Abstract
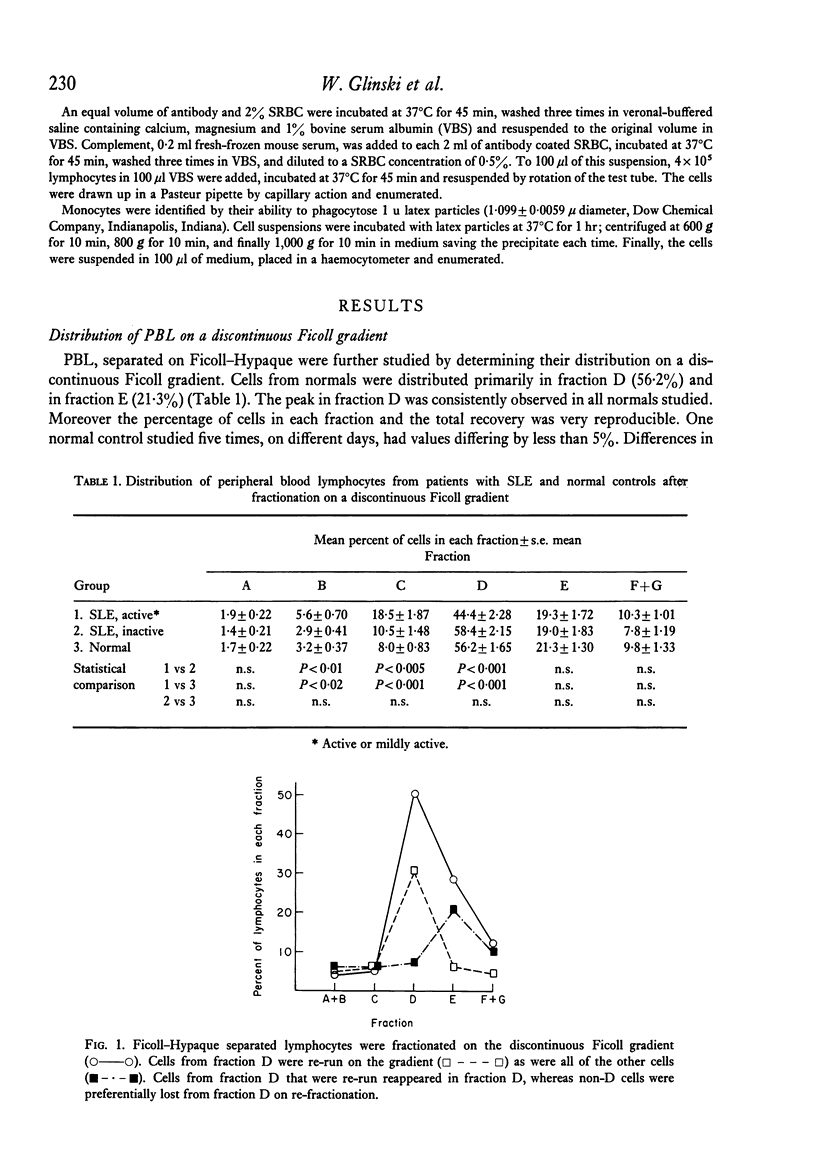
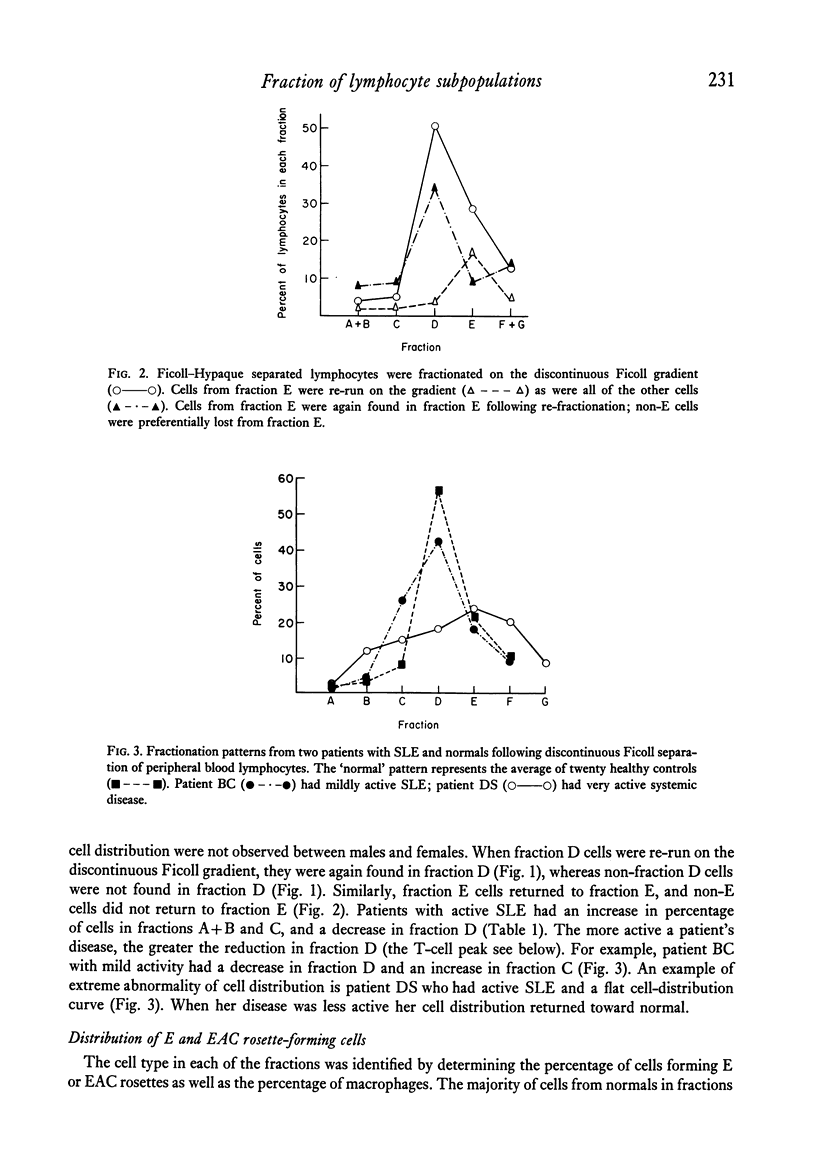
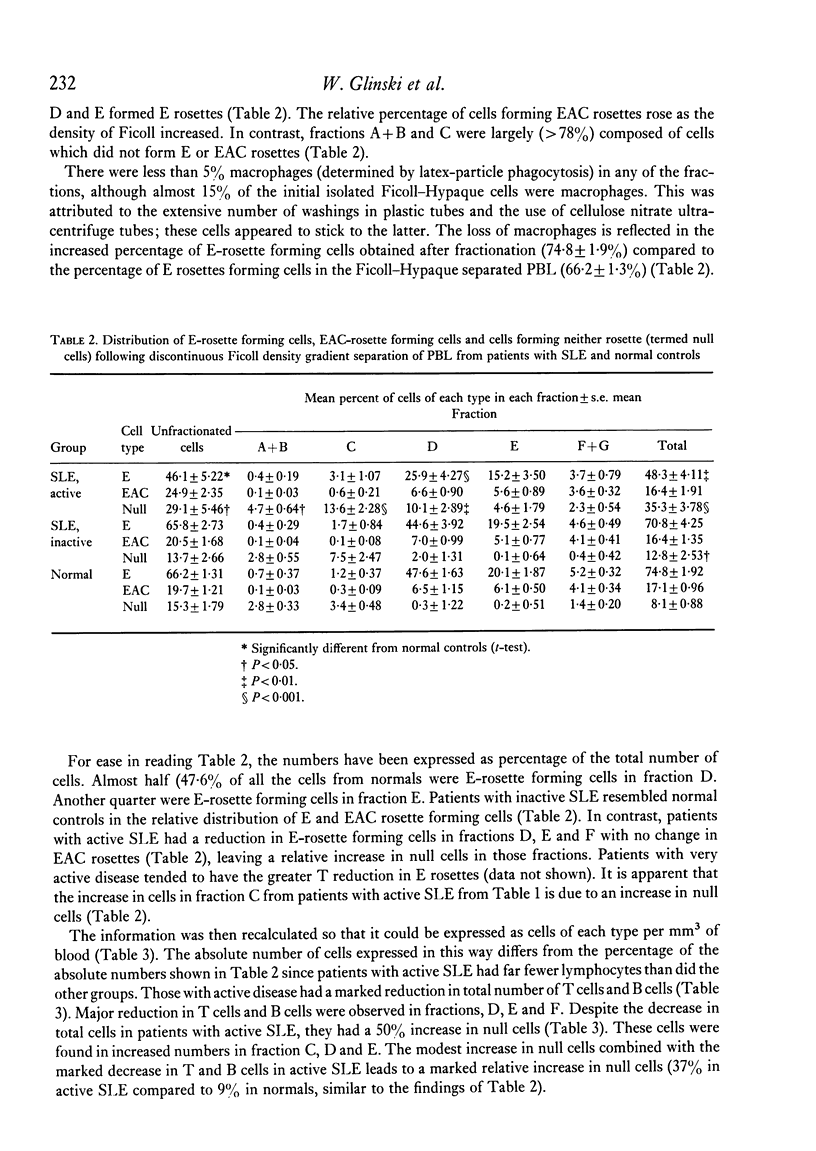
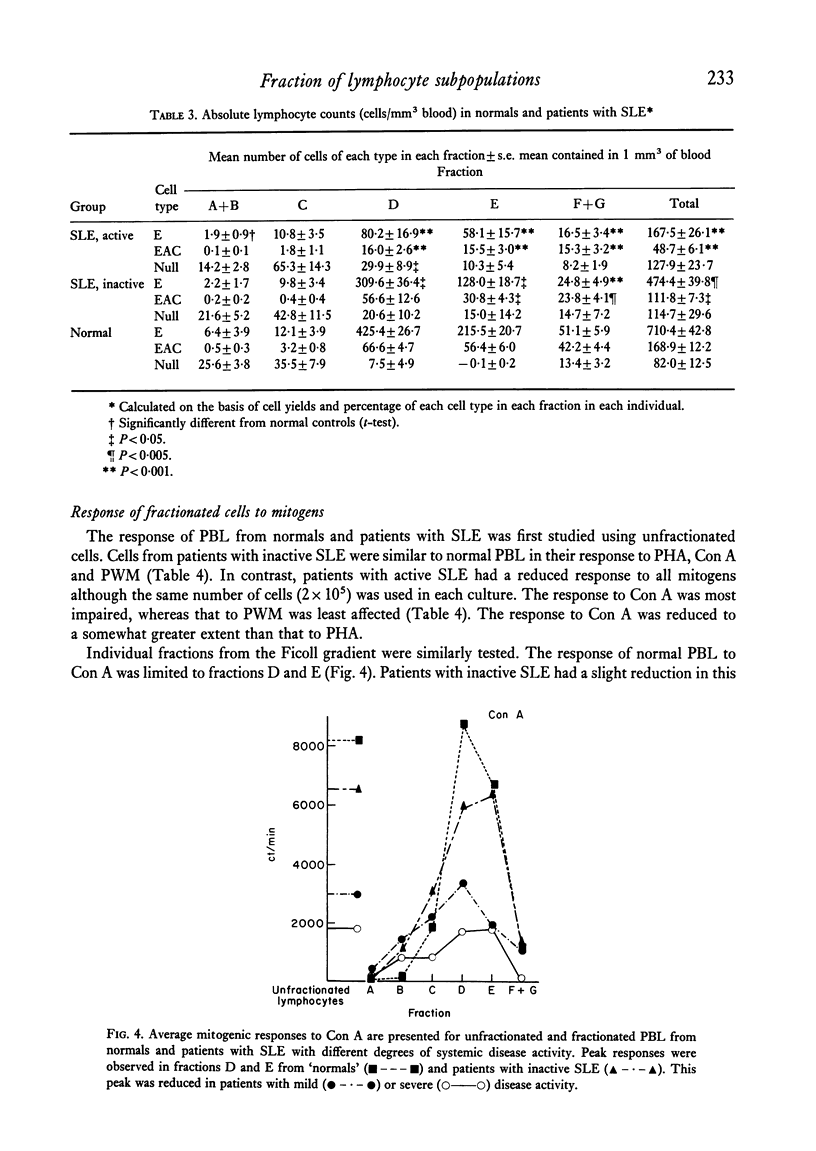
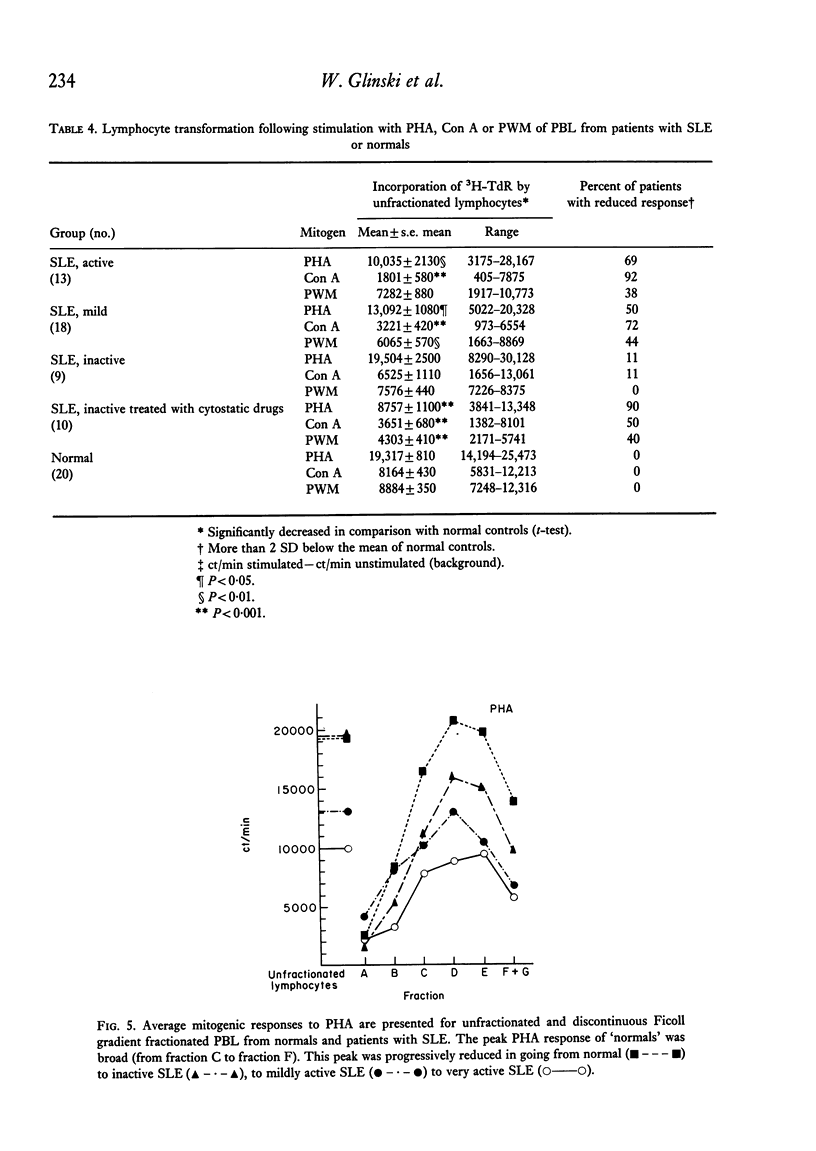
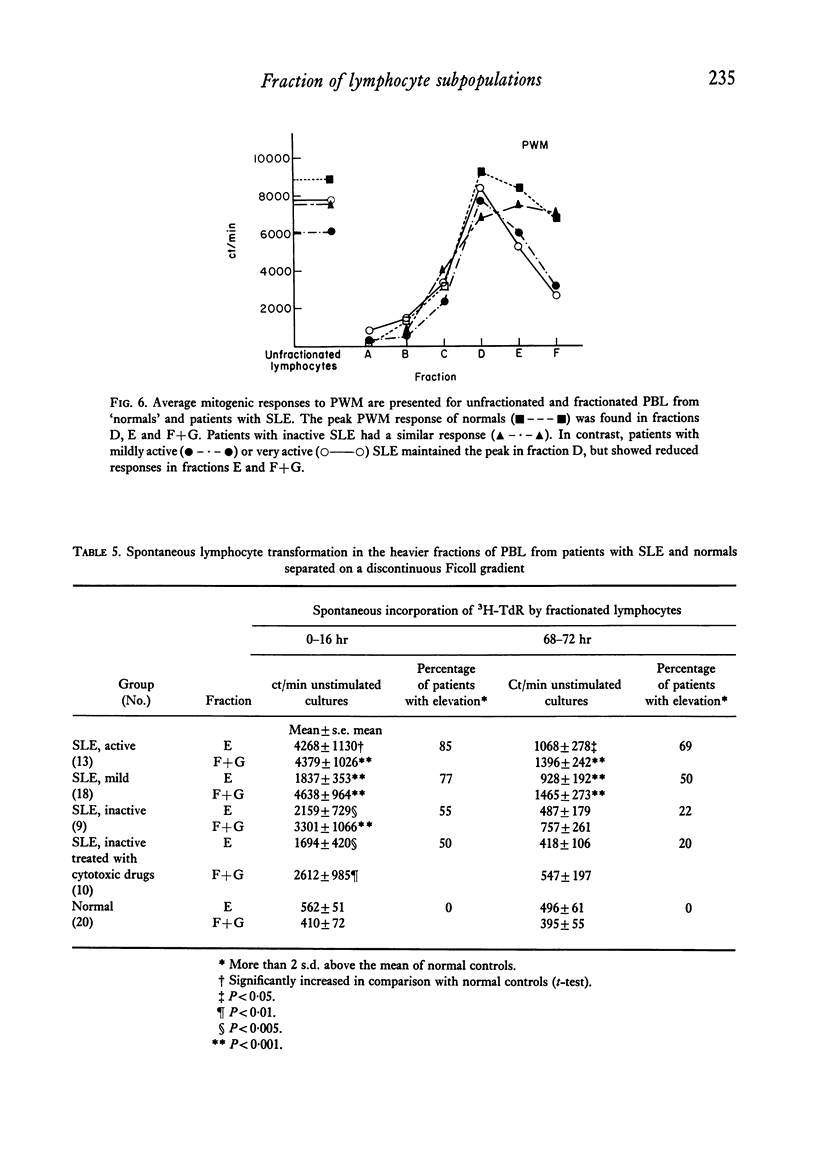
Peripheral blood lymphocytes of thirty normal volunteers and fifty-two patients with systemic lupus erythematosus were fractionated using a discontinuous (5-30 percent) Ficoll gradient. Such fractionation permitted the isolation, identification of study of null cells, T cells and B cells. Patients with inactive SLE were found to have a cell distribution and responsiveness to PHA, Con A and Pokeweed mitogen (PWM) similar to controls. In contrast, patients with active SLE showed a significant decrease in T-cell fractions as well as a relative increase in null cells, a normal distribution of B cells, a marked reduction in responsiveness to Con A, a lesser reduction to PHA and only a minor reduction to PWM. With increasing disease activity, the number of null cells increased despite lymphopenia. Spontaneous lymphocyte transformation was observed in patients with SLE. This occurred predominantly in the fractions enriched in B cells and was observed