Abstract
The bacterium Xenorhabdus nematophila is a mutualist of the entomopathogenic nematode Steinernema carpocapsae. During its life cycle, the bacterium exists both separately from the nematode and as an intestinal resident of a nonfeeding nematode form, the infective juvenile (IJ). The progression of X. nematophila from an ex vivo existence to a specific and persistent colonization of IJs is a model to understand the mechanisms mediating the initiation and maintenance of benign host-microbe interactions. To help characterize this process, we constructed an X. nematophila strain that constitutively expresses green fluorescent protein, which allowed its presence to be monitored within IJs. Using this strain, we showed that few bacterial cells initiate colonization of an individual IJ and that these grow inside the lumen of the IJ intestine in a reproducible polyphasic pattern during colonization. In accordance with these two observations, we demonstrated that the final population of bacteria in a nematode is of predominantly monoclonal origin, suggesting that only one or two bacterial clones initiate or persist during colonization of an individual nematode. These data suggest that X. nematophila initiates IJ colonization by competing for limited colonization sites or resources within the nematode intestine. This report represents the first description of the biological interactions occurring between X. nematophila and S. carpocapsae during the early stages of the colonization process, provides insights into the physiology of X. nematophila in its host niche, and will facilitate interpretation of future data regarding the molecular events mediating this process.
The mutualistic, monospecific partnership between the gram-negative enterobacterium Xenorhabdus nematophila and its entomopathogenic nematode host, Steinernema carpocapsae, is one of several emerging models to study benign host-microbe interactions (9, 13, 27). The natural life cycle of S. carpocapsae nematodes includes reproductive stages that occur exclusively within larval-stage insects and that are not colonized by X. nematophila and also includes a nonreproductive, nonfeeding, soil-dwelling stage known as the infective juvenile (IJ), which is colonized, at a discrete intestinal location termed the vesicle, by a monoculture of X. nematophila bacteria (9).
Colonized IJs exist in the soil until they invade a susceptible insect host, seeking the blood system or hemolymph. Once there, the IJs release their X. nematophila symbionts by defecation (19, 31; E. C. Martens and H. Goodrich-Blair, unpublished data). At this point in their life cycles, bacteria and nematodes exist separately although in close proximity to one another. The released X. nematophila bacteria contribute to the killing of the insect host and grow to high density in the resulting cadaver. These specific bacteria are essential for nematode growth and development presumably both by serving as a direct food source and by supplying nutrients through degradation of the insect carcass (2, 22).
When nematode numbers become high and nutrients become limiting in the insect cadaver, S. carpocapsae nematode progeny reassociate with X. nematophila bacteria and differentiate into the colonized, nonfeeding IJ form that emerges into the soil to forage for a new host (23). IJ formation, and concomitant colonization with X. nematophila, is a model of the process by which a specific and intimate association between a microbe and a eukaryotic host in a benign relationship develops (9, 27). A deeper understanding of X. nematophila-nematode interactions will provide insights into the general mechanisms mediating the development and maintenance of benign microbe-host interactions and also allow a comparison of benign interactions with those that are pathogenic (27).
We chose to characterize the early stages of nematode colonization by X. nematophila to better understand the process by which this mutualistic interaction is initiated and maintained. A characteristic of the IJ stage of the nematode, which develops within the spent insect cadaver prior to emergence into the soil, is its sealed mouth and anus (18). The IJ therefore does not ingest resources or excrete intestinal waste during its existence outside an insect and must be colonized by X. nematophila prior to development into this stage. Until now, experiments exploring the initiation of X. nematophila colonization of the pre-IJ nematode have not been reported. We considered two possible models to explain how mature IJs (i.e., those that have emerged from an insect host) obtain a full complement of colonizing bacteria (13, 19). In the simplest model, we postulated that a developing IJ ingests a large number of bacteria from its environment before it ceases feeding and that these bacteria, instead of being digested, are retained in the intestinal vesicle. A more complex model supposes that a pre-IJ nematode retains only one or a few bacteria in a selective manner and, after the IJ ceases ingesting external bacteria, these few bacteria grow until they reach the full density found in a mature IJ.
To test these models, we conducted experiments utilizing an in vitro culturing technique in which S. carpocapsae nematodes are grown on a lawn of X. nematophila bacteria (31). This technique allows monitoring of nematodes throughout their reproductive life cycle, and progeny IJs emerging from these lawns are colonized at levels that are comparable to those of IJs produced from insect infection (13). In addition to this technique, we developed and/or implemented several new tools that facilitated the examination of early stages in the association between nematodes and bacteria. We present data that document the process by which nematodes achieve the fully colonized state, differentiate between the hypothetical colonization models described above, and provide insight into mechanisms mediating early colonization events.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
