Abstract
Bacillus subtilis was recently reported to synthesize unsaturated fatty acids (UFAs) with a double bond at positions Δ5, Δ7, and Δ9 (M. H. Weber, W. Klein, L. Muller, U. M. Niess, and M. A. Marahiel, Mol. Microbiol. 39:1321-1329, 2001). Since this finding would have considerable importance in the double-bond positional specificity displayed by the B. subtilis acyl lipid desaturase, we have attempted to confirm this observation. We report that the double bond of UFAs synthesized by B. subtilis is located exclusively at the Δ5 position, regardless of the growth temperature and the length chain of the fatty acids.
Fatty acid desaturases are nonheme, iron-containing, oxygen-dependent enzymes involved in regioselective introduction of double bonds in fatty acyl aliphatic chains (12). They are present in all groups of organisms, i.e., bacteria, fungi, plants, and animals, and they play a key role in the maintenance of the proper structure and functioning of biological membranes. Two general classes of fatty acid desaturases have been identified. (i) The acyl carrier protein (ACP) desaturases, which uses acyl-ACPs as substrates, are soluble plant enzymes and plastid-localized (15); (ii) the membrane-bound desaturases are found in a wide range of taxa and use acyl-CoA or acyl lipid substrates (13-15). They show different consensus motifs (15). The soluble class contains a consensus motif consisting of carboxylate and histidine ligands that coordinate an active site di-iron cluster, as revealed by the X-ray structure of the castor Δ9 stearoyl-ACP desaturase (11). The integral membrane class contains a different consensus motif for the putative active site composed of three histidine-rich regions, which are presumably involved in iron binding (14). Unfortunately, information about substrate specificities, regioselectivities, and structure of membrane desaturases is scarce due to the technical limitations in obtaining large quantities of active forms of such enzymes.
In a previous work, it was reported that the Bacillus subtilis des gene codes for a unique desaturase, Des (1). Transcription of the des gene is tightly controlled by a two-component regulatory system composed of a membrane-associated kinase, DesK, and a soluble transcriptional regulator, DesR (3). Activation of this pathway takes place when cells are shifted to low growth temperatures (2, 6). The B. subtilis desaturase is a polytopic membrane protein with six transmembrane domains and one membrane-associated domain, which likely represents a substrate-binding motif (7). This desaturase, which contains a tripartite motif of His essential for catalysis, is an acyl lipid desaturase, since it is able to introduce a double bond into the acyl chain of membrane glycerolipids (1, 6). It was previously shown that expression of the des gene in Escherichia coli resulted in desaturation of the palmitic moieties of the membrane phospholipids to give cis-5-hexadecenoic acid, indicating that the B. subtilis desaturase is a Δ5 acyl lipid desaturase (1). However, Weber et al. (17) recently reported that four unsaturated fatty acid (UFA) species differing in length, branching pattern, and position of the double bond were synthesized in B. subtilis JH642. Under the same conditions, none of these fatty acids species was detected in a B. subtilis isogenic strain possessing a des null mutation, showing that their production is strictly dependent on the B. subtilis desaturase (10). On the basis of these data, Weber et al. (17) questioned whether the B. subtilis desaturase was a Δ5 desaturase and concluded that this enzyme was able to introduce double bonds into different positions (Δ5, Δ7, or Δ9) of fatty acids of chain lengths of 16 or 17 carbon atoms. Since these data raised the possibility that Des could have different specificity to introduce the double bond if it is expressed either in a B. subtilis (17) or in an E. coli (1) host, we reevaluated the structure of the UFAs synthesized by B. subtilis by using two different complementary techniques.
The B. subtilis strains used in this study were trpC2 phe-1 derivatives of JH642. Strain LC5, containing a kanamycin resistance gene cassette in the des gene, was constructed as described previously (3). Strain AKP20 overproduces the transcriptional activator DesR, resulting in constitutive expression of the desaturase gene (3). This strain overproduced UFAs compared with the wild-type strain JH642 (Table 1), greatly facilitating the determination of the double bond position of these fatty acids by gas chromatography-mass spectrometry (GC-MS) analysis.
TABLE 1.
