 3 | |
| 113 ica::Tc | Defective in production of PIA | 3 |
| 113 ica::Tc/pNC1 | Cmr | This study |
| 113 ica::Tc/pDU1212 | Cmr | This study |
| Plasmids | ||
| pDU1212 | pBR322 carrying hla+ and Cmr | 6 |
| pNC1 | Empty vector control; pBR322 carrying 3.0-kb HindIII fragment from pDU1212 encoding Cmr | This study |
PIA, encoded by the ica genes, has been shown to be required for biofilm formation by S. aureus (3, 14, 15); therefore, we investigated PIA production in the wild-type and hla::erm strains. PIA was extracted from cells grown in TSB supplemented with 0.2% glucose (the medium used for biofilm assays), serial twofold dilutions were spotted onto nitrocellulose, and Western blotting was performed as previously described by Cramton et al. (3) by using antibody to PIA/PNAG [β(1-6)-N-acetylglucosamine] (16). A wild-type PIA-producing strain (113) and an isogenic ica mutant (113 ica::Tc) served as controls. No difference in the levels of PIA production between the wild-type and alpha-toxin mutant strains was observed (data not shown). Furthermore, in 10 clinical S. aureus strains analyzed (22), no correlation between PIA production and alpha-hemolysis was observed. We also investigated the ability of a multicopy dose of hla (plasmid pDU1212) to rescue the biofilm formation defect of an ica mutant. Neither pDU1212 (hla+) nor the vector control (pNC1) had any effect on the biofilm formation phenotype of the ica mutant (data not shown).
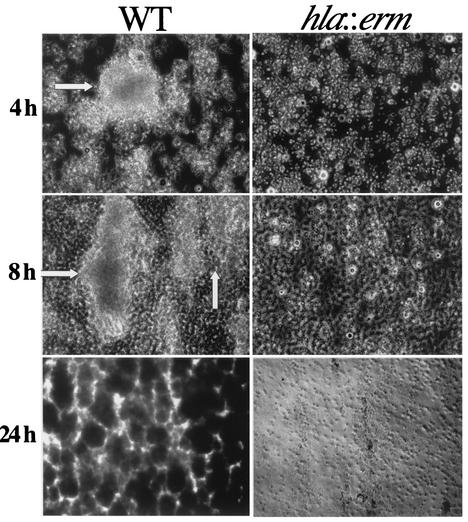
To better understand the nature of the biofilm-deficient phenotype of the hla::erm mutant, phase-contrast microscopy was employed to observe and compare levels of surface attachment at 8 h in 24-well polystyrene plates (Costar, Corning, N.Y.) (Fig. 2). This assay was similar to the 96-well plate assay (described in references 9 and 11) with the exception that nonadherent cells were removed by aspiration. For the wild-type strain, microcolonies (dark regions) were found scattered evenly throughout the field of view but were not present in the fields of view for the hla::erm mutant and the vector control. The strain carrying the plasmid pDU1212 (hla+) in the hla::erm background exhibited more robust biofilm formation than even the wild type—the entire surface was covered in a dense mass of microcolonies. Therefore, the crystal violet staining data presented in Fig. 1B correlates with the microscopy data presented in Fig. 2.
FIG. 2.
Direct visualization of attachment phenotypes. Bacteria were inoculated onto 24-well plates, incubated for 8 h at 37°C, and then analyzed by phase-contrast microscopy. Dark areas are the adherent bacteria, and the light grayish regions represent the surface of the 24-well plate. The magnification is ×1,050. WT, wild type.
