Abstract
Activity of secreted alkaline phosphatase (SEAP) produced by transfected cells is rapidly down-regulated by endoplasmic reticulum (ER) stress independent of transcriptional regulation. This phenomenon was observed in a wide range of cell types triggered by various ER stress inducers. The magnitude of the decrease in SEAP was proportional to the extent of ER stress and inversely correlated with the induction of endogenous ER stress markers grp78 and grp94. In contrast to SEAP, activity of secreted luciferase was less susceptible to ER stress. The decrease in SEAP activity by ER stress was caused by abnormal post-translational modification, accelerated degradation and reduced secretion of SEAP protein. In transgenic mice constitutively producing SEAP, systemic induction of ER stress led to reduction in serum SEAP. In these mice, administration with lipopolysaccharide caused rapid, transient decrease in serum SEAP activity, and it was correlated with up-regulation of grp78 in several organs including the spleen, lung, kidney, liver and heart. These results elucidated for the first time a possible involvement of transient, systemic ER stress in endotoxemia and provided evidence for usefulness of ER stress responsive alkaline phosphatase for real-time monitoring of ER stress in vitro and in vivo.
INTRODUCTION
Endoplasmic reticulum (ER) stress is implicated in a wide range of pathologies including viral infection, ischemic injury, neurodegenerative disorders and metabolic diseases (1,2). Detection, quantification and monitoring of ER stress are required for investigating molecular events involved in these pathologies. Assessment of ER stress is also essential for screening of agents for therapeutic intervention in ER stress-associated diseases. To date, several assay systems have been developed for the assessment of ER stress. Expression of endogenous biomarkers, e.g. grp78 and grp94, is most commonly used for this purpose (3). Phosphorylation of PERK and eIF2α, cleavage of ATF6 and procaspase 12, and splicing of XBP-1 mRNA are also used as endogenous indicators for ER stress (2,4). Alternatively, reporter systems using the ER stress response element (ERSE) combined with lacZ or luciferase have been used for monitoring ER stress (5,6). However, these systems require extraction of RNA or protein and do not allow for continuous or successive monitoring of ER stress in living cells and animals. Use of green fluorescent protein (GFP) may overcome this hurdle (7), but it is still not competent for quantitative, successive assessment of ER stress in internal organs.
The secreted alkaline phosphatase (SEAP) reporter system has been widely used to investigate activity of known or putative promoter/enhancer elements that drive gene expression (8). Normally, alkaline phosphatase is not secreted, but the recombinant SEAP derived from placental alkaline phosphatase is released efficiently from transfected cells. In transfectants, the level of SEAP activity in culture media is directly proportional to changes in intracellular SEAP mRNA and protein (8,9). This property allows SEAP to serve as a quantitative reporter for gene expression. As a reporter, SEAP has several important advantages over other molecules. Because preparation of cell lysates is not required, it is possible to monitor activity of certain genes continuously using identical cell cultures. Using chemiluminescent assays, SEAP activity can be quantified quickly and sensitively (9). Activity of endogenous alkaline phosphatases present in samples is able to be eliminated by preheating the samples and assaying in the presence of l-homoarginine without affecting SEAP activity (9). Furthermore, in contrast to secreted luciferase, activity of SEAP is not affected by serum, allowing this molecule as an in vivo reporter protein (10).
In eukaryotic cells, proteins to be secreted enter the subcellular pathway through the ER. In the ER, the proteins are folded into native conformation and undergo a multitude of post-translational modifications. Only correctly folded proteins are exported to the Golgi apparatus (11). Based on this current knowledge, perturbation of ER function (i.e. ER stress) could be monitored using a secreted reporter protein. In the present investigation, we tested this possibility using SEAP as an indicator molecule. We here show that SEAP can serve as a selective and sensitive indicator for ER stress in vitro and in vivo. Using mice transgenic with the ER stress responsive alkaline phosphatase (ES-TRAP), we provide in vivo evidence for transient, systemic induction of ER stress during acute endotoxemia.
MATERIALS AND METHODS
Reagents
MG132 was purchased from Peptide Institute (Osaka, Japan), and human transforming growth factor-β (TGF-β) was from Genzyme (Cambridge, MA). Human recombinant interleukin-1β (IL-1β) and human recombinant tumor necrosis factor-α (TNF-α) were generous gifts of Otsuka Pharmaceutical Co. Ltd (Tokushima, Japan) and Dr Katsuo Noguchi (Teikyo University School of Medicine, Tokyo), respectively. All other reagents were purchased from Sigma-Aldrich Japan (Tokyo, Japan).
Cells and transfectants
The porcine renal proximal tubular cell line LLCPK1, the murine hepatoma cell line Hepa-1c1c7 and the rat alveolar macrophage NR8383 were obtained from the American Type Culture Collection (Manassas, VA). The rat mesangial cell line SM43 was established as described before (12). LL/SEAP, LL/MLuc and LL/EGFP cells were established by stable transfection of LLCPK1 cells with pSEAP2-Control (BD Biosciences, Palo Alto, CA), pcDNA3-MLuc (provided by Dr Stefan Golz, Bayer Healthcare AG, Germany) (13) and pEGFP-N1 (Clontech, Palo Alto, CA), respectively. Hepa1/SEAP and SM/SEAP cells were generated by stable transfection of Hepa-1c1c7 and SM43 cells with pSEAP2-Control. Expression of transgenes and transgene products were confirmed by northern blot analyses, western blot analyses, chemiluminescent assays and fluorescent microscopy. LLCPK1 cells, SM43 cells, NR8383 cells and their derivatives were maintained in DMEM/F-12 (Gibco-BRL, Gaithersburg, MD) containing 5–10% fetal bovine serum (FBS). Hepa-1c1c7 and Hepa1/SEAP cells were maintained in α-MEM (Invitrogen, Carlsbad, CA) supplemented with 5% FBS. All experiments were performed in the presence of 1% FBS.
Preparation of recombinant SEAP
Confluent SM/SEAP cells in a 100 mm culture plate were incubated in 7 ml DMEM/F-12 containing 1% FBS for 48 h. After the incubation, conditioned medium was collected, centrifuged to remove insoluble components and used to examine direct effects of ER stress inducers on the activity of SEAP. The activity of recombinant SEAP prepared was ∼2.5 × 106 relative light unit (RLU).
Northern blot analysis
Total RNA was extracted by the single-step method, and northern blot analysis was performed as described before (14). cDNAs for SEAP (BD Biosciences), GRP78 (provided by Dr Kazunori Imaizumi, Nara Institute of Science and Technology, Nara, Japan) (15), GRP94 (provided by Dr Amy S. Lee, University of South California, Los Angeles, CA) (16), Metridia luciferase (MLuc) (13), and enhanced GFP (EGFP; Clontech) were used for preparation of radio-labeled probes. Expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control.
