Abstract
Thermotoga maritima tRNase Z cleaves pre-tRNAs containing the 74CCA76 sequence precisely after the A76 residue to create the mature 3′ termini. Its crystal structure has revealed a four-layer αβ/βα sandwich fold that is typically found in the metallo-β-lactamase superfamily. The well-conserved six histidine and two aspartate residues together with metal ions are assumed to form the tRNase Z catalytic center. Here, we examined tRNase Z variants containing single amino acid substitutions in the catalytic center for pre-tRNA cleavage. Cleavage by each variant in the presence of Mg2+ was hardly detected, although it is bound to pre-tRNA. Surprisingly, however, Mn2+ ions restored the lost Mg2+-dependent activity with two exceptions of the Asp52Ala and His222Ala substitutions, which abolished the activity almost completely. These results provide a piece of evidence that Asp-52 and His-222 directly contribute the proton transfer for the catalysis.
INTRODUCTION
Every single tRNA molecule terminates with the sequence CCA (1). This 3′ terminal sequence is essential for aminoacylation of the tRNAs (2) and for translation on the ribosome (3). The tRNAs are transcribed as larger precursors, which subsequently undergo various processing steps such as removal of 5′ and 3′ extra nucleotides to produce mature tRNAs (4). tRNA 3′ processing endoribonuclease (tRNase Z) is one of the tRNA-maturing enzymes, which removes a 3′ trailer from pre-tRNA (5–10). Most tRNase Zs cleave pre-tRNAs immediately downstream of a discriminator nucleotide, onto which the CCA residues are added to generate mature tRNA, while Thermotoga maritima tRNase Z cleaves pre-tRNAs containing the 74CCA76 sequence precisely after the A76 residue to create the mature 3′ termini (10).
tRNase Zs can be categorized into two groups: a short form (tRNase ZS) that consists of 300–400 amino acids and a long form (tRNase ZL) that contains 800–900 amino acids (11,12). Bacteria and archaea genomes contain a tRNase ZS gene only, while eukaryotic genomes encode either only tRNase ZL or both forms. The C-terminal half region of tRNase ZL has high similarity to the whole region of tRNase ZS, and these regions contain a well-conserved histidine motif, which has been shown to be essential for the Mg2+-dependent enzymatic activity by mutational analyses for T.maritima tRNase ZS (10) and Drosophila melanogaster tRNase ZL (13).
Sequence analysis has suggested that tRNase Zs belong to the metallo-β-lactamase superfamily, and the crystal structures of T.maritima, Bacillus subtilis and Escherichia coli tRNase Zs have revealed a four-layer αβ/βα sandwich fold that is typically found in the metallo-β-lactamase superfamily (14–16). From the structural and functional studies of the superfamily enzymes, it is assumed that the well-conserved six histidine and two aspartate residues together with metal ions also form the tRNase Z catalytic center (Figure 1A and B). It is known that metal ions such as Zn2+, Fe2+ and Mn2+ are required for activities of this superfamily's enzymes (17–20).
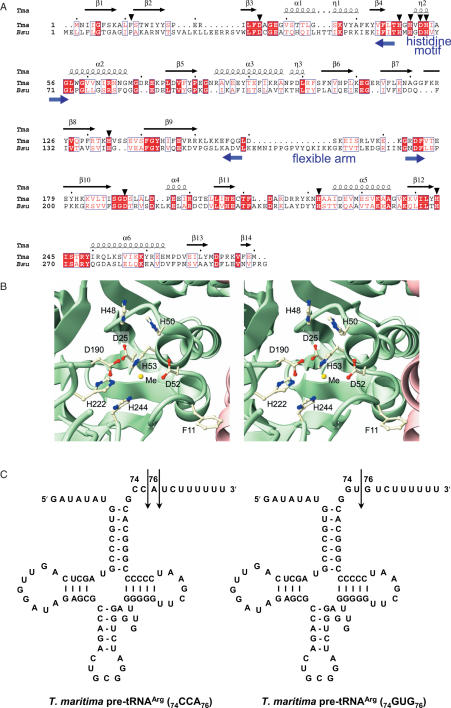
Figure 1.
Structures of T.maritima tRNase Z and pre-tRNAs. (A) The amino acid sequences of T.maritima (Tma) and B.subtilis (Bsu) tRNase Zs are aligned using ClustalW (25) and represented using ESPript (26). The T.maritima tRNase Z secondary structures for α-helices, β-strands and 310 helices are indicated by α, β and η, respectively. The red box and the blue rectangle denote the conserved and moderately conserved residues, respectively. The regions of the histidine motif, the flexible arm and the mutated amino acid residues (triangle) are shown. (B) Stereoview for the catalytic center of T.maritima tRNase Z (14). The two subunits are colored green and pink, respectively. The mutated residues, except His-134, which is disordered, are represented by ball-and-stick models. One unidentified metal ion is depicted as a yellow sphere. (C) T.maritima pre-tRNAArg(74CCA76) and pre-tRNAArg(74GUG76). The sequence 74CCA76 in the former is substituted with 74GUG76 in the latter. Cleavage sites are denoted by arrows.
