Abstract
Expression of the σD-dependent flagellin gene, hag, is repressed by the CodY protein in nutrient-rich environments. Analysis of a codY mutant bearing a hag-lacZ reporter suggests that the availability of amino acids in the environment is the specific signal that triggers this repression. Further, hag-lacZ expression appears to be sensitive to intracellular GTP levels, as demonstrated by increased expression upon addition of decoyinine. This result is consistent with the postulate that the availability of amino acids in the environment effects intracellular GTP levels through the stringent response. However, the levels of hag-lacZ measured upon the addition of subsets of amino acids suggest an additional mechanism(s). CodY is a DNA binding protein that could repress flagellin expression directly by binding to the hag promoter region, or indirectly by binding to the fla/che promoter region that governs expression of the σD transcriptional activator required for hag gene expression. Using an electrophoretic mobility shift assay, we have demonstrated that purified CodY protein binds specifically to both the hag and fla/che promoter fragments. Additionally, CodY acts as a nutritional repressor of transcription from the fla/che promoter region that contains two functional promoters. CodY binds to both the σD- and σA-dependent promoters in this region, as demonstrated by DNase I footprint analyses. Footprint analyses of the hag gene demonstrated that CodY binds downstream of its σD-dependent promoter. Taken together, these results identify new members of the CodY regulon that encode motility functions in Bacillus subtilis and are controlled by the σD alternate sigma factor.
The σD regulon of Bacillus subtilis is composed of genes encoding proteins for motility and chemotaxis functions (including the structural gene for flagellin, hag) (1, 16), which are maximally expressed at the end of exponential growth in complex, sporulation medium (11, 14, 20). In fact, σD-dependent gene expression has been shown to be subject to nutritional repression (15). In complex medium, expression of a σD-dependent reporter gene (hag-lacZ) is repressed early in exponential growth, increases as nutrients become limiting, and peaks early in stationary phase. In contrast, expression of this reporter remains high and constant throughout growth in minimal medium. The addition of Casamino Acids (CAA) to minimal medium results in a decreased level of hag-lacZ expression that remains constant throughout growth, whereas the addition of amino acids to minimal medium recapitulates the pattern of expression found in complex medium. Therefore, it appears that at least two classes of nutritional signals may control flagellin gene expression.
Interestingly, hag-lacZ expression in a codY mutant growing in complex medium is constitutively high, demonstrating that nutritional repression of flagellin gene expression is abolished in this strain (15). CodY controls the nutritional regulation of several genes involved in competence and the metabolism of nitrogen and acetate in response to growth rate (8). In S7 minimal medium, the development of competence is repressed by the addition of CAA (4). This repression is exerted throughout growth and is relieved in strains bearing a codY mutation (19). Furthermore, the highest levels of CodY-dependent repression of nitrogen metabolism genes occur in cells growing rapidly in a medium rich in amino acids, and this repression is relieved at the end of exponential growth (8). Since CodY has been shown to mediate the repressive effects of both CAA and amino acids in a manner consistent with their regulation of flagellin gene expression, its genetic and molecular role in the control of σD-dependent gene expression was analyzed in this study.
Recent studies have implicated CodY as a global regulator in B. subtilis that monitors the general nutritional state of the cell by sensing intracellular GTP levels (17). Under rich growth conditions when GTP levels are high, CodY acts as a transcriptional repressor, while carbon or nitrogen limitation results in poor growth and decreased GTP levels that appear to relieve CodY repression, allowing for expression of genes that comprise the CodY regulon. Ratnayake-Lecamwasam et al. have demonstrated that GTP binds to purified CodY and acts as a corepressor in an in vitro transcription assay, supporting the postulate that intracellular GTP levels are the molecular link between the nutritional state of the cell and CodY activity (17). This work has received much attention since it provides an elegant mechanism for sensing the nutritional environment of the bacterial cell and effecting global gene expression by CodY in B. subtilis and perhaps nutrient-dependent gene expression in other bacterial species where CodY homologs have been identified (5, 9, 17). In fact, the CodY homolog in Lactococcus lactis acts as a transcriptional repressor of genes involved in oligopeptide transport and appears to respond to the intracellular pool of branched chain amino acids (BCAA) (12). In this study we monitored the effects of varying intracellular GTP pools and BCAA availability on CodY repression of flagellin gene expression.
MATERIALS AND METHODS
Bacterial strains.
The Escherichia coli strains used were XL-1 Blue (recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10] [Tetr]) (Stratagene) and BL21λDE3 (hsdS gal [λcIts857 ind1 Sam7 nin5 lacUV5-T7] gene1) (23) (Novagen) containing pPS41, which has been previously described (18). The latter strain overproduces CodY protein, while XL-1 Blue cells were used for amplification of pTC99. The B. subtilis strains used in this study are listed in Table 1. All strains were maintained on solid medium on tryptose blood agar base (Difco) or Luria broth plates. Antibiotics (Sigma) were used at standard concentrations when necessary: 1 μg of erythromycin/ml, 25 μg of lincomycin/ml, 5 μg of chloramphenicol/ml, 5 μg of neomycin/ml, and 50 μg of ampicillin/ml.
TABLE 1.