Western blot analysis
Western blot analysis was performed as described before (17). Goat polyclonal anti-human placental alkaline phosphatase antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and horseradish peroxidase-conjugated donkey anti-goat IgG (Santa Cruz Biotechnology) were used to detect SEAP. SEAP was visualized using the enhanced chemiluminescence system (Amersham Biosciences, Buckinghamshire, UK). As a loading control, the level of β-actin was evaluated using anti-β-actin antibody (Sigma-Aldrich Japan). Densitometric analysis was performed using Scion Image Software (Scion Corporation, Frederick, MD).
Chemiluminescent assay
Activity of SEAP and MLuc was evaluated by chemiluminescent assays. The SEAP assay was performed using Great EscAPe SEAP Detection Kit (BD Biosciences), as described previously (10). In brief, 5 µl of samples (culture medium, serum or cellular extract) were mixed with 15 µl of 1× dilution buffer and incubated at 65°C for 30 min. After the incubation, the samples were mixed with 20 µl of assay buffer containing l-homoarginine, left at room temperature for 5 min and then added with 20 µl of chemiluminescent enhancer containing 1.25 mM CSPD substrate. After incubation in dark for 30 min, the samples were subjected to assays using a luminometer (Gene Light 55; Microtech Nition, Chiba, Japan). To prepare cellular extracts, cells were harvested by trypsinization, washed twice with PBS and sonicated for 20 s. After centrifugation, supernatants were subjected to SEAP assay. To prepare organ extracts, tissue samples were sonicated in PBS for 20 s, heated at 65°C for 15 min and subjected to the analysis. Activity of MLuc was evaluated by adding 5 µl of coelenterazine (1 × 10−3 M in DMSO, Wako, Osaka, Japan) to 20 µl of culture media. Immediately after mixing the samples, MLuc activity was measured by the luminometer.
Formazan assay
The number of viable cells was assessed by a formazan assay using Cell Counting Kit-8 (Dojindo Laboratory, Kumamoto, Japan). In brief, after collecting culture media for chemiluminescent assays, cells in 96-well plates were incubated at 37°C for 2 h in medium containing 10% Cell Counting Kit-8 assay solution. Absorbance (450 nm) of formazan generated from WST-8 was measured by Spectra Max 340 (Nihon Molecular Devices, Tokyo, Japan).
Fluorescence microscopy
Fluorescence microscopic analysis was performed using Olympus IX71 (Olympus, Tokyo, Japan). Fluorescence intensity of EGFP was measured using Spectra Max GEMINI EM (Nihon Molecular Devices).
Ex vivo gene transferred mice
SEAP-producing mice were created by intraperitoneal implantation of Hepa1/SEAP cells (originated from C57BL/6 mice; 2.5 × 105 cells/mouse) into C57BL/6 mice (body weight 25 g, total eight mice). In this experimental model, substantial levels of serum SEAP were detectable for at least 10 days (N. Hiramatsu, unpublished data). After 24–48 h, the mice were administered with thapsigargin to induce ER stress, as described later.
Transgenic mice
ES-TRAP mice were generated as follows. The dioxin responsive element-controlled SEAP gene (18) was microinjected into the pronuclei of fertilized oocytes of C57BL/6 mice, and the injected embryos were transferred into pseudopregnant mice using standard techniques. Transgenic pups were screened by PCR using the following primers; 1F: 5′-AACATGGACATTGACGTGATCCTAG-3′; 1R: 5′-TCTCGTATTTCATGTCTCCAGGCTC-3′. The ES-TRAP mouse, which constitutively expresses SEAP in various organs, was occasionally isolated from the pool of the transgenic offspring. To evaluate activity of SEAP in various organs, wild-type mice and ES-TRAP mice were perfused with PBS systemically to wash out blood, and brains, hearts, lungs, spleens, livers and kidneys were subjected to chemiluminescent assay, as described before. Activity of SEAP per 1 µg total protein was evaluated to compare the levels in different organs. Protein assay was performed using Micro BCA™ Protein Assay Kit (Pierce, Rockford, IL).
In vivo induction of ER stress
To induce systemic ER stress in vivo, cell-implanted mice and ES-TRAP mice (body weight 25 g; four mice, respectively) were administered with thapsigargin (1 mg/kg body weight) intraperitoneally, as described previously (19). Blood was collected periodically from the tail vein, and activity of SEAP in serum was evaluated. For the induction of endotoxemia, ES-TRAP mice (body weight 20 g; four mice) were administered with lipopolysaccharide (LPS; 200 µg/mouse; Escherichia coli 0111; B4, Sigma-Aldrich Japan) intraperitoneally. After the administration, serum was collected periodically and subjected to chemiluminescent assay. To confirm the induction of ER stress, organs were collected 8 h after the injection of LPS and subjected to northern blot analysis of grp78.
In vivo induction of heat shock response
ES-TRAP mice (body weight 22 g; four mice) were heated at 42°C for 15 min using two incandescent lamps set at 15 cm above the mice. After the induction of whole body hyperthermia, serum was sampled every 2 h until 6 h and subjected to chemiluminescent assay. Brains, lungs and livers were also collected before or 2 and 6 h after the heat shock and subjected to northern blot analysis of hsp70 and grp78.
Statistical analysis
All assays were performed in quadruplicate. Data were expressed as means ± SE. Statistical analysis was performed using the non-parametric Mann–Whitney U-test to compare data in different groups. P-value <0.05 was considered to indicate a statistically significant difference.
RESULTS
Suppression of SEAP activity by ER stress
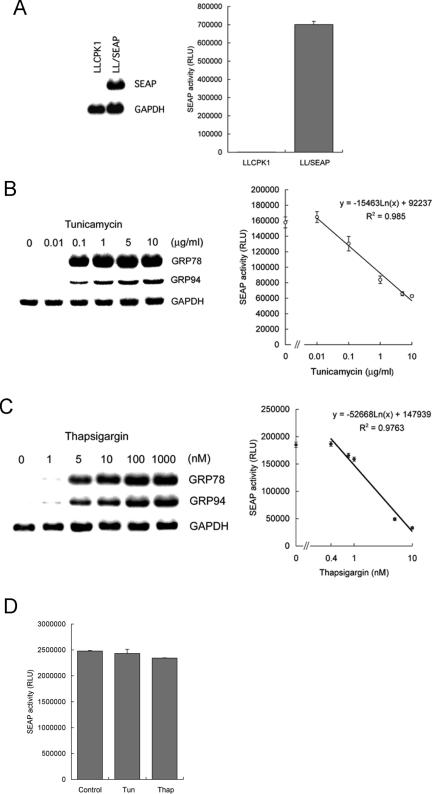
To examine whether SEAP serves as an indicator for ER stress, we established stably transfected LLCPK1 cells, LL/SEAP, that constitutively express and secrete SEAP under the control of the simian virus 40 (SV40) promoter (Figure 1A). As shown in Figure 1B, treatment of these cells with tunicamycin induced expression of endogenous ER stress markers, grp78 and grp94, and down-regulated SEAP activity in a dose-dependent manner. Linear, inverse correlation was observed between SEAP activity and concentration of tunicamycin (Figure 1B, right). The dose-dependent, suppressive response of SEAP was also observed in LL/SEAP cells treated with another ER stress inducer thapsigargin (Figure 1C). To exclude a possibility that tunicamycin and thapsigargin could directly interfere with enzyme activity of SEAP, recombinant SEAP was added with tunicamycin (10 µg/ml) or thapsigargin (1 µM) using the maximum concentration tested in Figure 1B and C. After incubation at 37°C for 6 h, the samples were subjected to chemiluminescent assay. As shown in Figure 1D, incubation with tunicamycin or thapsigargin did not affect activity of SEAP.