X. nematophila strains and plasmids used in this study are listed in Table 1. Permanent stocks of bacterial strains were maintained at −80°C in Luria-Bertani (LB) broth (14) supplemented with 25% glycerol (for Escherichia coli) or 10% dimethyl sulfoxide (for X. nematophila). Unless stated otherwise, X. nematophila and E. coli were grown at 30°C in LB broth or agar that either had not been exposed to light or to which 0.1% pyruvate had been added (32). When appropriate, media were supplemented with chloramphenicol (10 μg ml−1 for X. nematophila or 30 μg ml−1 for E. coli) and gentamicin (30 μg ml−1). Solid, lipid-agar medium for coculture of S. carpocapsae on lawns of X. nematophila was prepared as described elsewhere (27). E. coli S17-1 λ pir (25) was used to conjugally transfer plasmids into X. nematophila strains as previously described (10). S. carpocapsae (strain All) was propagated by passage through Galleria mellonella larvae (Vanderhorst Wholesale Inc., St. Marys, Ohio) and harvested in White traps as previously described (27, 30). As needed, axenic, J1 stage S. carpocapsae organisms were isolated by harvesting nematode eggs with bleach from gravid adult females as previously described (28), except that the eggs were rinsed with sterile LB broth instead of water or buffered salts solution.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant features | Source or reference |
|---|---|---|
| X. nematophila strains | ||
| HGB007 | ATCC 19061, wild type, Ampr | ATCC |
| HGB081 | Spontaneous Rifr mutant of X. nematophila ATCC 19061 | S. Forst |
| HGB340 | GFP+ derivative of HGB007, Cmlr | This study |
| HGB566 | Mini-Tn7-ECFP-containing derivative of HGB007, Gmr | This study |
| HGB567 | Mini-Tn7-DsRed gene-containing derivative of HGB007, Gmr | This study |
| STM-1 | HGB081 containing unique (probe A) signature-tagged mini-Tn5 | 13; this study |
| STM-2 | HGB081 containing unique (probe B) signature-tagged mini-Tn5 | 13; this study |
| STM-3 | HGB081 containing unique (probe C) signature-tagged mini-Tn5 | 13; this study |
| Plasmids | ||
| pNK2859 | Contains mini-Tn10-kan, source of PaphA | 29 |
| pQBI63 | Source of Super-Glo GFP gene | Qbiogene |
| pKR100 | oriR6K-based suicide vector, Cm1r | K. Visick |
| pKR100B | pKR100 with unique BgIII site | This study |
| pECM9 | pQBI63 with PaphA in place of PT7 | This study |
| pECM2 | PaphA-Super-Glo GFP gene fragment from pECM9 cloned into pKR100B | This study |
| pECM20 | pECM2 containing a 614-bp chromosomal insert from ATCC 19061 | This study |
| pBK-mini-Tn7(Gm)PA1/04/03-DsRed gene | Source of mini-Tn7-PA1/04/03-DsRed gene, Gmr | L. Lambertsen |
| pBK-mini-Tn7(Gm)PA1/04/03-ECFP gene | Source of mini-Tn7-PAl/04/03-ECFP gene, Gmr | L. Lambertsen |
| pUX-BF13 | Tn7 transposition helper plasmid (in E. coli S17-1 λ pir) | 5 |
The enhanced isoform of green fluorescent protein (GFP) used in this work was Super-Glo GFP (Qbiogene, Carlsbad, Calif.) and is hereafter referred to as GFP. To obtain constitutive expression of the GFP gene, it was fused to a 126-bp fragment containing the aphA promoter (PaphA) amplified from E. coli transposon Tn903 by using pNK2859 (29) as a template and the primers 5′-GGGGGGAGATCTCGTTGTGTCTCAAAATCTCTG-3′ and 5′-GGGTCTAGATGAATATGGCTCATAACACCC-3′. The resulting PaphA fragment was cut with BglII and XbaI and ligated into pQBI63 (Qbiogene) cut with BglII and XbaI, resulting in the plasmid pECM9. For subsequent transfer of this fusion into X. nematophila, a BglII-EcoRV PaphA-GFP gene fragment was subcloned from pECM9 into pKR100B to create the plasmid pECM2. Plasmid pKR100B is a suicide vector used for delivering constructs into X. nematophila and was constructed by cutting pKR100 (a derivative of pGP704 [pJM703.1] [15], kindly provided by Karen Visick, Loyola University of Chicago, Chicago, Ill.) with SalI and XbaI and inserting a linker comprised of the sequence 5′-GTCGACAGATCTAGA-3′, thereby generating a unique BglII site. To direct the PaphA-GFP gene fragment to the chromosome of X. nematophila, Sau3AI genomic fragments (average size, 0.4 to1.6 kb) were ligated into pECM2 cut with BglII. To generate an X. nematophila strain expressing the GFP gene, this library was electroporated as previously described (4) into E. coli S17-1 λ pir and conjugated en masse into X. nematophila ATCC 19061 (25). Exconjugants were selected on chloramphenicol. Since pECM2 replicates using the origin oriR6K, which is not functional in X. nematophila, these exconjugants must contain integrations, directed by homologous recombination with the cloned Sau3AI fragments, of pECM2 into the bacterial chromosome. Four exconjugants were selected based on their high fluorescence levels detected by flow cytometry (conducted at the University of Wisconsin—Madison CSC Flow Cytometry Facility). Of these, strain HGB340 was the brightest and was used for all subsequent studies. To determine the location of the plasmid insertion in strain HGB340, the original library fragment that was cloned in pECM2 and that directed integration into the X. nematophila chromosome was isolated and sequenced (24). The cloned fragment (GenBank accession number AY194223) is 614 bp and shares predicted amino acid sequence similarity with a putative potassium efflux system from Yersinia pestis. We have found that HGB340 colonizes S. carpocapsae similarly to HGB007 (the wild type) under all conditions tested (data not shown), indicating that integration of pECM20 and constitutive expression of the GFP gene do not exert an observable effect on the process of bacterial colonization of nematodes. Additionally, the E. coli S17-1 λ pir strain harboring pECM20 has proven suitable for labeling other X. nematophila strains and mutants with the GFP gene (data not shown).
Mini-Tn7 constructs containing the ECFP and DsRed genes (Clontech, Palo Alto, Calif.) were kind gifts from Lotte Lambertsen (Technical University of Denmark, Lyngby). These constructs were conjugated into HGB007 from E. coli S17-1 λ pir and delivered to the X. nematophila att Tn7 site (Martens and Goodrich-Blair, unpublished data) by triparental mating using the helper plasmid pUX-BF13 (5).
In vitro coculture of nematodes on bacterial lawns.
In vitro coculture of nematodes on bacterial lawns was performed as described previously (27) in 6-cm-diameter petri plates (Fisher Scientific, Pittsburgh, Pa.) containing lipid-agar medium, except that cocultures for preparation of immature IJs were carried out in 500-cm2 polystyrene plates (Corning, Corning, N.Y.) and inoculated with IJs instead of axenic J1 nematodes. Approximately 4 × 105 IJs were inoculated onto each 500-cm2 plate to initiate nematode growth. Culture progression was monitored by microscopic examination of nematodes (see below). Immature IJs typically formed 8 to 9 days after introduction of the IJ inoculum into cocultures.