B. subtilis strains used in this study
| Strain | Genotype | Source or derivation and reference |
|---|---|---|
| LMB7 | trpC2 pheA1 | J. Hoch, JH642b |
| LMB25 | trpC2 pheA1 hag-lacZ (Eryr) | Transform [LMB7:pDM632Ery, Eryr]a (15) |
| LMB96 | trpC2 pheA1 SPβc2Δ2::Tn917::pSK10Δ6 | P. Zuber, ZB307Ab, (26) |
| LMB207 | trpC2 pheA1 codY146::cat hag-lacZ (Eryr) | Transform [LMB25:FJS151 (21), Cmr]a (15) |
| LMB240 | trpC2 pheA1 SPβc2Δ2φ[fla/che-cat-lacZ] Neor | Transform [LMB96:pTC99, Neor]a (this study) |
| LMB254 | trpC2 pheA1 codY146::cat | Transform [LMB7:FJS51 (21), Cmr]a (this study) |
| LMB255 | trpC2 pheA1 SPβc2Δ2φ[fla/che-cat-lacZ] Neor | Transduce [LMB7:LMB240, Neor]c (this study) |
| LMB256 | trpC2 pheA1 codY146::cat SPβc2Δ2φ[fla/che-cat-lacZ] Neor | Transduce [LMB254:LMB240, Neor]c (this study) |
Bracketed information indicates recipient strain transformed:plasmid DNA (pTC99) or chromosomal DNA used for transformation, selecting for indicated resistance. The reference is shown with parentheses.
Previous name of strain.
Bracketed information indicates recipient strain transduced: source strain of transducing lysate, selecting for indicated resistance. The reference is shown with parentheses.
Construction of B. subtilis strains.
The LMB240 strain bearing the fla/che-cat-lacZ transcriptional fusion was constructed by transformation of LM96 with plasmid pTC99. pTC99 contains the fla/che promoter region residing on a 1.03-kb fragment of DNA amplified from genomic sequence found upstream of flgB, the first structural gene within the fla/che operon. This fragment of DNA was amplified from LMB7 chromosomal DNA by PCR using the upper primer OHW15 (5′-ACACTGCAGGAAACTCCTTGGGTATTCAA-3′) and the lower primer OLS2 (5′-CGGGATCCTCCACTTACCTCCATTTCAGT-3′), which were designed to contain target sites for PstI and BamHI restriction enzymes (underlined), respectively. The conditions for PCR are described below for preparation of probe fragment, except that an annealing temperature of 55°C was used. The resultant 1.03-kb fragment was digested with PstI and BamHI to generate compatible ends for subsequent insertion of the fragment into pJPM122 (22), resulting in pTC99.
For construction of fla/che-cat-lacZ reporter strains, a lysate from LMB240 was prepared as previously described (24). Strains LMB255 and LMB256 were generated by transducing strains LMB7 and LMB254, respectively, with this lysate as described previously (24). LMB7 is the wild-type strain, and LMB254 is an otherwise isogenic strain containing a null mutation in codY that was introduced by transformation of chromosomal DNA from FJS151 as described in Table 1.
Bacterial growth and measurement of β-galactosidase activity.
B. subtilis cultures for β-galactosidase assays were grown as described previously (15) in complex, sporulation (2XSG) or minimal medium (S7) with the addition of 100-μg/ml concentrations each of tryptophan and phenylalanine, since the reporter strains studied are auxotrophic for these requirements. In some experiments, the minimal medium was further supplemented with 0.32% CAA, or subsets of the same amino acids found in CAA were added as pure, sterile solutions to their final concentrations in 0.32% CAA. The effect of decoyinine on hag-lacZ expression was monitored by first growing the reporter strain (LMB25) in complex medium (2XSG) lacking decoyinine to a turbidity at 600 nm of 0.35 to 0.45. The culture was then split into two flasks, and decoyinine (dissolved at 100 mg/ml in 1 N KOH) was added to one flask to a final concentration of 500 μg/ml and an equal volume of 1 N KOH was added to the second flask, as described by others (17). β-Galactosidase activity as a result of hag-lacZ expression was determined as previously described (6), while the incubation time for hydrolysis of o-nitrophenyl-β-d-galactopyranoside was increased to 60 min to monitor fla/che-cat-lacZ expression.
CodY purification.
CodY was purified for an electrophoretic mobility shift assay (EMSA) and DNase I footprint analysis from an E. coli BL21λDE3 strain transformed with pPS41, an overexpression plasmid for codY. Recombinant CodY was purified from this strain essentially as described by others (18), except that the pulse time for sonication was reduced from 15 s to 6 s and the heparin agarose column used was a Hi-Trap column (Pharmacia). Cell extract from the BL21λDE3 strain, lacking the pPS41 plasmid, was similarly produced and used as a negative control.
Preparation of probe fragments.
DNA probes for EMSA and DNase I footprinting analysis were produced via PCR using primers specific to the regions to be amplified and the appropriate plasmids as templates (Table 2). EMSA probes were internally labeled through inclusion of 50 μCi of [α-32P]dCTP (NEN), whereas footprint probes were end labeled by first treating with kinase either the upper or lower primer in the PCR as described below. All PCR amplifications were accomplished in a 25-μl reaction volume containing 1× Taq buffer stock (50 mM KCl, 10 mM Tris-Cl [pH 9.0], 0.1% Triton X-100), 1.5 mM MgCl2, a 1 mM concentration of each deoxynucleoside triphosphate (Promega), a 0.5 μM concentration of each primer for EMSA and unlabeled primer for the footprint assay (Operon), 1 μg of plasmid DNA as template, and 5 U of Taq polymerase (Perkin-Elmer). The cycling parameters were an initial denaturation step of 94°C for 4 min; then 30 cycles at 94°C for 1 min, 50 to 52°C for 1 min, and 72°C for 1 min; and a final extension step of 72°C for 10 min. The annealing temperature for the hag-specific primers was 50°C, and 52°C was used for the remaining primers.
TABLE 2.
