Abstract
Regulatory inactivation of DnaA helps ensure that the Escherichia coli chromosome is replicated only once per cell cycle, through accelerated hydrolysis of active replication initiator ATP-DnaA to inactive ADP-DnaA. Analysis of Δhda strains revealed that the regulatory inactivation of DnaA component Hda is necessary for maintaining controlled initiation but not for cell growth or viability.
Highly regulated initiation of chromosomal replication is a critical component of cell cycle control in both prokaryotes and eukaryotes. DnaA protein initiates DNA replication in Escherichia coli by binding to 9-mer DnaA boxes within the chromosomal origin, oriC (reviewed in references 7, 17, and 23). The origin DNA wraps around the DnaA complex (5, 6) and, in the presence of protein HU or integration host factor, causes neighboring AT-rich 13-mers to unwind (1, 3). DnaA, aided by DnaC, directs loading of DnaB helicase onto the opened DNA (20, 25), which is ultimately followed by replisome assembly (15, 19).
It is important that chromosomal DNA is replicated completely once, and only once, for each cell division cycle (2, 8). E. coli bacteria have several mechanisms that restrict the ability of DnaA to reinitiate DNA replication at oriC.
These include the sequestration of newly replicated, hemimethylated origin DNA by SeqA protein (18) and regulation of available free DnaA in the cell, mainly through titration of DnaA by the datA locus, which possesses a high affinity for DnaA (13, 14).
Regulatory inactivation of DnaA (RIDA), a third mechanism, accelerates the hydrolysis of ATP-DnaA, the form active for initiation, to inactive ADP-DnaA (reviewed in reference 10). RIDA activity was originally found in a soluble cell extract that specifically inhibited in vitro replication from an oriC-containing plasmid (9, 16). This activity requires the β subunit of DNA polymerase III loaded as a sliding clamp on template DNA and a partially purified factor, IdaB. RIDA is further stimulated by DNA synthesis (16).
More recently, the novel Hda (for “homologous to DnaA”) protein was shown to possess IdaB activity (11). Hda has high sequence homology to the domain III ATPase region of DnaA, and both proteins belong to the AAA+ protein family. Proteins belonging to this family are prevalent throughout prokaryotes and eukaryotes and include many proteins known to be involved with the initiation of DNA replication, including various ORC, CDC, and MCM proteins in eukaryotes (21).
Hda was identified as a multicopy suppressor of the β-subunit mutant allele dnaN36. In that study, Hda was stated to be essential since a Δhda::Cmr allele was shown to transduce with approximately 200-fold-higher efficiency into strain C600 cells that harbored a plasmid containing an hda fusion construct than into C600 cells possessing the empty vector (11).
Independently, Hda was discovered through its link to RK2 plasmid DNA replication. This broad-host-range plasmid requires both host-encoded DnaA and self-encoding TrfA for initiation of DNA replication. Expression of the membrane-binding fragment of TrfA is lethal, and Hda was identified as a suppressor of this lethality (12).
Construction and screening of Δhda::Tetr, hda51::Tetr, and ΔgalK::Tetr strains.
Recombinant strains of E. coli possessing the mutation Δhda::Tetr (Fig. 1A), hda51::Tetr (Fig. 1B), or ΔgalK::Tetr were constructed in order to analyze the effects of Hda function on cell growth and viability, as well as initiation control. These constructs were created by using the λ recombination system (28). Recombinants were selected for on Luria-Bertani (LB)-tetracycline (10 μg/ml) medium (22) and grown in LB-tetracycline (10 μg/ml) liquid medium. Genomic DNA was screened by PCR with primers complementary to sequences immediately flanking the genomic hda coding sequence. These constructs were transduced into wild-type E. coli with P1 lysate.
FIG. 1.
Genomic constructs of hda mutations. Wild-type genomic hda nucleotide positions are numbered. The tetA-tetR (∼2 kb) inserted was amplified from a Tn10 template. (A) In the Δhda::Tetr deletion construct, the majority of the hda coding sequence (647 out of 747 nucleotides) has been replaced with tetA-tetR. (B) In the hda51::Tetr-disrupted construct, tetA-tetR has been inserted between nucleotides 50 and 51 of the hda coding sequence.