SDS isolation of immature S. carpocapsae IJs from mixtures of developmental stages.
Immature IJs were harvested when approximately 50% of the nematodes present in an in vitro coculture were microscopically observed to be IJs and were selectively isolated from the heterogeneous populations of other S. carpocapsae stages by treatment with sodium dodecyl sulfate (SDS; Sigma, St. Louis, Mo.). IJs are resistant to treatment with 1% SDS, while other stages are not. This treatment was based on the procedure described by Cassada and Russell (8) for the specific isolation of Caenorhabditis elegans dauerlarvae from mixed developmental stages. Nematodes were harvested by flooding the culture plates with ∼200 ml of distilled water (dH2O), resuspending the nematodes and remaining bacterial cells, and centrifuging the suspensions at 1,000 × g. Nematode suspensions were treated with 1% SDS for 20 min with periodic agitation and then washed a total of three times with dH2O (50 ml/wash). Nematodes were rinsed an additional two times with dH2O in a filter apparatus equipped with an 11-μm-pore-size nylon membrane (Fisher Scientific). Immature IJs recovered in this manner were stored in dH2O at 25°C during the period of bacterial outgrowth. Control experiments were conducted to determine if SDS treatment affects the number of bacteria in IJs. In one of these experiments, 0.5% bleach was employed to surface sterilize IJs and to remove other nematode stages from the population (see Results). Treatment with bleach achieved the same result that treatment with SDS did; however, IJ survival was poor after this treatment, so it was not used for selective isolation of IJs used for prolonged observation.
Isolation of nematodes from bacteria without SDS.
To monitor outgrowth of HGB340 in immature IJs, nematodes were harvested from heterogeneous populations without the use of SDS treatment. These nematodes were washed repeatedly with excess volumes of sterile dH2O to remove external bacteria and were subsequently stored in dH2O.
Microscopic examination of S. carpocapsae.
To observe S. carpocapsae microscopically, suspensions of nematodes were applied to the surface of a glass slide on which a 4% agarose pad containing 10 mM sodium azide (Sigma) had been applied. A coverslip was applied to the slide before the nematode suspension was allowed to dry completely, and the slide was observed under a Nikon Eclipse TE300 inverted microscope at ×600 magnification. Fluorescence microscopy of GFP-containing samples was performed by using fluorescein isothiocyanate, tetramethyl rhodamine isocyanate, and triple-band DAPI (4′,6′-diamidino-2-phenylindole)-FITC (fluorescein isothiocyanate)-TRITC (tetramethyl rhodamine isothiocyanate) filter sets (Chroma, Brattleboro, Vt.; items 31001, 31002, and 8200, respectively). GFP-expressing bacteria were differentiated from nematode intestinal autofluorescence by using filter set 8200 and appeared green, whereas autofluorescence appears white-yellow. Images were recorded electronically with a digital camera (Hamamatsu, Hamamatsu City, Japan; model C4742-95-10NR) and a personal computer equipped with MetaMorph version 4.5r6 software (Universal Imaging Corporation, West Chester, Pa.). Images were prepared for publication by using Adobe Photoshop version 6.0.1 and Deneba Canvas version 7.0.
Quantification of colonizing bacteria.
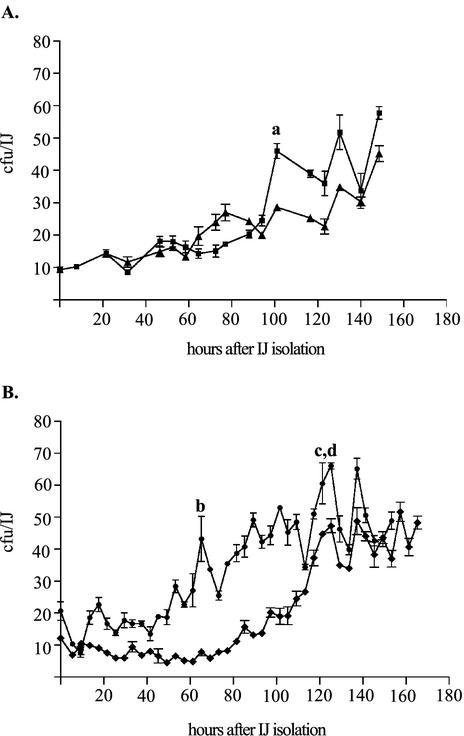
Quantification of X. nematophila CFU per IJ was performed as previously described (13). Samples of maturing IJs that had been isolated by use of the SDS isolation procedure described above were removed at various times after isolation, surface sterilized, enumerated, and sonicated in a water bath sonicator (Branson Ultrasonics, Danbury, Conn.) to liberate bacteria from the intestines of the IJs. Between 9 × 103 and 1.1 × 104 IJs were sonicated at each time point, and the number of CFU per IJ was determined by plating dilutions of sonicates on LB agar plus 0.1% pyruvate. The detection limit of this procedure was ≥0.003 CFU/IJ, and approximately 60 CFU/IJ were normally detected in IJs cultivated on wild-type X. nematophila (13). Because microscopic examination of nematodes containing HGB340 detected visible bacteria in the intestinal vesicle exclusively (see below), we believe that viable counts of CFU per IJ determined through homogenization and plating reflect bacteria that are liberated from this location only. Raw data from the curves shown below (see Fig. 3) were analyzed by analysis of variance and a t test to determine if significant reductions in recoverable CFU per IJ occurred during the course of growth. To calculate the maximum doubling time of X. nematophila bacteria in nematodes, a linear regression analysis was performed with data taken from four regions of the curves shown below (see Fig. 3). Regions containing three or more consecutive points of increasing value were selected for analysis, and the slopes of best-fit lines were used directly to determine the doubling time at each point.
