Abstract
We tested for hormonal and behavioral differences between Carolina chickadees (Poecile carolinensis) taken from a disturbed (recently logged) forest, an undisturbed forest, or a residential site. We measured fecal corticosterone and body mass levels in the field, and fecal corticosterone, body mass, and caching behavior in an aviary experiment. In the field, birds from the disturbed forest exhibited significantly higher fecal corticosterone levels than birds from either the undisturbed forest or from the residential site. Birds from the disturbed forest also exhibited lower body mass than those from the undisturbed forest but higher body mass than those from the residential site. Our aviary results suggest that these physiological differences between field sites are the result of short-term responses to ecological factors: Neither body mass nor fecal corticosterone levels varied between birds captured at different sites. Aviary sample sizes were sufficient to detect seasonal variation in fecal corticosterone (lowest in summer), body mass (highest in spring), and rate of gain in body mass (highest in winter). Under “closed-economy” aviary conditions (all food available from a feeder in the aviary), there were no site differences in the percent of seeds taken from the feeder that were cached. However, under “open-economy” conditions (food occasionally available ad libitum), significantly fewer seeds were cached by birds from the disturbed forest compared to the undisturbed or residential sites. On average, there was only a two-fold difference in population-levels of fecal corticosterone. This difference is about the same as an increase in fecal corticosterone induced by a two-hour increase in food deprivation, and can not be considered to be an acute stress response to disturbance.
Keywords: Chickadee, Poecile carolinensis, fecal corticosterone, forestry effects, caching, forest disturbance, stress, energy regulation
INTRODUCTION
Forest management practices, deforestation, and large-scale fragmentation of forests can have lasting impacts on community composition and species richness (Brawn et al. 2001; Hughes et al. 2002; Nardoo 2004). One obvious result of forest loss and increased fragmentation of forest tracks is the loss of, or change in, the habitat features on which many species depend (Murcia 1995), and this can result in declining numbers of affected species (Chalfoun et al. 2002; Hobson and Bayne 2000). While it is critical for us to understand the impact of habitat loss on the decline and extirpation of populations, certain physiological indices may provide more immediate measures of the effects of human-induced environmental disturbance and potentially of the ability of populations to adapt to these disturbances. Several studies demonstrate that extended periods of acute physiological “stress” can negatively affect both behavior (foraging or social behavior) and physiology (reproductive, nutrient processing or immune system). These effects could in turn be detrimental for a population’s survival (see Sapolsky 1996; Wingfield et al. 1997). Therefore, habitat alterations that cause elevated levels of stress may not decrease population size directly but might affect the population’s long- term viability in a host of ways (Hoffman & Hercus 2000).
In the face of increased global habitat loss and fragmentation, studies uncovering the relationship of these disturbances to physiological state (and thus individual fitness and population viability) are necessary. For example, Suorsa et al. (2004) demonstrated that “physiological stress” (measured as the heterophil-lymphocyte ratios) and cell-mediated immunocompetence are negatively related to forest patch area in Eurasian treecreeper chicks (Certhia familiaris). Eurasian treeceeper chicks from young-aged forests also exhibit higher plasma corticosterone (cort) levels than chicks from old-growth forests (Suorsa et al. 2003). Wasser et al. (1997) showed that male Northern spotted owls (Strix occidentalis caurina) in timber harvest areas had higher cort levels than males in areas undergoing minimal harvest; similar results were found comparing males close to major logging roads with males far from roads (but see Tempel and Gutierrez, 2004, for an example of no effect of management on California spotted owls, Strix occidentalis occidentalis). Note that each of these affected species can be characterized as “interior species” that specialize on deep forest habitat. These species should be particularly prone to forest fragmentation effects (Bender et al. 1998), and the physiological data suggest that this prediction is met (but see Mazerolle and Hobson 2002 who showed that increased territory defense in contiguous forests results in high energetic demand and reduced immunological condition compared with birds in fragmented forest).
This analysis leaves open the question of how forestation effects (as measured by physiological indices) are distributed among different components of the community. Bender et al. (1998) suggested that the effect of patch size on population density is negligible for generalist species (i.e., those that use both edge and interior habitats), as compared to interior or edge species. Here we ask whether the physiological response to recent forest management practices in a generalist species, Carolina chickadees (Poecile carolinensis), is similar to the patterns seen in interior species. We measured physiological state of individuals in both field and laboratory settings using fecal glucocorticoid metabolites (fCort) levels as our index of physiological stress (as in Creel et al. 2002; Millspaugh et al. 2001; Wasser et al. 1998). We also measured relative body mass (corrected for size) as an index of body condition. To measure foraging behavior, we conducted experiments on caching rates under laboratory conditions. Both field and aviary measurements were taken over the course of one year to give some understanding of seasonal variation in the behavioral and physiological traits we were measuring. The field measurements give us an indication of the stress-related (as measured by fCort levels) and energy-related (as measured by relative body mass) responses to the ecological conditions that exist at the site of capture. Our laboratory experiments provide some context for these field results because birds from all populations were housed under the same conditions. If birds from the different populations have different physiological or behavioral responses to our aviary conditions, these differences may be the result of relatively non-plastic adult characteristics, possibly stemming from genetic differences or acquired differences caused by development in different habitats. However, if birds from the different populations have similar physiological responses to our aviary conditions, we would conclude that the population differences seen in the field represent short-term reactive responses to the different environments. Our aviary experiments also included deprivation intervals of varying lengths (see Methods); these experiments give us some measure of the degree of deprivation required to induce an increase in fCort levels. These results should also give us some context with which to understand population differences in fCort levels.
METHODS
Sites and Field Samples
We collected at four sites, an undisturbed forest site, a disturbed forest site and two residential sites. The bulk of our samples are from two sites owned by Purdue University, the Ross Reserve Biological Station (“undisturbed forest” site) and Martell Forest (“disturbed forest” site). The sites are part of a ribbon of forest that runs along the Wabash River. These two sites are about 5 km from each other and about 12 km west of the city of West Lafayette, IN. Given that flock ranges are 10–20 ha at their maximum (Smith 1991), these populations can be considered to be functionally independent. This is underscored by the fact that the Ross and Martell forest populations have different dialects in their chick-a-dee calls (Freeberg et al. 2003). The Ross Reserve has not been logged for at least 70 years, whereas the Martell Forest has had both clear-cutting and selective logging as a part of the activities of the Department of Forestry and Natural Resources research program. The Martell Forest contains several stands of walnut trees and white pines, in addition to the native oak/hickory forest typical of the area (and typical of the Ross Reserve). Our sampling from December 2000 through February 2002 followed several clear-cuttings (2.5 hectares in November 1999, 1 hectare in January 2000, 1.5 hectares in January 2001, 1.5 hectares in September 2001, and 1 hectare in January 2002), disrupting about 30% of the forested area from which we collected birds. All birds were collected a maximum of 100m from a clear cut area.
In addition to the samples from these two forested sites, we collected field samples from marked birds from a residential area inside the city of West Lafayette (“West Lafayette residential” site), characterized by 50–100 year-old homes and mature oak and hickory trees on half-acre lots. We also obtained laboratory data from two birds collected from a home near Battleground, Indiana. The home is on a 2-acre wooded lot adjacent to the Wabash River 10 km northeast of West Lafayette (“Battleground residential” site) in an area characterized by 50–100 year-old homes. Birds at both of these sites are typically fed year-round from bird feeders.
All birds were captured with treadle traps baited with sunflower seeds. Temporary feeder stands were used in the two forested sites and were baited for about one week before birds were captured. At the two residential sites, birds were captured near permanent feeders.
