Abstract
In Streptococcus pneumoniae, CpsB, CpsC, and CpsD are essential for encapsulation, and mutants containing deletions of cpsB, cpsC, or cpsD exhibit rough colony morphologies. CpsD is an autophosphorylating protein-tyrosine kinase, CpsC is required for CpsD tyrosine phosphorylation, and CpsB is a phosphotyrosine-protein phosphatase. We have previously shown that autophosphorylation of CpsD at tyrosine attenuates its activity and consequently reduces the level of encapsulation and negatively regulates CPS production. In this study, we further investigated the role of the carboxy-terminal (YGX)4 repeat domain of CpsD in encapsulation. A CpsD truncation mutant in which the entire (YGX)4 repeat domain was removed was indistinguishable from a strain in which the entire cpsD gene had been deleted, indicating that the carboxy-terminal (YGX)4 tail is required for CpsD activity in capsular polysaccharide production. Double mutants having a single tyrosine residue at position 2, 3, or 4 in the (YGX)4 repeat domain and lacking CpsB exhibited a rough colony morphology, indicating that in the absence of an active protein-tyrosine phosphatase, phosphorylation of just one of the tyrosine residues in the (YGX)4 repeat was sufficient to inactivate CpsD. When various mutants in which CpsD had either one or combinations of two or three tyrosine residues in the (YGX)4 repeat domain were examined, only those with three tyrosine residues in the (YGX)4 repeat domain were indistinguishable from the wild-type strain. The mutants with either one or two tyrosine residues exhibited mucoid colony morphologies. Further analysis of the mucoid strains indicated that the mucoid phenotype was not due to overproduction of capsular polysaccharide, as these strains actually produced less capsular polysaccharide than the wild-type strain. Thus, the tyrosine residues in the (YGX)4 repeat domain are essential for normal functioning of CpsD.
Streptococcus pneumoniae (the pneumococcus) is an important cause of invasive disease in human populations throughout the world, resulting in high morbidity and mortality. An important feature of S. pneumoniae is its capacity to produce a polysaccharide capsule, which is structurally distinct for each of the 90 known serotypes of the organism (1, 14). The capsular polysaccharide (CPS) is essential for pneumococcal virulence (1); all fresh clinical isolates of S. pneumoniae are encapsulated (smooth), and spontaneous nonencapsulated (rough) derivatives of such strains are almost completely avirulent. Examination of the types of genes present in the pneumococcal cps loci characterized to date indicates that CPS is synthesized via lipid-linked repeat unit intermediates in a fashion similar to O-antigen biosynthesis in gram-negative bacteria (25, 34) except in types 3 and 37, in which CPS is synthesized by a processive transferase in the same fashion as hyaluronic acid synthesis in group A streptococci (5, 7, 18).
The capacity to regulate CPS production in S. pneumoniae may be very important for the survival of the pneumococcus in different host environments. Maximal expression of CPS is essential for systemic virulence because of its antiphagocytic properties (1). Invasive disease is invariably preceded by asymptomatic colonization of the nasopharynx, and the thickness of the capsule influences the degree of exposure of other important pneumococcal surface structures, such as the adhesins which are required during this initial colonization phase. However, the mechanisms involved in regulation of CPS production are complex and poorly understood. The first four genes (cpsA to cpsD) are common to all serotypes except types 3 and 37 and have been implicated in CPS regulation. CpsA has been shown to be a transcriptional activator of the cps locus in Streptococcus agalactiae (6). CpsB, CpsC, and CpsD function together to regulate CPS production (3, 20, 21). CpsC and CpsD are predicted to function together in polymerization and export of CPS, in a fashion similar to ExoP in exopolysaccharide production in Sinorhizobium meliloti (11, 13) and Wzc from Escherichia coli K-12 and K-30 (24, 30). We have shown that CpsD, like Wzc, is an autophosphorylating protein-tyrosine kinase and demonstrated that CpsC is required for CpsD tyrosine phosphorylation (21). CpsB is a manganese-dependent phosphotyrosine-protein phosphatase that is required to dephosphorylate CpsD (3, 20). Autophosphorylation of CpsD at tyrosine attenuates its activity, reduces the level of encapsulation, and hence negatively regulates CPS production.
Pneumococci have been shown to undergo a bidirectional phase variation between two distinct colonial morphologies, described as opaque and transparent. The opaque form is associated with massively increased virulence in animal models of systemic disease, which correlates with increased production of CPS relative to cell wall teichoic acid (15). The transparent phenotype exhibits an enhanced capacity to colonize the nasopharynx of infant rats (32), and this correlates with an apparent decrease in CPS production. Transcriptional regulation of the CPS locus may play a role in phase variation (33). However, the mechanism involved was not investigated. Weiser et al. (33) also showed that environmental oxygen levels affected the amount of both CPS and phosphorylated CpsD (CpsD∼P) detected. These results show that the balance of CpsD to CpsD∼P is altered by anaerobic growth conditions but does not exclude the possibility that CpsD has a higher specific activity under anaerobic growth conditions due to unknown factors.
We have shown previously that point mutations in cpsD affecting either the ATP-binding domain (Walker A motif) or the carboxy-terminal (YGX)4 repeat domain eliminated tyrosine phosphorylation of CpsD but affected CPS production differently. Whereas a mutation in the Walker A motif resulted in loss of encapsulation, mutation of the tyrosines in the (YGX)4 repeat domain resulted in a mucoid phenotype, suggesting an increase in CPS production (21). In the present study, we further investigated the role of the C-terminal (YGX)4 repeat domain of CpsD in CPS production in S. pneumoniae and investigated the basis for the mucoid phenotype associated with certain cpsD mutants.
MATERIALS AND METHODS
Bacterial strains and plasmids.
S. pneumoniae strain Rx1-19F is a derivative of Rx1 (a rough derivative of the type 2 strain D39) expressing type 19F capsule (19). The insertion-duplication mutants Rx1-19F-A and Rx1-19F-D, in which the cps19fA and cps19fD genes, respectively, were interrupted with pVA891, have been described previously (13). The defined in-frame deletion mutants Rx1-19F-BΔ and Rx1-19F-DΔ and the defined point mutants Rx1-19F-DY→F and Rx1-19F-BΔ:DY→F have also been described previously (21). Pneumococci were routinely grown in Todd-Hewitt broth (Oxoid, Basingstoke, Hampshire, England) with 0.5% yeast extract (Difco Laboratories, Detroit, Mich.) (THY) or on blood agar. When appropriate, erythromycin was added to the medium at a concentration of 0.2 μg/ml. The S. pneumoniae strains were transformed as described previously for strain D39 (4). Production of type 19F capsule by pneumococci was assessed by the Quellung reaction with monospecific antiserum obtained from the Statens Seruminstitut, Copenhagen, Denmark.
Construction of defined cpsD mutants.
Defined point mutations in cps19fD were constructed by overlap extension PCR as described previously (21). Complementary primers were designed to alter one, two, or three of the four Y residues in the C-terminal (YGX)4 repeat of Cps19fD to F. Their sequences are available on request. These primers were used in conjunction with CPS5′ (nucleotides 146 to 169 of the S. pneumoniae type 19F cps operon, GenBank accession number U09239) and J6 (complementary to nucleotides 5704 to 5724) to amplify this region of the cps locus. The final CPS5′/J6 PCR products incorporating the specific mutations were transformed directly into Rx1-19F-D. The transformants were initially screened for loss of erythromycin resistance, and the presence of the mutations was confirmed by sequencing of PCR products. These mutants were designated Rx1-19F-DY1, Rx1-19F-DY2, Rx1-19F-DY3, Rx1-19F-DY4, Rx1-19F-DY12, Rx1-19F-DY34, and Rx1-19F-DY123 to reflect the Y residues still present in their (YGX)4 repeat domains.