Figure 1.
Suppression of SEAP activity by endoplasmic reticulum (ER) stress in stably transfected cells. (A) Establishment of stable transfectants. LLCPK1 cells were stably transfected with a SEAP gene under the control of the simian virus (SV40) promoter, and established LL/SEAP cells were subjected to northern blot analysis (left) and chemiluminescent assay of culture medium (right). (B and C) Responses of LL/SEAP cells to tunicamycin (B) and thapsigargin (C). LL/SEAP cells were treated with tunicamycin (0.01–10 µg/ml) or thapsigargin (0.4–1000 nM) for 6 h, and expression of grp78 and grp94 (left) and activity of SEAP in culture media (right) were evaluated. (D) Recombinant SEAP was added with tunicamycin (Tun, 10 µg/ml) or thapsigargin (Thap, 1 µM) and incubation at 37°C for 6 h. Activity of SEAP was assessed by chemiluminescent assay. SEAP assays were performed in quadruplicate, and data are expressed as means ± SE. RLU, relative light unit. In northern blot analysis, expression of GAPDH was used as a loading control.
Characterization of the response of SEAP to ER stress
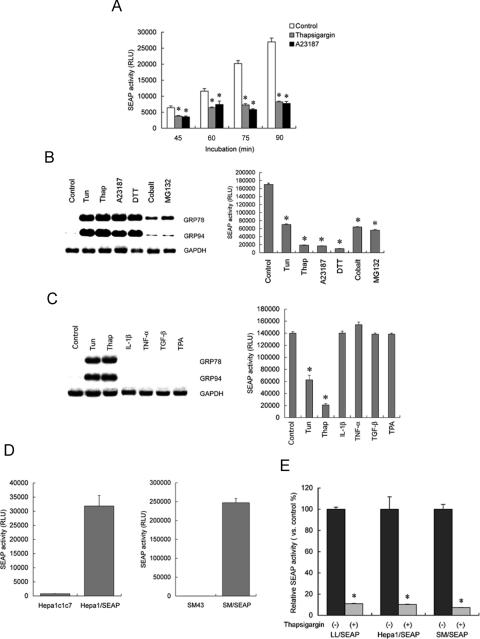
We examined how quickly SEAP activity is affected by ER stress. LL/SEAP cells were pretreated with thapsigargin or A23187 for 30 min. After changing medium, the cells were further incubated in the presence of the drugs for up to 60 min. Culture media were collected every 15 min and subjected to SEAP assay. The results revealed that exposure to the ER stress inducers for 45 min caused significant reduction in SEAP activity (Figure 2A).
Figure 2.
Characterization of the response of SEAP to ER stress. (A) Rapid reduction in SEAP activity by ER stress. LL/SEAP cells were pretreated with thapsigargin (1 µM) or A23187 (2 µM) for 30 min. After changing the medium, the cells were further incubated in the presence of the drugs for up to 60 min. Culture media were collected every 15 min and subjected to SEAP assay. (B) Responses to known ER stress inducers. LL/SEAP cells were treated with tunicamycin (Tun; 10 µg/ml), thapsigargin (Thap; 1 µM), A23187 (2 µM), dithiothreitol (DTT; 1 mM), cobalt chloride (Cobalt; 1 mM) or MG132 (50 µM) for 6 h and subjected to northern blot analysis of grps (left) and SEAP assay (right). (C) Lack of responses to ER stress-unrelated stimuli. LL/SEAP cells were treated with interleukin 1β (IL-1β; 20 ng/ml), tumor necrosis factor-α (TNF-α; 250 U/ml), transforming growth factor-β (TGF-β; 10 ng/ml) or 12-o-tetradecanoylphorbol-13-acetate (TPA; 50 ng/ml) for 6 h and subjected to analyses. Tunicamycin (10 µg/ml) and thapsigargin (1 µM) were used as positive controls. (D) Secretion of SEAP by Hepa1/SEAP and SM/SEAP cells. The murine hepatoma cell line Hepa-1c1c7 and the rat mesangial cell line SM43 were stably transfected with a SEAP gene under the control of the SV40 promoter, and Hepa1/SEAP and SM/SEAP cells were established. Activity of SEAP in culture media was evaluated by chemiluminescent assay. (E) Responses to ER stress of LL/SEAP and Hepa1/SEAP cells. LL/SEAP, Hepa1/SEAP and SM/SEAP cells were treated with (+) or without (−) thapsigargin (1 µM) for 6 h, and culture media were subjected to SEAP assay. Assays were performed in quadruplicate, and data are expressed as means ± SE. Asterisks indicate statistically significant differences (P < 0.05).
To evaluate selectivity and specificity of the response of SEAP, LL/SEAP cells were treated with known ER stress inducers including tunicamycin, thapsigargin, A23187, dithiothreitol (DTT), cobalt chloride and MG132, and expression of grps and activity of SEAP were evaluated (Figure 2B). All ER stress inducers significantly suppressed SEAP activity (Figure 2B, right), which was in parallel with induction of grp78 and grp94. (Figure 2B, left). It is worthwhile to note that modest induction of grps by cobalt chloride and MG132 was correlated with modest suppression of SEAP. Tunicamycin, thapsigargin, A23187, DTT, cobalt chloride and MG132 are known to induce ER stress through different mechanisms; e.g. suppression of protein glycosylation, disturbance of calcium homeostasis, inhibition of disulfide bond formation, hypoxia and intervention in protein degradation (20). The fact that SEAP activity was suppressed by all of these agents indicated that SEAP is down-regulated by ER stress regardless of triggers or underlying mechanisms. To further examine selectivity and specificity of the response of SEAP, LL/SEAP cells were treated with ER stress-unrelated bioactive substances including IL-1β, TNF-α, TGF-β and 12-o-tetradecanoylphorbol-13-acetate (TPA) and subjected to assays. Northern blot analysis confirmed that none of these agents induced ER stress in LLCPK1 cells (Figure 2C, left). Chemiluminescent assay showed that none of these agents suppressed SEAP activity (Figure 2C, right).