At the time of capture, birds were weighed to the nearest 0.1 g using a portable electronic balance, and wing chord (right wing) was measured to the nearest 0.5 mm. The birds were then transferred to a small wire cage with an aluminum foil floor and kept there about 15 min, which was usually long enough to collect a fecal sample. Fecal samples were stored in a chilled container for transport to the laboratory (usually within 45 min), then stored at −80°C until analyzed. All birds were banded with colored leg-bands to ensure identity on recapture. Relative age of birds (juvenile vs. adult) captured in late summer and fall was determined by the shape of the outer retrices (see Pyle 1997). Birds caught in late winter and spring were identified as “adults.” In this population, sex correlates with the size of the bird: birds with wing chords less than 62 mm are females, and those with larger wings are males (Thirakhupt 1985). An indirect test of this cutoff is offered by our aviary studies. Aviary birds were sacrificed at the end of the study for brain measurements (see Lucas et al. 2004) and each carcass was checked for the presence of testes. All 24 birds assumed to be males by wing chord measurements had testes.
Laboratory Experiments
We captured a total of 24 males (2 at the Battleground residence, 10 at Martell Forest, and 12 at the Ross Reserve) and kept each bird in an indoor aviary for 6 weeks. Given the limitation of our holding facilities (4 aviaries housing 1 bird each), we chose to test only one sex (males) to reduce the potential confounding variance caused by sex differences in behavior or hormone profiles. The birds were housed singly in 3 × 3 × 2.1 m aviaries. The birds could not see any of the other birds but could easily hear each other through mesh covering the ceiling of each aviary. Each aviary contained seven polyvinyl chloride “trees” with 10 evenly spaced cache sites (each 3.5 × 7.0 × 8.0 mm) in each tree and a perch under each cache site. An infrared sensor in each cache site was used to detect the time of caching. The sites were small enough that only one seed could be cached in a site. The birds readily use these sites as cache sites (see Lucas 1994; Lucas and Zielinski 1998; Pravosudov and Lucas 2000). Each aviary contained one computer-controlled feeder that delivered hulled sunflower seeds and two electronic balances, one mounted under the feeder and the other one in the middle of the aviary with a perch mounted on it. A computer recorded body mass and time of day every time a bird perched on either of the balances. Air temperature was constant throughout the experiment (19±1SD°C), and photoperiod was changed once a week to mimic natural light levels. All birds were given ad libitum access to grit, eggshell, and water containing vitamins. They also got one mealworm and 0.25 g carrot per day, in addition to sunflower seeds, peanuts, or turkey starter (see below) that they were given in the experiments.
The first experiment was designed to test for correlations in the seasonal patterns of caching behavior, body mass regulation, and fCort titers. We have four separate aviaries, and we ran four solitary birds at a time. Each set of four birds was tested under two conditions for a period of 6 weeks. For the first 3 weeks, the birds were run under “closed economy” conditions, where food (except for the mealworm and the carrot) was available only from an electronic feeder that delivered shelled sunflower seeds one at a time from a cassette into a small cup. Upon removal of the seed (detected with an infrared sensor monitored by the computer), a seed was delivered with a probability of 0.1 every second when the feeder is “on” and never when the feeder is “off.” For 5 of the 6 sets of birds, the feeder schedule was calculated as follows: we divided all but the last hour of the daylight interval into 15-min intervals and turned the feeder on in a randomly chosen 10% of these intervals (with the constraint that no two “on” times could occur within 30 min of each other). The feeder was then “on” for the first 12 min of an interval. The last hour of each day was used to clean each room and to restock the feeders — the feeders were always off at this time. Thus, for example, in an 11-hr day, the feeder would be on for 10% of 40 15-min intervals ( [11 − 1] hr × 4 intervals), for a total duration of 12 minutes × 4 intervals = 48 min. The first set of 4 birds we tested was given more intervals per day (12% of 15-min intervals) but less access per interval (10 min). The result is that this set had a lower variance in access to food and, consequently, shorter periods of food deprivation. This first set will be referred to as set 0. The other five are sets 1 – 5. We changed the feeder schedule after running set 0 to fewer but longer access intervals to increase the mean deprivation intervals between feedings and thereby promote caching behavior (see Lucas and Walter 1991; Lucas et al. 1993). In all cases, experiments were run 7 days per week.
The second experimental condition each set of birds faced was designated as an “open economy” condition because birds were periodically given ad libitum access to food. This experiment was designed to test for spatial memory, although the caching rates for the disturbed site were so low that we could not test for spatial memory in this experiment (see Results). For 11 consecutive days, we deprived the birds for 4 hr in a 1-m3 holding cage, then transferred them to their aviary containing a small bowl with 10 shelled sunflower seeds. The birds were then given 30-min access to those seeds, which they could eat, cache, or leave in the bowl. After the 30 minutes, we transferred the birds back to the holding cage. On one-half of the trials (randomly chosen), the birds were given 0.5-hr ad libitum access to turkey starter food and crushed peanut, then deprived 3.5 hr before they were put back into their aviary for 30 min to retrieve any cached seeds. On the other half of the trials, the birds were given 2.5-hr ad libitum access to turkey starter and crushed peanuts, followed by 1.5 hr of deprivation and then a 30-min retrieval trial. If the birds failed to cache any seeds, they would be given 5 sunflower seeds in randomly selected sites during the retrieval phase. Otherwise, they were given access to any seeds they cached during the caching phase. At all other times during the day, the birds were held in the 1−m3 holding cage and given ad libitum access to turkey starter and 0.2 g crushed peanut, plus one mealworm at the end of the day. Each day the birds were kept, we collected a fecal sample at dusk for fCort analysis.
Animal care in this experiment met NIH guidelines and was approved under Purdue’s PACUC protocol #97-058-00. Birds were caught under Indiana DNR license #2666 and U.S. Federal Fish and Wildlife Permit #MB721988-1.
Corticosterone Assays
Fecal droppings were weighed and dried to constant mass at 40° C in 1.5 ml Eppendorf tubes. Dried samples weighing more than 30mg were homogenized with a spatula and a sample of less than 30mg was put in a new 1.5mL Eppendorf tube. Samples were homogenized in 1mL of 75% ethanol using glass beads, vortex, and sonicator and incubated at least 3 hours at room temperature before they were briefly vortexed and centrifuged for 6 minutes at 11000 rpm. The ethanol supernatant was aspirated off and 300μL of ethanol extract were transferred into glass tubes and dried either in an oven or a water bath. We then added 400μL of acetate buffer, 20μL of chloroform, and 10μL of β-glucuronidase/arylsulfatase enzyme to each dried sample and incubated them at 39° C for 16 hours (± 2 hours). Steroids were then extracted over Extrelut NT columns with 4.5mL of diethyl ether. After drying at 35° C under nitrogen we added 500μL PBSg to each dry tube and used 100μL duplicates in a corticosterone radioimmunoassay (tritium-labeled cortocosterone: PerkinElmer Life Sciences NET 399; corticosterone antibody: Esoterix Endocrinology B3-163). Fecal corticosterone concentrations are expressed as ng/g dry feces. Estimates of assay variation were: intra-assay variation= 8.5 % and inter-assay variation= 12 %, with a detection limit of 0.1 ng.
There is minimal reported cross-reactivity with other hormones for the assay kit we used (Desoxycorticosterone: 3.3%; Aldosterone, Cortisol, Cortisone, Desoxycortisol, Desoxycortiosterone, Estradiol, Estrone, Progesterone, Testosterone, tetrahydrocortisol, tetrahydrocortisone, among others, all <1.0%).