Primers and plasmids used for probe preparation
| Probea | Regionb | Upper primerc | Lower primerc | Plasmidd (reference) |
|---|---|---|---|---|
| dppE | −172 to +84 | OFV1: GGACGTTTTTGATGAGGT | OFV2: CCGGAAGACCCGAAATAC | pFS48 (22) |
| hagE | −221 to +135 | OJI3: GTAAAAGTGATTGCGGTTG | OJI4: CGGTTCAGTGTGTTAAGC | pMG601 (14) |
| fla/cheE | −365 to +51 | OJI5: GGTACTAAACAACAAATTCC | OJI6: CCATTTCAGTTTTTTTCACCC | pWE1 (6) |
| PD-3F | −271 to −53 | OJ17:AATAAAGGAAATCAATAAGC | OFC2: GCAACTAGGTATAAAGTCCTAG | pWE1 (6) |
| fla/chePAF | −99 to +51 | OFC1: CTAGGACTTTATACCTAGTTGC | OJI6: CCATTTCAGTTTTTTTCACCC | pWE1 (6) |
| hagF | +30 to +194 | OHAG1/2: CAGTTACAAAATAAGG | OHAG2: CTTAACAACATATTCAGG | pMG601 (14) |
Identity of probe followed by application (E = EMSA; F = footprint analysis).
Region contained within probe relative to transcriptional start site = +1.
Name of primer: sequence.
Plasmid template used in PCR amplification.
The upper or lower primer for footprint analysis (upper to label nontemplate strand, lower to label template) was end labeled in a 30-μl kinase reaction mixture containing 3 μmol of primer, 1× kinase buffer (0.5 M Tris-Cl [pH 8.3], 0.1 M MgCl2, 1 mM spermidine, 1 mM EDTA [pH 5.0], 15 mM dithiothreitol), 50 μCi of [γ-32P]dATP, and 15 U of T4 polynucleotide kinase. The reaction was incubated at 37°C for 45 min, and the enzyme was inactivated by heat denaturation at 65°C for 10 min. Half of the kinase reaction mixture (15 μl) was added to the PCR described above for specific labeling of either the template or nontemplate strand. In order to isolate full-length probes and to remove unincorporated radionucleotides, the entire contents of each PCR were resolved by electrophoresis on an 8% polyacrylamide (30:1) nondenaturing gel. The full-length radiolabeled probes were purified from gel slices into double-distilled H2O following standard procedures, and the volume was reduced by repeated extraction with s-butanol. Typically, probe preparations of 40,000 cpm/μl, as determined by scintillation counter, were obtained for use in the footprint assay.
EMSA.
EMSAs for assessment of DNA binding activity were performed as described previously (18), with minor modifications. As opposed to using end-labeled DNA probes, the probes were generated by PCR and radiolabeled by the addition of [α-32P]dCTP as described above. Specific activities for the radiolabeled probes synthesized were approximately 108 cpm/μg, and 50,000 cpm was used in each assay. Crude extracts from the E. coli strain overproducing CodY, as well as from the same strain lacking the overexpression vector, were added at 89 ng unless otherwise noted. Additionally, the native gels were not dried before being subjected to autoradiography.
DNase I footprinting analysis.
This method was used to determine the specific regions on fla/che and hag bound by CodY. End-labeled probes were prepared and purified as described above, and CodY binding was allowed as described for the EMSA, except that a 10-μl volume was used. Magnesium and calcium chloride levels were adjusted to 1.5 and 0.5 mM, respectively, by the addition of activator buffer (5 mM MgCl2, 10 mM CaCl2), and DNase I digestion was performed by adding 1 U of RQ1 DNase I. The digestion reaction was allowed to proceed for 45 s at 25°C and terminated by the addition of 4 μl of stop solution (20 mM EDTA, 1% sodium dodecyl sulfate, 0.6 M NaOAC, 0.25 mg of tRNA/ml). The DNA products were extracted by phenol-chloroform, precipitated by the addition of ethanol, and resuspended in 3 μl of sequencing gel loading buffer (Amersham Life Science) and 3 μl of 0.2 N NaOH prior to loading on a 6-to-8% polyacrylamide gel electrophoresis-urea gel. The gels were dried and subjected to autoradiography.
RESULTS AND DISCUSSION
Repression of hag-lacZ expression by the availability of amino acids is mediated by CodY.
Previously, we demonstrated that CodY mediates nutritional repression of flagellin gene (hag) expression in complex medium. Specifically, hag-lacZ expression was found to be constant and high in a codY mutant, whereas hag-lacZ expression in a wild-type strain is repressed early in exponential growth (15). In fact, recent work has implicated the codY gene product in the nutritional regulation of several genes and operons and has demonstrated that it apparently responds to the availability of CAA, as well as to a mixture of amino acids in the environment (3, 7, 8, 10, 19, 25). Since we had previously determined that hag-lacZ expression is also repressed by the addition of CAA and amino acids (15), we sought to determine the role of CodY in mediating these specific responses.
Flagellin gene expression in response to CAA and amino acid addition was monitored in wild-type (LMB25) and codY mutant (LMB207) cells by measuring β-galactosidase activity throughout growth in minimal medium and in minimal medium supplemented with 0.32% CAA or with the same combination and concentration of purified amino acids. The pattern of hag-lacZ expression found in the wild-type background was nearly identical to the results previously obtained (Fig. 1A) (15). hag-lacZ expression is constant and high in cells growing in minimal medium and is repressed throughout growth (>3-fold) by the addition of CAA. A similar level of repression is achieved early in exponential growth by the addition of purified amino acids. However, this repression is relieved at the end of exponential phase, presumably as the amino acids are exhausted. These results demonstrate that a mixture of amino acids recreates the pattern of expression found for the flagellin gene in rich medium (15) and that an additional component of CAA (that is neither an amino acid nor exhausted by bacterial metabolism) exerts negative control of hag gene expression.
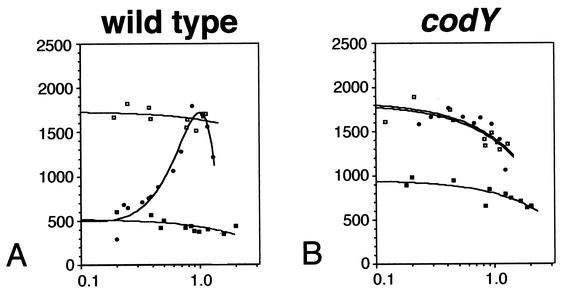
FIG. 1.