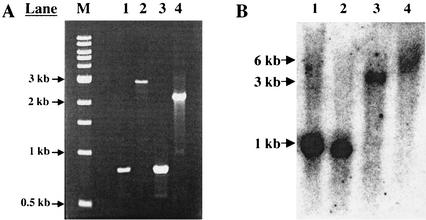
A culture of the recipient bacteria (MG1655 or MC1061) was grown in LB medium to mid-log phase, and cells were harvested. Bacterial suspensions were mixed with an equal volume of P1 lysate (5 × 107 PFU/ml) and a sterile mixture of 0.015 M CaCl2 and 0.03 M MgSO4 and incubated (37°C for 20 min) without agitation. A portion of each transduction mixture was spread onto LB-tetracycline (10 μg/ml) plates for selection. Candidate transductants were grown in LB-tetracycline (10 μg/ml) liquid medium, and the resulting strains were designated JE201 (MG1655 hda51::Tetr), JE101 (MC1061 hda51::Tetr), JE202 (MG1655 Δhda::Tetr), and JE102 (MC1061 Δhda::Tetr). Genomic DNA was screened by PCR with the same primers as mentioned previously. Results of PCR screening show that the wild-type strains MG1655 and MC1061 both produced the expected single 0.8-kb band (Fig. 2A, lanes 1 and 3), while JE101 (Fig. 2A, lane 2) and JE201 (data not shown) produced a single 2.8-kb band and JE102 (Fig. 2A, lane 4) and JE202 (data not shown) produced a single 2.1-kb band, consistent for the disrupted and deleted alleles, respectively. These results indicate that, in strains JE101, JE102, JE201, and JE202, the Tetr cassette is properly placed to interrupt or delete the hda coding sequence and is not inserted elsewhere in the genome, leaving the wild-type hda gene intact at its native locus. For further confirmation, these same strains were also screened by Southern blotting (22). The double-digested (PvuI and BglII) genomic DNA was resolved (22) and was probed with a 1.8-kb probe that encompasses hda plus an additional 500 bp in each direction. The bands obtained for MG1655, MC1061, JE101, and JE202 (Fig. 2B) were 1, 1, 3, and 6 kb, respectively, and substantiate the results from the PCR analysis.
FIG. 2.
(A) PCR screen of wild-type and hda mutant strains with primers from the regions immediately flanking genomic hda. Both wild-type strains, MG1655 and MC1061 (lanes 1 and 3, respectively); the hda51::Tetr-containing strain, JE101 (lane 2); and the Δhda::Tetr-containing strain, JE102 (lane 4), are shown. Relevant bands of the 1-kb ladder (lane M) are indicated on the left. (B) Southern blot of wild-type and hda mutant strains. Results for wild-type strains MG1655 and MC1061 (lanes 1 and 2, respectively), hda51::Tetr strain JE101 (lane 3), and the Δhda::Tetr strain JE202 (lane 4) are shown.
Growth rates of wild-type and mutant strains.
Overnight cultures of wild-type and recombinant strains were back-diluted into fresh LB medium and LB-tetracycline (10 μg/ml) medium, respectively, and grown at 37°C with shaking, and optical density readings, as an indication of growth, were recorded. Cultures of cells possessing the hda51::Tetr mutation were compared to their respective wild-type strains and found to have doubling times of 28 min (JE201) compared to 29 min (MG1655) and of 36 min (JE101) compared to 36 min (MC1061) (Fig. 3B). A very small growth rate difference was observed in comparing cells that completely lack hda (Δhda::Tetr) with their respective wild-type strains, with doubling times of 39 min (JE202) compared to 33 min (MG1655) and of 39 min (JE102) compared to 36 min (MC1061) (Fig. 3A). Additionally, the presence or absence of a functional hda gene had no effect on the final stationary-phase densities of the cell cultures. Similar absences of differences in growth between wild-type and mutant strains were seen with a minimal medium as well (data not shown). Therefore, a lack of Hda does not appear to have a significant effect on cell growth under these conditions.
FIG. 3.
Growth curves of wild-type and hda mutant strains in rich media at 37°C. (A) Strains MG1655 (♦), MC1061 (▴), JE202 (MG1655 Δhda::Tetr) (▪), and JE102 (MC1061 Δhda::Tetr) (•). (B) Strains MG1655 (♦), MC1061 (▴), JE201 (MG1655 hda51::Tetr) (□), and JE101 (MC1061 hda51::Tetr) (○).
Quantitative transduction of mutants into wild-type strains.