Statistics
We collected repeated measurements of fCort levels and body mass from birds measured in both the field and aviaries. We therefore used repeated measures ANCOVA (Proc Mixed, SAS 1994) to test for factors contributing to variation in these dependent variables. Body mass levels were divided by wing chord to account for differences in size between birds; we refer to this variable as “relative body mass”. Fecal corticosterone levels were log transformed to normalize residuals; percent of seeds cached in the aviary under closed-economy conditions was arcsine-square-root transformed to normalize residuals. The number of seeds cached in the open-economy experiment tended to be relatively low; we therefore used a poisson repeated measures ANCOVA model to test for site and season effects on this behavior (Proc GLMIMIX; Littell et al. 1996). The statistical models are described in more detail in the Results. In all cases, several independent variables were added to the models and all 2-way and 3-way interactions between variables were initially added to the models and deleted starting with the lowest F value until the remaining model had only significant (α=0.05) interaction terms and all the main variables. Where means of class variables were significantly different, we used multiple comparisons t-tests (“diff” command under Proc Mixed with α=0.05) to evaluate differences between treatments or time categories.
We compared body mass under aviary conditions for birds captured at the different sites. We used two measures of body mass: morning mass (1 hr after dawn) and the change in body mass from 1 hr after dawn to 1 hr before dusk. In both cases, we used relative body mass instead of absolute mass. We also included experimental day and its square in the model to account for systematic changes in body mass as the experiment progressed.
RESULTS
Field Corticosterone and Body Mass Levels
We collected 89 body mass measurements and 87 fecal samples from 77 individually marked birds (see Table 1). Each bird was assigned a relative age (juvenile or adult) and sex (based on wing chord). A total of 21 adult females, 7 juvenile females, 38 adult males and 11 juvenile males was sampled. Note that the excess number of males in our sample is caused by our focusing on males for the laboratory component of this study. Relative body mass was also used as a covariate in our repeated measures ANCOVA. None of these factors accounted for a significant amount of variation in fCort concentration (relative body mass: F1,75 = 1.52, P = 0.22; age: F1,75 = 0.13, P = 0.72; sex: F1,75 = 1.01, P = 0.32).
Table 1.
Sample sizes for (a) field and (b) aviary experiments. Sample sizes for the field results are number of mass estimates followed by the number of cort values. Samples sizes for the aviary are the number of birds run in each set that were taken from each site. See text for a description of seasons and aviary set.
| (a) Field sample sizes. | ||||
|---|---|---|---|---|
| season
|
||||
| Site | fall | spring | summer | Winter |
| Disturbed site | 20/20 | 1/1 | 5/4 | 13/13 |
| Undisturbed site | 23/22 | 3/3 | 9/8 | 9/9 |
| WL residential | 7/7 | 0/0 | 1/1 | 0/0 |
| (b) Aviary sample sizes | ||||||
|---|---|---|---|---|---|---|
| set
|
||||||
| Site | 0 | 1 | 2 | 3 | 4 | 5 |
| Disturbed site | 2 | 2 | 0 | 2 | 2 | 2 |
| Undisturbed site | 0 | 2 | 4 | 2 | 2 | 2 |
| Battleground residential site | 2 | 0 | 0 | 0 | 0 | 0 |
We also tested for seasonal variation in fCort levels. We have samples from 9 different months and divided the samples into the following seasons: winter (December and January), spring (February through May), summer (July through September) and fall (October and November). [Note, we include February in the “spring” season here because testosterone titers in this population are near their peak in this month and singing rates also rise in February (unpublished data)]. Fecal corticosterone levels varied significantly across seasons (F3,75 = 5.54, P = 0.002), with the lowest levels in summer and highest levels in fall and winter (Fig. 1). The seasonal patterns were similar between sites, as indicated by a non-significant season × site interaction (F4,72 = 0.72, P = 0.58).
Figure 1.
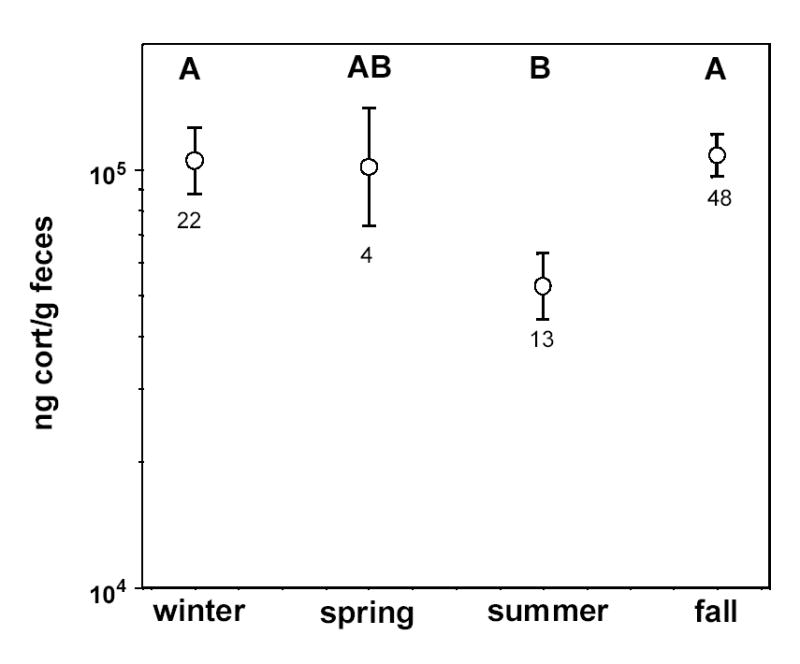
Field-derived least squares means (±SE) of the seasonal variation in fecal corticosterone concentrations (note log scale on Y axis). These values represent means across all three sites. Letters above each symbol represent the results of multiple comparisons tests (see Methods): symbols with different letters are significantly different from one another at α=0.05. Sample sizes are given under each error bar.
Controlling for season, body mass, sex, and age, fCort levels differed significantly between sites (F2,75 = 5.37, P = 0.007), with concentrations from the disturbed forest significantly higher than from either the undisturbed forest or the West Lafayette residential site (Fig. 2a).
Figure 2.
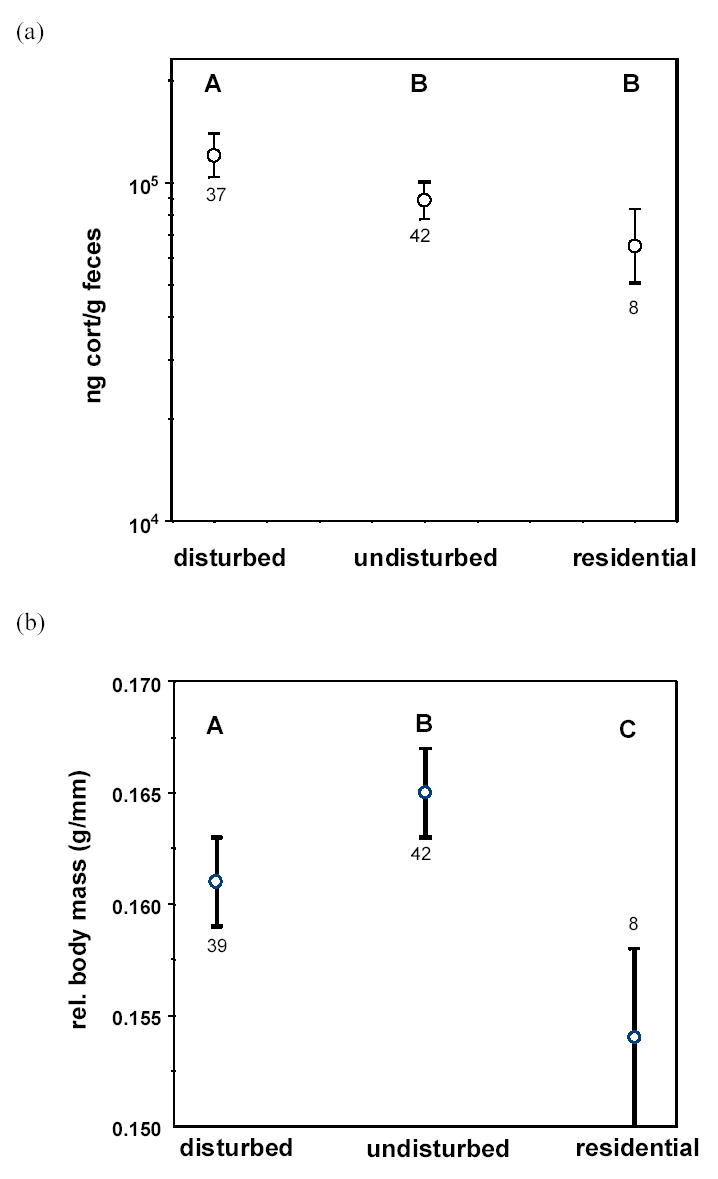
Field-derived least squares means (±SE) of (a) fecal corticosterone concentrations (note log scale on Y axis) and (b) relative body mass (mass/wingchord) at three capture sites. Letters above each symbol represent the results of multiple comparisons tests (see Methods): symbols with different letters are significantly different from one another at α=0.05. ‘disturbed’=Martell; ‘undisturbed’=Ross; ‘residential’=West Lafayette residential site. Sample sizes are given near each error bar.