Fatty acid composition of total membrane lipid extracts from B. subtilis strains JH642 and AKP20a
| Fatty acid(s) or ratio | % of total fatty acids for indicated strain and temp (°C)
|
|||||
|---|---|---|---|---|---|---|
| JH642
|
AKP20
|
|||||
| 37 | 25 | 15 | 37 | 25 | 15 | |
| Iso-C13:0 | 0.11 | 0.109 | 0.044 | 0.10 | 0.11 | 0.08 |
| Anteiso-C13:0 | 0.037 | 0.03 | 0.022 | 0.0357 | 0.033 | 0.02 |
| Iso-C14:0 | 2.176 | 2.01 | 2.98 | 1.797 | 1.53 | 2.24 |
| n-C14:0 | 0.787 | 0.486 | 1.08 | 1.10 | 0.83 | 1.41 |
| Iso-C15:0 | 23.72 | 20.71 | 13.5 | 25.58 | 19.94 | 15.02 |
| Anteiso-C15:0 | 29.80 | 30.80 | 24.8 | 38.0 | 39.99 | 41.21 |
| n-C15:0 | 2.44 | 1.36 | 2.19 | 2.35 | 1.1 | 0.94 |
| Iso-C16:1 | 0.15 | 1.11 | 0.275 | 0.77 | 2.086 | 2.30 |
| Iso-C16:0 | 4.65 | 6.4 | 8.26 | 2.09 | 1.14 | 0.88 |
| n-C16:1 | 1.69 | 3.45 | 1.036 | 6.88 | 9.06 | 12.60 |
| n-C16:0 | 10.17 | 7.85 | 21.88 | 5.25 | 2.04 | 4.32 |
| Iso-C17:1 | 0.47 | 2.7 | 0.456 | 3.03 | 7.19 | 5.19 |
| Anteiso-C17:1 | 0.09 | 0.655 | 0.117 | 0.96 | 3.94 | 4.44 |
| Iso-C17:0 | 14.6 | 12.05 | 10.66 | 5.16 | 2.58 | 2.59 |
| n-C17:1 | 0 | 0 | 0 | 1.23 | 1.93 | 0.66 |
| Anteiso-C17:0 | 7.10 | 8.8 | 6.96 | 4.49 | 5.30 | 4.58 |
| n-C17:0 | 1.06 | 0.52 | 2.13 | 0.59 | 0.36 | 0.35 |
| Iso-C18:0 | 0.060 | 0.14 | 0.022 | 0 | 0.44 | 0.36 |
| Iso-C18:1 0 | 0.065 | 0.18 | 0.19 | 0.005 | 0.021 | 0 |
| n-C18:0 | 0.82 | 0.82 | 3.38 | 0.53 | 0.38 | 0.81 |
| Iso/anteisob | 1.537 | 1.127 | 1.17 | 0.885 | 0.711 | 0.57 |
| UFAs | 2.465 | 8.1 | 2.074 | 12.875 | 24.227 | 25.19 |
Cells were grown in Spizizen minimal medium supplemented with glucose to exponential phase at 37°C and then shifted to 25 or 15°C. Total lipids were extracted and transesterified to yield fatty acids methylesters, and products were identified by GC-MS. Values are the means of results of three experiments and are expressed as percentage of total fatty acids.
Ratio of iso-branched fatty acids to anteiso fatty acids.
To determine the fatty acid composition, cells were grown at 37°C in Spizizen minimal salts (16) medium supplemented with 0.2% glucose and 50 μg each of tryptophan and phenylalanine/ml to exponential phase at 37°C and then shifted to 25 or 15°C. The cultures were harvested in stationary phase. Total cellular fatty acids were prepared by the method of Bligh and Dyer (4). The fatty acid methyl esters were prepared by transesterification of glycerolipids with 0.5 M of sodium methoxide in methanol (5) and then analyzed in a Perkin-Elmer Turbo Mass gas chromatography-mass spectrometer on a capillary column (30 mm by 0.25 mm in diameter) of 100% dimethylpolysiloxane (PE-1, Perkin-Elmer). Helium at 1 ml min−1 was used as the carrier gas, and the column temperature was programmed to rise by 4°C min−1 from 140°C to 240°C. Branched-chain fatty acids, straight-chain fatty acids, and UFAs used as reference compounds were obtained from Sigma Chemical Co.