To examine whether the down-regulation of SEAP by ER stress is not restricted to the particular cell type, we established a mouse hepatoma cell line Hepa1/SEAP and a rat mesangial cell clone SM/SEAP, both of which constitutively secreted SEAP under the control of the SV40 promoter (Figure 2D). In both cell types, treatment with thapsigargin markedly suppressed SEAP activity to the same extent as in LL/SEAP cells (Figure 2E). The suppression of SEAP activity by ER stress was also observed in glomerular mesangial cells and podocytes that express SEAP under the control of endogenous promoters including the κB enhancer element, the cAMP response element or the nephrin gene promoter (our unpublished data). These results suggested that suppression of SEAP by ER stress is observed generally regardless of stimuli, cell types or upstream regulatory elements that drive SEAP expression.
Responses of other secreted and non-secreted reporter proteins to ER stress
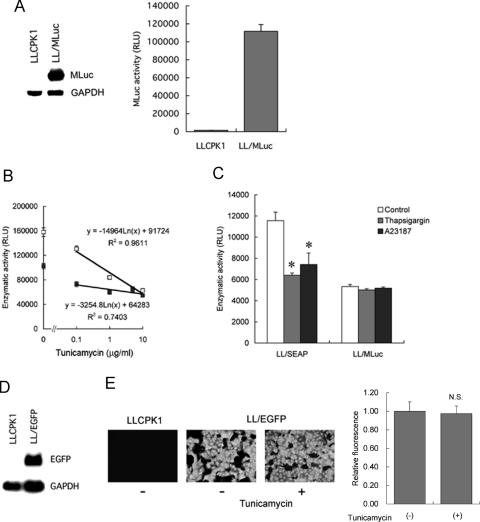
We examined responses of other reporter proteins to ER stress. For this purpose, another secreted reporter protein MLuc (13), a secreted luciferase, was tested. LLCPK1 cells were stably transfected with MLuc, and LL/MLuc cells were established. Abundant, constitutive expression of the transgene and luciferase activity in culture media were confirmed by northern blot analysis (Figure 3A, left) and chemiluminescent assay (Figure 3A, right). The cells were then treated with tunicamycin, and culture media were subjected to MLuc assay. Like SEAP, the tunicamycin-induced ER stress significantly suppressed MLuc activity, but the magnitude of suppression was modest. In contrast to SEAP, dose-dependent response was not evident at concentrations from 0.1 to 10 µg/ml (Figure 3B). Furthermore, ER stress induced by 1 h exposure to 1 µM thapsigargin or 2 µM A23187 significantly reduced SEAP activity, whereas the same ER stress did not affect activity of MLuc (Figure 3C). These results indicated that, although activity of MLuc was affected by ER stress, its response was less sensitive than that of SEAP.
Figure 3.
Responses of other secreted and non-secreted reporter proteins to ER stress. (A) Establishment of LL/MLuc cells. LLCPK1 cells were stably transfected with a gene encoding secreted Metridia luciferase (MLuc), and LL/MLuc cells were established. left, northern blot analysis of MLuc mRNA in LLCPK1 cells and LL/MLuc cells. right, MLuc activity in culture media of LLCPK1 cells and LL/MLuc cells. (B) Dose-dependent responses of MLuc and SEAP to tunicamycin. LL/MLuc cells (closed square) and LL/SEAP cells (open square) were treated with tunicamycin (0.1–10 µg/ml) for 6 h, and culture media were subjected to chemiluminescent assays. (C) Susceptibility of MLuc and SEAP to ER stress triggered by thapsigargin and A23187. LL/SEAP and LL/MLuc cells were pretreated with thapsigargin (1 µM) or A23187 (2 µM) for 30 min. After changing the medium, the cells were further incubated in the presence of the drugs for 30 min and subjected to chemiluminescent assays. (D) Establishment of LL/EGFP cells. LLCPK1 cells were stably transfected with a gene encoding enhanced green fluorescent protein (EGFP), and LL/EGFP cells were established. Northern blot analysis of EGFP mRNA in LLCPK1 cells and LL/EGFP cells is shown. (E) Effect of ER stress on the activity of EGFP. Fluorescence microscopic analysis of LLCPK1 cells and LL/EGFP cells was performed before (−) and after (+) the treatment with tunicamycin (10 µg/ml) for 8 h. Fluorescence intensity of EGFP was evaluated, and relative fluorescence was shown on the right. All assays were performed in quadruplicate, and data are expressed as means ± SE. Asterisks indicate statistically significant differences (P < 0.05). N.S., not significant.
To further examine responses of other reporter proteins to ER stress, EGFP was also tested. LLCPK1 cells were stably transfected with a gene coding for non-secreted protein EGFP, and LL/EGFP cells were established (Figure 3D). LL/EGFP cells were then treated with tunicamycin (10 µg/ml) for 8 h and subjected to fluorescence microscopy. As shown in Figure 3E, treatment with tunicamycin did not affect intensity of fluorescence in LL/EGFP cells (0.98 ± 0.08 in tunicamycin-treated cells versus 1.00 ± 0.10 in tunicamycin-untreated cells, means ± SE, not statistically significant). Non-secreted reporter proteins including β-galactosidase, luciferase and GFP have been used in ERSE-based reporter assays for detection of ER stress (5–7). Our results, together with the previous reports, suggested that, in contrast to secreted proteins, activity of non-secreted reporter proteins is not susceptible to ER stress.