If cells harboring the disrupted and deleted hda mutations require other compensatory mutations in order to survive, Δhda::Tetr and hda51::Tetr should transduce into a wild-type background with lower efficiency than that of the transduction of a mutated nonessential gene, such as ΔgalK::Tetr. For quantitative transduction, the same P1-mediated transduction procedure was followed as outlined above. P1 lysates grown on strains containing Δhda::Tetr, hda51::Tetr, or ΔgalK::Tetr were diluted to 5 × 107 PFU/ml in LB medium. Recipient strains (MG1655 and MC1061) were grown in LB medium to mid-log phase and treated with the P1 lysates (multiplicity of infection, 0.05). After transduction, cells were plated on LB-tetracycline (10 μg/ml) and incubated at 37°C. Transductants were counted on the second day after transduction, and efficiencies were calculated as transductants per cells plated. P1-mediated transduction of Δhda::Tetr, hda51::Tetr, and ΔgalK::Tetr into MG1655 or MC1061 occurred with similar frequencies (Table 1). Since Δhda::Tetr and hda51::Tetr are as efficiently transferred into a wild-type E. coli background as is ΔgalK::Tetr, this suggests that hda is not essential for cell viability. Although less quantitative, the frequencies with which the mutant alleles were initially generated via the λ recombination system (data not shown) are consistent with the transduction frequency results and support the conclusion that hda is not essential for cell viability.
TABLE 1.
Transduction frequency of mutant constructs
| Mutation | Recipient strain | Transduction frequency (10−7 transductants/cells plated) |
|---|---|---|
| ΔgalK::Tetr | MG1655 | 1.7 |
| MC1061 | 3.5 | |
| Δhda::Tetr | MG1655 | 4.4 |
| MC1061 | 5.8 | |
| hda51::Tetr | MG1655 | 3.5 |
| MC1061 | 5.6 | |
| Δhda::Tetr | MC1061 | 12.0 |
| MC1061/pET19b | 5.9 | |
| MC1061/pPK101 | 5.5 | |
| C600 | 9.0 | |
| C600/pET19b | 4.7 | |
| C600/pPK101 | 5.6 |
Since it had been reported previously that a Δhda::Cmr construct transduced with higher efficiency into a C600 background in the presence of an hda-containing vector than in its absence (11), we performed a similar experiment by first transforming C600 and MC1061 cells with a pET17b-derived vector containing a T7-tagged version of hda (pPK101). Previous work with this plasmid (12) demonstrated that this form of Hda is active as an inhibitor of replication in vivo and in vitro. RK2 plasmid is less stable in cells with copies of pPK101 and, conversely, more stable in cells lacking Hda (JE202). Furthermore, increased inner membrane-associated RK2 plasmid replication in vitro increases in membrane extracts prepared from JE202, and this enhanced replication can be inhibited by addition of purified, T7-tagged Hda protein (12). The Hda encoded by pPK101 contains a conserved point mutation (V2A) and seven additional amino acids fused to the C terminus of the protein. Similarly, the Hda utilized in the previously mentioned study of Kato and Katayama (11) was part of a fusion protein (MBP-Hda-Myc′His).
Our results show that Δhda::Tetr transduces with similar efficiencies into the MC1061 and C600 strains carrying an empty vector as into the same strains carrying an hda expression vector (Table 1). Also, there was not a significant difference in transduction efficiencies between the MC1061 and C600 strains, so strain background differences do not appear to account for the differences between our results and those previously published (11). The transduction efficiency differences may be due to differences in the Δhda constructs themselves or to the P1 transduction procedure. The use of different drug resistance genes, and perhaps their orientation, to delete hda may result in varied polar effects on the expression of neighboring genes, making it seem as though hda is an essential gene in one case but not the other. With regard to our transduction procedure, it involves a short incubation period (20 min), so it is unlikely that mutations complementary to Δhda would develop during such incubation, leading to an increase in total transductants.
Flow cytometry analysis.
For flow cytometry measurements, cells were grown in AB minimal medium (4) supplemented with thiamine (10 μg/ml), glucose (0.2%) and Casamino Acids (0.5%). Exponentially growing cells (optical density at 450 nm, 0.15) were treated with rifampin (150 μg/ml), which inhibits transcription and therefore also replication initiation, and cephalexin (10 μg/ml), which inhibits cell division. Growth was continued for four to five generations to allow ongoing replication to finish. The treated cells were fixed as described previously (27). Fixed cells were stained in fluorescein isothiocyanate (1.5 μg/ml) overnight (27). The DNA within these cells was stained with Hoechst 33258 (1.5-μg/ml final concentration). Flow cytometry analysis was performed with a FACStar instrument (Becton Dickinson) equipped with an argon ion laser and a krypton laser (both from Spectra Physics), as described previously (26).