In the structures of the three bacterial tRNase Zs, a unique long flexible arm extending from the core domain has been uncovered, which is thought to be essential for pre-tRNA binding [(15,16,21,22), R. Ishii, M. Nashimoto and S. Yokoyama, unpublished data]. However, little is known about how the exact cleavage site in pre-tRNA is selected and what makes the T.maritima enzyme cleave pre-tRNA after the 74CCA76 sequence not after the discriminator.
Many catalytic mechanisms have been proposed for the metallo-β-lactamase superfamily (17–20) and metallo-phosphatases (23), in which nucleophilic attack of the carbonyl group of the β-lactam ring or the phosphoryl group by a hydroxyl generated from a metal-ligated water molecule commonly triggers the catalytic reaction. Although a reaction mechanism for pre-tRNA cleavage by tRNase Z has been proposed on the basis of these precedents (15), no direct evidence was shown.
In this report, we examined T.maritima tRNase Z variants containing single amino acid substitutions in the catalytic center for pre-tRNA cleavage. Cleavage by each variant in the presence of Mg2+ was hardly detected, although it bound to pre-tRNA. Surprisingly, however, Mn2+ ions rescued the lost Mg2+-dependent tRNase Z activity with two exceptions of the Asp52Ala and His222Ala substitutions. This observation suggests that Asp-52 and His-222 may directly contribute the proton transfer for the catalysis.
MATERIALS AND METHODS
Preparation of the tRNase Z variants
Based on pQE-80L (Qiagen), the expression plasmids for 10 T.maritima tRNase Z variants containing a single amino acid substitution, Phe11Pro, His134Ala, Asp190Ala, His222Ala, His244Ala, Asp52Ser, Asp52Glu, His222Cys, His222Ser or His222Lys, were constructed using specific primer pairs (Supplementary Table 1) as described before (10). Histidine-tagged T.maritima tRNase Z variants were produced in E.coli cells and purified using nickel–agarose beads as described previously (10).
Pre-tRNA synthesis
The T.maritima pre-tRNAs were synthesized in vitro with T7 RNA polymerase (Takara Shuzo) from the synthetic pre-tDNAs containing its promoter. The transcription reactions were carried out under the conditions recommended by the manufacturer (Takara Shuzo), and the transcribed pre-tRNAs were gel-purified. The pre-tRNAs were subsequently labeled with fluorescein according to the manufacturer's protocol (Amersham Pharmacia Biotech). Briefly, after the removal of the 5′-phosphates of the transcripts with bacterial alkaline phosphatase (Takara Shuzo), the transcripts were phosphorylated with ATPγS and T4 polynucleotide kinase (Takara Shuzo). Then a single fluorescein moiety was appended onto the 5′-phosphorothioate site. The resulting pre-tRNAs with fluorescein were gel-purified before assays.
In vitro tRNA 3′ processing assay
The 3′ processing reactions for fluorescein-labeled pre-tRNA were performed with the T.maritima tRNase Z variants basically in a mixture (6 μl) containing 10 mM Tris–HCl (pH 7.5), 1.5 mM DTT, 25 mM NaCl and 10 mM MgCl2 (or 0.2 mM MnCl2) at 60°C. In several assays, pH and temperature were changed or different divalent metal ions were used. After resolution of the reaction products on a 10% polyacrylamide-8 M urea gel, the gel was analyzed with a Typhoon 9210 (Amersham Pharmacia Biotech).
Gel-shift assay
To determine the dissociation constant of a tRNase Z/pre-tRNA complex, fluorescein-labeled pre-tRNA (3.8–50 nM) was incubated at 25°C for 20 min with various amounts (0.005–2 μM) of the tRNase Z variants in a buffer (6 μl) containing 10 mM Tris–HCl (pH 7.5), 1.5 mM DTT, 25 mM NaCl and 10 mM MgCl2 (or 0.2 mM MnCl2). After the incubation, the sample was mixed with the same volume of a loading buffer (10 mM Tris–HCl, pH 7.5, 1 mM EDTA and 50% glycerol) and electrophoresed on a 5% non-denaturing polyacrylamide gel with TBE buffer (90 mM Tris base, 90 mM boric acid and 1.5 mM EDTA, pH 8.3). After electrophoresis, the labeled RNAs were quantified with the Typhoon 9210.