The four cpsBΔ cpsD double mutants, designated Rx1-19F-BΔ:DY1, Rx1-19F-BΔ:DY2, Rx1-19F-BΔ:DY3, and Rx1-19F-BΔ:DY4, which contained a deleted cpsB gene and only Y1, Y2, Y3, or Y4 in the (YGX)4 repeat domain of cpsD were constructed as described above except that chromosomal DNA from Rx1-19F-BΔ was used as the template for the overlap extension PCRs.
Four defined mutants in which the cpsD gene was truncated by replacing the codon for Y1, Y2, Y3, or Y4 with the TAA stop codon were constructed by overlap extension PCR as described above. The sequences of the primers used are available on request. The presence of the correct mutations was confirmed by sequencing of PCR products, and the mutants were designated Rx1-19F-DT0, Rx1-19F-DT1 Rx1-19F-DT2, and Rx1-19F-DT3, respectively, to reflect the number of YGX repeats present in the corresponding CpsD protein.
Construction of an insertion-duplication mutant.
The cps19fF gene in the mucoid strain Rx1-19F-DY→F was interrupted by insertion-duplication mutagenesis as previously described (13) with pVA891 containing a small internal fragment of cps19fF (nucleotides 5225 to 5725). The resultant transformants were initially screened for erythromycin resistance, and the presence of pVA891 was confirmed by PCR. The mutant was designated Rx1-19F-DY→F:F.
Preparation of cell wall-associated CPS from pneumococci for Western immunoblotting.
The method used to extract cell wall-associated CPS was based on the methods used by Sorensen et al. (27) and Fischer and Tomasz (10) to extract pneumococcal cell walls. Pneumococci were grown in 200 ml of THY to an A600 of 0.45. The cells were pelleted by centrifugation at 5,000 × g for 15 min. Samples of the culture supernatant (1 ml) were stored at −20°C. The pellets were resuspended in 10 ml of phosphate-buffered saline containing 2% (wt/vol) sodium dodecyl sulfate (SDS) (Sigma, St. Louis, Mo.) and immediately heated to 100°C for 30 min to inactivate autolysin and dissolve the cell membranes. The samples were cooled to room temperature, washed three times with phosphate-buffered saline, and then resuspended in 10 ml of phosphate-buffered saline. The cell walls were disrupted by two passages through a French pressure cell operated at 12,000 lb/in2. The lysates were centrifuged at 100,000 × g for 35 min, and the pellets were resuspended in 0.5 ml of 150 mM Tris-HCl-1 mM MgSO4, pH 7.0. Then 200 U of mutanolysin (Sigma) was added, and the preparations were incubated at 37°C for 18 h. Finally, the CPS preparation was treated with 10 μg of proteinase K (Roche Applied Science, Mannheim, Germany) and incubated at 56°C for 4 h prior to storage at −20°C.
Western immunoblotting.
Bacterial cell lysates from 1 ml of log-phase culture (A600 of 0.25; containing 5 × 108 CFU/ml) were solubilized in 20 μl of sample buffer prior to separation on sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-12% PAGE) gels (17) and transfer onto an Immobilon-P polyvinylidene difluoride membrane (Millipore Corporation, Bedford, Mass.) (29). Filters were probed with either mouse antiphosphotyrosine monoclonal antibody clone PY99 (Santa Cruz Biotechnology, Santa Cruz, Calif.), used at a dilution of 1:1,000, or mouse anti-CpsD antiserum, used at 1:100, followed by a 1:5,000 dilution of goat anti-mouse immunoglobulin G conjugated to alkaline phosphatase (Bio-Rad Laboratories, Hercules, Calif.). Enzyme-labeled bands were visualized with a nitroblue tetrazolium-X-phosphate substrate system (Roche Applied Science). A Benchmark prestained protein ladder (Life Technologies, Gaithersburg, Md.) was used as molecular weight markers.
Samples consisting of either 100 μl of CPS preparation or 200 μl of culture supernatant were separated on 12% polyacrylamide gels as described by Tikkanen et al. (28) prior to transfer to a Hybond N+ membrane (Amersham Biosciences, Buckinghamshire, England). Filters were probed with polyclonal rabbit anti-group 19 antiserum used at a dilution of 1:5,000, followed by a 1:5,000 dilution of goat anti-rabbit immunoglobulin G conjugated to alkaline phosphatase (Bio-Rad Laboratories). The filters were then developed as described above.
Immunofluorescence.
The presence of surface-exposed CPS in pneumococci was assessed by indirect immunofluorescence as described by Klauser et al. (16). The bound polyclonal rabbit anti-type 19F antiserum was detected with a goat anti-rabbit immunoglobulin antibody conjugated to fluorescein isothiocyanate (Sigma). Phase contrast and epifluorescence microscopy was performed with an Olympus BH2 microscope with fluorescein isothiocyanate filters and a 100× oil immersion objective.
Measurement of CPS with a Stains-all assay.
The CPS preparation method described above could not be used in this assay because the residual SDS present in the samples interfered with the color development. To determine the total amount of CPS produced by each strain, CPS preparations were made by resuspending pneumococci grown on blood agar plates in 150 mM Tris-HCl (pH 7.0)-1 mM MgSO4 so that the A600 was 5. This is equivalent to 5 × 109 pneumococci/ml. An aliquot of 1 ml was pelleted in a microcentrifuge at 13,000 rpm. The “wash supernatant” was transferred to a clean 1.5-ml reaction tube, and the pellet was resuspended in 0.5 ml of 150 mM Tris-HCl (pH 7.0)-1 mM MgSO4. Autolysis of the bacteria was induced by the addition of 0.1% (wt/vol) deoxycholate (Sigma) and incubation at 37°C for 15 min. The samples were then incubated with 100 U of mutanolysin, 50 μg of DNase I (Roche Applied Science), and 50 μg of RNase A (Roche Applied Science) at 37°C for 18 h. The wash supernatants were also incubated with 50 μg of DNase I and 50 μg of RNase A at 37°C for 18 h. The samples were then incubated with 50 μg of proteinase K at 56°C for 4 h prior to storage at −20°C.
The amount of CPS in each sample was determined by mixing either the supernatant (250 μl) or the pellet (50 μl plus 200 μl of H2O) with 1 ml of Stains-all solution [20 mg of 1-ethyl-2-[3-(1-ethylnaphtho-[1,2-d]thiazolin-2-ylidene)-2-methylpropenyl]naphtho-[1,2-d]thiazolium bromide (Stains-all; Sigma) and 60 μl of glacial acetic acid in 100 ml of 50% formamide] and measuring the absorbance at 640 nm (26). Absorbance values were compared with a standard curve generated with known concentrations of purified type 19F CPS; the relationship was linear between 20 μg and 300 μg of CPS per ml. The total amount of Stains-all-reactive material per 5 × 109 pneumococci was then calculated.
Size exclusion chromatography of CPS.
CPS was prepared as described for the Stains-all assay so that the amount of CPS in the final preparation was approximately 1 mg/ml. The CPS preparations (250 μg) were then fractionated on a column containing Sephacryl S-400 (1.6 by 40 cm; Amersham Biosciences). The samples were eluted with 150 mM Tris-HCl (pH 7.0)-150 mM NaCl, and 0.5-ml fractions were collected. CPS was detected by mixing 250 μl of each fraction with 1 ml of Stains-all solution (as described above) and measuring the absorbance at 640 nm. At least two independent CPS preparations were tested, and the tests were repeated at least three times for each strain. The elution profiles obtained from the repeated samples from each strain were superimposable, demonstrating that the profiles obtained were reproducible. The voided volume (V0) of the column was determined with a heat-killed suspension of Serratia marcescens and found to be 13 ml.
RESULTS
Characterization of cpsD mutants containing one, two, or three tyrosine residues in the (YGX)4 repeat domain.