FIG. 3.
X. nematophila growth in isolated immature IJs. Populations of immature IJs were assayed at 4- or 6-h intervals to determine the average number of X. nematophila cells associated with IJs. Two replicates each from two separate experiments (A and B) are shown. Each point is the average result for three individual assays ± standard error (measured in CFU per IJ). Lowercase letters a to d indicate the regions of growth where data were used to determine the maximum growth rate (see Materials and Methods). Peaks from both curves under the region labeled c and d were used.
Analysis of signature-tagged clones in single nematodes.
To determine which signature-tagged clone(s) was present in individual nematodes, IJs produced on a mixed lawn of strains STM-1, STM-2, and STM-3 were surface sterilized and individual IJs were ground in 100 μl of LB broth in a Duall 20 homogenizer (Kimble-Kontes, Vineland, N.J.). The entire 100-μl volume of homogenate was plated on LB agar, and only homogenates from which ≥88 CFU were recovered were analyzed. Individual X. nematophila colonies were patched onto LB agar plates overlaid with sterilized 10-cm-diameter Hybond nylon membranes (Amersham-Pharmacia, Piscataway, N.J.). Colony blot and hybridization analyses to detect signature-tagged strains were performed as previously described (12, 13), with the exception that bacterial DNA was cross-linked to nylon membranes by using a UV cross-linker (Stratagene, La Jolla, Calif.).
RESULTS
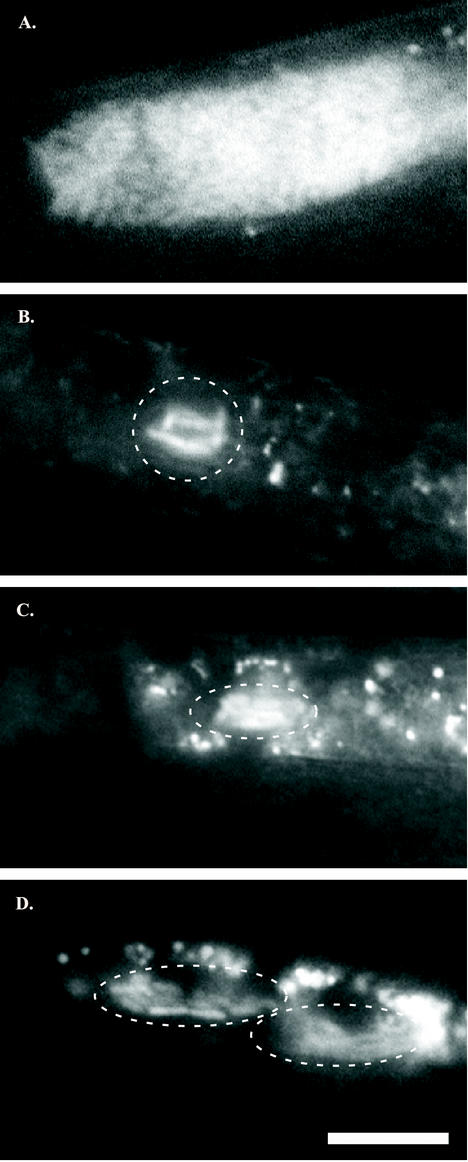
To observe interactions between X. nematophila and S. carpocapsae during early stages of the colonization process, we constructed a derivative of wild-type X. nematophila HGB007 that constitutively expresses an enhanced isoform of GFP (see Materials and Methods; Table 1). This strain, HGB340, is readily detectable inside mature IJs by epifluorescence microscopy (Fig. 1A) and colonizes IJs to levels similar to those of the parent (data not shown).
FIG. 1.
Bacterial colonization of immature IJs. Vesicles of mature IJs may contain 40 to >100 X. nematophila cells (A), whereas the vesicles of immature IJs contain only a few X. nematophila cells (indicated by enclosure in a dashed white line) (B to D). In panel B, six to seven rod-shaped X. nematophila cells cluster in close proximity to each other in the vesicle. The vesicle shown in panel C contains slightly more X. nematophila cells, and that in panel D contains even more than the IJ shown in panel B, but each has noticeably fewer than the full complement of cells found in a mature IJ (A). GFP-labeled X. nematophila cells were distinguished from nematode intestinal autofluorescence by virtue of bacterial cell shape and differential spectral emission under appropriate fluorescence filters (see Materials and Methods). In all images, nematodes are oriented with heads off the left side of the panel. Magnification, ×600. Bar, 10 μm.
Premigratory IJs are colonized by few bacteria.
S. carpocapsae nematodes were cultured on lawns of HGB340, and the resulting IJ progeny were microscopically examined for the presence of bacteria in the vesicle. Two types of IJs were examined: those that had migrated away from the lawn on which they were cultured (mature IJs) and those that had recently formed on the lawn but had not yet migrated (immature IJs). The latter were isolated from the mixed population based on the exclusive resistance of IJs to 1% SDS (see Materials and Methods) (8). Both types of IJs were observed by using epifluorescence microscopy to detect GFP (Fig. 1). As expected, mature IJs had brightly fluorescent vesicles with numerous individual bacterial cells distinguishable, which is indicative of complete colonization (Fig. 1A). In mature IJs, HGB340 bacteria were observed to be present in the anterior region of the intestine only and not in the posterior intestine, the pharynx, or on the surface of the nematode (data not shown). In contrast to these mature IJs, immature IJs were usually colonized by only a few bacteria (“oligocolonization”) (Fig. 1B to D). To address the possibility that SDS treatment directly affects levels of bacteria inside IJs, a heterogeneous mixture of S. carpocapsae developmental stages cultivated on lawns of HGB340 was microscopically examined without prior exposure to SDS (see below). Oligocolonization was also observed in immature IJs in this population. Nematode samples containing immature IJs were also surface sterilized with bleach (see Materials and Methods), and the number of CFU per IJ was enumerated before SDS treatment. Counts of CFU per IJ both before and after SDS treatment for identical batches of nematodes were found to be the same (data not shown).