RESULTS AND DISCUSSION
Metal ion requirements for the T.maritima tRNase Z activity
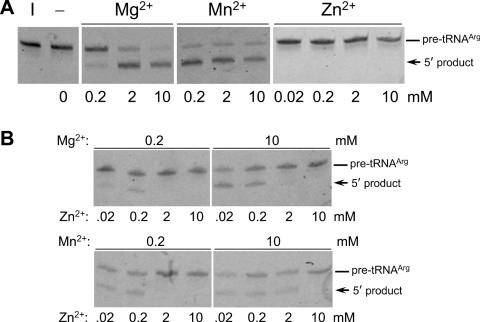
First of all, we examined metal ion requirements for the T.maritima tRNase Z activity. T.maritima pre-tRNAArg(74CCA76) 5′ end labeled with fluorescein (Figure 1C) was reacted with the wild-type enzyme in the presence of several metal ions. Mg2+ and Mn2+ ions activated the enzyme in the range of 0.2–10 mM, while Zn2+ did not at all activate in the range of 0.02–10 mM (Figure 2A). Although this enzyme was active in the presence of 0.2 or 10 mM Mg2+ alone (Figure 2A), the activity decreased with the increase in the added Zn2+ amount and vanished in the presence of high amounts of Zn2+ (Figure 2B). Similar result was obtained with respect to the Mn2+-dependent activity (Figure 2B). These data indicate that Mg2+ or Mn2+ ions are essential for the T.maritima tRNase Z activity and that Zn2+ is inhibitory to the activity in high concentrations. A similar observation with respect to Arabidopsis thaliana tRNase Z has been reported (24).
Figure 2.
Mg2+ or Mn2+ ions are essential for the T.maritima tRNase Z activity. The assays for in vitro 3′ processing of T.maritima pre-tRNAArg(74CCA76) by T.maritima wild-type tRNase Z in the presence of Mg2+, Mn2+ or Zn2+ alone (A), and in the presence of Mg2+ or Mn2+ together with Zn2+ (B). The pre-tRNA (0.1 pmol) was incubated with histidine-tagged tRNase Z (4 pmol) at 60°C for 10 min. The cleavage products were analyzed on a 10% polyacrylamide-8 M urea gel. I, input RNA.
We have identified two zinc ions in the active site of the crystal structure of T.maritima tRNase Z [R. Ishii, M. Nashimoto and S. Yokoyama, unpublished data]. Similarly, two zinc ions in the active site have been detected in tRNase Zs from B.subtilis and E.coli (15,16). One zinc ion was also coordinated in the B.subtilis enzyme/tRNA complex recently uncovered (21). Although these observations appear to be discrepant with the above results, the coordinated Zn2+ ions may be needed for the activity. Alternatively, Zn2+ ions may be dispensable for the active form of tRNase Z. In any case, the roles of Mg2+ or Mn2+ ions would be to help fold pre-tRNA properly, to induce tRNase Z to form the active conformation, and/or to assist tRNase Z to interact with pre-tRNA correctly.
Mn2+ rescue of the tRNase Z activity
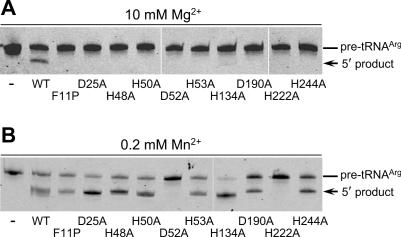
Next, we examined how single amino acid substitutions with Ala in the catalytic center other than the histidine motif affect pre-tRNA cleavage by T.maritima tRNase Z (Figure 1A and B). Recombinant histidine-tagged tRNase Z variants containing a single substitution, His134Ala, Asp190Ala, His222Ala or His244Ala (Figure 1A and B) were generated, and T.maritima pre-tRNAArg(74CCA76) or pre-tRNAArg(74GUG76) 5′ end labeled with fluorescein (Figure 1C) was reacted with these variants. The previously tested variants containing His48Ala, His50Ala, Asp52Ala or His53Ala (Figure 1A and B) were also examined (10). As shown in Figure 3A, all these variants hardly cleaved pre-tRNAArg(74CCA76) in the presence of Mg2+, although they bound to the substrate with varying Kd values (0.02–1.40 μM) (Table 1). The cleavage of pre-tRNAArg(74GUG76) in the presence of Mg2+ was also hardly detected, and the enzymes bound to the substrate with varying Kd values (0.02–1.66 μM) (Table 1). These results confirm that the six histidines (amino acids 48, 50, 53, 134, 222 and 244) and two aspartates (amino acids 52 and 190) are important for catalysis, and suggest that these residues are not essential for pre-tRNA binding although some of these may participate in some of the important interactions.
Figure 3.
The Mn2+-rescued cleavage of pre-tRNAArg by T.maritima tRNase Z variants. The assays for in vitro 3′ processing of T.maritima pre-tRNAArg(74CCA76) by T.maritima tRNase Z variants in the presence of 10 mM Mg2+ (A) or 0.2 mM Mn2+ (B). The pre-tRNA (0.1 pmol) was incubated with histidine-tagged tRNase Z variants (4 pmol) at 60°C for 10 min. The cleavage products were analyzed on a 10% polyacrylamide–8 M urea gel.
Table 1.