To distinguish the four individual tyrosine (Y) residues in the (YGX)4 repeat domain of CpsD, they were designated Y1 (Y215), Y2 (Y218), Y3 (Y221), and Y4 (Y224). A series of CpsD mutants in which various Y's were replaced with phenylalanine (F) were constructed as described in Materials and Methods. The inferred amino acid sequences of the C-terminal tyrosine-rich domain of CpsD in the four S. pneumoniae Rx1-19F mutants containing only one Y (designated Rx1-19F-DY1, Rx1-19F-DY2, Rx1-19F-DY3, and Rx1-19F-DY4), two mutants containing two Y's (Rx1-19F-DY12 and Rx1-19F-DY34), and one containing three Y's (Rx1-19F-DY123) are shown in Fig. 1.
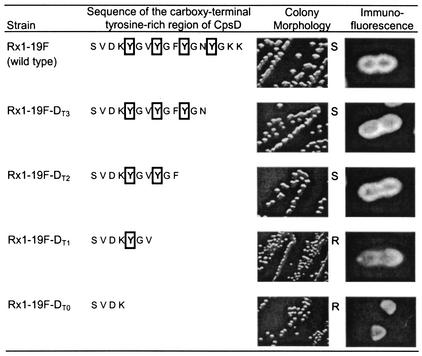
FIG. 1.
Phenotype of mutants containing various numbers of tyrosines in the C-terminal tyrosine-rich (YGX)4 repeat domain of CpsD. The sequences of the C-terminal tyrosine-rich (YGX)4 repeat domain of CpsD for Rx1-19F and various cpsD mutants are shown in the first column. The Y residues are shown in boldface with a solid box, and the F residues are in boldface with a dotted box. The designations and colony morphology of strains containing these mutations are shown in the next two columns. The strain designations, colony morphology, and immunofluorescence of Rx1-19F-BΔ, which has an in-frame deletion of the cpsB gene, and several double mutants containing both the depicted mutations in cpsD and an in-frame deletion of the cpsB gene are shown in the last three columns. The immunofluorescent image shows a typical bacterium.
The colony morphology of the four cpsD mutants with single Y residues (Rx1-19F-DY1, Rx1-19F-DY2, Rx1-19F-DY3, and Rx1-19F-DY4) and the two mutants with two Y residues (Rx1-19F-DY12 and Rx1-19F-DY34) was identical to that of the mucoid mutant Rx1-19F-DY→F, which lacks all four Y's (Fig. 1). However, when three Y's were present, as in Rx1-19F-DY123, the colony morphology was indistinguishable from that of the wild-type strain Rx1-19F (Fig. 1). These results suggest that the presence of one or two Y residues in the (YGX)4 repeat domain of CpsD is insufficient for wild-type CPS production in S. pneumoniae.
Effect of deletion of cpsB gene on mutants containing a single tyrosine residue in the (YGX)4 repeat domain.
Four double mutants in which the cpsB gene was deleted to prevent dephosphorylation of CpsD in addition to the mutations in the cpsD gene (as shown in Fig. 1) were constructed as described in Materials and Methods and designated Rx1-19F-BΔ:DY1, Rx1-19F-BΔ:DY2, Rx1-19F-BΔ:DY3, and Rx1-19F-BΔ:DY4. Whereas Rx1-19F-BΔ:DY1 exhibited a smooth colony morphology, Rx1-19F-BΔ:DY2, Rx1-19F-BΔ:DY3, and Rx1-19F-BΔ:DY4 were rough, which contrasts with the mucoid phenotype of the single cpsD mutants Rx1-19F-DY1, Rx1-19F-DY2, Rx1-19F-DY3, and Rx1-19F-DY4 and the double mutant Rx1-19F-BΔ:DY→F, which has been described previously (Fig. 1) (21). When Rx1-19F-BΔ:DY2, Rx1-19F-BΔ:DY3, and Rx1-19F-BΔ:DY4 were examined by immunofluorescence microscopy, they were indistinguishable from Rx1-19F-BΔ, having a small amount of CPS-related material evenly distributed over the cell surface (Fig. 1). These results indicate that the presence of a single phosphorylated Y at position 2, 3, or 4 in the (YGX)4 repeat domain was sufficient to inactivate CpsD when CpsB (the protein-tyrosine phosphatase) is absent, resulting in the observed rough phenotype of these double mutants. In contrast, the Rx1-19F-BΔ:DY1 double mutant was smooth, suggesting that wild-type levels of CPS were produced and indicating that CpsDY1 is still functional even in the absence of CpsB.
Characterization of cpsD mutants truncated at positions Y1, Y2, Y3, and Y4 of the (YGX)4 repeat domain.
Four mutants in which the cpsD gene was truncated by replacing the codon for Y1, Y2, Y3, or Y4 with the TAA stop codon were constructed by overlap extension PCR, as described in Materials and Methods. The resultant mutants, designated Rx1-19F-DT0, Rx1-19F-DT1 Rx1-19F-DT2, and Rx1-19F-DT3, contained zero, one, two, and three YGX repeats in CpsD, respectively, as shown in Fig. 2. A single base change in the cpsD gene in Rx1-19F-DT3 introduced a stop codon at the Y4 position. However, the DNA between the introduced stop codon and the end of cpsD was deleted for Rx1-19F-DT0, Rx1-19F-DT1, and Rx1-19F-DT2 to ensure that there were no polar effects due to a larger intergenic region between cpsD and cpsE.
FIG. 2.
Phenotype of truncated cpsD mutants. The protein sequences of the C-terminal (YGX)4 region of CpsD for Rx1-19F and the four truncated mutants are shown. The Y residues are boxed. S indicates a smooth colony morphology, and R indicates rough colonies. The immunofluorescent image shows a typical bacterium.
The colony morphology of mutants Rx1-19F-DT2 and Rx1-19F-DT3, which contained CpsD with two and three YGX repeats, respectively, was smooth, suggesting that they produced wild-type levels of CPS (Fig. 2), whereas mutants Rx1-19F-DT0 and Rx1-19F-DT1 containing CpsD with zero and one YGX repeat, respectively, were rough. Interestingly, when examined by immunofluorescence microscopy to detect surface-expressed CPS, Rx1-19F-DT0 and Rx1-19F-DT1 had different phenotypes (Fig. 2). The Rx1-19F-DT1 mutant had a small amount of CPS-related material evenly distributed over the cell surface, suggesting that CpsDT1 retains some activity. In comparison, CPS-related material could only be detected at the poles of some of the Rx1-19F-DT0 bacteria, as seen with Rx1-19F-DΔ, a mutant in which the entire cpsD gene had been deleted (21). These results indicate that CpsD is not functional if the entire (YGX)4 tail is deleted and that at least two YGX repeats are required for normal function. This suggests that the C-terminal tyrosine-rich region of CpsD must perform a specific function in CPS production.
Detection of CpsD and CpsD∼P by Western immunoblotting.
The presence of CpsD∼P in cell lysates from Rx1-19F-DY→F, Rx1-19F-DY1, Rx1-19F-DY2, Rx1-19F-DY3, Rx1-19F-DY4, Rx1-19F-DY12, Rx1-19F-DY34, Rx1-19F-DY123, and Rx1-19F was investigated by Western immunoblotting with a mouse antiphosphotyrosine monoclonal antibody (Fig. 3A). CpsD∼P was readily detected in cell lysates from Rx1-19F, Rx1-19F-DY123, Rx1-19F-DY12, and Rx1-19F-DY34 and only weakly in cell lysates from Rx1-19F-DY2, Rx1-19F-DY3, and Rx1-19F-DY4, but not at all in cell lysates from Rx1-19F-DY→F and Rx1-19F-DY1. The intensity of the bands detected was related to the number of tyrosines present in the (YGX)4 repeat domain except for CpsDY1, which suggests that Y1 either does not become phosphorylated or is phosphorylated at a low level that cannot be detected by Western immunoblotting. However, Y1 does appear to become phosphorylated in CpsDY12, as relatively less CpsDY2∼P was detected with the antiphosphotyrosine monoclonal antibody compared to CpsDY12∼P, and there was no difference between the intensity of the CpsDY12∼P and CpsDY34∼P bands detected (Fig. 3A). These results suggest that tyrosine phosphorylation occurs at position Y2, Y3, or Y4 before it can occur at Y1. All lysates contained similar amounts of CpsD, as detected with a polyclonal mouse anti-CpsD antiserum (Fig. 3B).