Mechanisms involved in the suppression of SEAP by ER stress
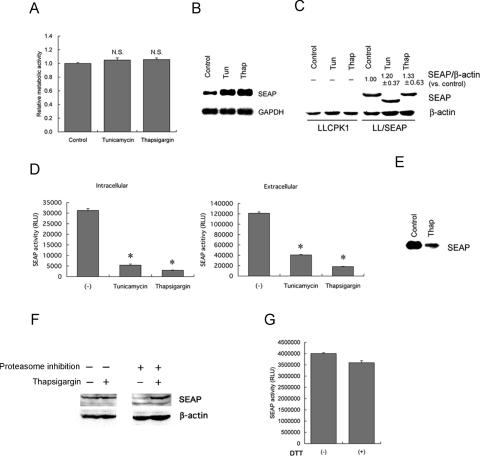
Several possibilities may be raised for down-regulation of SEAP activity in the cells exposed to ER stress. Those include; non-specific cellular damage, transcriptional suppression, translational inhibition, abnormal post-translational modification, enhanced degradation and/or suppression of secretion. To test the first possibility, LL/SEAP cells were treated with tunicamycin or thapsigargin for 6 h and subjected to formazan assay (21) to determine the number of viable cells. As shown in Figure 4A, both agents did not affect the number of viable cells. Northern blot analysis also showed that expression of SEAP mRNA was not suppressed but rather increased by the treatment with tunicamycin or thapsigargin, excluding the second possibility (Figure 4B). Similarly, western blot analysis revealed that the intracellular level of SEAP protein was not decreased by the treatment with tunicamycin or thapsigargin (Figure 4C). The decrease in molecular size of SEAP in tunicamycin-treated cells is owing to inhibition of protein glycosylation. These results indicated that neither transcription nor translation of SEAP was the target of ER stress. However, using the same samples used for western blot analysis, we detected that the activity of intracellular SEAP was markedly reduced in tunicamycin-treated (83% reduction) and thapsigargin-treated (91% reduction) LL/SEAP cells (Figure 4D, left). The extent of suppression was correlated with the degree of reduction in extracellular SEAP activity (66% reduction in tunicamycin-treated cells and 85% reduction in thapsigargin-treated cells) (Figure 4D, right). Figure 4E shows levels of SEAP protein in culture media of untreated and thapsigargin-treated LL/SEAP cells. In addition to the suppression of intracellular SEAP activity, secretion of SEAP protein was also reduced by the treatment with thapsigargin (Figure 4E). The fact that the secretion of SEAP was inhibited whereas the level of intracellular SEAP was unaffected by ER stress raised another possibility that, like bacterial alkaline phosphatase (22), SEAP with inappropriate post-translational modification may be degraded rapidly. Indeed, inhibition of the proteasome pathway resulted in accumulation of intracellular SEAP in thapsigargin-treated cells, which was not observed in cells without proteasome inhibition (Figure 4F). These results suggested that abnormal post-translational modification, enhanced proteasome-mediated degradation and reduced secretion of SEAP protein were caused by ER stress and responsible for the reduction of SEAP activity.
Figure 4.
Mechanisms involved in the suppression of SEAP activity by ER stress. (A–E) LL/SEAP cells were treated with tunicamycin (10 µg/ml) or thapsigargin (1 µM) for 6 h and subjected to analyses. (A) Cell viability evaluated by formazan assay. (B) Northern blot analysis of SEAP mRNA. (C) Western blot analysis of intracellular levels of SEAP. As a loading control, the level of β-actin was examined (bottom), and relative values of SEAP against β-actin (SEAP/β-actin) were shown (n = 3). (D) Activity of intracellular (left) and extracellular (right) SEAP. (E) Western blot analysis of extracellular levels of SEAP. (F) Intracellular levels of SEAP in thapsigargin-treated cells with or without blockade of the proteasome pathway. LL/SEAP cells were treated with thapsigargin in the absence (−) or presence (+) of proteasome inhibition (25 µM MG132) for 12 h, and cellular protein was subjected to western blot analysis. (G) Lack of involvement of disulfide bonds formation in SEAP activation. SEAP protein was treated with (+) or without (−) 100 mM DTT for 1 h. DTT was washed out using cut-off membranes (M.W. < 50 kDa), and the resultant samples were subjected to SEAP assay. All SEAP assays were performed in quadruplicate, and data are expressed as means ± SE. Asterisks indicate statistically significant differences (P < 0.05). N.S., not significant.
In bacterial alkaline phosphatase, disulfide bonds are necessary for its appropriate conformation and activation (23). In SEAP, disulfide bonds could also be critical for its enzyme activity, which might be targeted by ER stress. To examine this possibility, recombinant SEAP protein was treated with or without DTT, and its activity was retested. As shown in Figure 4G, treatment with DTT did not obviously affect SEAP activity. This result indicated that the abnormal post-translational modification of SEAP by ER stress may not be owing to failure in the disulfide bond formation.
In vivo response of SEAP in transgenic mice following systemic induction of ER stress
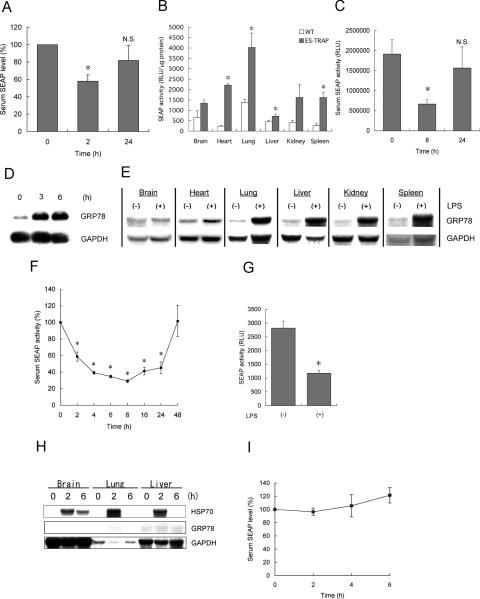
We recently reported that SEAP is a useful in vivo reporter molecule that can be detected easily in serum (10). In mice, the half-life of SEAP in serum is ∼2 h (N. Hiramatsu, unpublished data), and altered production and secretion of SEAP may result in alteration in the level of serum SEAP within a few hours. To investigate whether in vivo induction of ER stress leads to down-regulation of serum SEAP in animals, an ex vivo gene transfer approach was used. In brief, Hepa1/SEAP cells were transplanted into the peritoneal cavity of C57BL/6 mice, and after 24–48 h, thapsigargin was administered into mice to induce systemic ER stress (19). Serum was sampled from the tail vain for up to 24 h, and activity of SEAP was evaluated. As shown in Figure 5A, the serum level of SEAP was rapidly and significantly decreased within 2 h by the systemic induction of ER stress. The decrease in the serum SEAP was partially recovered after 24 h. This result suggested that rapid suppression of SEAP by ER stress was caused not only in vitro but also in vivo, and the ES-TRAP-based approach would be useful for monitoring of ER stress in living animals.
Figure 5.