We evaluated differences in relative body mass between sites. Neither relative age (F1,72 = 0.02, P = 0.90) nor sex (F1,72 = 0.17, P = 0.68) had an effect on relative mass. However, controlling for season, age, and sex, the relative mass levels were significantly different between sites (F2,72 = 7.57, P = 0.001), with highest body mass levels at the undisturbed forest site and the lowest levels at the West Lafayette residential site (Fig. 2b). Relative mass did not vary among seasons (F3,72 = 1.64, P = 0.19), nor was there a significant season × site interaction (F4,68 = 1.79, P = 0.14).
Aviary Corticosterone and Body Mass Levels: Closed Economy
We collected an average of 21 fCort measurements (1 per day) per bird in the closed-economy experiment. For this experiment, we estimated the effects on fCort levels of four independent variables: body mass, change in body mass from dawn to dusk, experimental set (of 4 birds), and site of origin. Not surprisingly, fCort levels (log10 transformed) declined with an increase in daily mass gain (β = −0.32 ± 0.005, F1,23 = 45.2, P < 0.0001), and fCort levels overall declined with an increase in the birds’ mean daily body mass, although the effect of mean relative body mass was non-linear (βmass= −1.88 ± 0.63, F1,23 = 8.9, P = 0.007; βmass × mass = 0.075 ± 0.028, F1,23 = 7.1, P = 0.014).
Fecal corticosterone levels also varied significantly with experimental set (F5,16 = 7.3, P = 0.001). Birds run in February with the lowest variability in access to food (set 0) had significantly lower fCort levels than any other set (multiple comparisons t-test, α = 0.05, Fig. 3). For the other 5 sets run on the same feeder schedule, those tested in winter and spring had higher fCort levels than birds tested in summer (Fig. 3), which was similar to the fCort patterns observed in field-caught birds. Finally, with relative body mass and set held constant, there was no significant site effect on fCort level (F2,16 = 2.15, P = 0.15).
Figure 3.
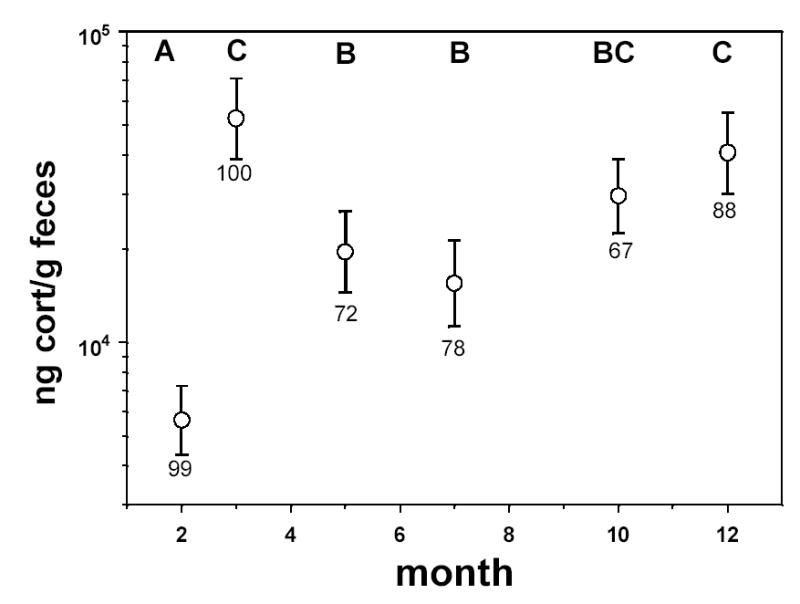
Seasonal variation in fecal corticosterone concentrations (least squares means ±SE) under closed-economy aviary conditions (note log scale on Y axis). The six symbols represent 6 experimental sets of birds: set 0 is month=2, sets 1–4 are months 5–12 respectively; set 5 is month 3. Note that these values represent means across all sites (see Results). Letters above each symbol represent the results of multiple comparisons tests; symbols with different letters are significantly different from one another at α=0.05. Seasons in the text are winter (December – January), spring (February–May), summer (July–September), and fall (October–November). Sample sizes are given under each error bar.
For dawn body mass, relative body mass increased non-linearly over the course of the experiment (βexp-day = 0.0010 ± 0.0002, F1,23 = 30.3, P < 0.0001, βexp-day × exp-day = −0.00002 ± 0.000005, F1,23 = 15.0, P = 0.0008). There was also a significant experimental-set effect (F5,16 = 4.0, P = 0.015), with the lowest relative body mass levels shown by birds in the set 0 (i.e. least variability in access to food), and, among the other sets, the highest relative body mass levels shown by birds run in spring (Fig. 4a). The effect of site of capture on relative dawn body mass was not significant (F2,16 = 3.0, P = 0.08).
Figure 4.
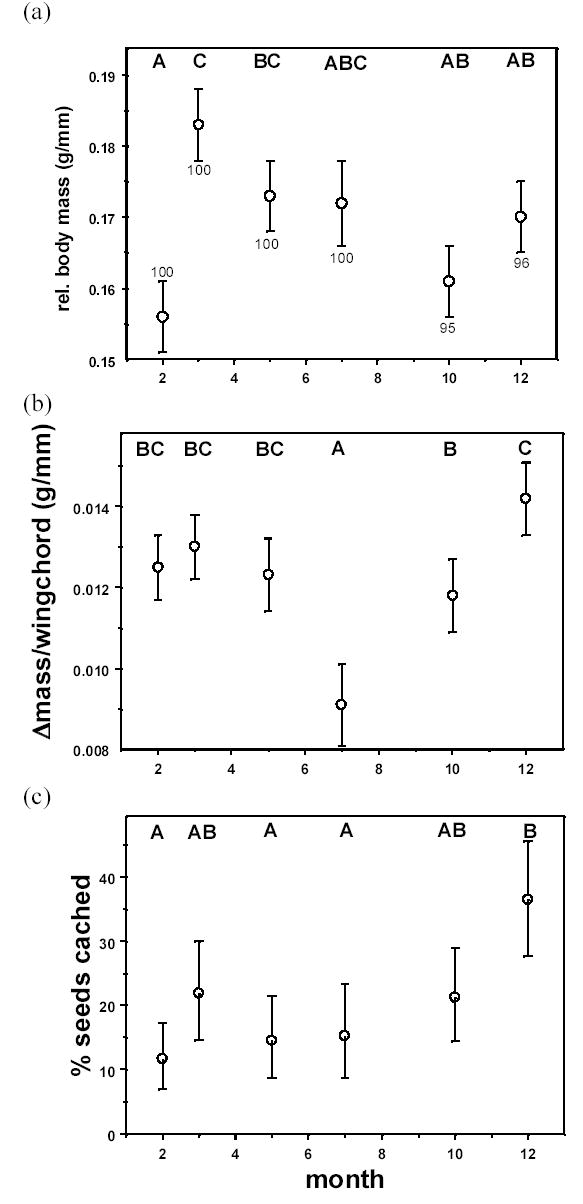
Effect of time-of-year on aviary-derived least squares means (±SE) of (a) relative body mass, (b) daily relative change in mass, and (c) percent of seeds taken from the feeder that were cached under closed-economy aviary conditions. The six symbols represent 6 experimental sets of birds: set 0 is month=2; sets 1–4 are months 5–12 respectively; set 5 is month 3. Note that these values represent means across all capture sites (see Results). Letters above each symbol represent the results of multiple comparisons tests: symbols with different letters are significantly different from one another at α=0.05. Sample sizes for all 3 figures are given in 4a.