FIG. 3.
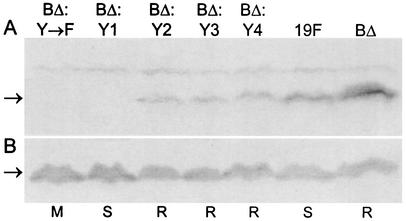
Detection of CpsD and CpsD≈P in S. pneumoniae cpsD point mutants. Western immunoblot of whole-cell lysates from strains Rx1-19F-DY→F (Y→F), Rx1-19F-DY1 (Y1), Rx1-19F-DY2 (Y2), Rx1-19F-DY3 (Y3), Rx1-19F-DY4 (Y4), Rx1-19F-DY12 (Y12), Rx1-19F-DY34 (Y34), Rx1-19F-DY123 (Y123), and Rx1-19F (19F) probed with mouse antiphosphotyrosine monoclonal antibody PY99 (A) and probed with mouse anti-CpsD antiserum (B). The expected mobility of the CpsD protein is indicated by an arrow. S indicates strains with a smooth colony morphology, and M indicates those which are mucoid.
The presence of CpsD∼P in cell lysates from Rx1-19F, Rx1-19F-BΔ:DY→F, Rx1-19F-BΔ:DY1, Rx1-19F-BΔ:DY2, Rx1-19F-BΔ:DY3, Rx1-19F-BΔ:DY4, and Rx1-19F-BΔ was also investigated by Western immunoblotting with the mouse antiphosphotyrosine monoclonal antibody (Fig. 4A). As expected, CpsD∼P could easily be detected in Rx1-19F and Rx1-19F-BΔ, and a lower level of CpsD∼P could be detected in Rx1-19F-BΔ:DY2, Rx1-19F-BΔ:DY3, and Rx1-19F-BΔ:DY4. However, no CpsD∼P could be detected in Rx1-19F-BΔ:DY1 or Rx1-19F-BΔ:DY→F. These results suggest that when only Y1 was present in the (YGX)4 repeat domain of CpsD, it was not efficiently phosphorylated, whereas Y2, Y3, and Y4 were phosphorylated. All lysates contained similar amounts of CpsD, as detected with a polyclonal mouse anti-CpsD antiserum (Fig. 4B).
FIG. 4.
Detection of CpsD and CpsD∼P protein in S. pneumoniae cpsBΔ cpsD double mutants. Western immunoblot of whole-cell lysates from strains Rx1-19F-BΔ:DY→F (BΔ:Y→F), Rx1-19F-BΔ:DY1 (BΔ:Y1), Rx1-19F-BΔ:DY2 (BΔ:Y2), Rx1-19F-BΔ:DY3 (BΔ:Y3), Rx1-19F-BΔ:DY4 (BΔ:Y4), Rx1-19F (19F), and Rx1-19F-BΔ (BΔ) probed with mouse antiphosphotyrosine monoclonal antibody PY99 (A) and with mouse anti-CpsD antiserum (B). The expected mobility of the CpsD protein is indicated by an arrow. S indicates strains with a smooth colony morphology, R indicates those which are rough, and M indicates those which are mucoid.
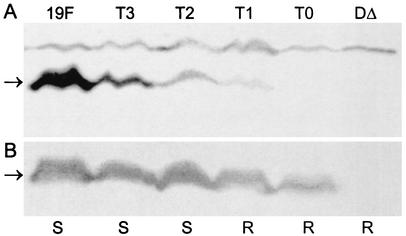
The presence of CpsD in cell lysates from Rx1-19F, Rx1-19F-DT0, Rx1-19F-DT1, Rx1-19F-DT2, Rx1-19F-DT3, and Rx1-19F-DΔ was investigated by Western immunoblotting with a polyclonal mouse anti-CpsD antiserum (Fig. 5B). Similar amounts of CpsD were detected in all cell lysates except Rx1-19F-DΔ, which does not produce the CpsD protein. The presence of CpsD∼P in these cell lysates was also investigated by Western immunoblotting with the mouse antiphosphotyrosine monoclonal antibody (Fig. 5A). CpsD∼P could be detected in cell lysates from Rx1-19F, Rx1-19F-DT1, Rx1-19F-DT2, and Rx1-19F-DT3 but not in cell lysates from Rx1-19F-DT0 and Rx1-19F-DΔ. These results show that when CpsD contains only one YGX repeat, as in CpsDT1, Y1 can become phosphorylated. This contrasts with the observed results for CpsDY1, as CpsDY1∼P could not be detected by Western immunoblotting, suggesting that the C-terminal (FGX)3 repeats may interfere with phosphorylation of Y1.
FIG. 5.
Detection of CpsD and CpsD∼P protein in S. pneumoniae truncated cpsD mutants. Western immunoblot of whole-cell lysates from strains Rx1-19F (19F), Rx1-19F-DT3 (T3), Rx1-19F-DT2 (T2), Rx1-19F-DT1 (T1), Rx1-19F-DT0 (T0), and Rx1-19F-DΔ (DΔ) probed with mouse antiphosphotyrosine monoclonal antibody PY99 (A) and with mouse anti-CpsD antiserum (B). The expected mobility of the CpsD protein is indicated by an arrow. S indicates strains with a smooth colony morphology, and R indicates those which are rough.
Analysis of cell wall-associated CPS production by Western immunoblotting.
To determine if mutations in the (YGX)4 repeat domain affected the length of the CPS polymer, cell wall-associated CPS was extracted from the wild-type strain Rx1-19F and the mutants described above and compared by Western immunoblotting with a rabbit anti-group 19 CPS antiserum, as described in Materials and Methods.
CPS preparations from wild-type Rx1-19F and the two truncated cpsD mutants Rx1-19F-DT2 and Rx1-19F-DT3 appeared to contain similar amounts of CPS (Fig. 6), suggesting that these mutants were similar to the wild-type strain. In contrast, CPS preparations from Rx1-19F-DT0 and Rx1-19F-DΔ did not contain any detectable CPS, but CPS preparations from Rx1-19F-DT1 contained a reduced amount of high-molecular-weight CPS relative to Rx1-19F (Fig. 6). These results confirm that when its entire (YGX)4 tail is deleted, CpsD is not functional. CpsD retains some function if a single YGX repeat unit is present, and at least two YGX repeats are required for CpsD to be fully functional.
FIG. 6.
Detection of cell-associated CPS from S. pneumoniae truncated cpsD mutants. Western immunoblot of cell-associated CPS preparations from strains Rx1-19F (19F), Rx1-19F-DT0 (T0), Rx1-19F-DT1 (T1), Rx1-19F-DT2 (T2), Rx1-19F-DT3 (T3), and Rx1-19F-DΔ (DΔ) probed with rabbit anti-group 19 antiserum. S indicates strains with a smooth colony morphology, and R indicates those which are rough.
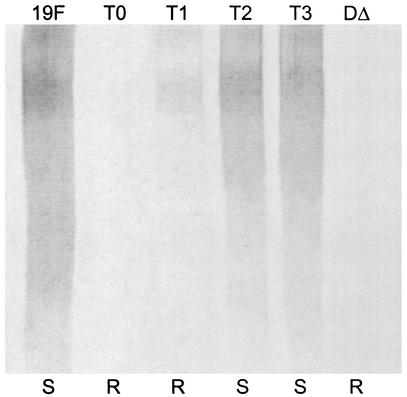
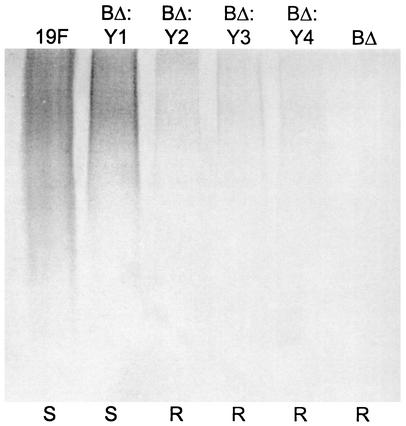
When the CPS produced by wild-type Rx1-19F and Rx1-19F-BΔ:DY1 was compared, Rx1-19F-BΔ:DY1 produced almost as much high-molecular-weight CPS as Rx1-19F (Fig. 7). Whereas Rx1-19F-BΔ did not produce any high-molecular-weight CPS, the rough mutants Rx1-19F-BΔ:DY2, Rx1-19F-BΔ:DY3, and Rx1-19F-BΔ:DY4 produced a reduced amount of high-molecular-weight CPS (Fig. 7). These results indicate that, in the absence of CpsB, CpsDY1 is functional, while CpsDY2, CpsDY3, and CpsDY4 retain a very low level of activity and CpsD is not functional.