Bacterial growth occurs in the vesicle of maturing IJs.
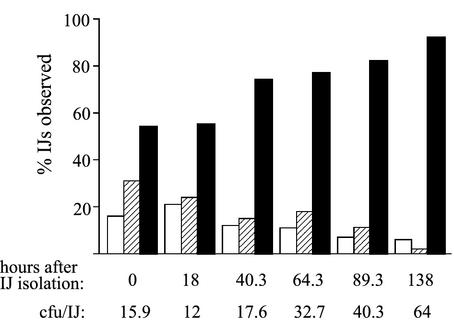
The observation that immature IJs contain few bacteria, even though their mouths and anuses are already sealed, indicates that the fully colonized vesicle typical of a mature IJ results from bacterial growth within the intestinal vesicle. To monitor this process, a population of nematodes was cultivated on HGB340 until immature IJs began to form and were removed from exogenous X. nematophila by rinsing with large volumes of distilled water (see Materials and Methods). These nematodes were intermittently observed by fluorescence microscopy for 138 h after isolation. Individual IJs in this nematode population were distinguished by IJ-specific morphological features (20) and scored according to the number of green fluorescent bacteria that each appeared to contain (Fig. 2). IJs were scored as belonging to one of three groups: (i) those containing no observable fluorescent bacteria, (ii) those in which the vesicle was oligocolonized (Fig. 1B and C), and (iii) those with full or nearly full vesicles (approximately one-third or more fully colonized) (Fig. 1A). The percentages of both oligocolonized IJs and those with no observable bacteria decreased over time, while the number of near fully or fully colonized IJs increased over time. At each observation time, the average number of CFU per IJ was quantified (see Materials and Methods) and found to increase over time, which correlated with the increase in the number of IJs with brightly fluorescing vesicles. Furthermore, oligocolonized IJs are not observed in populations of mature IJs (i.e., >150 h postisolation), suggesting that the trend toward increased colonization is not reversible (data not shown).
FIG. 2.
Microscopic analysis of colonization density of GFP-labeled X. nematophila in nematode IJs. Nematodes were cultivated on lawns of HGB340, and immature IJs were isolated by repeated rinsing with sterile dH2O (see Materials and Methods). At various times after isolation (indicated below each set of bars), nematodes were observed by fluorescence microscopy and rated as belonging to one of three classes: not visibly colonized (open bars), oligocolonized (hatched bars), or fully colonized (filled bars). Between 170 and 303 IJs were observed for each time point, and at each time point a sample of IJs was used to quantify the average CFU per IJ (values shown below graph).
To examine the growth characteristics of X. nematophila in IJs in more detail, we quantified the number of CFU per IJ over time in three separate populations of immature IJs derived from wild-type (nonfluorescent) X. nematophila lawns and isolated by SDS treatment. After isolation, samples were taken from the immature IJ populations at 4- or 6-h intervals over approximately a 150-h period and the average number of CFU per IJ was quantified (Fig. 3). The data indicate that the bacterial population within IJs fluctuates, with an overall trend toward increasing bacterial density. Several of the decreases in detectable CFU per IJ were determined to be statistically significant relative to previous peaks in CFU per IJ. Four regions of individual growth curves were chosen to determine the maximum growth rate of X. nematophila in the intestinal vesicle. The doubling time was determined to be 10 ± 0.5 h (average ± standard error) per doubling.
X. nematophila populations in individual IJs are often clonal.
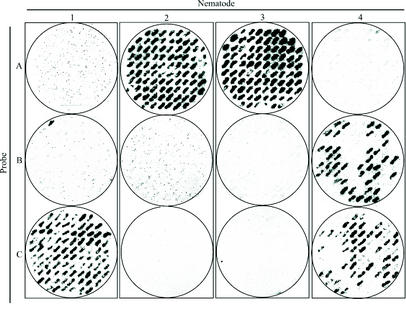
The results presented thus far are consistent with the hypothesis that limited colonization sites are available for X. nematophila within the forming IJ and that competition for these sites may occur. To address this possibility, we assessed the number of X. nematophila clones that were present inside individual IJs by using two approaches (Fig. 4 and 5). First, we cultivated IJs on a lawn containing a mixture of three strains that each contain a uniquely tagged mini-Tn5 insertion (strains STM-1, -2, and -3). These strains were generated in a previously published signature-tagged mutagenesis study conducted by our lab and were selected because they do not have a competitive colonization defect (13). These three strains can be distinguished by hybridization to a unique probe (A, B, or C, respectively). Four individual IJs derived from the mixed bacterial lawn were homogenized, and the homogenates were plated to recover liberated X. nematophila bacteria. Individual bacterial colonies isolated from each nematode were grown on nylon membranes, and colony blot analyses were separately performed with probes A, B, and C (Fig. 4). Of the four nematodes, three yielded bacteria that hybridized to one of the three probes. The fourth nematode had been colonized by a mixture of bacteria that hybridized to two probes. In the case of this nematode, 49% (43 of 88) of the clones were detected by probe B, whereas the remaining 51% (45 of 88) were detected by probe C.
FIG. 4.
Competition of signature-tagged X. nematophila strains for nematode colonization. X. nematophila bacteria recovered from individual IJs (numbered 1 to 4) were probed to determine if they hybridized to probe A, B, or C. All 88 colonies recovered from IJ 1 hybridized to probe C. All 88 colonies recovered from IJs 2 and 3 hybridized to probe A. For IJ 4, 49% (43 of 88) of the clones hybridized to probe B, whereas the remaining 51% (45 of 88) hybridized to probe C.