both early (0-16 hr) and late (68-72 hr) in the lymphocyte cultures. The method of discontinuous Ficoll gradients is both versatile and reproducible with good correlations between isolated lymphoid subpopulations and disease activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- August C. S., Merler E., Lucas D. O., Janeway C. A. The response in vitro of human lymphocytes to phytohemagglutinin and to antigens after fractionation on discontinuous density gradients of albumin. Cell Immunol. 1970 Dec;1(6):603–618. doi: 10.1016/0008-8749(70)90026-2. [DOI] [PubMed] [Google Scholar]
- Bach M. K., Brashler J. R. Isolation of subpopulations of lymphocytic cells by the use of isotonically balanced solutions of Ficoll. I. Development of methods and demonstration of the existence of a large but finite number of subpopulations. Exp Cell Res. 1970 Aug;61(2):387–396. doi: 10.1016/0014-4827(70)90462-3. [DOI] [PubMed] [Google Scholar]
- COOPER I. A., FIRKIN B. G. DEOXYRIBONUCLEIC ACID SYNTHESIZING CELLS IN THE PERIPHERAL BLOOD OF PATIENTS WITH "AUTO-IMMUNE" DISORDERS. Australas Ann Med. 1965 May;14:142–145. doi: 10.1111/imj.1965.14.2.142. [DOI] [PubMed] [Google Scholar]