Flagellin gene expression in wild-type and codY mutant strains. β-Galactosidase activity was monitored in strains bearing a hag-lacZ reporter construct throughout growth in minimal medium and minimal medium supplemented with either 0.32% CAA or the same combination and concentration of purified amino acids. For each panel, the y axis is β-galactosidase activity expressed in Miller units and the x axis is absorbance at 600 nm. Symbols: open squares, minimal medium; closed squares, minimal medium plus 0.32% CAA; closed circles, minimal medium plus amino acids. (A) Wild-type strain (LMB25); (B) codY mutant strain (LMB207). The data presented are the results of two experiments.
Expression of the hag-lacZ reporter in a codY mutant grown in the presence or absence of amino acids was constant and high (Fig. 1B), demonstrating that CodY is required for nutritional repression of flagellin gene expression in response to the availability of amino acids in the environment. However, the effect of CAA on hag-lacZ expressions appears to be largely independent of CodY. The pattern of hag-lacZ expression in the codY mutant grown in the presence of CAA was identical to the wild-type strain grown in this medium and was repressed approximately twofold throughout growth (Fig. 1B). These results are consistent with previous work demonstrating that at least two classes of nutritional signals repress flagellin gene expression (15). Whereas CodY is absolutely required for amino acid repression of flagellin gene expression, it appears to play a small role in governing the level of the response to CAA.
CodY repression of hag-lacZ appears to be governed by intracellular GTP levels.
A recent study has implicated intracellular GTP levels as the signal that governs CodY activity in response to amino acid availability via the stringent response (17). We therefore studied the effect of decoyinine addition and differential activation of the stringent response on hag-lacZ expression.
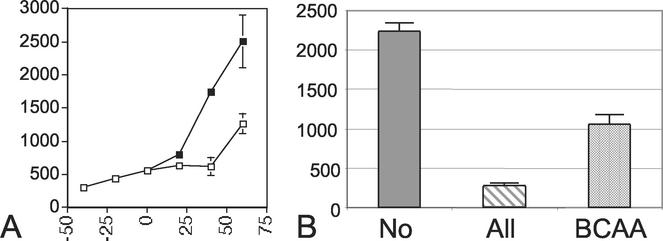
Decoyinine addition decreases intracellular GTP levels due to its role as a GMP synthetase inhibitor. Cells bearing the hag-lacZ translational fusion (LMB25) were grown in complex medium to early exponential phase in the absence of decoyinine. The culture was split, and decoyinine was added to one flask. Within 20 min a detectable increase in hag-lacZ expression was observed in cells growing in the presence of decoyinine. At 1 h following decoyinine addition, maximum expression of the reporter was obtained and levels were nearly twofold greater than the levels of β-galactosidase measured for cells grown in the absence of decoyinine (Fig. 2A). The effect of decoyinine addition on hag-lacZ expression was abolished in a reporter strain bearing a codY null mutation (LMB207) (data not shown). These results are consistent with previous studies demonstrating increased expression of CodY targets following decoyinine addition (9) and implicate intracellular GTP levels in the regulation of flagellar gene expression by CodY.
FIG. 2.
Effects of decoyinine and subsets of amino acids on flagellin expression. β-Galactosidase activity was monitored in a wild-type strain bearing a hag-lacZ reporter construct. (A) hag-lacZ expression in complex, sporulation medium (open squares) and in complex medium with the addition of 500 μg of decoyinine/ml (closed squares). The y axis is β-galactosidase activity expressed in Miller units, and the x axis is time in minutes. At the zero time point the culture was split and decoyinine added as described in Materials and Methods. The data presented are the results of two experiments. (B) hag-lacZ expression early in logarithmic growth (A600 = 0.5) in minimal medium and in minimal medium supplemented with the purified amino acids listed on the x axis at the same concentrations found in 0.32% CAA. No = no amino acid supplementation; All = all amino acids found in CAA; BCAA = leucine, isoleucine, and valine. The y axis is β-galactosidase activity expressed in Miller units. The data presented are averages of replicate experiments.
Intracellular GTP pools are apparently affected by amino acid availability through the stringent response (2). As a result of amino acid limitation, the ratio of uncharged to charged tRNA in the cell increases, triggering the stringent response and the synthesis of ppGpp from existing GTP pools by the stringent response regulator, RelA. Furthermore, production of ppGpp inhibits IMP dehydrogenase, the first enzyme in the GMP synthesis pathway. Therefore, activation of the stringent response by amino acid limitation decreases intracellular GTP by at least two separate mechanisms and is predicted to inhibit CodY activity in the cell. This hypothesis is based on recent biochemical studies demonstrating that CodY is a GTP binding protein and that the GTP cofactor is required for maximum repression of one of its target genes (dpp) in an in vitro transcription reaction (17).
We sought to quantify the level of repression exerted by CodY on flagellin expression due to activation of the stringent response, since the high levels of hag-lacZ expression (>2,000 Miller units when maximally expressed) provide a sensitive monitor of CodY activity. The hag-lacZ reporter strain was grown in S7 minimal medium containing 2 mM glucose. Under these conditions, glucose is utilized as the energy source, and the addition of 0.32% CAA is nearly limiting for growth and has a maximal effect on the repression of hag-lacZ expression (15). The hag-lacZ reporter strain (LMB25) was grown in S7 medium in the presence or absence of amino acids, and their effect on flagellin gene expression was measured during early exponential growth (A600 = 0.5). The lack of amino acid supplementation was presumed to activate the stringent response, and a time point early in exponential growth was chosen for this analysis since maximal CodY repression of hag expression is found at this time (Fig. 1A). Flagellin gene expression in the reporter strain resulted in an eightfold decrease in β-galactosidase activity (285 versus 2,242 Miller units) when the reporter cells were grown in the presence of amino acids (Fig. 2B). These results support the hypothesis that activation of the stringent response by the depletion of amino acids in the medium leads to inactivation of CodY and an eightfold increase in hag-lacZ expression.