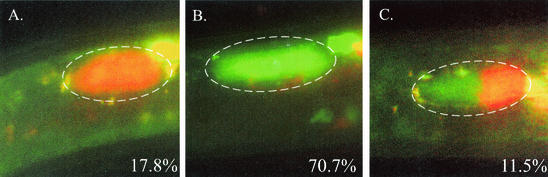
FIG. 5.
Competition of HGB566 and HGB567 for nematode colonization. IJs raised on a lawn inoculated with equal amounts of HGB566 and HGB567 were analyzed to determine whether they contained only strain HGB567 (A), only strain HGB566 (B), or a mixture of both fluorescent strains (C). The frequency of each colonization type is indicated in the lower right corner of each panel. A total of 481 IJs were examined. The reason for the disproportionately high percentage of nematodes colonized by ECFP-expressing bacteria only may be due to the unequal competition of these strains for growth and survival during the ∼10-day coculture period. This inequality in strain representation further exemplifies the tendency toward monoclonal colonization because the 17.8% of nematodes that contained only DsRed-expressing bacteria would have been fully colonized by those bacteria despite the fact that they were a minority population.
We wished to directly observe the apparent monoclonal and biclonal colonization phenomenon suggested by the experiment described above in a larger sample size. To do this, we constructed two X. nematophila strains, HGB566 and HGB567, that are differentially detectable in vivo because they constitutively express the genes for enhanced cyan fluorescent protein (ECFP) and a red fluorescent protein (DsRed). IJs were cultured on a mixed lawn that was initially established by mixing equal amounts of HGB566 and HGB567. Resultant IJs were analyzed by epifluorescence microscopy to determine if they contained only ECFP- or DsRed-labeled bacteria or a mixture of both. We found that of 481 IJs examined, 88.5% contained only one type of labeled bacteria (70.7% with ECFP, 17.8% with DsRed) (Fig. 5A and B), whereas the remaining 11.5% of the nematodes contained a mixture of both ECFP- and DsRed-labeled bacteria (Fig. 5C). In nematodes that contained a mixture of both ECFP- and DsRed-labeled bacteria, bacterial growth occurred in distinct sectors (e.g., Fig. 5C), suggesting that bacterial movement may be physically restricted during growth in the nematode intestine. The percentage of IJs containing both DsRed- and ECFP-labeled bacteria (11.5%) can be used to estimate the actual percentage of biclonal colonization. Because equivalent percentages of IJs should be biclonally colonized by two ECFP-labeled strains alone or by two DsRed-labeled strains alone, we estimated the percentage of biclonal colonization for this experiment to be approximately 35% (3 × 11.5%). This estimate agrees with the percentage of biclonal colonization from the previous experiment, which was 25% (one in four IJs).
DISCUSSION
Previous work from many laboratories has established that the IJ stage of Steinernematid nematodes is specifically colonized by a single species of Xenorhabdus bacteria (2, 11; C. E. Cowles and H. Goodrich-Blair, unpublished data) and that this colonization is limited to the intestinal vesicle (6, 21, 22). However, until the present study was done, an examination of early events in the colonization process had not been reported. Our results have allowed us to differentiate between the two hypothetical colonization models that we have proposed and to demonstrate the existence of two previously undescribed early stages in the association between S. carpocapsae and X. nematophila. The first is a competitive initiation event that typically results in colonization by a monoclonal or oligoclonal population of X. nematophila. This stage was demonstrated by two experiments in which differentially labeled wild-type strains were competing for colonization (Fig. 4). The observation that X. nematophila populations are frequently of oligoclonal origin corresponds with the direct observation that early in the colonization process very few GFP-labeled bacteria can be seen occupying the vesicle (Fig. 1) and supports the hypothesis that a small number of X. nematophila bacteria initiate vesicle colonization and subsequently grow inside the host. The second early colonization stage we have identified in this work is an outgrowth phase during which bacteria reproduce inside the intestine of an IJ and ultimately achieve their maximum population density. Evidence for this stage includes the fact that immature IJs derived from lawns of fluorescent X. nematophila bacteria contain noticeably fewer fluorescent bacteria (Fig. 1B to D) than those IJs that have been allowed to mature (Fig. 1A). The second line of evidence for an outgrowth stage comes from the fact that a pure population of nonfeeding IJs exhibits a quantitative increase in the average number of CFU per IJ over time (Fig. 2 and 3) and that this increase in bacterial number can be microscopically localized to the intestinal vesicle (Fig. 2). Since the only source of bacteria within the IJ population is that within the intestinal vesicle, this increase in bacterial cell counts over time must result from outgrowth of the initial colonizing bacteria within the vesicle. A reproducible feature of the in vivo growth patterns observed for X. nematophila is repeated waves of population rise and decline. This observation demonstrates the dynamic nature of the X. nematophila population within the nonfeeding IJ and suggests that the nematode may be controlling the bacterial population in some way (see below).
The clarification of the processes through which uncolonized nematodes become persistently colonized by X. nematophila will provide a useful foundation for proposing and testing hypotheses regarding the molecular mechanisms that mediate bacterium-nematode interactions. For example, the colonization competition assays (Fig. 4 and 5) and the ability to label X. nematophila mutant strains with autofluorescent proteins described in this report can help reveal the precise stage(s) of colonization that is blocked in recently described colonization mutants (13, 27). Furthermore, our work provides insights into the early events in the development of the colonized IJ stage that will be useful in the mass production and field application of nematodes for biological control.
A highly efficient colonization initiation event results in oligocolonized vesicles.
In a remarkably efficient process, few initiating X. nematophila bacteria per S. carpocapsae IJ results in greater than 90% of a given population of IJs (isolated from either insects or in vitro) being colonized (3, 27; Martens and Goodrich-Blair, unpublished data). This finding elicits the following questions: how is colonization initiation limited to a few bacteria and what mechanisms account for its efficiency? One possibility is that there is a specific, physical contact between the bacteria and the nematode, such as a receptor-adhesin-mediated interaction, that is anatomically restricted to the region at which initiation takes place. If an essential physical interaction between the bacteria and the nematode exists, then we predict that colonization could be inhibited by the presence of competing bacteria that are capable of binding the initiation region and perhaps also by the presence of small molecules, pure protein, or antibodies that interfere with the initial interaction.