Cells treated as described above end up with an integral number of chromosomes, which represents the number of origins at the time of drug treatment (24). In a culture of cells with synchronous initiation, the integral number of chromosomes is 2n (n = 1, 2…). Asynchronous initiation results in cells with an integral number of chromosomes different from 2n. Most of the cells in the culture of a wild-type strain (MG1655) contained four origins, and some contained two, while a few contained eight origins (Fig. 4). This shows that initiation of replication under these conditions occurs early in the cell cycle at two origins. A similar distribution of origins was found for cells containing the disrupted hda51::Tetr allele (JE201). In the Δhda::Tetr deletion strain (JE202), cells contained three, five, six, and seven origins in addition to two, four, and eight, indicating that initiation of replication occurs asynchronously in these cells. The average number of origins per cell was about four in the two former strains while it was about six in JE202. The increased average number of origins indicates that cells tend to overinitiate, meaning that more than the normal number of origins per cell are initiated per cycle, and this is presumably the reason for the asynchrony phenotype seen here. Similar results were obtained with strain MC1061 and its derivatives (data not shown).
FIG. 4.
DNA histograms of cells grown in glucose-CAA medium (4) at 30°C and treated with rifampin and cephalexin. Distinct peaks represent the accumulation of cells with integral numbers of chromosomes that reflect the numbers of origins at the time of drug action. Top, MG1655 wild-type strain; middle, hda51::Tetr strain; bottom, Δhda::Tetr strain.
The extra initiations caused by the lack of Hda protein presumably occur due to increased levels of ATP-DnaA, and it is reasonable to suppose that they occur on fully methylated origins after sequestration is over. Normally the initiation potential is reduced during sequestration by (i) titration of free DnaA by newly replicated datA sites (13, 14) and (ii) conversion of ATP-DnaA to ADP-DnaA by RIDA activity of the replication fork. The results presented here show that datA titration is not sufficient to suppress overinitiation and that inactivation of active ATP-DnaA is also required.
Interestingly, the disrupted hda51::Tetr strain did not show an overinitiation and asynchrony phenotype. Orientation of the tetA-tetR insertion between bp 50 and 51 of hda is such that transcription of tetR is in the same direction as the 5′-to-3′ remaining 697 bp of hda (Fig. 1A). It is therefore possible, via transcriptional read-through and translational restart, that an amino-terminally truncated Hda protein is synthesized in cells of this strain. If so, that would indicate that the first 16 amino acids of the Hda protein are not necessary for regulation of initiation frequency.
Previous investigations have established that proper timing requires both sequestration by SeqA protein (18) and titration of DnaA by the datA site (14). Here we show that the third known mechanism of preventing secondary initiations, RIDA, is also indispensable for controlled genomic replication during exponential growth.
Acknowledgments
We are grateful to Kirsti Solberg Landsverk at the Department of Biophysics flow cytometry facility for expert running of the flow cytometer and to Anne Wahl for excellent technical assistance.
The studies, in part, utilized the Macromolecular Analysis Shared Resource of the Lombardi Cancer Center (P30CA51008). This work was supported in part by a grant from the National Institutes of Health (R01GM49700) to E.C. and by one from the Norwegian Cancer Society to K.S.
REFERENCES
- 1.Baker, T. A., K. Sekimizu, B. E. Funnell, and A. Kornberg. 1986. Extensive unwinding of the plasmid template during staged enzymatic initiation of DNA replication from the origin of the Escherichia coli chromosome. Cell 45:53-64. [DOI] [PubMed] [Google Scholar]
- 2.Boye, E., A. Løbner-Olesen, and K. Skarstad. 2000. Limiting DNA replication to once and only once. EMBO Rep. 1:479-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bramhill, D., and A. Kornberg. 1988. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell 52:743-755. [DOI] [PubMed] [Google Scholar]
- 4.Clark, D. J., and O. Maaløe. 1967. DNA replication and the division cycle in Escherichia coli. J. Mol. Biol. 23:99-112. [Google Scholar]