FIG. 7.
Detection of cell-associated CPS from S. pneumoniae cpsBΔ cpsD double mutants. Western immunoblot of cell-associated CPS preparations from strains Rx1-19F (19F), Rx1-19F-BΔ:DY1 (BΔ:Y1), Rx1-19F-BΔ:DY2 (BΔ:Y2), Rx1-19F-BΔ:DY3 (BΔ:Y3), Rx1-19F-BΔ:DY4 (BΔ:Y4), and Rx1-19F-BΔ (BΔ) probed with rabbit anti-group 19 antiserum. S indicates strains with a smooth colony morphology, and R indicates those which are rough.
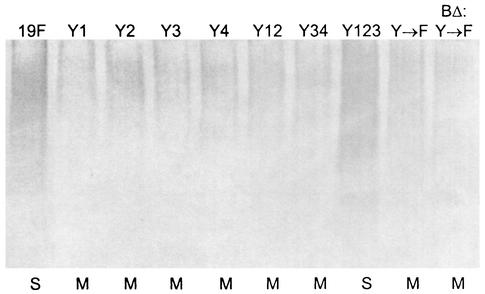
Interestingly, CPS preparations from the mucoid mutant strains Rx1-19F-DY1, Rx1-19F-DY2, Rx1-19F-DY3 Rx1-19F-DY4, Rx1-19F-DY12, Rx1-19F-DY34, Rx1-19F-DY→F, and Rx1-19F-BΔ:DY→F contained less high-molecular-weight CPS than Rx1-19F (Fig. 8A). The smooth strain Rx1-19F-DY123 contained about as much CPS as Rx1-19F (Fig. 8A). These results suggest that the observed mucoid phenotype in the mutants Rx1-19F-DY1, Rx1-19F-DY2, Rx1-19F-DY3 Rx1-19F-DY4, Rx1-19F-DY12, Rx1-19F-DY34, Rx1-19F-DY→F, and Rx1-19F-BΔ:DY→F is not due to overproduction of CPS. The culture supernatants from these strains were examined to determine if the mucoid strains released more CPS from the cell surface into the extracellular medium than the wild-type strain. Less CPS was recovered from the supernatant of the mucoid strains than from the supernatant of wild-type Rx1-19F (data not shown), confirming that the mucoid strains actually produced less CPS than the wild-type strain, as shown above.
FIG. 8.
Detection of cell-associated CPS from S. pneumoniae cpsD mutants containing one, two, or three tyrosine residues in the (YGX)4 repeat domain. Western immunoblot of CPS preparations from strains Rx1-19F (19F), Rx1-19F-DY1 (Y1), Rx1-19F-DY2 (Y2), Rx1-19F-DY3 (Y3), Rx1-19F-DY4 (Y4), Rx1-19F-DY12 (Y12), Rx1-19F-DY34 (Y34), Rx1-19F-DY123 (Y123) Rx1-19F-DY→F (Y→F), and Rx1-19F-BΔ:DY→F (BΔ:Y→F) probed with rabbit anti-group 19 antiserum. S indicates strains with a smooth colony morphology, and M indicates those which are mucoid.
Quantitation of amount of CPS produced with Stains-all assay.
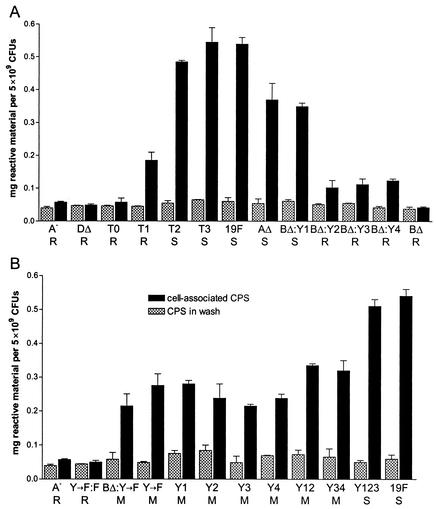
Stains-all is known to bind to acid polysaccharides (26). As type 19F CPS is a highly acidic polysaccharide, we used Stains-all, as described in Materials and Methods, to quantitate the amount of CPS produced by Rx1-19F and the cpsD mutants described above. The results are shown in Fig. 9. Strain Rx1-19F-A, which contains a polar insertion-duplication mutation in cpsA and has been shown previously not to have any detectable CPS-related material on its cell surface (21), was included as a negative control. This strain showed a background level of 0.06 mg of reactive material per 5 × 109 pneumococci, whereas smooth strains had up to 0.55 mg of reactive material per 5 × 109 pneumococci. The amount of material detected in the wash supernatant of all strains ranged from 0.04 mg to a maximum of 0.1 mg of reactive material per ml, indicating that the CPS material is closely associated with the pneumococcal cell. These CPS preparations were also checked by Western immunoblotting with a polyclonal rabbit anti-group 19 antiserum as described above. The relative properties of anti-CPS-reactive material in these preparations from the various mutants grown on blood agar were the same as that previously shown for preparations from THY cultures (data not shown).
FIG. 9.
Detection of CPS from Rx1-19F and various S. pneumoniae cps mutants with Stains-all. The graphs show the amount of Stains-all reactive material per 5 × 109 pneumococci in CPS preparations (black bars) and in the wash supernatant (hatched bars), determined as described in Materials and Methods. Measurements of two independent samples were taken, and error bars represent standard errors. The strains measured are indicated as follows. A: Rx1-19F-A (A−), Rx1-19F-DΔ (DΔ), Rx1-19F-DT0 (T0), Rx1-19F-DT1 (T1), Rx1-19F-DT2 (T2), Rx1-19F-DT3 (T3), Rx1-19F (19F), Rx1-19F-AΔ (AΔ), Rx1-19F-BΔ:DY1 (BΔ:Y1) Rx1-19F-BΔ:DY2 (BΔ:Y2) Rx1-19F-BΔ:DY3 (BΔ:Y3) Rx1-19F-BΔ:DY4 (BΔ:Y4), and Rx1-19F-BΔ (BΔ). B: Rx1-19F-A (A−), Rx1-19F-DY→F:F (Y→F:F), Rx1-19F-BΔ:DY→F (BΔ:Y→F), Rx1-19F-DY→F (Y→F), Rx1-19F-DY1 (Y1), Rx1-19F-DY2 (Y2), Rx1-19F-DY3 (Y3), Rx1-19F-DY4 (Y4), Rx1-19F-DY12 (Y12), Rx1-19F-DY34 (Y34), Rx1-19F-DY123 (Y123), and Rx1-19F (19F). S indicates strains with a smooth colony morphology, R indicates those which are rough, and M indicates those which are mucoid.
Whereas the smooth strain Rx1-19F-BΔ:DY1 was found to contain 65% of the amount of cell-associated CPS detected with Rx1-19F; the rough strains Rx1-19F-BΔ:DY2, Rx1-19F-BΔ:DY3, and Rx1-19F-BΔ:DY4 contained only approximately 20% of the amount of CPS present in Rx1-19F (Fig. 9A). Only a background level of 0.045 mg of reactive material per 5 × 109 pneumococci could be detected for Rx1-19F-BΔ with this method. These results confirm the results obtained by Western immunoblotting described above (Fig. 7).