There was no significant change in daily body mass gain the longer each bird was in the aviary (F1,23 = 0.12, P = 0.74), but there was a significant set effect (F5,16 = 5.1, P = 0.006). The lowest weight gain was shown by birds tested in the summer months, and the highest body mass gain by birds tested in winter and early spring (Fig. 4b). There was also a significant site effect (F2,16 = 6.2, P = 0.010), with the Battleground residential birds showing significantly higher body mass gains than birds from either the undisturbed forest site or the disturbed forest site and no difference between birds from the undisturbed and disturbed forest sites (multiple comparisons t-test, α = 0.05).
Aviary Caching Intensity: Closed Economy
We asked whether birds from different sites or from different experimental sets differed in the percent of seeds taken from the feeder that were cached. Relative body mass at dawn was used as a covariate in the model, although it did not have a significant effect on the percent of seeds cached (F1,23 = 0.16, P = 0.70).
Experimental set had a marginally significant effect on percent of seeds cached (F5,16 = 2.8, P = 0.053), with winter birds caching more intensely than late spring and summer birds, and birds in the February set 0 (those with the least variable access to food) caching least of all (Fig. 4c). There was also a significant site effect (F2,16 = 4.4, P = 0.030). The two Battleground residential birds cached significantly more than birds from either of the forested sites, but there was no difference between birds from the undisturbed and disturbed sites (multiple range t-test, α = 0.05).
Aviary Corticosterone and Body mass Levels: Open Economy
We measured fCort levels for 20 birds from sets 1 through 5 (i.e., no birds from set 0 or from the Battleground residential site), with an average of 10 samples per bird. All birds were tested on two deprivation intervals, 1.5 and 3.5 hr. In addition to deprivation interval, we included relative body mass, experimental set, and site of origin in the repeated measures analysis of covariance model.
Relative mass did not account for a significant amount of variation in fCort levels (F1,13 = 0.32, P = 0.58). Birds from the disturbed site did not have significantly different fCort levels than birds from the undisturbed site (F1,11 = 0.02, P = 0.89).
In contrast, birds given the longer deprivation interval had higher (log10 transformed) fCort levels than those with the shorter deprivation interval (F1,11 = 17.5, P = 0.0015; 3.5-hr deprivation: ± SE, 4.35 ± 0.07; 1.5-hr deprivation: 3.95 ± 0.08 ng/g feces). Birds in different sets also differed in their fCort levels (F4,11 = 7.5, P = 0.004). A multiple comparisons test suggests that late fall and winter birds exhibit the highest fCort levels, and summer birds exhibit the lowest levels (α = 0.05, Fig. 5), a pattern that mimics the field results.
Figure 5.
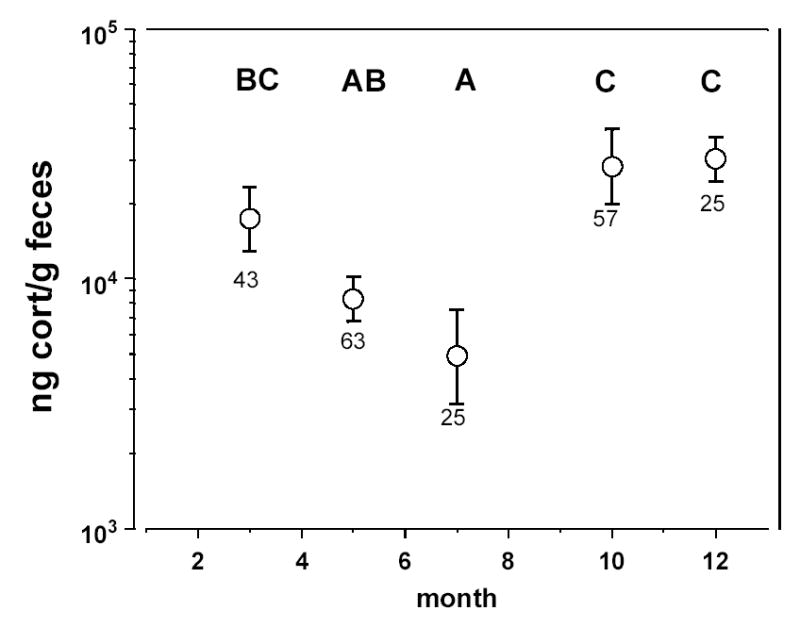
Least squares means (±SE) fecal corticosterone concentrations representing seasonal variation under open-economy aviary conditions (note log scale on Y axis). The five symbols represent 5 experimental sets of birds: sets 1–4 are months 5–12 respectively; set 5 is month 3. Note that these values represent means across all sites (see Results). Letters above each symbol represent the results of multiple comparisons tests: symbols with different letters are significantly different from one another at α=0.05. Sample sizes are given under each error bar.
We have body mass levels for all 24 birds run in the aviary. Neither experimental set (F5,13 = 1.07, P = 0.42), deprivation interval (F3,25 = 0.08, P = 0.97) nor site of origin (F2,13 = 1.06, P = 0.37) accounted for a significant amount of variation in relative mass.
Aviary Caching Rates: Open Economy
Using a Poisson repeated measures ANCOVA model, we asked how many of the 10 seeds offered to the birds during the caching trial were cached by each bird (based on 10 days and therefore 10 behavioral measurements per bird). This was taken as a function of experimental set, deprivation interval, site of origin, and relative mass. Experimental set did not account for a significant amount of variation in the number of seeds cached (F5,13 = 2.05, P = 0.14). In contrast, birds subject to longer deprivation intervals cached fewer seeds than those given short intervals (β = −0.18 ± 0.08, F1,18 = 4.75, P = 0.043) and birds that were that were relatively lightweight cached significantly more than those that were heavy (β = −22.8 ± 5.4, F1,20 = 17.7, P = 0.0004). Site of origin also accounted for a significant amount of variation in the number of seeds cached (F2,13 = 7.28, P = 0.008), with birds from the disturbed site caching significantly fewer seeds overall than either the Battleground residential birds or birds from the undisturbed site. The latter two were not significantly different from one another (multiple comparisons t-test, α = 0.05, Fig. 6).
Figure 6.