In a physiological setting, such as the surface of a catheter, biofilms may exist and persist under conditions of flow. To mimic these conditions in vitro, S. aureus biofilms were grown under conditions of constant flow (40 ml/h) by using 0.1× TSB as the growth medium in the flow cell system described by Christensen et al. (2). Overnight cultures of S. aureus were diluted 1:1,000 in 0.1× TSB, 300 μl of diluted cells was injected into the flow cell chamber, and the cells were allowed to acclimate for 15 min before being subjected to flow. Figure 3A shows that by 4 h the wild type had attached to the surface of the flow chamber and begun to form large macrocolonies. After 8 h of constant flow, the wild-type macrocolonies became larger and more numerous. In addition, the surface area between macrocolonies was completely covered by a monolayer of cells. By 24 h, wild-type macrocolonies had increased in size and density to the point of completely filling the flow chamber. The architecture of the wild-type biofilm at 24 h consisted of densely packed circular macrocolonies outlined by narrow, light regions that were the channels between the macrocolonies. In contrast to the wild type, the hla::erm mutant attached to the surface as a sparse monolayer, failed to exhibit macrocolony formation even at 24 h, and lacked any discernible architecture.
FIG. 3.
Phenotypes of cells under flow conditions. Biofilms of the wild-type (WT; S. aureus 8325-4) and the hla::erm mutant (S. aureus DU1090) strains were grown in flow cell chambers. At the times indicated, biofilms were observed from a top-down perspective by using phase-contrast microscopy. In images from 4 and 8 h, light regions represent bacterial macrocolonies (indicated by the white arrows) and dark areas are the surface of the flow cell chamber. The magnification is ×675 for images from 4 and 8 h. At 24 h, the images were recorded at an original magnification of ×230. (They are shown at a magnification of ×173.) In these images from 24 h, the very dense macrocolonies formed by the wild type appear as dark regions and the light areas define macrocolony borders or channels between the macrocolonies. Small clusters of cells, but no macrocolonies, were observed for the hla mutant at all time points.
In this study, we show a role for alpha-hemolysin in S. aureus biofilm formation, and in particular, this toxin appears to be required for cell-to-cell interactions. We were initially surprised to find that a secreted toxin had such a dramatic impact on biofilm formation; however, other examples exist in which secreted toxins may play a role in biofilm formation (12, 13, 22). The fact that cells carrying a mutant allele of hla are capable of initially colonizing a surface but never organize into multicellular macrocolonies indicates a defect in cell-to-cell interactions. Based on the data presented in this study, we propose that alpha-hemolysin plays a role primarily in cell-to-cell interactions during biofilm formation.
Alpha-hemolysin is, in part, controlled by the agr system. It has been shown that an agr mutant produces less alpha-hemolysin but is a hyper-biofilm-forming strain (22). However, the agr system regulates a wide array of virulence factors, including those involved in surface binding and surface-associated virulence. Thus, even though alpha-hemolysin production is reduced in an agr mutant, other surface-associated virulence factors may be overexpressed, functionally compensating for the lack of alpha-hemolysin. Furthermore, in vivo studies of device-related infections have shown that alpha-hemolysin is not regulated by agr but that its expression is predominately controlled by the two-component regulator sae (7). Therefore, alpha-hemolysin may be produced in an agr-independent fashion when S. aureus colonizes in-dwelling devices in the biofilm mode of growth.
Acknowledgments
We thank A. Chueng and M. Palma for helpful advice and Richard J. O'Callaghan for sending strains DU1090 and DU1090/pDU1212. We also thank Jerry Pier for providing the PIA/PNAG antibodies and Cuong Vuong and Michael Otto for providing clinical S. aureus isolates.
This work was supported by grants from Microbia, Inc., by the American Cancer Society institutional research grant #IRG-82-003-17, and by the Pew Charitable Trusts to G.A.O. G.A.O. is a Pew Scholar in the Biomedical Sciences.