The smooth CpsD truncation mutants Rx1-19F-DT2 and Rx1-19F-BΔ:DT3 produced 90% and 101% of Rx1-19F CPS levels, respectively, and only background levels of reactive material could be detected for the rough strains Rx1-19F-DT0 and Rx1-19F-DΔ with this method, confirming the Western immunoblotting results (Fig. 6). However, the rough strain Rx1-19F-BΔ:DT1 contained 34% of the amount of reactive material detected in Rx1-19F. A small amount of CPS was also detectable in this strain by Western immunoblotting (Fig. 6). Rx1-19F-AΔ, a partially encapsulated strain with colonies that are approximately half the size of those of Rx1-19F (21), contained 69% of the amount of reactive material detected for Rx1-19F and approximately double that for Rx1-19F-BΔ:DT1 (Fig. 9A). This suggests that 34% of wild-type CPS levels is insufficient to confer a smooth colony morphology in pneumococci growing on blood agar.
When the amount of CPS associated with the mucoid cpsD mutants containing zero, one, or two Y residues in the (YGX)4 repeat domain was examined, they were found to contain between 40% and 62% of wild-type CPS levels (Fig. 9B). Rx1-19F-DY123, which contains three Y residues in the (YGX)4 repeat domain of CpsD and exhibits a smooth colony morphology, produced an amount of CPS similar to that found in Rx1-19F (94%; Fig. 9B). The amount of CPS produced by the mutants exhibiting mucoid colony morphologies was consistent with the results obtained by Western immunoblotting (Fig. 8). This suggests that the mucoid phenotype must be due to the physical and rheological properties of the CPS and not to overexpression of CPS, as would have been predicted for a mucoid phenotype.
Chromatographic analysis of CPS from wild-type and mucoid strains.
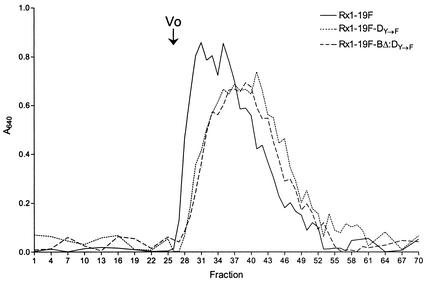
To determine if the chain lengths of CPS from wild-type strain Rx1-19F and mucoid strains Rx1-19F-DY→F and Rx1-19F-BΔ:DY→F were different, CPS preparations from these strains were subjected to size exclusion chromatography as described in Materials and Methods. The presence of type 19F CPS in fractions 27 to 55 was confirmed by Western immunoblotting with a rabbit anti-group 19 CPS antiserum (data not shown). The CPS from Rx1-19F (wild-type CPS) eluted earlier than the CPS from the mucoid strains Rx1-19F-DY→F and Rx1-19F-BΔ:DY→F (Fig. 10), suggesting that the molecular weight of wild-type CPS was larger than that of mucoid CPS.
FIG. 10.
Size exclusion chromatography of wild-type CPS and mucoid CPS with Sephacryl S-400. The elution profile of the wild-type strain Rx1-19F and the mucoid strains Rx1-19F-DY→F and Rx1-19F-BΔ:DY→F are shown. The volume of each fraction was 0.5 ml, and the voided volume (V0; fraction 26) is marked. The amount of CPS in each fraction was quantitated with the Stains-all reagent and measurement of the A640.
Insertion-duplication mutagenesis of cps19fF.
To determine if the mucoid phenotype is due solely to expression of type 19F CPS, the cps19fF gene (which encodes the putative N-acetylmannosamine transferase) in the mucoid strain Rx1-19F-DY→F was interrupted by insertion-duplication mutagenesis as described in Materials and Methods. The resultant transformant was designated Rx1-19F-DY→F:F and exhibited a rough colony morphology, which was confirmed by Quellung reaction (data not shown). When examined by immunofluorescence microscopy, no surface-expressed CPS could be detected (data not shown), as seen previously for Rx1-19F-A, which contains a polar mutation in cpsA, eliminating type 19F CPS production (21). When the amount of CPS produced by Rx1-19F-DY→F:F was quantified with the Stains-all assay, only a background level of reactive material was detected, as for Rx1-19F-A (Fig. 9B). These results indicate that the mucoid phenotype is due entirely to the expression of type 19F CPS.
DISCUSSION
We have previously shown that CpsD is an autophosphorylating protein-tyrosine kinase and that tyrosine phosphorylation in the C-terminal (YGX)4 repeat domain attenuates its activity (21). We demonstrated that the absence of the phosphotyrosine-protein phosphatase (CpsB) resulted in high levels of CpsD∼P and eliminated CPS production. However, in strains containing CpsDY→F, which cannot be phosphorylated, the absence of CpsB had no effect on CPS production (21). Thus, tyrosine phosphorylation of CpsD has a negative effect on CPS production in S. pneumoniae. We demonstrate here that CpsD requires a C-terminal YGX repeat domain consisting of at least two repeats to be functional and that the Y residues also play an important role in CpsD activity.
The truncated CpsD mutant Rx1-19F-DT0 demonstrated clearly that the (YGX)4 repeat domain is required for CpsD function in S. pneumoniae. CpsDT0, which lacks the entire (YGX)4 repeat domain, is nonfunctional, and the phenotype of the mutant Rx1-19F-DT0 is indistinguishable from that of Rx1-19F-DΔ, in which the entire cpsD gene has been deleted (Fig. 2). CpsDT1, which has a single YGX repeat, had a low level of activity resulting in production of 34% of wild-type CPS levels but was rough, as determined by colony morphology (small, dull colonies) and Quellung reaction (Fig. 6, Fig. 9A). Although 34% of the wild-type CPS level appears to be a substantial level of CPS expression, it is clearly insufficient to confer a smooth macroscopic phenotype. Previously, Dillard and Yother (8) showed that the well-documented rough S. pneumoniae strain Rx1 produces 40% of normal type 3 CPS levels. CpsDT2 and CpsDT3, which have two and three YGX repeats, respectively, functioned normally, as the mutants Rx1-19F-DT2 and Rx1-19F-DT3 were smooth and produced wild-type levels of CPS (Fig. 9A). Thus, a minimum of two YGX repeats are absolutely required for CpsD function. These findings are consistent with the phenotype of a truncated Escherichia coli WzcCPS protein (35); when the C-terminal tyrosine-rich domain was deleted, the truncated WzcCPS protein was unable to function in K-30 CPS assembly.
Interestingly, tyrosine phosphorylation of homologous Wzc proteins from E. coli K-12 and K-30 was found to have opposite effects on polysaccharide biosynthesis. Colanic acid biosynthesis in E. coli K-12 requires the nonphosphorylated form of WzcCA (31). When Wzb (the protein-tyrosine phosphatase) was absent, WzcCA was highly phosphorylated and no colanic acid was formed, and when Wzb was overproduced, very little WzcCA was phosphorylated and an increased amount of colanic acid was produced (31). Conversely, phosphorylation of WzcCPS was determined to be essential for K-30 CPS assembly (35). When all seven Y's in the C terminus of WzcCPS were replaced with F's, the mutant WzcCPS protein was identical to that of a C-terminal deletion mutant; it was not functional, and no K-30 CPS was assembled. In fact, there is a requirement for two to five Y residues in the C terminus of WzcCPS to be phosphorylated for function (24). Overexpression of Wzb, the phosphotyrosine-protein phosphatase, also reduced K-30 CPS assembly. Paradoxically, the loss of phosphotyrosine-protein phosphatase activity due to deletion of the wzb gene also resulted in loss of K-30 CPS assembly (35). The function of CpsD in pneumococcal CPS production is more consistent with the function of WzcCA than that of WzcCPS.
The mucoid phenotype observed in the mutants expressing CpsD proteins containing zero, one, or two Y residues in the (YGX)4 repeat domain is not due to overexpression of CPS, as previously proposed (21); these strains actually produced less CPS than the wild-type strain (Fig. 9). The mucoid CPS produced by the mucoid type 19F strains also had a lower molecular weight than wild-type CPS (Fig. 10).