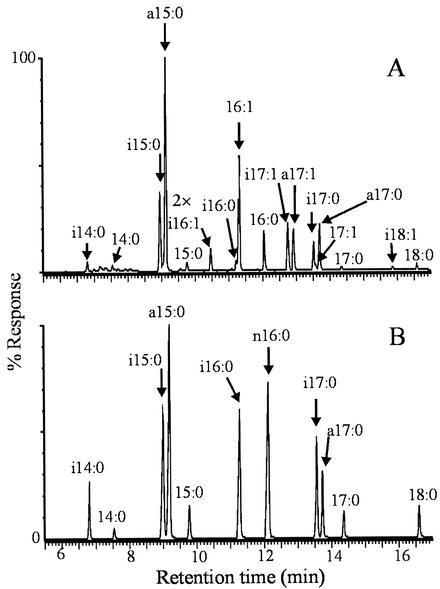
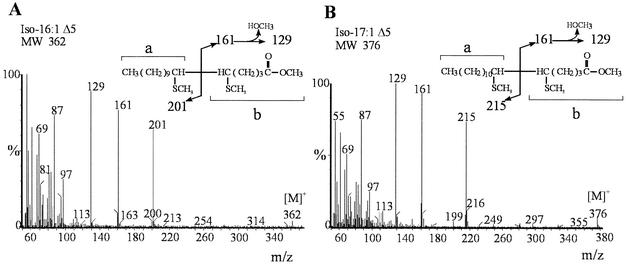
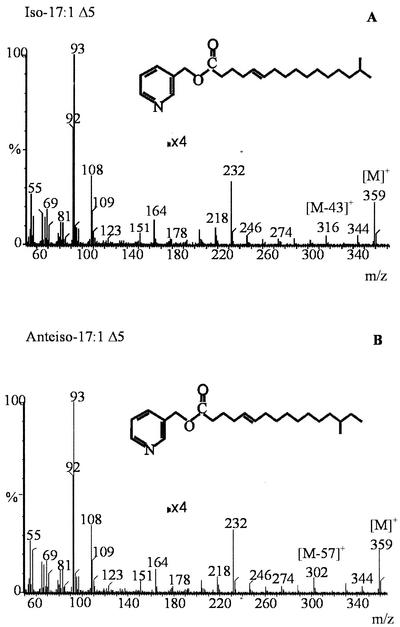
As shown in Fig. 1A and Table 1, in addition to the major branched-chain fatty acids described in B. subtilis (9), six different UFA products differing in length and branching pattern were identified at all tested temperatures in strains JH642 and AKP20. The positions of the double bonds in these UFAs were determined by GC-MS. Fatty acid methyl esters were converted to dimethyl disulfide (DMDS) adducts, as described previously (18), and then separated on a PE-1 column ramped from 140 to 280°C at 4°C min−1. The spectra were recorded in the electron impact mode at 70 eV, using 1-s scans of the m/z 40-400. The addition of DMDS to the mixture displaces the peak of the monounsaturated components to longer retention times. In Fig. 2A and 2B are illustrated examples of the electron impact mass spectrum of the DMDS adducts of iso-C16:1 and iso-C17:1 fatty acids. The mass spectrum of the adducts shows weak ions [M]+ at m/z 362 (Fig. 2A) and m/z 376 (Fig. 2B), corresponding to the theoretical mass of molecular ions of the DMDS adducts of C16 and C17 monosaturated fatty acids, respectively. Two prominent ions are formed by cleavage between the methylthio-substituted (CH3S) carbons located at the original site of the unsaturation. The strong ions at m/z 161 and m/z 201 indicate the position of the double bond at Δ5 in the iso-C16:1 fatty acid (Fig. 2A). The ions observed at m/z 161 and m/z 215 indicate a Δ5 double bond in the iso-C17:1 fatty acid (Fig. 2B). A major ion at m/z 129 is due to loss of methanol (CH3OH) from ion b, as shown in Fig. 2A and 2B. The fragmentation pattern obtained for the DMDS adducts of n-C16:1, anteiso-C17:1, n-C17:1, and iso-C18:1 also established the location of the double bond at the Δ5 position (data not shown). These results were confirmed by analyzing the GC-MS spectrum of the picolynil ester derivatives of UFAs. An additional advantage of this technique is that the spectra of 3-picolinyl esters contained information about the position of the methyl branching in these UFAs. 3-Picolinyl esters were prepared from unesterified fatty acids as described previously (8) and then examined by GC-MS in a PE-1 column programmed at 2.5°C min−1 from 180 to 240°C. Figure 3 shows an example of the mass spectra of the picolinyl esters of iso-C17:1 and anteiso-C17:1 fatty acids. These spectra show prominent ions at m/z 92, 108, 151, and 164 characteristic of picolinyl esters (8) and a less abundant [M]+ at 359 m/z (Fig. 3). The cleavage adjacent to the branch point resulted in the absence of the ions at [M-29]+ (Fig. 3A) and [M-43]+ (Fig. 3B), indicating the loss of a substituted carbon fragment. The enhanced abundance of the ion of 14 atomic mass units (amu) below these diagnostic ions, [M-43]+ (Fig. 3A) and [M-57]+ (Fig. 3B) also indicates the presence of a methyl branching at the ω-1 (iso-) or ω-2 (anteiso-) positions of these fatty acids. The picolinyl derivatives from iso-C17:1 (Fig. 3A) and anteiso-C17:1 (Fig. 3B) fatty acids showed a gap of 26 amu (204 to 178), a doublet with a prominent m/z 232, and a less prominent m/z 218, indicating that these UFAs