Dissociation constants and observed rate constants for T.maritima tRNase Z variants
| Enzyme | Ion | Pre-tRNAArg (74CCA76) | Pre-tRNAArg (74GUG76) | ||||
|---|---|---|---|---|---|---|---|
| Cleavage | Kd (μM)a | kobs (min−1)b | Cleavage | Kd (μM)a | kobs (min−1)b | ||
| Wild type | Mg2+ | CCA| | 0.05 ± 0.012 | 0.15 ± 0.010 | GU|G | 0.17 ± 0.035 | 0.08 ± 0.001 |
| Mn2+ | CC|A | 0.09 ± 0.010 | 0.24 ± 0.031 | GU|G | 0.16 ± 0.009 | 0.20 ± 0.028 | |
| F11P | Mg2+ | — | 0.27 ± 0.069 | — | — | 0.41 ± 0.032 | — |
| Mn2+ | CC|A | 0.15 ± 0.036 | 0.13 ± 0.004 | GU|G | 0.12 ± 0.025 | 0.12 ± 0.006 | |
| D25A | Mg2+ | — | 0.30 ± 0.074 | — | — | 0.29 ± 0.081 | — |
| Mn2+ | CC|A | ND | 0.26 ± 0.024 | GU|G | ND | 0.15 ± 0.048 | |
| H48A | Mg2+ | — | 0.03 ± 0.004 | — | — | 0.11 ± 0.021 | — |
| Mn2+ | CCA| | ND | 0.16 ± 0.049 | GU|G | ND | <0.01 | |
| H50A | Mg2+ | — | 0.29 ± 0.082 | — | — | 0.35 ± 0.006 | — |
| Mn2+ | CCA| | ND | 0.16 ± 0.033 | GU|G | ND | <0.01 | |
| D52A | Mg2+ | — | 0.44 ± 0.082 | — | — | 1.34 ± 0.392 | — |
| Mn2+ | — | 0.16 ± 0.095 | — | — | 0.19 ± 0.020 | — | |
| H53A | Mg2+ | — | 0.06 ± 0.010 | — | — | 0.12 ± 0.018 | — |
| Mn2+ | CC|A | ND | 0.22 ± 0.024 | GU|G | ND | 0.13 ± 0.031 | |
| H134A | Mg2+ | — | 1.40 ± 0.069 | — | — | 1.66 ± 0.286 | — |
| Mn2+ | CC|A | ND | 0.42 ± 0.015 | GU|G | ND | 0.28 ± 0.019 | |
| D190A | Mg2+ | — | 0.25 ± 0.110 | — | — | 0.70 ± 0.192 | — |
| Mn2+ | CCA| | 0.18 ± 0.032 | 0.08 ± 0.021 | GU|G | 0.20 ± 0.026 | <0.01 | |
| H222A | Mg2+ | — | 0.02 ± 0.003 | — | — | 0.02 ± 0.002 | — |
| Mn2+ | — | 0.05 ± 0.025 | — | — | 0.07 ± 0.013 | — | |
| H244A | Mg2+ | — | 0.17 ± 0.015 | — | — | 0.33 ± 0.138 | — |
| Mn2+ | CCA| | ND | 0.20 ± 0.029 | GU|G | ND | 0.03 ± 0.003 | |
|, primary cleavage site; ND, not determined; —, cleavage not detected or hardly detected (Supplementary Figure 1).
aData are the means ± SD of three independent experiments (Supplementary Figure 2).
bEach fluorescein-labeled pre-tRNA (20 nM) was reacted with each variant (700 nM) at 60°C for 3 min. Data are the means ± SD of three independent experiments.
These variants were also tested for cleavage of these pre-tRNAs in the presence of Mn2+. To our surprise, Mn2+ ions restored the Mg2+-dependent tRNase Z activity lost by single His or Asp substitutions with two exceptions (Asp52Ala and His222Ala), although the observed rate constant values differed depending on the variants and the substrates (Figure 3B and Table 1). We confirmed that the Mn2+-rescued activity of these variants is endoribonucleolytic not exoribonucleolytic (data not shown).
Zn2+, Cu2+ and Co2+ ions cannot rescue the lost tRNase Z activity
We also examined other metal ions for the ability to rescue the lost Mg2+-dependent activity of the tRNase Z variants. Two representative tRNase Z variants, His50Ala and Asp52Ala, were assayed in the presence of Zn2+, Cu2+ or Co2+. None of these metal ions rescued the lost tRNase Z activity in the ion concentration of 0.2 mM (data not shown). These metal ions did not rescue also in the range of 0.1–10 mM (data not shown).
Metal ion coordination in tRNase Z
Together with the observation that Mg2+ or Mn2+ ions are indispensable for the T.maritima tRNase Z activity, the Mn2+-rescue phenomena imply that, instead of Zn2+ ions, Mg2+ or Mn2+ ions may be coordinated in the catalytic center of T.maritima tRNase Z. This coordination may be limited in the active enzyme/substrate complex. Without the substrate, Zn2+ ions may be weakly coordinated in the catalytic center, and, upon pre-tRNA binding, Zn2+ ions may be replaced with Mg2+ or Mn2+ ions. This supposition appears to be consistent with the observation that only one Zn2+ ion is coordinated in the active site of the crystal structure of the B.subtilis tRNase Z/tRNA complex, whereas two Zn2+ ions are coordinated in the active site of the free protein structure (15,21). Alternatively, Mg2+ or Mn2+ ions may be coordinated near the catalytic center to induce an active enzyme/substrate conformation. These possibilities are not mutually exclusive. High resolution crystal structure of the pre-tRNA/tRNase Z complex in the presence of the Mg2+ or Mn2+ ions would clarify this issue.