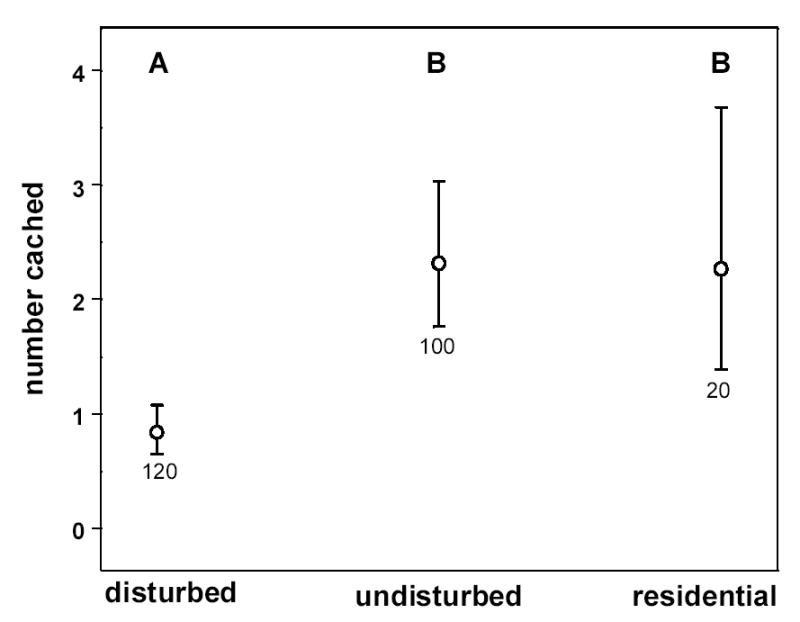
Aviary-derived least squares means (±SE) of the number of seeds cached (out of a maximum of 10) by birds from three capture sites under open economy conditions. Least squares means and error bars represent back-transformed estimates from Poisson ANCOVA (see text). Letters above each symbol represent the results of multiple comparisons tests (see Methods): symbols with different letters are significantly different from one another at α=0.05. ‘disturbed’=Martell; ‘undisturbed’=Ross; ‘residential’=Battleground residential site. Sample sizes are given under each error bar.
DISCUSSION
Carolina chickadees from the disturbed forested area had higher fCort levels and lower body mass than birds from the undisturbed forested site. In contrast, birds from the West Lafayette residential site had minimal levels of both fCort and body mass. These results suggest that the stress response to disturbance shown by deep-woods specialists (Suorsa et al. 2003; Wasser et al. 1997) can be generalized to include habitat generalists such as Carolina chickadees. How might these forest disturbances affect “stressful” physiological and potentially behavioral changes in chickadees? Considerable research has indicated that areas of habitat heterogeneity due to forest fragmentation frequently alter patterns of solar radiation, wind, and hydrological cycles (reviewed in Murcia 1995; Saunders et al. 1991). Further, research has indicated that carnivorous predators high in trophic levels are extremely vulnerable to fragmentation effects, typically declining in numbers in fragmented areas. The result is that lower-level carnivores often increase in numbers in these areas, and these predators exert stronger predation pressures on small birds and mammals (Crooks and Soulé 1999). Thus, human-induced changes to forested areas may have a cascade of influences impacting both the biotic and abiotic environment in ways detrimental to even generalist species such as chickadees (see also Romero 2004).
No differences between populations in fCort or body mass were recorded under identical aviary conditions. Our sample sizes in the aviary experiments were necessarily lower than those from the field component of this study. Therefore, the power to detect differences in the aviaries is lower than our ability to detect differences in the field. Nonetheless, our aviary experiments were powerful enough to pick up seasonal trends in fCort that mirror changes observed in the field (e.g., lowest levels in summer), and fCort levels exhibited a predictable rise in response to increased deprivation intervals and to an overall reduction in food delivery rates (see Goymann and Wingfield 2004; Kitaysky et al. 1999; McEwen 1998). Indeed, we detected seasonal variation in relative body mass in the aviary but not in the field. We conclude that differences in fCort levels and body mass between populations that we measured in the field are short-term responses to ecological conditions, and not permanent differences in physiology characteristic of these populations.
Our field results suggest that body mass and fCort levels do not necessarily track each other. Body mass is high and fCort levels are low in the undisturbed site, whereas body mass is low and fCort levels are low in the West Lafayette residential site. Birds at the residential site have year-round access to food at feeders. We expect birds with predictable and high levels of food to down-regulate fat levels (Lucas and Walter 1991; McNamara and Houston 1990; Pravosudov and Grubb 1997; Pravosudov and Lucas 2001). This should, in turn, reduce the “allostatic load” of these birds and thereby reduce fCort levels (Goymann and Wingfield 2004; McEwen and Wingfield 2003). In contrast, an unpredictable food supply will select for increased mass levels, but only if the mean food abundance is high enough to enable a strategic regulation of body mass (Lucas and Walter 1991). We suggest that high body mass coupled with relatively low fCort levels exhibited by the birds from the undisturbed forested site could be the result of unpredictable but otherwise relatively abundant food levels.
Several factors could result in the combination of relatively low body mass and high fCort levels in the forested, disturbed site. First, food availability could be reduced to the point where chickadees are simply incapable of maintaining higher body mass levels. This in turn can increase allostatic load and therefore cort levels (see Kitaysky et al. 1999; Suorsa et al. 2002). Alternatively, recent forestry activity (i.e., clear-cutting, forest thinning) is likely to fragment territories which in turn should affect the social interactions between birds. Chickadees are territorial throughout the year with pair-maintained territories in the breeding season and flock-maintained territories in fall and winter (Smith 1972; Smith 1991); clear-cutting (as opposed to targeted thinning) in particular is likely to disrupt territoriality. There is recent evidence that forestry practices can indeed disrupt territoriality in black-capped chickadees: during the spring, a pair’s territory was more likely to be intruded upon if the pair was in a disturbed habitat relative to an undisturbed habitat (Fort and Otter 2004). An increase in aggressive interactions can act as an allostatic load that can cause an increase in cort levels (Romero 2004) and a reduction in body mass (Lange and Leimar 2004). Finally, birds may exhibit an increase in cort levels as a direct response to the noise and disruption caused by the heavy machinery used in logging (e.g., see Creel et al. 2002; Millspaugh et al. 2001).
While our aviary results suggest that our study populations do not differ in fCort levels or body mass under identical aviary conditions, differences in caching intensity point to more permanent physiological differences between populations. Nonetheless, these caching trends are consistent with the response to social disruption discussed above. For example, caching behavior in species belonging to the family Paridae (the family to which Carolina chickadees belong) is found only in species that are territorial all year long (Ekman 1989). Similar findings (i.e. caching only in stable social groups) between populations of black-capped chickadees showed the same trend (A. Brodin, pers. comm.). By extension, a reduction in territoriality caused by forestry practice should reduce caching intensities in the field (see Ekman 1989). Interestingly, this difference in energy regulation patterns is the only difference we observed under aviary conditions, and here only in open-economy conditions. In our open-economy conditions, deprivation is imposed on the birds irrespective of their caching rates, whereas in the closed-economy conditions, birds can still eat when the feeder is closed if they have cached. The implication from our results is that caching rates in the face of imposed deprivation (as would occur, for example, with severe weather) are a more sensitive estimate of the behavioral regulation of energy stores than caching rates in the face of variation in the rate at which new resources are discovered.
Our analysis of fecal glucocorticoids addresses only one component of the regulation of these “stress” hormones: specifically, regulation of basal levels of glucocorticoids. In fact, we know more about the acute stress response, essentially aspects of the “fight-or-flight” response (Wingfield et al. 1998) and the detrimental aspects of prolonged acute levels of these hormones (McEwen 1999, 2000; Sapolsky 1996) than we know about adaptive aspects of variation in basal levels of glucocorticoids. The magnitude of the population differences we report here is unlikely to result in the maladaptive response caused by long-term maintenance of acute levels of glucocorticoids. Our results suggest that a fairly broad range of food and body mass related factors will generate small increases in fCort levels. Indeed, these effects are similar to the population differences we discovered in the field. For example, an increase in deprivation interval from 1.5 to 3.5 hr increased fCort levels 2.5 times. The maximal population difference (disturbed forest site compared to the West Lafayette residential site) represented an increase of 1.7 times. This is similar to the seasonal differences measured in the field (fall levels are 2.1 times summer levels). The changes are also similar to the approximate 1.5 times increase in cort shown in mountain chickadees to unpredictable and restricted food access compared to ad libitum access to food (Pravosudov et al. 2001). Nonetheless, changes in cort levels of 1.5 times significantly alter caching behavior and spatial memory in mountain chickadees (Pravosudov 2003). The changes we see between populations should therefore generate population differences in cort-induced behaviors such as movement rates or differences in energy regulation patterns (Pravosudov 2003).