have the double bond at a Δ5 position. Similar results were obtained with picolinyl derivatives of the other four UFAs present in B. subtilis (data not shown). Our assignment of the double bond position of the UFAs synthesized by B. subtilis JH642 does not agree with the UFAs structures reported by Weber et al. (17). Our most straightforward interpretation of this difference is that Weber et al. (17) deduced the double bond position of the B. subtilis UFAs by comparing the fatty acid profiles obtained by gas chromatographic analysis with a microbial identification library generation software (Microbial ID). Here, we located the double bond position of the UFAs by GC-MS, which is one of the most powerful tools used for lipid analysis (5). None of the UFAs synthesized by strain JH642 were found in the isogenic des strain LC5, as shown in Fig. 1B, confirming that Des is the sole desaturase present in B. subtilis and that this enzyme has exclusively Δ5 activity.
FIG. 1.
Chromatogram of fatty acids synthesized by strains AKP20 (A) and LC5 (B) at 25°C. The peaks corresponding to the identified fatty acids are indicated by arrows. i, iso-branched chain fatty acids; a, anteiso-branched chain fatty acids. The peaks of the chromatogram shown in panel A and located at the right of the 2× label were twofold magnified.
FIG. 2.
Mass spectrum of dimethyl disulfide derivatives separated by GC of iso-C16:1 (A) and iso-C17:1 (B). The aliphatic fragments a and b containing the carboxyl group are indicated by braces. [M]+, molecular ion.
FIG. 3.
Mass spectra of 3-picolinyl esters. [M]+, molecular ion. The peaks of the mass spectra located at the right of the 4× label were fourfold magnified. The fragments at intervals of 43 and 57 amu ([M-43]+ and [M-57]+, respectively) are indicated. Details of interpretation of the spectrum are given in the text.
In order to gain some insight into the substrate requirements of the B. subtilis desaturase in a qualitative sense, a range of possible fatty acids substrates were supplied to the E. coli strain AK7/pDM10 (1). This strain carries a fabB null mutation that is functionally complemented by the B. subtilis des gene carried by plasmid pDM10 (1). In conjunction with this, analysis of the desaturation products was undertaken to determine the regioselectiviy of the enzyme. Cultures of AK7/pDM10 cells grown to exponential phase at 30°C on Luria-Bertani medium were supplemented with 50 μM of the straight-chain fatty acids tetradecanoic acid (C14:0), pentadecanoic acid (C15:0), and octadecanoic acid (C18:0) and the branched-chain fatty acids 14-methyl pentadecanoic acid (iso-C16:0), 15-methylhexadecanoic acid (iso-C17:0), and 14-methylhexadecanoic acid (anteiso-C17:0). The positions of the double bond of the UFA products derived from the exogenous supplemented fatty acids were determined by GC-MS of the corresponding fatty-acyl DMDS. The results of these experiments demonstrated the position of the double bond to be the Δ5 position in all tested substrates (data not shown). Thus, we conclude that the B. subtilis desaturase catalyzes the introduction of a cis-double bond at the Δ5 position of a wide range of saturated fatty acids, and we have named this protein Δ5-Des.