The Mn2+-rescue phenomena would be based primarily on the property that Mn2+ ions have higher affinity to the active site and/or a nearby site than Mg2+. Mn2+ ions would be able to be coordinated even in the catalytic site and/or the nearby site distorted due to the lack of one of the important residual groups.
Existence of specific interactions with the CCA bases
The Mn2+-rescued cleavage of pre-tRNAArg(74GUG76) by the variants occurred at the same site as by the wild type, while the cleavage site of pre-tRNAArg(74CCA76) by each enzyme fluctuated by one nucleotide (Table 1). The cleavage by the variants His48Ala, His50Ala, Asp190Ala, and His244Ala occurred primarily after CCA, and the cleavage by the other variants was primarily after CC as in the case of the wild type (Table 1). The cleavage of pre-tRNAArg(74GUG76) by the former variants was ∼7- to >16-fold less efficient than that of pre-tRNAArg(74CCA76), suggesting that these residues are important for the correct interaction with the nucleotide residues near the scissile bond of CCA-less pre-tRNA and that there would exist other residues that interact specifically with the bases of the CCA nucleotides.
His-134 may be important to grasp pre-tRNA
The observation that the cleavage site changes by one nucleotide depending on the mutation site, the nucleotide residues near the scissile bond, and the metal ion species suggests that, upon binding of pre-tRNA, the enzyme would change its conformation into the activated form accommodating the two metal ions. Furthermore, together with the fact that His-134 is disordered in the crystal (14), the observation that the His134Ala variant has a 28-fold higher Kd value than the wild type in the interaction with pre-tRNAArg(74CCA76) (Table 1) implies that, in the Mg2+-dependent reaction, His-134 may play an important role in clamping pre-tRNA.
Mn2+-rescue of Phe11Pro and Asp25Ala variants
We examined how a single substitution of Phe-11 or Asp-25, which resides in the peripheral region of the active site, affects the tRNase Z activity. Phe-11 was substituted with Pro that is highly conserved among the other tRNase Zs and Asp-25, which is also highly conserved and appears to fix the active site pocket, was replaced with Ala. These variants were not able to cleave both pre-tRNAArg substrates in the presence of Mg2+ although they bound to the substrates with Kd values of 0.27–0.41 μM (Figure 3A and Table 1). Interestingly, the Mg2+-dependent activity lost by either single substitution was also restored by Mn2+ ions (Figure 3B and Table 1). The other metal ions Zn2+, Cu2+ and Co2+ did not rescue the lost tRNase Z activity of the Asp25Ala variant in the ion concentrations of 0.1–10 mM (data not shown). Their kobs values (0.12–0.26 min−1) were comparable to those (0.20 and 0.24 min−1) of the wild type and their cleavage sites were the same as those of the wild type (Table 1). This observation indicates that the Mn2+-rescue phenomena are not restricted to the variants containing active site residue substitutions and occurs also in the variants with substitutions in the peripheral region of the active site. Mn2+ ions but not Mg2+ ions would be able to be coordinated even in the catalytic site and/or the nearby site distorted due to the substitution of one of the peripheral residues that affect the active site structure.
Asp-52 and His-222 may directly contribute the proton transfer
Curiously, the Asp-52 or His-222 substitution with Ala abolished the enzymatic activity almost completely even in the presence of Mn2+ (Figure 3A and B and Table 1). This abolished activity was not due to the loss of the pre-tRNA binding ability because the His222Ala variant bound to both pre-tRNAs very stably and the Asp52Ala did so fairly well (Table 1). These results imply that Asp-52 and His-222 not only support the active center structure but also may directly contribute the proton transfer for the catalysis.
The indispensability of Asp-52 is consistent with a catalytic mechanism proposed for B.subtilis tRNase Z, in which Asp-67 (Asp-52 in the T.maritima enzyme) acts as a general base to create a hydroxide ion that attacks the phosphate group (15). In this model, a proton is donated from a water molecule to stabilize the 3′ oxygen generated by the phosphodiester bond cleavage. However, the absence of the Mn2+ rescue for the His222Ala variant together with the observations that His-247 (His-222 in the T.maritima enzyme) of B.subtilis tRNase Z is located near the scissile bond and that His-222 of the T.maritima enzyme is positioned similarly suggests that this histidine may act as a proton donor. Similarly, it is proposed that the equivalent His in the phosphorylcholine esterase domain of choline-binding protein E plays a catalytic role (20).