- 5.Crooke, E., R. Thresher, D. S. Hwang, J. Griffith, and A. Kornberg. 1993. Replicatively active complexes of DnaA protein and the Escherichia coli chromosomal origin observed in the electron microscope. J. Mol. Biol. 233:16-24. [DOI] [PubMed] [Google Scholar]
- 6.Funnell, B. E., T. Baker, and A. Kornberg. 1987. In vitro assembly of a prepriming complex at the origin of the Escherichia coli chromosome. J. Biol. Chem. 262:10327-10334. [PubMed] [Google Scholar]
- 7.Kaguni, J. M. 1997. Escherichia coli DnaA protein: the replication initiator. Mol. Cells 7:145-157. [PubMed] [Google Scholar]
- 8.Katayama, T. 2001. Feedback controls restrain the initiation of Escherichia coli chromosomal replication. Mol. Microbiol. 41:9-17. [DOI] [PubMed] [Google Scholar]
- 9.Katayama, T., and E. Crooke. 1995. DnaA protein is sensitive to a soluble factor and is specifically inactivated for initiation of in vitro replication of the Escherichia coli minichromosome. J. Biol. Chem. 270:9265-9271. [DOI] [PubMed] [Google Scholar]
- 10.Katayama, T., and K. Sekimizu. 1999. Inactivation of Escherichia coli DnaA protein by DNA polymerase III and negative regulations for initiation of chromosomal replication. Biochimie 81:835-840. [DOI] [PubMed] [Google Scholar]
- 11.Kato, J., and T. Katayama. 2001. Hda, a novel DnaA-related protein, regulates the replication cycle in Escherichia coli. EMBO J. 20:4253-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, D. K., T. Banack, D. M. Lerman, J. C. Tracy, J. E. Camara, E. Crooke, D. Oliver, and W. Firshein. 2003. Identification of a novel membrane-associated gene product that suppresses toxicity of a TrfA peptide from plasmid RK2 and its relationship to the DnaA host initiation protein. J. Bacteriol. 185:1817-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitagawa, R., H. Mitsuki, T. Okazaki, and T. Ogawa. 1996. A novel DnaA protein-binding site at 94.7 min on the Escherichia coli chromosome. Mol. Microbiol. 19:1137-1147. [DOI] [PubMed] [Google Scholar]
- 14.Kitagawa, R., T. Ozaki, S. Moriya, and T. Ogawa. 1998. Negative control of replication initiation by a novel chromosomal locus exhibiting exceptional affinity for Escherichia coli DnaA protein. Genes Dev. 12:3032-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kornberg, A., and T. A. Baker. 1992. DNA replication. W. H. Freeman and Co., New York, N.Y.
- 16.Kurokawa, K., T. Mizushima, T. Kubota, T. Tsuchiya, T. Katayama, and K. Sekimizu. 1998. A stimulation factor for hydrolysis of ATP bound to DnaA protein, the initiator of chromosomal DNA replication in Escherichia coli. Biochem. Biophys. Res. Commun. 243:90-95. [DOI] [PubMed] [Google Scholar]
- 17.Langer, U., S. Richter, A. Roth, C. Weigel, and W. Messer. 1996. A comprehensive set of DnaA-box mutations in the replication origin, oriC, of Escherichia coli. Mol. Microbiol. 21:301-311. [DOI] [PubMed] [Google Scholar]
- 18.Lu, M., J. L. Campbell, E. Boye, and N. Kleckner. 1994. SeqA: a negative modulator of replication initiation in E. coli. Cell 77:413-426. [DOI] [PubMed] [Google Scholar]
- 19.Marians, K. 1992. Prokaryotic DNA replication. Annu. Rev. Biochem. 61:673-719. [DOI] [PubMed] [Google Scholar]
- 20.Marszalek, J., and J. M. Kaguni. 1994. DnaA protein directs the binding of DnaB protein in initiation of DNA replication in Escherichia coli. J. Biol. Chem. 269:4883-4890. [PubMed] [Google Scholar]
- 21.Neuwald, A. F., L. Aravind, J. L. Spouge, and E. V. Koonin. 1999. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9:27-43. [PubMed] [Google Scholar]
- 22.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., vol. 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Skarstad, K., and E. Boye. 1994. The initiator protein DnaA: evolution, properties, and function. Biochim. Biophys. Acta 1217:111-120. [DOI] [PubMed] [Google Scholar]
- 24.Skarstad, K., E. Boye, and H. B. Steen. 1986. Timing of initiation in individual Escherichia coli cells. EMBO J. 5:1711-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutton, M. D., K. M. Carr, M. Vicente, and J. M. Kaguni. 1998. Escherichia coli DnaA protein: the N-terminal domain and loading of DnaB helicase at the E. coli chromosomal origin. J. Biol. Chem. 273:34255-34262. [DOI] [PubMed] [Google Scholar]
- 26.Torheim, N. K., E. Boye, A. Lobner-Olesen, T. Stokke, and K. Skarstad. 2000. The Escherichia coli SeqA protein destabilizes mutant DnaA204 protein. Mol. Microbiol. 37:629-638. [DOI] [PubMed] [Google Scholar]
- 27.Wold, S., K. Skarstad, H. B. Steen, T. Stokke, and E. Boye. 1994. The initiation mass for DNA replication in Escherichia coli K-12 is dependent on growth rate. EMBO J. 13:2097-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]