Acknowledgments
We are grateful to Monica Hourcade for technical support and to Antonio Uttaro for critically reading the manuscript.
This work was supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Agencia Nacional de Promoción Científica y Tecnológica (FONCYT). P. Aguilar was a fellow from CONICET, and D. de Mendoza is a Career Investigator from CONICET and an International Research Scholar from Howard Hughes Medical Institute.
REFERENCES
- 1.Aguilar, P. S., J. E. Cronan, Jr., and D. de Mendoza. 1998. A Bacillus subtilis gene induced by cold shock encodes a membrane phospholipid desaturase. J. Bacteriol. 180:2194-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar, P. S., P. Lopez, and D. de Mendoza. 1999. Transcriptional control of the low-temperature-inducible des gene encoding the Δ5 desaturase of Bacillus subtilis. J. Bacteriol. 181:7028-7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguilar, P. S., A. M. Hernandez-Arriaga, L. E. Cybulsky, A. C. Erazo, and D. de Mendoza. 2001. Molecular basis of thermosensing: a two component transduction thermometer in Bacillus subtilis. EMBO J. 20:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 31:911-917. [DOI] [PubMed] [Google Scholar]
- 5.Christie, W. 1989. Gas chromatography and lipids. The Oily Press, Ayr, Scotland.
- 6.de Mendoza, D., G. Schujman, and P. S. Aguilar. 2002. Biosynthesis and function of membrane lipids, p. 43-55. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its relatives: from genes to cells. ASM Press, Washington D.C.
- 7.Diaz, A. R., M. C. Mansilla, A. J. Vila, and D. de Mendoza. 2002. Membrane topology of the acyl-lipid desaturase from Bacillus subtilis. J. Biol. Chem. 277:48099-48106. [DOI] [PubMed] [Google Scholar]
- 8.Harvey, D. J. 1982. Picolinyl esters as derivatives for the structural determination of long chain branched and unsaturated fatty acids. Biomed. Mass Spectrom. 9:33-38. [Google Scholar]
- 9.Kaneda, T. 1991. Iso- and anteiso- fatty acids in bacteria: biosynthesis, function and taxonomic significance. Microbiol. Rev. 55:288-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein, W., M. H. W. Weber, and M. A. Marahiel. 1999. Cold shock response of Bacillus subtilis: isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 181:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindquist, Y., W. Huang, G. Schneider, and J. Shanklin. 1996. Crystal structure of delta9 stearoyl-acyl carrier protein desaturase from castor seed and its relationship to other di-iron proteins. EMBO J. 15:4081-4092. [PMC free article] [PubMed] [Google Scholar]
- 12.Meesapydsuk, D., D. W. Reed, C. H. Savile, P. Buist, S. Ambrose, and P. S. Covello. 2000. Characterization of the regiochemistry and cryptoregiochemistry of a Caenorhabditis elegans fatty acid desaturase (FAT-1) expressed in Saccharomyces cerevisiae. Biochemistry 39:11948-11954. [DOI] [PubMed] [Google Scholar]
- 13.Murata, N., and H. Wada. 1995. Acyl-lipid desaturases and their importance in the tolerance and acclimation to cold of cyanobacteria. Biochem. J. 308:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shanklin, J., E. Whittle, and B. G. Fox. 1994. Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry 33:12787-12794. [DOI] [PubMed] [Google Scholar]
- 15.Shanklin, J., and E. B. Cahoon. 1998. Desaturation and related modifications of fatty acids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49:611-641. [DOI] [PubMed] [Google Scholar]
- 16.Spizizen, J. 1958. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc. Natl. Acad. Sci. USA 44:1072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber, M. H. W., W. Klein, L. Muller, U. M. Niess, and M. A. Marahiel. 2001. Role of the Bacillus subtilis fatty acid desaturase in membrane adaptation during cold shock. Mol. Microbiol. 39:1321-1329. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto, K., A. Shibahara, T. Nakayama, and G. Kajimoto. 1991. Determination of double-bond positions in methylene-interrupted dienoic fatty acids by GC-MS as their dimethyl disulfide adducts. Chem. Phys. Lipids 60:39-50. [Google Scholar]