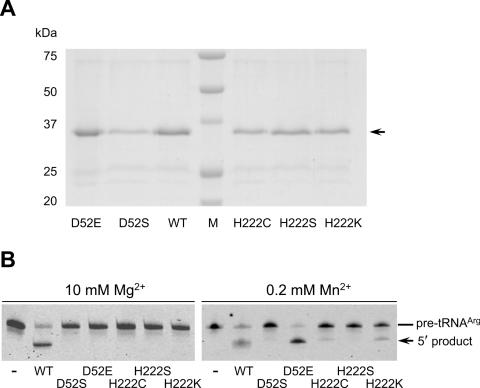
Furthermore, if this supposition is correct, the enzymes containing substitutions of Asp-52 with Glu (pKa 4.2) and of His-222 with Cys (pKa 8.3) or Lys (pKa 10.0) should be active in the presence of Mn2+ even though they would have no activity in the presence of Mg2+ ions. These variants, which were expressed in E.coli and purified with nickel agarose (Figure 4A), were tested for cleavage of pre-tRNAArg (74CCA76), and this was indeed the case (Figure 4B and Table 2). As expected, Mn2+-rescued activity was hardly detected with respect to substitutions of Asp-52 with Ser and His-222 with Ser, although these variants bound to the substrate fairly well (Figure 4B and Table 2).
Figure 4.
The Mn2+-rescue assays for T.maritima tRNase Z variants containing single substitutions at Asp-52 or His-222. (A) Protein profiles of histidine-tagged T.maritima tRNase Z variants. Each purified protein (0.5−1 μg) was analyzed on an SDS–10% polyacrylamide gel, and visualized by staining the gel with Coomassie brilliant blue R-250. (B) The assays for in vitro 3′ processing of T.maritima pre-tRNAArg(74CCA76) by T.maritima tRNase Z variants. The pre-tRNA (0.1 pmol) was incubated with the tRNase Z variants (4 pmol) at 60°C for 10 min. The cleavage products were analyzed on a 10% polyacrylamide–8 M urea gel.
Table 2.
Dissociation constants and observed rate constants for T.maritima tRNase Z variants
| Enzyme | Ion | Pre-tRNAArg (74CCA76) | ||
|---|---|---|---|---|
| Cleavage | Kd (μM)a | kobs (min−1)b | ||
| D52S | Mg2+ | — | 0.35 ± 0.083 | — |
| Mn2+ | — | 0.29 ± 0.018 | — | |
| D52E | Mg2+ | — | 0.29 ± 0.160 | — |
| Mn2+ | CC|A | ND | 0.36 ± 0.004 | |
| H222C | Mg2+ | — | 0.39 ± 0.095 | — |
| Mn2+ | CCA| | ND | < 0.01 | |
| H222S | Mg2+ | — | 0.36 ± 0.094 | — |
| Mn2+ | — | 0.25 ± 0.087 | — | |
| H222K | Mg2+ | — | 0.23 ± 0.020 | — |
| Mn2+ | CCA| | ND | 0.06 ± 0.003 | |
|, primary cleavage site; ND, not determined; —, cleavage not detected or hardly detected (Supplementary Figure 1).
aData are the means ± SD of three independent experiments (Supplementary Figure 2).
bEach fluorescein-labeled pre-tRNA (20 nM) was reacted with each variant (700 nM) at 60°C for 3 min. Data are the means ± SD of three independent experiments.
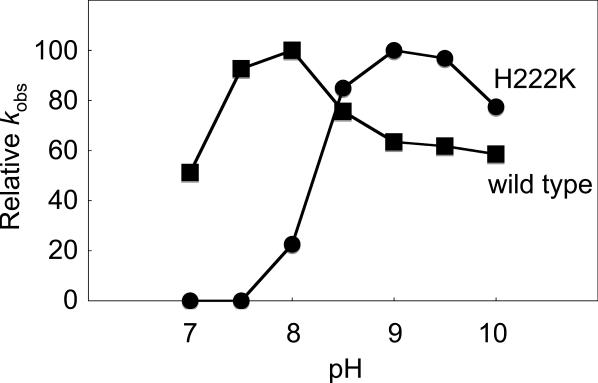
The pKa value of Glu (4.2) is almost the same as that of Asp (3.9), whereas the pKa values of Cys (8.3) and Lys (10.0) are much higher than that of His (6.0). Thus, pH-spectra of the tRNase Z activity for the His222Cys and His222Lys variants would be changed and the optimal pH would be shifted to a higher value, if His-222 is a proton donor. We measured the pH-spectra for the wild type and the His222Lys variant. As expected, the pH-spectrum for the variant drastically changed and the optimal pH value shifted from 8.0 for the wild type to 9.0 for the variant (Figure 5). The activity of the His222Cys variant was too low to accurately obtain a pH-spectrum. Taken together, these data strongly suggest that Asp-52 and His-222 may act as a general base and a proton donor, respectively.
Figure 5.
pH-spectra of the tRNase Z activity. Relative kobs values for the T.maritima pre-tRNAArg(74CCA76) cleavage reaction by T.maritima wild-type tRNase Z and its H222K variant are plotted against pH. The fluorescein-labeled pre-tRNA (20 nM) was reacted with each variant (700 nM) at pH 7–10 at 25°C.