Interestingly, the addition of BCAA results in a twofold decrease in hag-lacZ expression in the reporter strain (1,063 versus 2,242 Miller units) when compared to flagellin expression in the absence of amino acid supplementation (Fig. 2B). While BCAA addition may result in partial activation of the stringent response and a decrease in CodY repressor activity, it appears that these amino acids may play a role in governing CodY function independent of the stringent response. The CodY homolog in L. lactis responds to the availability of BCAA in the environment to mediate repression of its target genes (12). The addition of BCAA in our system leads to a twofold repression of hag-lacZ expression (Fig. 2B), consistent with a similar regulatory mechanism in B. subtilis.
Taken together, the results of our supplementation studies support the proposed role of the stringent response in indirectly controlling CodY activity by affecting intracellular GTP levels and suggest that additional signaling mechanisms exist. Additional environmental signals may include the monitoring of BCAA levels as seen in L. lactis and a non-amino-acid component found in CAA.
CodY binds specifically to hag and fla/che promoter fragments.
Having further characterized the nature of the nutritional signal responsible for repression of flagellin gene expression by CodY, we sought to analyze the molecular mechanism by which it exerts this control. CodY is a DNA binding protein that binds specifically to the srf, comK, and dpp promoters (18) and therefore appears to exert its repressive effect on transcription by inhibiting association of holoenzyme with promoter DNA. Expression of the flagellin gene initiates from a σD-dependent promoter and requires expression of the σD structural gene, sigD (14), encoded within the fla/che transcription unit (13). The effect of CodY on flagellin gene expression could be exerted directly by binding to the hag promoter or indirectly by binding to the fla/che promoter region, which contains dual promoters recognized by the σA and σD forms of the holoenzyme (6).
EMSA was used to monitor the ability of CodY to bind to the hag and fla/che promoter regions. Radiolabeled probes for both regions were prepared by PCR in the presence of [32P]dCTP as described in Materials and Methods. Radiolabeled dpp promoter probe was similarly prepared as a positive control for CodY binding, whereas the polC promoter fragment was prepared as a negative control (18). In all cases, radiolabeled probe was incubated in the absence of protein extract, with crude extract from the BL21λDE3 E. coli strain lacking the CodY-overproducing plasmid (pPS41), or with crude extract from the same strain bearing pPS41 and overproducing CodY.
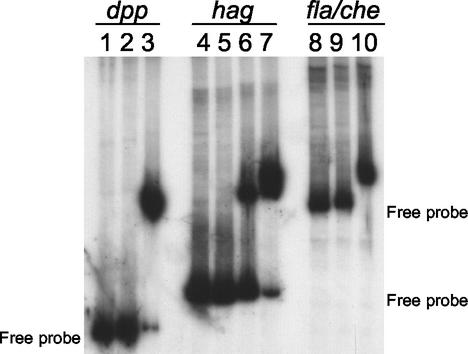
Our preparation of CodY protein was found to bind to the dpp promoter probe (Fig. 3, lane 3) and failed to bind to the polC probe (data not shown), confirming the results of others (18). Moreover, CodY was found to bind to both the hag and fla/che promoter probes (Fig. 3, lanes 6, 7, and 10). The retardation of electrophoretic mobility was shown to be consistent with binding of CodY protein, since no retardation was observed when crude extract from the E. coli strain lacking the codY overexpression vector (pPS41) was used (Fig. 3, lanes 2, 5, and 9).
FIG. 3.
Gel retardation of 32P-labeled promoter fragments by crude extracts from E. coli cells overproducing CodY. Lanes 1 to 3, dpp promoter probe; lanes 4 to 7, hag promoter probe; lanes 8 to 10, fla/che promoter probe. Lanes 1, 4, and 8, radiolabeled probe alone; lanes 2, 5, and 9, 89 ng of crude extract from a strain lacking the codY overexpression vector; lanes 3, 6, and 10, 89 ng of crude extract from CodY-overproducing strain; lane 7, 445 ng of crude extract from CodY-overproducing strain.
Therefore, the effect of CodY on flagellin gene expression appears to be exerted directly by binding to the hag promoter and indirectly by binding to the fla/che promoter region required for normal expression of sigD (6). Interestingly, a fivefold increase in CodY (445 ng) crude extract was required to obtain the same shift for the hag promoter as found for the dpp promoter (Fig. 3, compare lanes 3 and 7), whereas the same amount of CodY crude extract completely retarded the electrophoretic mobility of the fla/che promoter probe (Fig. 3, lane 10). These results suggest a similar affinity for CodY binding to the dpp and fla/che promoter regions that is relatively stronger than that of CodY binding to the hag promoter. In fact, competition assays using cold dpp promoter DNA as competitor (data not shown) demonstrated an intrinsic binding affinity of CodY for fla/che > dpp ≫ hag promoter DNA under the EMSA conditions used, further confirming the specificity of CodY binding to the fla/che and hag promoters.
CodY appears to control expression from the fla/che promoter region in vivo.
Having demonstrated that CodY binds to fla/che promoter DNA in vitro, we determined whether it controls fla/che expression in the cell. A fla/che-cat-lacZ reporter fusion was introduced into the SPβ site of wild-type and codY mutant cells, and fla/che expression was monitored by measuring β-galactosidase activity. Since fla/che promoter function is subject to feedback regulation by its gene products (6, 24), the fla/che reporter was introduced at the SPβ site. In this way the endogenous fla/che operon remains undisturbed, permitting us to study CodY regulation of fla/che expression under conditions in which the fla/che gene products are normally expressed.