- Decker J. L., Steinberg A. D., Gershwin M. E., Seaman W. E., Klippel J. H., Plotz P. H., Paget S. A. Systemic lupus erythematosus. Contrasts and comparisons. Ann Intern Med. 1975 Mar;82(3):391–404. doi: 10.7326/0003-4819-82-3-391. [DOI] [PubMed] [Google Scholar]
- Garner G. M., Gershwin M. E., Steinberg A. D. Properties of fractionated spleen cells from NZB/W mice. Cell Immunol. 1975 Jan;15(1):129–142. doi: 10.1016/0008-8749(75)90170-7. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Rosen F. S., Merler E. Identification and characterization of subpopulations of lymphocytes in human peripheral blood after fractionation on discontinuous gradients of albumin. The cellular defect in X-linked agammaglobulinemia. J Clin Invest. 1973 Jul;52(7):1726–1734. doi: 10.1172/JCI107354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershwin M. E., Steinberg A. D. Qualitative characteristics of anti-DNA antibodies in lupus nephritis. Arthritis Rheum. 1974 Nov-Dec;17(6):947–954. doi: 10.1002/art.1780170605. [DOI] [PubMed] [Google Scholar]
- Glinski W., Gershwin M. E., Steinberg A. D. Fractionation of cells on a discontinuous Ficoll gradient. Study of subpopulations of human T cells using anti-T-cell antibodies from patients with systemic lupus erythematosus. J Clin Invest. 