A possible catalytic mechanism of tRNase Z
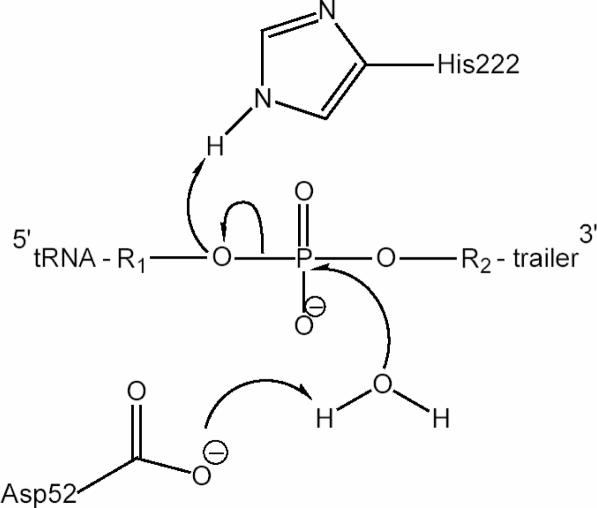
Based on the present results, we propose a mechanism for the tRNase Z catalysis (Figure 6). The chemistry of the phosphodiester bond cleavage reaction would begin with deprotonation and activation of a metal-coordinated water molecule by the Asp-52 residue to create a nucleophile. The resulting hydroxide ion would attack the phosphate group that connects tRNA and 3′ trailer sequences, and would cleave the phosphodiester bond leaving the phosphate at the 5′ terminus of the trailer. The reaction would finish with protonation of the 3′ oxygen of the tRNA molecule by the His-222 residue. Mg2+ or Mn2+ ions would help position the scissile phosphate properly in the active site through bridging oxygens of the pre-tRNA backbone phosphates and carbonyl oxygens of tRNase Z.
Figure 6.
A possible mechanistic scheme for pre-tRNA cleavage by T.maritima tRNase Z. Asp-52 works as a proton acceptor and His-222 acts as a proton donor. R1, upstream tRNA residue; R2, downstream trailer residue. See text for a detailed description.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
Acknowledgments
We thank M. Takeda for technical assistance. This work was supported by the Science Research Promotion Fund and the Academic Frontier Research Project Grant from the Promotion and Mutual Aid Corporation for Private Schools of Japan and by the Grant-in-Aid for Young Scientists from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT). Funding to pay the Open Access publication charges for this article was provided by MEXT.
Conflict of interest statement. None declared.
REFERENCES
- 1.Sprinzl M., Horn C., Brown M., Ioudovitch A., Steinberg S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1998;26:148–153. doi: 10.1093/nar/26.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamura K., Nameki N., Hasegawa T., Shimizu M., Himeno H. Role of the CCA terminal sequence of tRNA(Val) in aminoacylation with valyl-tRNA synthetase. J. Biol. Chem. 1994;269:22173–22177. [PubMed] [Google Scholar]
- 3.Green R., Noller H.F. Ribosomes and translation. Annu. Rev. Biochem. 1997;66:679–716. doi: 10.1146/annurev.biochem.66.1.679. [DOI] [PubMed] [Google Scholar]
- 4.Mörl M., Marchfelder A. The final cut. The importance of tRNA 3′-processing. EMBO Rep. 2001;2:17–20. doi: 10.1093/embo-reports/kve006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nashimoto M. Distribution of both lengths and 5′ terminal nucleotides of mammalian pre-tRNA 3′ trailers reflects properties of 3′ processing endoribonuclease. Nucleic Acids Res. 1997;25:1148–1155. doi: 10.1093/nar/25.6.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohan A., Whyte S., Wang X., Nashimoto M., Levinger L. The 3′ end CCA of mature tRNA is an antideterminant for eukaryotic 3′-tRNase. RNA. 1999;5:245–256. doi: 10.1017/s1355838299981256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiffer S., Rosch S., Marchfelder A. Assigning a function to a conserved group of proteins: the tRNA 3′-processing enzymes. EMBO J. 2002;21:2769–2777. doi: 10.1093/emboj/21.11.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takaku H., Minagawa A., Takagi M., Nashimoto M. A candidate prostate cancer susceptibility gene encodes tRNA 3′ processing endoribonuclease. Nucleic Acids Res. 2003;31:2272–2278. doi: 10.1093/nar/gkg337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubrovsky E.B., Dubrovskaya V.A., Levinger L., Schiffer S., Marchfelder A. Drosophila RNase Z processes mitochondrial and nuclear pre-tRNA 3′ ends in vivo. Nucleic Acids Res. 2004;32:255–262. doi: 10.1093/nar/gkh182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minagawa A., Takaku H., Takagi M., Nashimoto M. A novel endonucleolytic mechanism to generate the CCA 3′ termini of tRNA molecules in Thermotoga maritima. J. Biol. Chem. 2004;279:15688–15697. doi: 10.1074/jbc.M313951200. [DOI] [PubMed] [Google Scholar]