Detection and real-time monitoring of ER stress during endotoxemia using ES-TRAP. (A) Effect of systemic ER stress on the level of serum SEAP in ex vivo gene transferred mice. Hepa1/SEAP cells (2.5 × 105 cells) were implanted into the peritoneal cavity of C57BL/6 mice. After 24–48 h, thapsigargin (1 mg/kg body weight) was administered into eight mice. Serum was sampled at 2 and 24 h, and activity of SEAP was evaluated. (B) Activity of SEAP in organs of transgenic ES-TRAP mice. Tissue extracts were prepared from perfused organs of wild-type mice (WT) and ES-TRAP mice and subjected to chemiluminescent assay, as described in Materials and Methods. Activity of SEAP per 1 µg total protein was evaluated and compared among different organs. (C) Effect of systemic ER stress on the level of serum SEAP in ES-TRAP mice. Thapsigargin (1 mg/kg body weight) was administered into four mice (body weight 25 g) intraperitoneally, and serum was sampled at 8 and 24 h. (D) Induction of ER stress by LPS in macrophages. NR8383 alveolar macrophages were exposed to LPS (1 µg/ml) for 3–6 h, and expression of grp78 was examined by northern blot analysis. (E–G) Effects of endotoxemia in ES-TRAP mice on induction of ER stress in various organs (E), serum SEAP activity (F) and tissue SEAP activity in the spleen (G). ES-TRAP mice (body weight 20 g, n = 8) were administered without (−) or with (+) LPS (200 µg/mouse) intraperitoneally, and organs (8 h) and serum (2–48 h) were collected for northern blot analysis of grp78 (E) and SEAP assay (F and G). In (G), activity of SEAP in the spleen before [LPS(−)] and 8 h after the administration with LPS [LPS(+)] is shown. (H and I) Lack of in vivo response of SEAP to hyperthermia. ES-TRAP mice (body weight 22 g, n = 4) were heated at 42°C for 15 min, and brains, lungs and livers were collected before or 2 and 6 h after the whole body hyperthermia. Expression of hsp70 and grp78 was examined by northern blot analysis (H). Serum was also sampled every 2 h until 6 h and subjected to chemiluminescent assay to evaluate activity of SEAP (I). Data are expressed as means ± SE. Asterisks indicate statistically significant differences (P < 0.05). N.S., not significant.
In the ex vivo gene transfer model described above, the number of implanted cells may be increased or decreased spontaneously or in response to pharmacological or pathologic manipulation. We therefore used transgenic mice (ES-TRAP mice) that produce SEAP constitutively in various organs. Figure 5B shows the level of SEAP in several blood-free organs in wild-type mice and ES-TRAP mice. The background activity of SEAP in organ extracts was ∼240–1380 RLU/µg protein. In the heart, lung, liver and spleen, the levels of SEAP were significantly higher in ES-TRAP mice than in wild-type mice. Although the differences were not statistically significant, 2- to 3-fold increase in the level of SEAP activity was also observed in the brain and kidney. The established ES-TRAP mice exhibited very high levels of serum SEAP activity (2.1 ± 0.3 × 106 RLU, mean ± SE) compared with wild-type mice (8.3 ± 0.5 × 102 RLU). Using these mice, in vivo response of SEAP to systemic ER stress was evaluated. Consistent with the result in the ex vivo gene transferred mice (Figure 5A), ES-TRAP mice administered with thapsigargin exhibited significant decrease in the level of serum SEAP at 8 h (Figure 5C). The reduction in SEAP was partially recovered after 24 h. These results further supported feasibility of using ES-TRAP-based systems for in vivo monitoring of ER stress.
Detection and real-time monitoring of ER stress during endotoxemia using ES-TRAP mice
A recent report showed that systemic administration with LPS induced up-regulation of 150 kDa oxygen-regulated protein (ORP150), an ER-located molecular chaperone, in the lung (24). ER stress may be induced in some organs during the course of endotoxemia. Among various cell types, macrophages are most sensitive to LPS, and numerous alveolar macrophages are present in the lung. We speculated that LPS might directly induce ER stress in macrophages. To examine this possibility, we first investigated expression of grp78 in LPS-stimulated NR8383 alveolar macrophages. Northern blot analysis showed that, after the stimulation with LPS, grp78 was rapidly induced within 3 h and sustained for at least 6 h (Figure 5D).
Resident macrophages and macrophage-like cells are present in various organs. Other cells of non-macrophage lineages may also respond to LPS. We hypothesized that endotoxemia may induce ER stress not only in the lung but also in other organs. To examine this possibility, mice were administered with LPS systemically, and induction of grp78 was evaluated in several organs. As shown in Figure 5E, expression of grp78 was substantially induced in the lung, liver, kidney and spleen and modestly in the heart. Only in the brain, induction of grp78 was not detectable. This result evidenced that endotoxemia caused systemic ER stress. Based on this result, we tested whether systemic ER stress induced by LPS can be detected and monitored continuously using the ES-TRAP mice. ES-TRAP mice were injected with LPS systemically, and serum was collected periodically up to 48 h. Chemiluminescent assay revealed that activity of serum SEAP was rapidly reduced to 58.8 ± 5.5% within 2 h, further declined until 8 h (29.1 ± 1.8%) and gradually recovered thereafter. Forty-eight hours after the administration with LPS, the level of SEAP was recovered to the initial level (Figure 5F). The down-regulation of serum SEAP was associated with decrease in the level of active SEAP in the spleen, the organ that expressed grp78 most abundantly in response to LPS (Figure 5G). These results revealed that endotoxemia induced transient, reversible ER stress in various, but selective organs and that the ES-TRAP-based system allowed for continuous, real-time monitoring of ER stress during the course of the disease.
Lack of in vivo response of SEAP to hyperthermia
Endogenous ER stress markers GRPs belong to the family of heat shock proteins (2). It indicates that, among various stress responses, ER stress and heat shock may be close and might trigger similar subcellular events. However, a previous report showed that whole body hyperthermia led to induction of hsp70 and hsp27, but not grp78 (25), suggesting that heat shock in vivo can selectively induce heat shock response, but not ER stress response. To examine whether SEAP can distinguish ER stress from other stress responses in vivo, hyperthermia would be an ideal model. We therefore examined whether or not activity of serum SEAP is affected by whole body hyperthermia in vivo. ES-TRAP mice were heated at 42°C for 15 min as described in Materials and Methods. Following the induction of hyperthermia, brains, lungs and livers were collected before or 2 and 6 h after the heat shock and subjected to northern blot analysis of hsp70 and grp78. As shown in Figure 5H, thermal stress markedly induced expression of hsp70 in all organs tested with a peak at 2 h. Under this experimental condition, induction of grp78 was undetectable. It was contrastive to the result in the endotoxemia model showing significant up-regulation of grp78 in various organs (Figure 5E). Using this experimental setting, serum was sampled every 2 h until 6 h and subjected to chemiluminescent assay. In contrast to the endotoxemia model showing rapid and significant depression of serum SEAP (Figure 5F), the activity of serum SEAP was not affected by systemic hyperthermia (Figure 5I). This result further supported our idea that SEAP serves as an indicator for specific detection of ER stress.
DISCUSSION
Monitoring of ER stress is required for investigation of a broad range of pathophysiologic events in vitro and in vivo. However, the majority of current approaches require extraction of protein or RNA from cells and tissues. It is a crucial limitation especially for in vivo studies where successive monitoring of ER stress is required during the course of diseases. Recently, Iwawaki and co-workers reported monitoring for ER stress using a variant of GFP as a reporter and splicing of XBP-1 mRNA as a sensor (7). However, by this approach, successive in vivo monitoring of ER stress is not feasible, because tissue sampling or exposure of internal organs is essential for quantitative evaluation of fluorescence. In the present report, we developed a novel approach, ES-TRAP, for the assessment of ER stress in vitro and in vivo. Our results showed that this approach allowed for rapid, quantitative, sensitive and continuous monitoring of ER stress in identical living cells. We also demonstrated that it was also useful for real-time monitoring of ER stress in animals under pathologic situations.