1976 Mar;57(3):604–614. doi: 10.1172/JCI108316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J. A., Litwin A., Adams L. E., Krueger R. C., Hess E. V. Cellular immunity to nuclear antigens in systemic lupus erythematosus. J Clin Invest. 1972 Oct;51(10):2669–2677. doi: 10.1172/JCI107085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lies R. B., Messner R. P., Williams R. C., Jr Relative T-cell specificity of lymphocytotoxins from patients with systemic lupus erythematosus. Arthritis Rheum. 1973 May-Jun;16(3):369–375. doi: 10.1002/art.1780160312. [DOI] [PubMed] [Google Scholar]
- Messner R. P., Kennedy M. S., Jelinek J. G. Antilymphocyte antibodies in systemic lupus erythematosus. Effect on lymphocyte surface characteristics. Arthritis Rheum. 1975 May-Jun;18(3):201–206. doi: 10.1002/art.1780180302. [DOI] [PubMed] [Google Scholar]
- Messner R. P., Lindström F. D., Williams R. C., Jr Peripheral blood lymphocyte cell surface markers during the course of systemic lupus erythematosus. J Clin Invest. 1973 Dec;52(12):3046–3056. doi: 10.1172/JCI107503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal C. J., Franklin E. C. Depression of cellular-mediated immunity in systemic lupus erythematosus. relation to disease activity. Arthritis Rheum. 1975 May-Jun;18(3):207–217. doi: 10.1002/art.1780180303. [DOI] [PubMed] [Google Scholar]