We propose that substitution of Y residues with F in the C-terminal (YGX)4 repeat domain results in local conformational changes that interfere with CpsD function, reducing the amount of CPS produced. In fact, the data in Fig. 9 show a correlation between the number of tyrosines present in the (YGX)4 repeat domain and the amount of CPS produced. CpsD is predicted to function together with CpsC in polymerization and export of CPS, and mutations which affect the (YGX)4 repeat domain of CpsD appear to affect its role in CPS polymerization, resulting in mucoid CPS production. We have shown that the mucoid phenotype is entirely due to production of type 19F CPS, as disruption of the downstream cps19fF gene in Rx1-19F-DY→F:F resulted in loss of the mucoid phenotype and loss of type 19F CPS production. We recently constructed similar cpsD mutations in S. pneumoniae D39, a type 2 strain, and found that these mutants were also mucoid, indicating that the mucoid phenotype is not limited to type 19F CPS (unpublished data). We speculate that the difference in molecular size between wild-type CPS and mucoid CPS is sufficient to alter the physical and rheological properties of the mucoid CPS and account for the observed mucoid colony morphology of these mutants.
We propose that CPS biosynthesis requires the switching of CpsD between its active (nonphosphorylated) state and its inactive (phosphorylated state). In its active state, we hypothesize that CpsD interacts with CpsC and possibly other proteins, such as the polysaccharide polymerase (Cps19fI), promoting polymerization of the polysaccharide repeat units. When CpsD becomes phosphorylated, the interaction between CpsD and other proteins changes so that CPS polymerization ceases, promoting transfer of the CPS polymer to the undefined cell wall-CPS ligase. Dephosphorylation of CpsD∼P by CpsB then allows the cycle to be repeated. The mechanism that times this cycle is unknown, but cellular ATP levels may have an impact.
It has been demonstrated that the binding of ATP to CpsD and its homologues ExoP and WzcCPS is essential for the function of these proteins (9, 21, 23, 24, 35). This hypothesis is consistent with CpsC and CpsD functioning as polysaccharide copolymerases (class PCP2b), as described by Morona et al. (22). A similar model was proposed by Bastin et al. (2) to describe the function of Wzz and the O-antigen polymerase (Wzy) in determining the O-antigen modal chain length in lipopolysaccharide biosynthesis in E. coli and Salmonella enterica. Wzz interacts with Wzy and has two states: state E, which favors extension of the polymer by the polymerase, and state T, which favors transfer of the polymer to the ligase. The mechanism involved in changing the state of Wzz from E to T was not determined. We suggest that the mechanism, or switch, which changes the state of CpsC/CpsD in CPS production in S. pneumoniae and WzcCA in colanic acid assembly in E. coli is tyrosine phosphorylation. Wzz proteins (or PCP1 proteins) are not associated with an autophosphorylating tyrosine kinase, and a different mechanism must be involved in switching between the two states in these proteins.
There is an apparent difference in the functions of CpsDT2 compared to CpsDY12 and CpsDY34 even though all three proteins contain two Y's. Mutants containing CpsDY12 and CpsDY34 were mucoid and produced reduced levels of CPS (Fig. 1, Fig. 8, and Fig. 9B), whereas the mutant containing CpsDT2 was smooth and had wild-type levels of CPS (Fig. 2, Fig. 6, and Fig. 9A). One explanation for this could be that the truncated (YGX)2 repeat region in CpsDT2 can still function efficiently but the activity of CpsDY12 and CpsDY34 is affected by the presence of the two FGX repeats that cannot be phosphorylated.
All four C-terminal Y residues are implicated in phosphorylation of CpsD. Whereas Y2, Y3, and Y4 are readily phosphorylated, there is limited direct evidence to show phosphorylation of Y1 in the intact (YGX)4 repeat domain. When only a single YGX repeat was present in CpsD, as in CpsDT1, the single Y residue (equivalent to Y1) could be phosphorylated. CpsDT1∼P was detected in the lysate of Rx1-19F-DT1 by Western immunoblotting with the mouse antiphosphotyrosine monoclonal antibody (Fig. 5A), but CpsDY1∼P was not detected even in the absence of the phosphatase CpsB (in strains Rx1-19F-DY1 and Rx1-19F-BΔ:DY1, respectively; Fig. 3A and Fig. 4A). Additionally, the results suggest that multiple Y residues may be phosphorylated at one time in a single CpsD∼P protein. The amount of phosphorylated Y detected by Western immunoblotting was considerably higher for CpsDY12∼P and CpsDY34∼P than for CpsDY2∼P, CpsDY3∼P, and CpsDY4∼P, with relative increases for both CpsDY123∼P and CpsD∼P, indicating that more than one Y may be phosphorylated in CpsDY12∼P, CpsDY34∼P, CpsDY123∼P, and CpsD∼P (Fig. 3A).
Although these results do not determine conclusively how many Y residues become phosphorylated on an individual CpsD∼P protein, phosphorylation of Y1, Y2, Y3, and Y4 appears to be partly cooperative. Hence, initial phosphorylation at Y2, Y3, or Y4 could influence the phosphorylation state of other Y residues. Bender and Yother (3) demonstrated that CpsD∼P is able to transphosphorylate an exogenous substrate. Thus, we suggest that transphosphorylation between the Y residues in the (YGX)4 repeat domains of CpsD proteins may also play a role in the tyrosine phosphorylation/dephosphorylation cycle. A cooperative effect for tyrosine phosphorylation has also been suggested for WzcCA in E. coli (12). These authors suggest that initial phosphorylation of Y569, which is located between the Walker A and B motifs, enhances subsequent phosphorylation of the six Y residues in the C-terminal cluster of WzcCA.
The three double mutants Rx1-19F-BΔ:DY2, Rx1-19F-BΔ:DY3 and Rx1-19F-BΔ:DY4 were all rough (Fig. 1), indicating that a single phosphorylated Y at position Y2, Y3, or Y4 is sufficient to inactivate CpsD when it cannot be dephosphorylated. These data support the hypothesis that tyrosine phosphorylation of CpsD negatively regulates CPS production. However, unlike Rx1-19F-BΔ, these three mutants all produced a small amount of high-molecular-weight CPS (Fig. 7). We suggest that CpsDY2, CpsDY3, and CpsDY4 are transiently active, as we predict that they are phosphorylated more slowly than CpsD, and hence produce some CPS before becoming inactivated by phosphorylation on Y2, Y3, or Y4. Additionally, significantly less CpsDY2∼P, CpsDY3∼P, and CpsDY4∼P was detected in Rx1-19F-BΔ:DY2, Rx1-19F-BΔ:DY3, and Rx1-19F-BΔ:DY4 lysates compared to CpsD∼P from Rx1-19F-BΔ lysates (Fig. 4A). This supports the hypothesis that multiple Y residues are phosphorylated in wild-type CpsD even though a single phosphorylated Y at position Y2, Y3, or Y4 is sufficient to inactivate CpsD when it cannot be dephosphorylated. This suggests that the mechanisms that control the tyrosine phosphorylation/dephosphorylation cycle of CpsD are complex, and further investigations into these mechanisms are required. Future studies will also focus on the interactions of CpsD with CpsC and other proteins, such as the polysaccharide polymerase, and the role of tyrosine phosphorylation in this process.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council of Australia and the Channel 7 Children's Research Foundation.