- 11.Tavtigian S.V., Simard J., Teng D.H., Abtin V., Baumgard M., Beck A., Camp N.J., Carillo A.R., Chen Y., Dayananth P., et al. A candidate prostate cancer susceptibility gene at chromosome 17p. Nature Genet. 2001;27:172–180. doi: 10.1038/84808. [DOI] [PubMed] [Google Scholar]
- 12.Takaku H., Minagawa A., Takagi M., Nashimoto M. The N-terminal half-domain of the long form of tRNase Z is required for the RNase 65 activity. Nucleic Acids Res. 2004;32:4429–4438. doi: 10.1093/nar/gkh774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zareen N., Yan H., Hopkinson A., Levinger L. Residues in the conserved His domain of fruit fly tRNase Z that function in catalysis are not involved in substrate recognition or binding. J. Mol. Biol. 2005;350:189–199. doi: 10.1016/j.jmb.2005.04.073. [DOI] [PubMed] [Google Scholar]
- 14.Ishii R., Minagawa A., Takaku H., Takagi M., Nashimoto M., Yokoyama S. Crystal structure of the tRNA 3′ processing endoribonuclease tRNase Z from Thermotoga maritima. J. Biol. Chem. 2005;280:14138–14144. doi: 10.1074/jbc.M500355200. [DOI] [PubMed] [Google Scholar]
- 15.de la Sierra-Gallay I.L., Pellegrini O., Condon C. Structural basis for substrate binding, cleavage and allostery in the tRNA maturase RNase Z. Nature. 2005;433:657–661. doi: 10.1038/nature03284. [DOI] [PubMed] [Google Scholar]
- 16.Kostelecky B., Pohl E., Vogel A., Schilling O., Meyer-Klaucke W. The crystal structure of the zinc phosphodiesterase from Escherichia coli provides insight into function and cooperativity of tRNase Z-family proteins. J. Bacteriol. 2006;188:1607–1614. doi: 10.1128/JB.188.4.1607-1614.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z., Fast W., Benkovic S.J. On the mechanism of the metallo-β-lactamase from Bacteroides fragilis. Biochemistry. 1999;38:10013–10023. doi: 10.1021/bi990356r. [DOI] [PubMed] [Google Scholar]
- 18.Vogel A., Schilling O., Meyer-Klaucke W. Identification of metal binding residues for the binuclear zinc phosphodiesterase reveals identical coordination as glyoxalase II. Biochemistry. 2004;43:10379–10386. doi: 10.1021/bi049703+. [DOI] [PubMed] [Google Scholar]
- 19.Garrity J.D., Carenbauer A.L., Herron L.R., Crowder M.W. Metal binding Asp-120 in metallo-β-lactamase L1 from Stenotrophomonas maltophilia plays a crucial role in catalysis. J. Biol. Chem. 2004;279:920–927. doi: 10.1074/jbc.M309852200. [DOI] [PubMed] [Google Scholar]
- 20.Garau G., Lemaire D., Vernet T., Dideberg O., Di Guilmi A.M. Crystal structure of phosphorylcholine esterase domain of the virulence factor choline-binding protein E from Streptococcus pneumoniae: New structural features among the metallo-β-lactamase superfamily. J. Biol. Chem. 2005;280:28591–28600. doi: 10.1074/jbc.M502744200. [DOI] [PubMed] [Google Scholar]
- 21.de la Sierra-Gallay I.L., Mathy N., Pellegrini O., Condon C. Structure of the ubiquitous 3′ processing enzyme RNase Z bound to transfer RNA. Nat. Struct. Mol. Biol. 2006;13:376–377. doi: 10.1038/nsmb1066. [DOI] [PubMed] [Google Scholar]
- 22.Schilling O., Späth B., Kostelecky B., Marchfelder A., Meyer-Klaucke W., Vogel A. Exosite modules guide substrate recognition in the ZiPD/ElaC protein family. J. Biol. Chem. 2005;280:17857–17862. doi: 10.1074/jbc.M500591200. [DOI] [PubMed] [Google Scholar]
- 23.Jackson M.D., Denu J.M. Molecular reactions of protein phosphatases—Insights from structure and chemistry. Chem. Rev. 2001;101:2313–2340. doi: 10.1021/cr000247e. [DOI] [PubMed] [Google Scholar]
- 24.Spath B., Kirchner S., Vogel A., Schubert S., Meinlschmidt P., Aymanns S., Nezzar J., Marchfelder A. Analysis of the functional modules of the tRNA 3′ endonuclease (tRNase Z) J. Biol. Chem. 2005;280:35440–35447. doi: 10.1074/jbc.M506418200. [DOI] [PubMed] [Google Scholar]
- 25.Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gouet P., Courcelle E., Stuart D.I., Metoz F. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics. 1999;15:305–308. doi: 10.1093/bioinformatics/15.4.305. [DOI] [PubMed] [Google Scholar]