REFERENCES
- 1.Bayer, A. S., M. D. Ramos, B. E. Menzies, M. R. Yeaman, A. J. Shen, and A. L. Cheung. 1997. Hyperproduction of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: a host defense role for platelet microbicidal proteins. Infect. Immun. 65:4652-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christensen, B. B., C. Sternberg, J. B. Andersen, R. J. Palmer, Jr., A. T. Nielsen, M. Givskov, and S. Molin. 1999. Molecular tools for study of biofilm physiology. Methods Enzymol. 310:20-42. [DOI] [PubMed] [Google Scholar]
- 3.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nicols, and F. Gotz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cucarella, C., C. Solano, J. Valle, B. Amorena, I. Lasa, and J. R. Penades. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham, R., and J. Cheesbrough. 1992. Comparative activity of glycopeptide antibiotics against coagulase-negative staphylococci embedded in fibrin clots. J. Antimicrob. Chemother. 30:321-326. [DOI] [PubMed] [Google Scholar]
- 6.Fairweather, N., S. Kennedy, T. J. Foster, M. Kehoe, and G. Dougan. 1983. Expression of a cloned Staphylococcus aureus alpha-hemolysin determinant in Bacillus subtilis and Staphylococcus aureus. Infect. Immun. 41:1112-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goerke, C., U. Fluckiger, A. Steinhuber, W. Zimmerli, and C. Wolz. 2001. Impact of the regulatory loci agr, sarA and sae of Staphylococcus aureus on the induction of alpha-toxin during device-related infection resolved by direct quantitative transcript analysis. Mol. Microbiol. 40:1439-1447. [DOI] [PubMed] [Google Scholar]
- 8.Gross, M., S. E. Cramton, F. Gotz, and A. Peschel. 2001. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 69:3423-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heilmann, C., C. Gerke, F. Perdreau-Remington, and F. Gotz. 1996. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect. Immun. 64:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heilmann, C., M. Hussain, G. Peters, and F. Gotz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013-1024. [DOI] [PubMed] [Google Scholar]
- 11.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Gotz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 12.Kachlany, S. C., D. H. Fine, and D. H. Figurski. 2000. Secretion of RTX leukotoxin by Actinobacillus actinomycetemcomitans. Infect. Immun. 68:6094-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kachlany, S. C., P. J. Planet, M. K. Bhattacharjee, E. Kollia, R. DeSalle, D. H. Fine, and D. H. Figurski. 2000. Nonspecific adherence by Actinobacillus actinomycetemcomitans requires genes widespread in bacteria and archaea. J. Bacteriol. 182:6169-6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intracellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mack, D., M. Nedelmann, A. Krokotsch, A. Schwarzkopf, J. Heesemann, and R. Laufs. 1994. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intracellular adhesin. Infect. Immun. 62:3244-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maira-Litran, T., A. Kropec, C. Abeygunawardana, J. Joyce, G. Mark III, D. A. Goldmann, and G. B. Pier. 2002. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect. Immun. 70:4433-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrie, T. J., and J. W. Costerton. 1984. Scanning and transmission electron microscopy of in situ bacterial colonization of intravenous and intraarterial catheters. J. Clin. Microbiol. 19:687-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marrie, T. J., J. Nelligan, and J. W. Costerton. 1982. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation 66:1339-1341. [DOI] [PubMed] [Google Scholar]
- 19.Novick, R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155-166. [DOI] [PubMed] [Google Scholar]
- 20.O'Reilly, M., J. C. de Azavedo, S. Kennedy, and T. J. Foster. 1986. Inactivation of the alpha-haemolysin gene of Staphylococcus aureus 8325-4 by site-directed mutagenesis and studies on the expression of its haemolysins. Microb. Pathog. 1:125-138. [DOI] [PubMed] [Google Scholar]
- 21.Song, L., M. R. Hobaugh, C. Shustak, S. Cheley, H. Bayley, and J. E. Gouaux. 1996. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 274:1859-1866. [DOI] [PubMed] [Google Scholar]
- 22.Vuong, C., H. L. Saenz, F. Gotz, and M. Otto. 2000. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J. Infect. Dis. 182:1688-1693. [DOI] [PubMed] [Google Scholar]
- 23.Walker, B., M. Krishnasastry, L. Zorn, and H. Bayley. 1992. Assembly of the oligomeric membrane pore formed by Staphylococcal alpha-hemolysin examined by truncation mutagenesis. J. Biol. Chem. 267:21782-21786. [PubMed] [Google Scholar]