REFERENCES
- 1.Austrian, R. 1981. Some observations on the pneumococcus and on the current status of pneumococcal disease and its prevention. Rev. Infect. Dis. 3(Suppl.):S1-S17. [DOI] [PubMed] [Google Scholar]
- 2.Bastin, D. A., G. Stevenson, P. K. Brown, A. Haase, and P. R. Reeves. 1993. Repeat unit polysaccharides of bacteria: a model for polymerization resembling that of ribosomes and fatty acid synthetase, with a novel mechanism for determining chain length. Mol. Microbiol. 7:725-734. [DOI] [PubMed] [Google Scholar]
- 3.Bender, M. H., and J. Yother. 2001. CpsB is a modulator of capsule-associated tyrosine kinase activity in Streptococcus pneumoniae. J. Biol. Chem. 276:47966-47974. [DOI] [PubMed] [Google Scholar]
- 4.Berry, A. M., J. Yother, D. E. Briles, D. Hansman, and J. C. Paton. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57:2324-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartee, R. T., W. T. Forsee J. W. Jensen, and J. Yother. 2001. Expression of the Streptococcus pneumoniae type 3 synthase in Escherichia coli. Assembly of type 3 polysaccharide on a lipid primer. J. Biol. Chem. 276:48831-48839. [DOI] [PubMed] [Google Scholar]
- 6.Cieslewicz, M. J., D. L. Kasper, Y. Wang, and M. R. Wessels. 2001. Functional analysis in type Ia group B Streptococcus of a cluster of genes involved in extracellular polysaccharide production by diverse species of Streptococci. J. Biol. Chem. 276:139-146. [DOI] [PubMed] [Google Scholar]
- 7.DeAngelis, P. L., and P. H. Weigel. 1994. Immunochemical confirmation of the primary structure of streptococcal hyaluronan synthase and synthesis of high molecular weight product by the recombinant enzyme. Biochemistry 33:9033-9039. [DOI] [PubMed] [Google Scholar]
- 8.Dillard, J. P., and J. Yother. 1994. Genetic and molecular characterization of capsular polysaccharide biosynthesis in Streptococcus pneumoniae type 3. Mol. Microbiol. 12:959-972. [DOI] [PubMed] [Google Scholar]
- 9.Doublet, P., C. Grangeasse, B. Obadia, E. Vaganay, and A. J. Cozzone. 2002. Structural organization of the protein-tyrosine autokinase Wzc within Escherichia coli cells. J. Biol. Chem. 277:37339-37348. [DOI] [PubMed] [Google Scholar]
- 10.Fischer, H., and A. Tomasz. 1985. Peptidoglycan cross-linking and teichoic acid attachment in Streptococcus pneumoniae. J. Bacteriol. 163:46-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glucksmann, M. A., T. L. Reuber, and G. C. Walker. 1993. Genes needed for the modification, polymerisation, export, and processing of succinoglycan by Rhizobium meliloti: a model for succinoglycan biosynthesis. J. Bacteriol. 175:7045-7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grangeasse, C., P. Doublet, and A. J. Cozzone. 2002. Tyrosine phosphorylation of protein kinase Wzc from Escherichia coli K-12 occurs through a two-step process. J. Biol. Chem. 277:7127-7135. [DOI] [PubMed] [Google Scholar]
- 13.Guidolin, A., J. K. Morona, R. Morona, D. Hansman, and J. C. Paton. 1994. Nucleotide sequence analysis of genes essential for capsular polysaccharide biosynthesis in Streptococcus pneumoniae type 19F. Infect. Immun. 62:5384-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henrichsen, J. 1995. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 33:2759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, J. O., and J. N. Weiser. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177:368-377. [DOI] [PubMed] [Google Scholar]
- 16.Klauser, T., J. Pohlner, and T. F. Meyer. 1990. Extracellular transport of cholera toxin B subunit with Neisseria IgA protease beta-domain: conformation-dependent outer membrane translocation. EMBO J. 9:1991-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 18.Llull, D., E. Garcia, and R. Lopez. 2001. Tts, a processive beta-glucosyltransferase of Streptococcus pneumoniae, directs the synthesis of the branched type 37 capsular polysaccharide in Pneumococcus and other gram-positive species. J. Biol. Chem. 276:21053-21061. [DOI] [PubMed] [Google Scholar]
- 19.Morona, J. K., A. Guidolin, R. Morona, D. Hansman, and J. C. Paton. 1994. Isolation, characterization, and nucleotide sequence of IS1202, an insertion sequence of Streptococcus pneumoniae. J. Bacteriol. 176:4437-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morona, J. K., R. Morona, D. C. Miller, and J. C. Paton. 2002. Streptococcus pneumoniae capsule biosynthesis protein CpsB is a novel manganese-dependent phosphotyrosine-protein phosphatase. J. Bacteriol. 184:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morona, J. K., J. C. Paton, D. C. Miller, and R. Morona. 2000. Tyrosine phosphorylation of CpsD negatively regulates capsular polysaccharide biosynthesis in Streptococcus pneumoniae. Mol. Microbiol. 35:1431-1442. [DOI] [PubMed] [Google Scholar]
- 22.Morona, R., L. Van Den Bosch, and C. Daniels. 2000. Evaluation of Wzz/MPA1/MPA2 proteins based on the presence of coiled-coil regions. Microbiology 146:1-4. [DOI] [PubMed] [Google Scholar]
- 23.Niemeyer, D., and A. Becker. 2001. The molecular weight distribution of succinoglycan produced by Sinorhizobium meliloti is influenced by specific tyrosine phosphorylation and ATPase activity of the cytoplasmic domain of the ExoP protein. J. Bacteriol. 183:5163-5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paiment, A., J. Hocking, and C. Whitfield. 2002. Impact of phosphorylation of specific residues in the tyrosine autokinase, Wzc, on its activity in assembly of group 1 capsules in Escherichia coli. J. Bacteriol. 184:6437-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paton, J. C., and J. K. Morona. 1999. Streptococcus pneumoniae capsular polysaccharide, p. 201-213. In V. A. Fischetti et al. (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, D.C.
- 26.Schrager, H. M., J. G. Rheinwald, and M. R. Wessels. 1996. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J. Clin. Investig. 98:1954-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorensen, U. B., J. Henrichsen H. C. Chen, and S. C. Szu. 1990. Covalent linkage between the capsular polysaccharide and the cell wall peptidoglycan of Streptococcus pneumoniae revealed by immunochemical methods. Microb. Pathog. 8:325-334. [DOI] [PubMed] [Google Scholar]
- 28.Tikkanen, K., J. Hayrinen, S. Pelkonen, and J. Finne. 1995. Immunoblot analysis of bacterial polysaccharides: application to the type-specific polysaccharides of Streptococcus suis and Streptococcus agalactiae. J. Immunol. Methods 187:233-244. [DOI] [PubMed] [Google Scholar]
- 29.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent, C., P. Doublet, C. Grangeasse, E. Vaganay, A. J. Cozzone, and B. Duclos. 1999. Cells of Escherichia coli contain a protein-tyrosine kinase, Wzc, and a phosphotyrosine-protein phosphatase, Wzb. J. Bacteriol. 181:3472-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent, C., B. Duclos, C. Grangeasse, E. Vaganay, M. Riberty, A. J. Cozzone, and P. Doublet. 2000. Relationship between exopolysaccharide production and protein-tyrosine phosphorylation in gram-negative bacteria. J. Mol. Biol. 304:311-321. [DOI] [PubMed] [Google Scholar]
- 32.Weiser, J. N., R. Austrian, P. K. Sreenivasan, and H. R. Masure. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 62:2582-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiser, J. N., D. Bae, H. Epino, S. B. Gordon, M. Kapoor, L. A. Zenewicz, and M. Shchepetov. 2001. Changes in availability of oxygen accentuate differences in capsular polysaccharide expression by phenotypic variants and clinical isolates of Streptococcus pneumoniae. Infect. Immun. 69:5430-5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitfield, C. 1995. Biosynthesis of lipopolysaccharide O antigens. Trends Microbiol. 3:178-185. [DOI] [PubMed] [Google Scholar]
- 35.Wugeditsch, T., A. Paiment, J. Hocking, J. Drummelsmith, C. Forrester, and C. Whitfield. 2001. Phosphorylation of Wzc, a tyrosine autokinase, is essential for assembly of group 1 capsular polysaccharides in Escherichia coli. J. Biol. Chem. 276:2361-2371. [DOI] [PubMed] [Google Scholar]