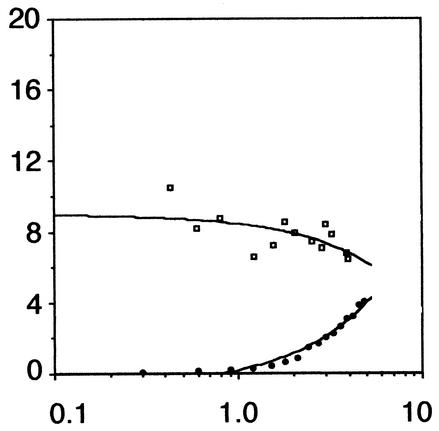
The results found in Fig. 4 demonstrate that while expression of fla/che-cat-lacZ is repressed early in growth in the wild-type strain, fla/che expression is released from nutritional repression in the codY strain. These results suggest that CodY is required for nutritional repression of fla/che expression in the cell. The fla/che promoter region contains two functional promoters, PD-3 and fla/chePA, recognized by the σD and σA forms of RNA polymerase, respectively (6). The results of the fla/che-cat-lacZ experiment failed to differentiate between CodY control of an individual promoter or its possible regulation of both promoter elements.
FIG. 4.
fla/che expression in wild-type and codY mutant strains. β-Galactosidase activity was monitored in strains bearing a fla/che-cat-lacZ reporter construct throughout growth in complex, sporulation medium. The y axis is β-galactosidase activity expressed in Miller units, and the x axis is absorbance at 600 nm. Symbols: closed circles, wild type (LMB255); open squares, codY (LMB256). The data presented are the results of two experiments.
Footprint analyses demonstrate that CodY binds to each of the fla/che promoters.
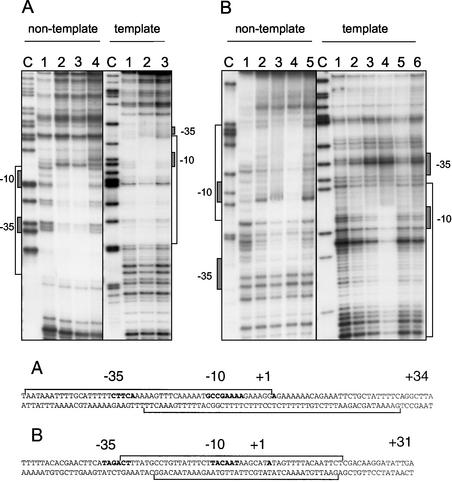
Since the fla/che promoter region contains both σD- and σA-dependent promoters (6), fla/che promoter probes were independently generated for each promoter (Table 2). The PD-3F probe was designed to contain DNA specific to the σD-dependent promoter, whereas the fla/chePAF probe contained sequences specific for the σA-dependent promoter. The nontemplate or template strand was labeled for each promoter probe, and the areas of CodY protection were determined by DNase I footprint analysis in the presence or absence of GTP.
Our results (Fig. 5) demonstrate that CodY binds to both the PD-3 and fla/chePA promoters on both the nontemplate and template strands, protecting the −10, −35, and nearby sequences from DNase I digestion. Similar levels of CodY protein (32.5 to 75 nM CodY) allowed for detectable levels of CodY binding to both promoters, suggesting a similar affinity of CodY for both the σA- and σD-dependent promoter sequences. However, the addition of 150 nM CodY to the PD-3 probe resulted in extended areas of protection not found for the fla/chePA probe. The latter result is particularly interesting given that only fla/chePA plays an important role in the expression of the fla/che operon (24). The extended area of protection seen for both the nontemplate and template strands of fla/chePA when 150 nM CodY was used (Fig. 5B, lane 4 in each panel), compared to the area of protection found when 75 nM CodY was used (Fig. 5B, lane 3 in each panel), may indicate cooperative binding of CodY. In fact, footprint analyses of other CodY targets have defined areas of protection ranging from 35 to 99 nucleotides (18, 19), consistent with cooperative binding of CodY to its target sequences.
FIG. 5.
DNase I footprint analyses of the fla/che promoter region that contains dual promoter elements dependent on σD and σA holoenzymes. The σD-dependent promoter (PD-3) lies 133 bp upstream of the σA-dependent promoter (fla/che PA), which lies 70 bp upstream of the translational start codon for the first gene in the fla/che operon, flgB (6). Individual probe fragments were prepared for each promoter and either the nontemplate or template strand radiolabeled as described in Materials and Methods. For each set of lanes, a sequencing reaction containing ddCTP was generated, and the products were resolved to produce a sequencing ladder labeled as the C lane. (A) Results of CodY binding to PD-3, the σD-dependent promoter. Nontemplate lane 1, no CodY protein; lanes 2 and 3, 32.5 and 75 nM CodY; lane 4, 32.5 nM CodY in the presence of 2 mM GTP. Template lane 1, no CodY protein; lane 2, 75 nM CodY; lane 3, 75 nM CodY in the presence of 2 mM GTP. (B) Results of CodY binding to fla/chePA, the σA-dependent promoter. Nontemplate lane 1, no CodY protein; lanes 2 to 4, increasing amounts of CodY protein (32.5, 75, and 150 nM); lane 5, 32.5 nM CodY in the presence of 2 mM GTP. Template lane 1, no CodY protein; lanes 2 to 4, increasing amounts of CodY protein (32.5, 75, and 150 nM); lanes 5 and 6, 32.5 and 75 nM CodY in the presence of 2 mM GTP. The sequence of the protected regions on each strand is given at the bottom, and the −10, −35 promoter sequences are indicated in boldface, along with the +1 transcriptional start sites that were determined in previous studies (6).