- Scheinberg M. A., Cathcart E. S. B cell and T cell lymphopenia in systemic lupus erythematosus. Cell Immunol. 1974 May;12(2):309–314. doi: 10.1016/0008-8749(74)90083-5. [DOI] [PubMed] [Google Scholar]
- Senyk G., Hadley W. K., Attias M. R., Talal N. Cellular immunity in systemic lupus erythematosus. Arthritis Rheum. 1974 Sep-Oct;17(5):553–562. doi: 10.1002/art.1780170509. [DOI] [PubMed] [Google Scholar]
- Steinberg A. D., Decker J. L. A double-blind controlled trial comparing cyclophosphamide, azathioprine and placebo in the treatment of lupus glomerulonephritis. Arthritis Rheum. 1974 Nov-Dec;17(6):923–937. doi: 10.1002/art.1780170602. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Talal N., Paul W. E. Lymphocyte classes in New Zealand mice. II. Decreased frequency of immunoglobulin-bearing lymphocytes and increased frequency of lymphocytes lacking detectable theta or immunoglobulin determinants. J Immunol. 1972 Oct;109(4):701–710. [PubMed] [Google Scholar]
- Stutman O. Lymphocyte subpopulations in NZB mice: deficit of thymus-dependent lymphocytes. J Immunol. 1972 Sep;109(3):602–611. [PubMed] [Google Scholar]
- Suciu-Foca N., Buda J. A., Thiem T., Reemtsma K. Impaired responsiveness of lymphocytes in patients with systemic lupus erythematosus. Clin Exp Immunol. 1974 Nov;18(3):295–301. [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Winfield J. B., Siegal F., Wernet P., Bentwich Z., Kunkel H. G. Analyses of lymphocytes from patients with rheumatoid arthritis and systemic lupus erythematosus. Occurrence of interfering cold-reactive antilymphocyte antibodies. J Clin Invest. 1974 Nov;54(5):1082–1092. doi: 10.1172/JCI107852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D. T., Peter B. J., Paulus H. E., Machleder H. I. Lymphocyte populations: separation by discontinuous density gradient centrifugation. J Immunol. 1973 Jun;110(6):1615–1622. [PubMed] [Google Scholar]
- Yu D. T., Peter J. B., Stratton J. A., Paulus H. E., Machleder H. I. Lymphocyte dynamics: change in density profiles and response to phytohemagglutinin of human lymphocytes during prolonged thoracic duct drainage. Clin Immunol Immunopathol. 1973 Jul;1(4):456–462. doi: 10.1016/0090-1229(73)90003-2. [DOI] [PubMed] [Google Scholar]