Generally, in a system for monitoring of a particular subcellular event, specificity of its response to the target event is important. In the present report, we demonstrated that activity of SEAP was depressed specifically by ER stress-inducing agents (tunicamycin, thapsigargin, A23187, DTT, cobalt chloride and MG132), but not by ER stress-unrelated stimuli including IL-1β, TNF-α, TGF-β and phorbol ester. To further confirm selectivity and specificity of the response, we examined responses of SEAP to endogenous, ER stress-unrelated differentiation factors (retinoic acid, glucocorticoid and vitamin D3) in glomerular podocytes and confirmed lack of responses (26). We also examined effects of ER stress-unrelated pathological stresses such as (i) mitogenic stress triggered by platelet-derived growth factor, (ii) inflammatory stress triggered by LPS, (iii) xenobiotic stress triggered by cigarette smoke and (iv) thermal stress triggered by heat shock, using several different cell types including rat mesangial cells, murine podocytes, murine hepatoma cells and porcine renal tubular epithelial cells, respectively. We found that individual cells responded to the individual pathological stimuli, but the levels of SEAP were never affected, except for heat shocked LLCPK1 cells that exhibited both modest up-regulation of grp78 and modest depression of SEAP activity [(26,27) and our unpublished data]. In addition to the in vitro evidence, we also provided in vivo evidence that activity of SEAP was depressed by ER stress caused by endotoxemia (Figure 5F), but not by ER stress-uninducible thermal stress (Figure 5I). Taken together, these results suggested that, as far as we have examined, the depressive response of SEAP is specific to ER stress, and ER stress-unrelated stimuli do not suppress SEAP activity.
In this report, we revealed that endotoxemia induced ER stress in various organs. Administration with LPS rapidly reduced serum levels of SEAP in ES-TRAP mice. The facts that the half-life of serum SEAP in mice is ∼2 h and that serum SEAP was reduced to 58.8 ± 5.5% within 2 h after the administration with LPS (Figure 5F) indicated that ER stress was induced immediately after the induction of endotoxemia. The level of SEAP was further declined until 8 h, suggesting that ER stress was sustained for at least several hours. However, the stress was found to be transient, and the level of SEAP was recovered to the initial level within 48 h. Interestingly, in endotoxemia, ER stress was induced not in all organs but preferentially in particular organs including the spleen, lung, liver and kidney. It indicated that susceptibility to endotoxin-triggered ER stress was different from organ to organ. The reason for the difference is unclear, but based on the fact that these organs contain substantial numbers of resident macrophages and macrophage-like cells, LPS could induce ER stress preferentially in macrophages. Indeed, treatment of NR8383 alveolar macrophages with LPS rapidly induced expression of grp78 within 3 h, as shown in this report. In contrast, treatment of rat mesangial cells with LPS did not trigger ER stress, although activation of NF-κB was evident (N. Hiramatsu, unpublished observation). Our findings may be consistent with a recent report showing intense expression of ORP150, an ER-located molecular chaperone, in alveolar macrophages in endotoxin-induced acute lung injury (24).
The ES-TRAP-based sensing of ER stress has several advantages over the previously reported approaches. The first is that activity of SEAP can be detected sensitively and quantitatively using conventional chemiluminescent systems (9). Only 5 µl of culture medium or serum is sufficient for the assessment. The response of ES-TRAP is very quick, and, in culture cells, exposure to triggers for <45 min was found to be sufficient to detect ER stress, as shown in this report. It allows for rapid detection of ER stress in vitro. Similarly, in ES-TRAP mice, simple blood sampling without sacrificing animals is sufficient to monitor ER stress. It means that the kinetics of ER stress can be easily monitored in identical animals during the course of diseases. If ES-TRAP mice expressing SEAP under the control of tissue- or cell type-specific promoters are generated, continuous monitoring of ER stress in particular tissues or organs will also be feasible in vivo via simple blood sampling. It is worthwhile to note that high levels of SEAP expression in either LLCPK1 cells or SM43 cells did not induce grp78 expression (N. Hiramatsu, unpublished data). It suggested that, in contrast to some other cellular proteins, overexpression of SEAP per se does not trigger ER stress in mammalian cells.
Although secreted luciferase can also serve as an indicator for ER stress, its detection sensitivity and dose-dependent response were inferior to those of SEAP (Figure 3). The preferential susceptibility of SEAP to ER stress may be based on our findings that multiple steps involved in the intracellular pathway for SEAP were the targets of ER stress. That is, ER stress disturbs post-translational modification required for SEAP activation, causes accelerated degradation of SEAP via the proteasome pathway and blocks secretion of SEAP to the extracellular space. Furthermore, an obvious limitation will be present in the in vivo use of secreted luciferase as an indicator of ER stress. That is, like bacterial luciferase, secreted luciferase MLuc is immediately inactivated by serum albumin and cannot serve as an in vivo reporter protein, as we recently reported (10). Because our in vitro experiments were performed in the presence of 1% FBS, interference with MLuc activity by serum could cause low sensitivity of this molecule to ER stress. Of note, activity of SEAP is very stable even in the presence of serum (10), and its half-life in culture is ∼500 h (28).
Our current data propose that ES-TRAP is a novel and unique indicator for ER stress in vitro and in vivo. The systems described here would be powerful especially for monitoring of ER stress in vivo under various pathophysiological conditions.
Acknowledgments
We appreciate Drs Stefan Golz (Bayer Healthcare AG), Kazunori Imaizumi (Nara Institute of Science and Technology) and Amy S. Lee (University of South California) for providing with plasmids. This work was supported, in part, by Grant-in-Aids for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No.16390243, No.17651026) to M.K. Funding to pay the Open Access publication charges for this article was provided by the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Conflict of interest statement. None declared.