CodY has been shown to be a GTP binding protein, and 2 mM GTP was found to increase repression of the dpp target gene in an in vitro transcription assay. However, binding of CodY to the dpp and srfA target genes is relatively unaffected (17). We therefore postulated that the addition of 2 mM GTP might affect the CodY footprint found on the fla/che promoters, indicative of a conformational change upon GTP binding. Perhaps GTP binding by CodY promotes a change in its conformation that increases its ability to act as a transcriptional repressor. No change in the footprint was observed upon GTP addition (Fig. 5A and B, last lanes in each panel). Further, it appeared that the addition of this nucleotide partially inhibited DNase I activity as evidenced by the greater representation of higher-molecular-weight DNA fragments in the reaction containing GTP. Therefore, our results suggest that the addition of 2 mM GTP does not significantly affect the footprint of CodY on either of the fla/che promoters, as has been found for the dpp and srfA targets (A. Sonenshein, personal communication).
CodY binds downstream of the σD-dependent hag promoter.
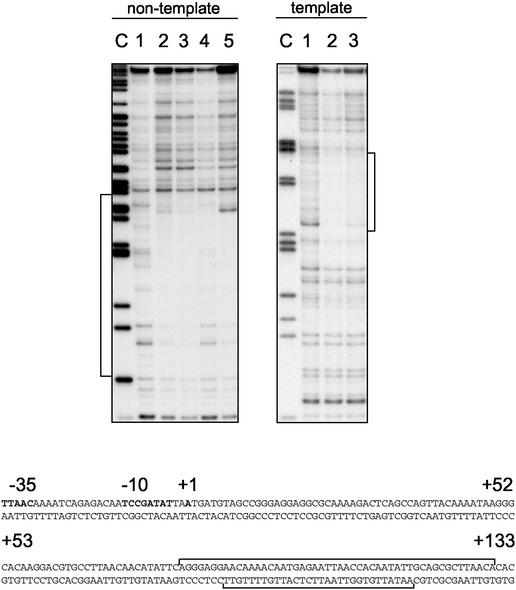
Initial attempts to localize the region of CodY binding to the hag promoter by DNase I footprint analysis demonstrated that the protected region was downstream of the σD-dependent promoter. As a result, new hag probes were synthesized that contained sequences downstream of the promoter (Table 2). Binding of CodY to these probes was comparable to binding of CodY to the fla/che probes, requiring 75 to 150 nM CodY for visible detection of the protected region. It appears that the relatively weak affinity of CodY to the hag probe used for EMSA may be due to the fact that the protected region is located at the 3′-most region of that probe, and additional residues downstream of this region may be required for stabilization of CodY binding.
The CodY footprint of the hag probe begins approximately 80 bp downstream of the transcriptional start site on both the nontemplate strand and template strands (Fig. 6). Protection of the nontemplate strand includes the sequences encoding the ribosome binding site and start codon (Fig. 6). Furthermore, as was found for CodY binding to the fla/che promoters, 2 mM GTP failed to promote a significant change in the CodY footprint found for the hag probe (Fig. 6, lanes 5 and 3 in the nontemplate and template panels, respectively).
FIG. 6.
DNase I footprint analyses of the 5′ end of the hag gene. Individual probe fragments were prepared for either the nontemplate or template strand radiolabeled as described in Materials and Methods. For each set of lanes a sequencing reaction containing ddCTP was generated, and the products were resolved to produce a sequencing ladder labeled as the C lane. Nontemplate lane 1, no CodY protein; lanes 2 to 4, increasing amounts of CodY protein (75, 150, and 300 nM); lane 5, 75 nM CodY in the presence of 2 mM GTP. Template lane 1, no CodY protein; lane 2, 150 nM CodY; lane 3, 150 nM CodY in the presence of 2 mM GTP. The sequence of the protected regions on each strand is given at the bottom, and the −10, −35 σD-dependent promoter is indicated in boldface along with the +1 transcriptional start site that was determined previously (14). The ribosome binding site and translational start codon are underlined and in boldface.
Binding of CodY to the srfA, comK, and dpp promoter regions includes, or is near, the σA-dependent promoters found within these regions (18, 19). CodY protection of the srfA promoter includes both the −10 and −35 regions, while only the −35 region of comK is protected, and protection of dpp begins immediately downstream of the −10 region and includes the transcriptional start site. CodY protection of the sequences encoding the ribosome binding site and start codon appears to be unique to the hag gene. No consensus target site has been identified for CodY binding (8), and the addition of the fla/che and hag sequences protected from DNase I digestion in an alignment search failed to identify highly conserved sequence motifs (data not shown). Instead, it has been proposed that a DNA structure formed by AT-rich DNA sequences is recognized and bound by CodY (9, 19). In fact, the sequences bound by CodY in both the fla/che promoter and hag gene are AT rich, although no conserved DNA structure was identified for these targets and other members of the CodY regulon by computer-based DNA-folding algorithms (data not shown).
While the specific molecular determinants for CodY binding to target DNA remain elusive, our results have led to the identification and study of additional members of the CodY regulon. Expression of the hag gene and fla/che operon was found to be subject to nutritional repression by CodY, and this activity was linked to intracellular GTP levels and perhaps the availability of BCAA and small peptides. These data confirm and extend our understanding of CodY, an important global regulator in B. subtilis that is highly conserved in other bacterial species (9, 12, 17). CodY appears to monitor the general nutritional state of the cell and govern the expression of gene products required for adaptation to nutritional stress, including competence factors and nitrogen utilization proteins (9). This work demonstrates that motility functions are another adaptive response controlled by CodY, since the hag gene and fla/che operon are required for flagellar assembly and expression of the flagellum-specific alternate sigma factor, SigD (1, 16). Moreover, the first biochemical evidence is presented for control of a SigD-dependent gene by the CodY protein.
Acknowledgments
We are grateful to Abraham L. Sonenshein and members of his laboratory for providing the CodY-overproducing strain and for technical advice necessary for completion of this work. We thank Laura Burrus and Joyce West for their insightful guidance in preparing CodY protein extracts and effectively implementing the EMSA, respectively. We acknowledge Ivan Moszer for amino acid utilization data in B. subtilis and the CCSF Bridges summer students of 1999 for construction of pTC99.