There has been some debate lately about the validity of fecal hormone analysis as a surrogate for serum hormone measurements (Goymann 2005; Millspaugh & Washburn 2004; Touma & Palme 2005). For example, Klasing (2005) and Goymann (2005) showed that high variation in the cellulose content of the diet can have a significant impact on excreted hormone levels independent of variation in serum titers. Since diet can change seasonally, these patterns call into question the basis of annual hormone profiles as measured by fecal analysis. As Klasing (2005) notes, variation in diet is particularly problematic for birds that have a well developed cecum, such as the Anseriformes. Fortunately, chickadees eat a diet (seeds and insects, Smith 1992) that is low in cellulose, and they have a relatively simple gut morphology (Klasing 2005); both of these factors should mitigate the problem with diet-induced variation in fecal hormone titers. In our study, aviary-based fCort levels can be used as validation of the seasonal pattern we observed in the field, because birds in our aviaries were given the same food throughout the year. The fact that we obtained the same patterns in the field that we observed in the laboratory gives us some confidence in our field results. It is also very unlikely that our site differences were caused by diet differences; seasonal variation in diet (insects in summer, seeds in winter) is likely to be much more pronounced than site differences within any given season. Again, seasonal hormone patterns shown under invariant diets in the lab suggest that the hormone profiles measured in the field are a true reflection of site and seasonal hormone patterns and not an artifact of diet on fecal hormone titers. Having said this, we acknowledge the caveats offered by a number of authors (e.g. Goymann 2005; Millspaugh & Washburn 2004; Touma & Palme 2005) that several levels of validation are important to verify the validity of fCort measurements. In particular, it would be useful to tract radioactively labeled corticosterone and determine the byproducts that are excreted (see for example Goymann et al. 2002). To our knowledge, this has not been done with Carolina chickadees.
One advantage of capturing birds in treadle traps (as opposed to mist nets) is that the birds are not subject to an acute stressor when captured (e.g., Wingfield 2003). In addition, Carere et al. (2003) suggested that fCort in great tits (Parus major) increases 30 min to 140 min after the onset of a social stress. Even if fCort increased in our birds in response to their capture, the results we report here (on feces collected within fifteen minutes of capture) are most likely to reflect fCort levels under conditions that did not result in an acute stress response.
Our results, along with those published by Fort and Otter (2004), present a framework for how forestry practices can impact local populations of species that occupy a broad range of habitats. The problem is that these impacts are multi-dimensional, and it is not at all trivial to understand how all the important pieces of these systems fit together (Fig. 7). As noted above, there is direct evidence that disturbance can alter territorial behavior (Fort and Otter 2004), and this in turn can cascade into several factors that affect allostatic load. This cascade includes effects on social interactions and effects on energy regulation (e.g. caching behavior). Of course, forest disturbance can influence food supplies directly (e.g. Suorsa et al 2003; Zanette et al 2000), which in turn affects cort levels (Goymann and Wingfield 2004; McEwen and Wingfield 2003). Disturbance generated by deforestation, in itself, and the unpredictable nature of forestry practices can also directly impact cort levels (see Romero 2004 for a general discussion of unpredictability and cort levels). Figure 7 represents only a subset of causal relationships that exist between these factors. Nonetheless, we are beginning to develop a much clearer picture of this process, and chickadees may prove to be an excellent model system to help us develop a synoptic view of the impact that forest disturbance has on the physiology and behavior of resident populations.
Figure 7.
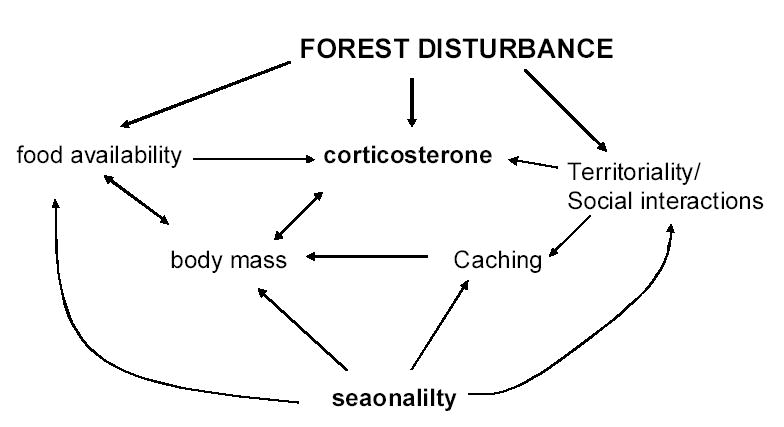
Flow diagram of effects discussed in this manuscript. Note that this is only a subset of causal relationships that exist between these factors (e.g. food availability affects and is affected by territoriality).
Acknowledgments
This research was funded by an NIMH grant to JRL, HS and Dr. Nicola Clayton (#1RO1MH62602-01). We thank Kristin Bledsoe, Ben and Kerry Fanson, Ken Henry, and Mark Nolen for comments on the manuscript.
References
- Brawn JD, Robinson SK, Thompson FR. The role of disturbance in the ecology and conservation of birds. Ann Rev Ecol Syst. 2001;32:251–276. [Google Scholar]
- Bender DJ, Contreras TA, Fahrig L. Habitat loss and population decline: a meta-analysis of the patch size effect. Ecology. 1998;79:517–533. [Google Scholar]
- Carere C, Groothuis TGG, Möstl E, Daan S, Koolhaus JM. Fecal corticosteroids in a territorial bird selected for different personalities: daily rhythm and the response to social stress. Horm Behav. 2003:540–548. doi: 10.1016/s0018-506x(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Chalfoun AD, Thompson FR, III, Ratnaswamy MJ. Nest predators and fragmentation: a review and meta-analysis. Conserv Biol. 2002;16:306–318. [Google Scholar]
- Creel S, Fox JE, Hardy A, Sands J, Garrott B, Peterson RO. Snowmobile activity and glucocorticoid stress responses in wolves and elk. Conserv Biol. 2002;16:809–814. [Google Scholar]
- Crooks KR, Soulé ME. Mesopredator release and avifaunal extinctions in a fragmented system. Nature. 1999;400:563–566. [Google Scholar]
- Ekmann J. Ecology of non-breeding systems of Parus. Wilson Bull. 1989;101:263–288. [Google Scholar]
- Fort KT, Otter KA. Territorial breakdown of black-capped chickadees, Poecile atricapillus, in disturbed habitats. Anim Behav. 2004;68:407–415. [Google Scholar]
- Freeberg TM, Lucas JR, Clucas B. Variation in chick-a-dee calls of a population of Carolina chickadees, Poecile carolinensis: identity and redundancy within note types. J Acoustical Soc Am. 2003;113:2127–213. doi: 10.1121/1.1559175. [DOI] [PubMed] [Google Scholar]
- Goymann W, Möstl E, Gwinner E. Non-invasive methods to measure androgen metabolites in excrements of European stonechats, Saxicola torquata rubicola. Gen Comp Endocrinol. 2002;129:80–87. doi: 10.1016/s0016-6480(02)00520-8. [DOI] [PubMed] [Google Scholar]
- Goymann W, Wingfield JC. Allostatic load, social status, and stress hormones: the costs of social status matter. Anim Behav. 2004;67:591–602. [Google Scholar]
- Goymann W. Noninvasive monitoring of hormones in bird droppings. Ann NY Acad Sci. 2005;1046:35–53. doi: 10.1196/annals.1343.005. [DOI] [PubMed] [Google Scholar]
- Hobson KA, Bayne E. Effects of forest fragmentation by agriculture on avian communities in the southern boreal mixed woods of western Canada. Wilson Bull. 2000;112:373–387. [Google Scholar]
- Hoffmann AA, Hercus MJ. Environmental stress as an evolutionary force. Bioscience. 2000;50:217–226. [Google Scholar]
- Hughes JB, Daily GC, Ehrlich PR. Conservation of tropical forest birds in countryside habitats. Ecol Lett. 2002;5:121–129. [Google Scholar]
- Klasing KC. Potential impact of nutritional strategy on noninvasive measurements of hormones in birds. Ann NY Acad Sci. 2005;1046:5–16. doi: 10.1196/annals.1343.003. [DOI] [PubMed] [Google Scholar]
- Kitaysky AS, Wingfield JC, Piatt JF. Dynamics of food availability, body condition, and physiological stress response in breeding black-legged kittiwakes. Func Ecol. 1999;13:577–584. [Google Scholar]
- Lange H, Leimar O. Social stability and daily body mass gain in great tits. Behav Ecol. 2004;15:549–554. [Google Scholar]
- Littell, R.C., Milliken, G.A., Stroup, W.W., Wolfinger, R.D., 1996. SAS system for mixed models. SAS Institute, Cary, North Carolina.