A second possible explanation for the predominantly clonal nature of bacterial populations in IJs is that multiple cells initiate colonization but that, most frequently, either one cell outcompetes the others in establishing a fully colonized vesicle or the phenomenon of repetitive rounds of population rise and decline observed during X. nematophila in vivo growth (Fig. 3) eventually eliminates all but a few clonal bacterial lineages. Future experiments that monitor the percentage of polyclonal bacterial populations (Fig. 5C) in nematodes over time will determine whether this percentage changes and should differentiate between these possibilities and the model suggesting that few cells actually initiate nematode colonization.
Initiating bacterial colonizers grow to full density within the IJ.
Once nematode colonization has been initiated, X. nematophila cells reproduce in the intestinal vesicle until a maximum bacterial cell density is achieved (Fig. 2 and 3). Based on this finding, we can conclude that the vesicular environment is conducive to bacterial growth and that the bacteria have access to a nutrient source, possibly from the nematode. X. nematophila may have evolved mechanisms to scavenge nutrients from the nematode, as do pathogens from their hosts. Alternatively, the nematode might actively provide nutrients to the growing X. nematophila monoculture. In light of the possibility of either passive or active transfer of nutrients from the nematode to the bacteria, it is interesting that axenic S. carpocapsae IJs survive longer at ambient temperatures than colonized IJs do (17). This suggests that maintenance of bacterial symbionts is an energy drain for the nematode and is consistent with the idea that the nematode provides food for its bacterial symbionts.
It is notable that when multiple clones of X. nematophila colonize an individual IJ, these bacteria grow in distinct sectors (Fig. 5C). The reason for this spatial limitation is unknown, but it implies that the vesicular environment may contain a matrix in which bacteria grow and which limits bacterial movement. This idea is in agreement with the findings of Bird and Akhurst (6), who observed that in electron micrographs, Xenorhabdus spp. appear to be embedded in an amorphous matrix inside the intestinal vesicle.
In the present experiments, X. nematophila reproduced in the intestinal vesicle with an overall trend toward a maximum capacity of approximately 45 to 70 CFU/IJ (Fig. 3). However, over the ≥150 h of the experiment, the detectable population size repeatedly increased and decreased, and during a discrete rise in population, X. nematophila numbers could be observed to double within as few as 10 h. Although this doubling time is lengthy compared to the 1-h doubling time of X. nematophila cells grown in LB broth at 30°C (Martens and Goodrich-Blair, unpublished data), it should be noted that immature IJ populations exhibit some amount of asynchrony regarding the state of bacterial populations within individuals (for an example, see Fig. 2). Therefore calculated rates of population growth based on growth dynamics of an entire population may underestimate the actual rate of growth of X. nematophila in the intestinal vesicles of individuals.
X. nematophila population declines may indicate a selective pressure imposed by the nematode to limit the size of its symbiont colony (e.g., through the production of antimicrobial compounds or vesicular structural rearrangements). Such a mechanism occurs in the symbiosis between the Hawaiian bobtail squid and its bacterial symbiont, Vibrio fischeri, in which the squid daily purges its light organ of 90% of bacteria and the remaining 10% of the bacterial population then grows to repopulate this niche (26). We are currently investigating the mechanism(s) by which S. carpocapsae may periodically restrict growth or eliminate viability of X. nematophila in the intestinal vesicle.
The observation that overall X. nematophila population size does not surpass a maximal level within the vesicle is important from the perspective of benign host-microbe interactions because it implies that the nematode efficiently limits the growth potential of X. nematophila inside its intestine. Bacteria such as Salmonella enterica, Enterococcus faecalis, Pseudomonas aeruginosa, Streptococcus pneumoniae, Staphylococcus aureus, and Burkholderia pseudomallei can be lethal intestinal pathogens of nematodes such as C. elegans (1). Furthermore, several examples exist in which ordinarily benign bacteria or fungi gain the ability to overcome host defenses and exhibit pathogenic qualities as they exploit new niches in the host (7, 16). Unchecked growth of X. nematophila within the intestine of S. carpocapsae could lead to pathogenesis and death of the IJ, but the complete absence of X. nematophila from the vesicle would block the reproductive fitness of an IJ. Thus, S. carpocapsae nematodes must have evolved exquisitely balanced mechanisms to promote X. nematophila colonization while concomitantly limiting it to a specific area, allowing these two organisms to coevolve as mutualists rather than as host and pathogen. By using the details of colonization initiation revealed by this study, our future goal is to understand the mechanisms that underlie the balanced X. nematophila-S. carpocapsae association. Such work will likely reveal general mechanisms by which hosts attempt to limit growth of microbial flora as well as how bacteria circumvent such attempts.
Acknowledgments
We thank Murray Clayton for assistance in statistical analysis and members of the H.G.-B. laboratory, as well as Steven Finkel, Katrina Forest, and Jon Woods, for comments on the manuscript.
This work was supported by NIH grant GM59776 and USDA/CRES grant CRHF-0-6055, both awarded to H.G.-B.
REFERENCES
- 1.Aballay, A., and F. M. Ausubel. 2002. Caenorhabditis elegans as a host for the study of host-pathogen interactions. Curr. Opin. Microbiol. 5:97-101. [DOI] [PubMed] [Google Scholar]
- 2.Akhurst, R. J. 1983. Neoaplectana species: specificity of association with bacteria of the genus Xenorhabdus. Exp. Parasitol. 55:258-263. [DOI] [PubMed] [Google Scholar]
- 3.Akhurst, R. J., and N. Boemare. 1990. Biology and taxonomy of Xenorhabdus, p. 75-87. In R. Gaugler and H. K. Kaya (ed.), Entomopathogenic nematodes in biological control. CRC Press, Inc., Boca Raton, Fla.