This research was supported by NSF-CAREER grant MCB-9600932 and NIH MBRS SCORE grant S06 GM52588 to L.M.-M. and by NIH-MBRS GM52588-03 support to J.I. and J.C.P.
REFERENCES
- 1.Aizawa, C., I. Zhulin, L. M. Márquez-Magaña, and G. Ordal. 2002. Motility and chemotaxis, p. 437-452. In A. Sonenshein, R. Losick, and J. Hoch (ed.), Bacillus subtilis and its relatives: from genes to cells. ASM Press, Washington, D.C.
- 2.Cashel, M., D. R. Gentry, V. J. Hernandez, and D. Vinella. 1996. The stringent response, p. 1458-1496. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 3.Debarbouille, M., R. Gardan, M. Arnaud, and G. Rapoport. 1999. Role of BkdR, a transcriptional activator of the SigL-dependent isoleucine and valine degradation pathway in Bacillus subtilis. J. Bacteriol. 181:2059-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubnau, D., J. Hahn, L. Kong, M. Roggiani, and Y. Weinrach. 1991. Genetic competence as a post-exponential global response. Semin. Dev. Biol. 2:3-11. [Google Scholar]
- 5.Dworkin, J., and R. Losick. 2001. Linking nutritional status to gene activation and development. Genes Dev. 15:1051-1054. [DOI] [PubMed] [Google Scholar]
- 6.Estacio, W. E., S. Santa Anna-Arriola, M. Adedipe, and L. M. Márquez-Magaña. 1998. Dual promoters are responsible for transcription initiation of the fla/che operon of Bacillus subtilis. J. Bacteriol. 180:3548-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferson, A. E., L. V. Wray, Jr., and S. H. Fisher. 1996. Expression of the Bacillus subtilis gabP gene is regulated independently in response to nitrogen and amino acid availability. Mol. Microbiol. 22:693-701. [DOI] [PubMed] [Google Scholar]
- 8.Fisher, S. 1999. Regulation of nitrogen metabolism in Bacillus subtilis: vive la différence! Mol. Microbiol. 32:223-232. [DOI] [PubMed] [Google Scholar]
- 9.Fisher, S. H., and M. Debarbouille. 2002. Nitrogen source utilization and its regulation, p. 181-191. In A. Sonenshein, R. Losick, and J. Hoch (ed.), Bacillus subtilis and its relatives: from genes to cells. ASM Press, Washington, D.C.
- 10.Fisher, S. H., K. Rohrer, and A. E. Ferson. 1996. Role of CodY in regulation of the Bacillus subtilis hut operon. J. Bacteriol. 178:3779-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilman, M., and M. J. Chamberlin. 1983. Developmental and genetic regulation of Bacillus subtilis genes transcribed by sigma-28 RNA polymerase. Cell 35:285-293. [DOI] [PubMed] [Google Scholar]
- 12.Guédon, E., P. Serror, S. Dusko Ehrlich, P. Renault, and C. Deforme. 2001. Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol. Microbiol. 40:1227-1239. [DOI] [PubMed] [Google Scholar]
- 13.Márquez-Magaña, L. M., and M. J. Chamberlin. 1994. Characterization of the sigD transcription unit of Bacillus subtilis. J. Bacteriol. 176:2427-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirel, D. B., and M. J. Chamberlin. 1989. The Bacillus subtilis flagellin (hag) gene is transcribed by the σ28 form of RNA polymerase. J. Bacteriol. 174:3095-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirel, D. B., W. F. Estacio, M. Mathieu, E. Olmsted, J. Ramirez, and L. M. Márquez-Magaña. 2000. Environmental regulation of Bacillus subtilis σD-dependent gene expression. J. Bacteriol. 182:3055-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ordal, G. W., L. M. Márquez-Magaña, and M. J. Chamberlin. 1993. Motility and chemotaxis, p. 765-784. In A. Sonenshein, J. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. ASM Press, Washington, D.C.
- 17.Ratnayake-Lecamwasam, M., P. Serror, K.-W. Wong, and A. L. Sonenshein. 2001. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev. 15:1093-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serror, P., and A. L. Sonenshein. 1996. Interaction of CodY, a novel Bacillus subtilis DNA-binding protein, with the dpp promoter region. Mol. Microbiol. 20:843-852. [DOI] [PubMed] [Google Scholar]
- 19.Serror, P., and A. L. Sonenshein. 1996. CodY is required for nutritional repression of Bacillus subtilis genetic competence. J. Bacteriol. 178:5910-5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer, V. L. 1987. Characterization of promoters and genes controlled by Bacillus subtilis sigma-28 RNA polymerase. Ph.D. thesis. University of California, Berkeley.
- 21.Slack, F. J., P. Serror, E. Joyce, and A. L. Sonenshein. 1995. A gene required for nutritional repression of the Bacillus subtilis dipeptide permease operon. Mol. Microbiol. 15:689-702. [DOI] [PubMed] [Google Scholar]
- 22.Slack, F. J., J. P. Mueller, and A. L. Sonenshein. 1993. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide transport operon. Mol. Microbiol. 175:4605-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-130. [DOI] [PubMed] [Google Scholar]
- 24.West, J. T., W. Estacio, and L. M. Márquez-Magaña. 2000. Relative roles of the fla/che PA, PD-3, and PsigD promoters in regulating motility and sigD expression in Bacillus subtilis. J. Bacteriol. 182:4841-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wray, L. V., Jr., A. E. Ferson, and S. H. Fisher. 1997. Expression of the Bacillus subtilis ureABC operon is controlled by multiple regulatory factors including CodY, GlnR, TnrA, and Spo0H. J. Bacteriol. 179:5494-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuber, P., and R. Losick. 1987. Role of abrB in spoOA- and spoOB-dependent utilization of a sporulation promoter in Bacillus subtilis. J. Bacteriol. 169:2223-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]