REFERENCES
- 1.Aridor M., Balch W.E. Integration of endoplasmic reticulum signaling in health and disease. Nature Med. 1999;5:745–751. doi: 10.1038/10466. [DOI] [PubMed] [Google Scholar]
- 2.Schroder M., Kaufman R.J. ER stress and the unfolded protein response. Mutat. Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 3.Kozutsumi Y., Segal M., Normington K., Gething M.J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B.A., Yuan J. Caspase-12 mediates endoplasmic reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature. 2003;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 5.He H., McColl K., Distelhorst C.W. Involvement of c-Fos in signaling grp78 induction following ER calcium release. Oncogene. 2000;19:5936–5943. doi: 10.1038/sj.onc.1203994. [DOI] [PubMed] [Google Scholar]
- 6.Mao C., Dong D., Little E., Luo S., Lee A.S. Transgenic mouse model for monitoring endoplasmic reticulum stress in vivo. Nature Med. 2004;10:1013–1014. doi: 10.1038/nm1004-1013. [DOI] [PubMed] [Google Scholar]
- 7.Iwawaki T., Akai R., Kohno K., Miura M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nature Med. 2004;10:98–102. doi: 10.1038/nm970. [DOI] [PubMed] [Google Scholar]
- 8.Berger J., Hauber J., Hauber R., Geiger R., Cullen B.R. Secreted placental alkaline phosphatase: A powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988;66:1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- 9.Cullen B.R., Malim M.H. Secreted placental alkaline phosphatase as a eukaryotic reporter gene. Methods Enzymol. 1992;216:362–368. doi: 10.1016/0076-6879(92)16033-g. [DOI] [PubMed] [Google Scholar]
- 10.Hiramatsu N., Kasai A., Meng Y., Hayakawa K., Yao J., Kitamura M. Alkaline phosphatase vs. luciferase as secreted reporter molecules in vivo. Anal. Biochem. 2005;339:249–256. doi: 10.1016/j.ab.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Taxis C., Vogel F., Wolf D.H. ER–Golgi traffic is a prerequisite for efficient ER degradation. Mol. Biol. Cell. 2002;13:1806–1818. doi: 10.1091/mbc.01-08-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitamura M., Taylor S., Unwin R., Burton S., Shimizu F., Fine L.G. Gene transfer into the rat renal glomerulus via mesangial cell vector: site specific delivery, in situ amplification, and sustained expression of an endogenous gene in vivo. J. Clin. Invest. 1994;94:497–505. doi: 10.1172/JCI117361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markova S.V., Golz S., Frank L.A., Kolthof B., Vysotski E.S. Cloning and expression of cDNA for a luciferase from the marine copepod Metridia longa. A novel secreted bioluminescent reporter enzyme. J. Biol. Chem. 2004;279:3212–3217. doi: 10.1074/jbc.M309639200. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura M., Suto T., Yokoo T., Shimizu F., Fine L.G. Transforming growth factor-β1 is the predominant paracrine inhibitor of macrophage cytokine synthesis produced by glomerular mesangial cells. J. Immunol. 1997;156:2964–2971. [PubMed] [Google Scholar]
- 15.Katayama T., Imaizumi K., Honda A., Yoneda T., Kudo T., Takeda M., Mori K., Rozmahel R., Fraser P., George-Hyslop P.S., Tohyama M. Disturbed activation of endoplasmic reticulum stress transducers by familial Alzheimer's disease-linked presenilin-1 mutations. J. Biol. Chem. 2001;276:43446–43454. doi: 10.1074/jbc.M104096200. [DOI] [PubMed] [Google Scholar]
- 16.Gazit G., Lu J., Lee A.S. De-regulation of GRP stress protein expression in human breast cancer cell lines. Breast Cancer Res. Treat. 1999;54:135–46. doi: 10.1023/a:1006102411439. [DOI] [PubMed] [Google Scholar]
- 17.Yao J., Hiramatsu N., Zhu Y., Morioka T., Takeda M., Oite T., Kitamura M. Nitric oxide-mediated regulation of connexin43 expression and gap junctional intercellular communication in mesangial cells. J. Am. Soc. Nephrol. 2005;16:58–67. doi: 10.1681/ASN.2004060453. [DOI] [PubMed] [Google Scholar]
- 18.Kasai A., Hiramatsu N., Meng Y., Yao J., Takeda M., Maeda S., Kitamura M. DRESSA: biosensing of dioxin and dioxin-like chemicals using secreted alkaline phophatase. Anal. Biochem. 2004;335:73–80. doi: 10.1016/j.ab.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Kondoh M., Tsukada M., Kuronaga M., Higashimoto M., Takiguchi M., Himeno S., Watanabe Y., Sato M. Induction of hepatic metallothionein synthesis by endoplasmic reticulum stress in mice. Toxicol. Lett. 2004;148:133–139. doi: 10.1016/j.toxlet.2003.12.066. [DOI] [PubMed] [Google Scholar]
- 20.Lee A.S. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem. Sci. 2001;26:504–510. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 21.Ishiyama M., Tominaga H., Shiga M., Sasamoto K., Ohkura Y., Ueno K. A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol. Pharm. Bull. 1996;19:1518–1520. doi: 10.1248/bpb.19.1518. [DOI] [PubMed] [Google Scholar]
- 22.Sone M, Kishigami S., Yoshihisa T., Ito K. Roles of disulfide bonds in bacterial alkaline phosphatase. J. Biol. Chem. 1997;272:6174–6178. doi: 10.1074/jbc.272.10.6174. [DOI] [PubMed] [Google Scholar]
- 23.Derman A.I., Beckwith J. Escherichia coli alkaline phosphatase fails to acquire disulfide bonds when retained in the cytoplasm. J. Bacteriol. 1991;173:7719–7722. doi: 10.1128/jb.173.23.7719-7722.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakagomi T., Kitada O., Yoshikawa K., Ozawa K., Ogawa S., Matsuyama T. The 150-Kilodalton oxygen-regulated protein ameliorates lipopolysaccharide-induced acute lung injury in mice. Am. J. Pathol. 2004;165:1279–1288. doi: 10.1016/S0002-9440(10)63387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tashiro M., Ernst S.A., Edwards J., Williams J.A. Hyperthermia induces multiple pancreatic heat shock proteins and protects against subsequent arginine-induced acute pancreatitis in rats. Digestion. 2002;65:118–126. doi: 10.1159/000057713. [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi K., Takano Y., Kasai A., Hayakawa K., Hiramatsu N., Enomoto N., Yao J., Kitamura M. Screening and identification of substances that regulate nephrin gene expression using engineered reporter podocytes. Kidney Int. 2006 doi: 10.1038/sj.ki.5001625. (in press) [DOI] [PubMed] [Google Scholar]
- 27.Kasai A., Hiramatsu N., Hayakawa K., Yao J., Maeda S., Kitamura M. High levels of dioxin-like potential in cigarette smoke evidenced by in vitro and in vivo biosensing. Cancer Res. 2006;66:7143–7150. doi: 10.1158/0008-5472.CAN-05-4541. [DOI] [PubMed] [Google Scholar]
- 28.Schlatter S., Rimann M., Kelm J., Fussenegger M. SAMY, a novel mammalian reporter gene derived from Bacillus stearothermophilus α-amylase. Gene. 2002;282:19–31. doi: 10.1016/s0378-1119(01)00824-1. [DOI] [PubMed] [Google Scholar]