- 4.Ausubel, F. A., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1998. Current protocols in molecular biology. Wiley and Sons, New York, N.Y.
- 5.Bao, Y., D. P. Lies, H. Fu, and G. P. Roberts. 1991. An improved Tn7-based system for the single-copy insertion of cloned genes into chromosomes of gram-negative bacteria. Gene 109:167-168. [DOI] [PubMed] [Google Scholar]
- 6.Bird, A. F., and R. J. Akhurst. 1983. The nature of the intestinal vesicle in nematodes of the family Steinernematidae. Int. J. Parasitol. 13:599-606. [Google Scholar]
- 7.Casadevall, A., and L. Pirofski. 2001. Host-pathogen interactions: the attributes of virulence. J. Infect. Dis. 184:337-344. [DOI] [PubMed] [Google Scholar]
- 8.Cassada, R. C., and R. L. Russell. 1975. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46:326-342. [DOI] [PubMed] [Google Scholar]
- 9.Forst, S., and D. Clarke. 2002. Bacteria-nematode symbioses, p. 57-77. In R. Gaugler (ed.), Entomopathogenic nematology. CABI Publishing, Wallingford, United Kingdom.
- 10.Forst, S. A., and N. Tabatabai. 1997. Role of the histidine kinase, EnvZ, in the production of outer membrane proteins in the symbiotic-pathogenic bacterium Xenorhabdus nematophilus. Appl. Environ. Microbiol. 63:962-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grewal, P. S., M. Matsuura, and V. Converse. 1997. Mechanisms of specificity of association between the nematode Steinernema scapterisci and its symbiotic bacterium. Parasitology 114:483-488. [DOI] [PubMed] [Google Scholar]
- 12.Grunstein, M., and D. S. Hogness. 1975. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc. Natl. Acad. Sci. USA 72:3961-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heungens, K., C. E. Cowles, and H. Goodrich-Blair. 2002. Identification of Xenorhabdus nematophila genes required for mutualistic colonization of Steinernema carpocapsae nematodes. Mol. Microbiol. 45:1337-1353. [DOI] [PubMed] [Google Scholar]
- 14.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 15.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min, K. T., and S. Benzer. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. USA 94:10792-10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitani, D. K. 2002. Master's thesis. University of California—Davis, Davis, Calif.
- 18.Mracek, Z., J. Weiser, and S. Gerdin. 1981. Head and cuticular structures of some species in the family Steinernematidae (Nematoda). Nematologica 27:443-448. [Google Scholar]
- 19.Poinar, G. O. 1966. The presence of Achromobacter nematophilus in the infective stage of a Neoaplectana sp. (Steinernematidae: Nematoda). Nematologica 12:105-108. [Google Scholar]
- 20.Poinar, G. O., Jr. 1990. Taxonomy and biology of Steinernematidae and Heterorhabditidae, p. 23-61. In R. Gaugler and H. K. Kaya (ed.), Entomopathogenic nematodes in biological control. CRC Press, Boca Raton, Fla.
- 21.Poinar, G. O., Jr., and R. Leutenegger. 1968. Anatomy of the infective and normal third-stage juvenile of Neoaplectana carpocapsae Weiser (Steinernematidae: Nematoda). J. Parasitol. 54:340-350. [PubMed] [Google Scholar]
- 22.Poinar, G. O., Jr., and G. M. Thomas. 1966. Significance of Achromobacter nematophilus Poinar and Thomas (Achromobacteraceae: Eubacteriales) in the development of the nematode, DD-136 (Neoaplectana sp. Steinernematidae). Parasitology 56:385-390. [DOI] [PubMed] [Google Scholar]
- 23.Popiel, I., D. L. Grove, and M. J. Friedman. 1989. Infective juvenile formation in the insect parasitic nematode Steinernema feltiae. Parasitology 99:77-81. [Google Scholar]
- 24.Rainey, P. B., D. M. Heithoff, and M. J. Mahan. 1997. Single-step conjugative cloning of bacterial gene fusions involved in microbe-host interactions. Mol. Gen. Genet. 256:84-87. [DOI] [PubMed] [Google Scholar]
- 25.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Biotechnology 1:784-791. [Google Scholar]
- 26.Visick, K. L., and M. J. McFall-Ngai. 2000. An exclusive contract: specificity in the Vibrio fischeri-Euprymna scolopes partnership. J. Bacteriol. 182:1779-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vivas, E. I., and H. Goodrich-Blair. 2001. Xenorhabdus nematophilus as a model for host-bacterium interactions: rpoS is necessary for mutualism with nematodes. J. Bacteriol. 183:4687-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volgyi, A., A. Fodor, A. Szentirmai, and S. Forst. 1998. Phase variation in Xenorhabdus nematophilus. Appl. Environ. Microbiol. 64:1188-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Way, J. C., M. A. Davis, D. Morisato, D. E. Roberts, and N. Kleckner. 1984. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene 32:369-379. [DOI] [PubMed] [Google Scholar]
- 30.Woodring, J. L., and H. K. Kaya. 1988. Steinernematid and Heterorhabditid nematodes: a handbook of biology and techniques. Southern Cooperative Series Bulletin 331. Arkansas Agricultural Experiment Station, Fayetteville, Ark.
- 31.Wouts, W. M. 1984. Nematode parasites of Lepidopterans, p. 655-696. In W. R. Nickle (ed.), Plant and insect nematodes. Marcel Dekker, New York, N.Y.
- 32.Xu, J., and R. E. Hurlbert. 1990. Toxicity of irradiated media for Xenorhabdus spp. Appl. Environ. Microbiol. 56:815-818. [DOI] [PMC free article] [PubMed] [Google Scholar]