- Lucas JR. Regulation of cache stores and body mass in Carolina chickadees (Parus carolinensis) Behav Ecol. 1994;5:171–181. [Google Scholar]
- Lucas JR, Brodin A, de Kort SR, Clayton NS. Does hippocampal size correlate with the degree of caching specialization? Proc Royal Soc B. 2004;271:2423–2429. doi: 10.1098/rspb.2004.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JR, Peterson LJ, Boudinier RL. The effects of time constraints and changes in body mass and satiation on the simultaneous expression of caching and diet-choice decisions. Anim Behav. 1993;45:639–658. [Google Scholar]
- Lucas JR, Walter LR. When should chickadees hoard food? Theory and experimental results Anim Behav. 1991;41:579–601. [Google Scholar]
- Lucas JR, Zielinski DL. Seasonal variation in the effect of cache pilferage on cache and body mass regulation in Carolina chickadees: what are the trade-offs? Behav Ecol. 1998;9:193–200. [Google Scholar]
- Mazerolle DF, Hobson KA. Physiological ramifications of habitat selection in territorial male oven birds: consequences of landscape fragmentation. Oecologia. 2002;130:356–363. doi: 10.1007/s00442-001-0818-z. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New Engl J Med. 1998;338:171–180. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- McNamara JM, Houston AI. The value of fat reserves and the tradeoff between starvation and predation. Acta Biotheoretica. 1990;38:37–61. doi: 10.1007/BF00047272. [DOI] [PubMed] [Google Scholar]
- Millspaugh JJ, Woods RJ, Hunt KE, Raedeke KJ, Brundige GC, Washburn BE, Wasser SK. Fecal glucocorticoid assays and the physiological stress response in elk. Wildlife Soc Bull. 2001;29:899–907. [Google Scholar]
- Millspaugh JJ, Washburn BE. Use of fecal glucocorticoid metabolite measures in conservation biology research: considerations for application and interpretation. Gen Comp Endocrinol. 2004;138:189–199. doi: 10.1016/j.ygcen.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Murcia C. Edge effects in fragmented forests: implications for conservation. TREE. 1995;10:58–62. doi: 10.1016/S0169-5347(00)88977-6. [DOI] [PubMed] [Google Scholar]
- Nardoo R. Species richness and community composition of songbirds in a tropical forest-agricultural landscape. Anim Conserv. 2004;7:93–105. [Google Scholar]
- Pravosudov VV. Long-term moderate elevation of corticosterone facilitates avian food-caching behaviour and enhances spatial memory. Proc Roy Soc Lond B. 2003;270:2599–2604. doi: 10.1098/rspb.2003.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravosudov VV, Kitaysky AS, Saldanha C, Wingfield JC, Clayton NS. Long-term unpredictable foraging conditions and physiological stress response in mountain chickadees (Poecile gambeli) Gen Comp Endocrinol. 2001;126:242–248. doi: 10.1006/gcen.2001.7684. [DOI] [PubMed] [Google Scholar]
- Pravosudov VV, Grubb TC., Jr Management of fat reserves and food caches in tufted titmice (Parus bicolor) in relation to unpredictable food. Behav Ecol. 1997;8:332–339. doi: 10.1006/anbe.1998.0739. [DOI] [PubMed] [Google Scholar]
- Pravosudov VV, Lucas JR. The effect of social dominance on fattening and food-caching behavior in Carolina chickadees, Poecile carolinensis. Anim Behav. 2000;60:483–493. doi: 10.1006/anbe.2000.1506. [DOI] [PubMed] [Google Scholar]
- Pravosudov VV, Lucas JR. A dynamic model of short-term energy management in small food-caching and non-caching birds. Behav Ecol. 2001;12:207–218. [Google Scholar]
- Pyle, P., 1997. Identification Guide to North American Birds. Slate Creek Press, Bolinas, California.
- Romero LM. Physiological stress in ecology: lessons from biomedical research. TREE. 2004;19:249–255. doi: 10.1016/j.tree.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- SAS Institute. 1994. SAS/STAT Software. Release 6.09 and Release 6.08 maintenance enhancements for PROC MIXED. SAS Institute, Inc., Cary, North Carolina.
- Saunders DA, Hobbs RJ, Margules CR. Biological consequences of ecosystem fragmentation: a review. Conserv Biol. 1991;5:18–32. [Google Scholar]
- Smith, S. M. 1991. The black-capped chickadee: behavioral ecology and natural history. Comstock, Ithaca, New York.
- Smith ST. Communication and other social behavior in Parus carolinensis. Publ Nuttall Ornithol Club. 1972;11:1–125. [Google Scholar]
- Suorsa P, Huhta E, Nikula A, Nikinman M, Jantti J, Helle H, Hakkarainen H. Forest management is associated with physiological stress in an old-growth forest passerine. Proc Roy Soc Lond B. 2003;270:963–969. doi: 10.1098/rspb.2002.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suorsa P, Helle H, Koivunen V, Huhta E, Nikula A, Hakkarainen H. Effects of forest patch size on physiological stress and immune competence in an area-sensitive passerine, the Eurasian treecreeper (Certhia familiaris): an experiment. Proc Roy Soc Lond B. 2004;271:435–440. doi: 10.1098/rspb.2003.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempel DJ, Gutierrez RJ. Factors related to fecal corticosterone levels in California spotted owls: implications for assessing chronic stress. Conserv Biol. 2004;18:538–547. [Google Scholar]
- Thirakhupt, K., 1985. Foraging ecology of sympatric parids: individual and population responses to winter food scarcity. Ph.D. thesis. Purdue University, West Lafayette, Indiana.
- Touma C, Palme R. Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann NY Acad Scie. 2005;1046:54–74. doi: 10.1196/annals.1343.006. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Bevis K, King G, Hanson E. Non-invasive physiological measures of disturbance in the Northern spotted owl. Conserv Biol. 1997;11:1019–1022. [Google Scholar]
- Wingfield, J.C., Hunt, K., Breuner, C., Dunlap, K., Fowler, G.S., Freed, L., Lepson, J., 1997. Environmental stress, field endocrinology and conservation biology. In: J.R. Clemmons and R. Buchholz (Eds.), Behavioural Approaches to Conservation in the Wild. Cambridge University Press, Cambridge, pp. 95–131
- Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. Ecological basis of hormone-behavior interactions: the emergency life history stage! Am. Zool. 1998;38:191–206. [Google Scholar]
- Wingfield JC. Control of behavioural strategies for capricious environments. Anim Behav. 2003;66:807–816. [Google Scholar]
- Zanette L, Doyle P, Tremont SM. Food shortage in small fragments: evidence from an area-sensitive passerine. Ecology. 2004;81:1654–1666. [Google Scholar]


