Abstract
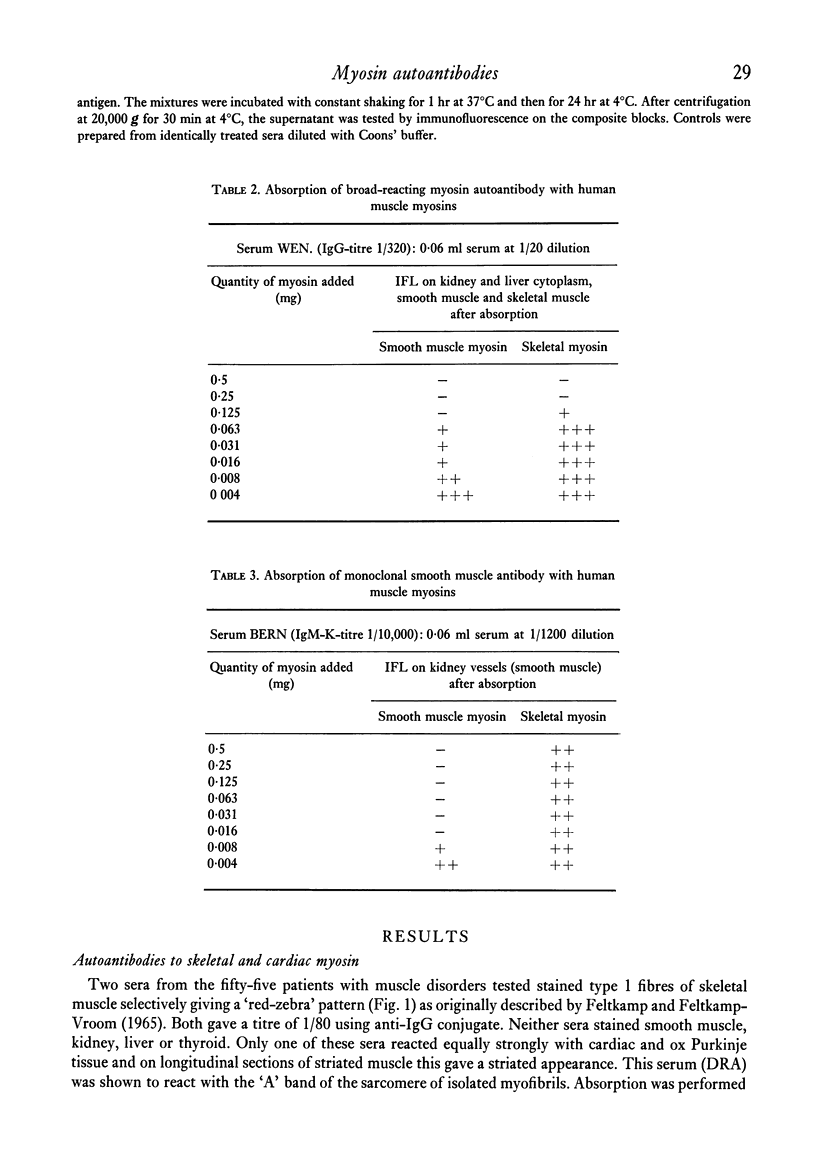
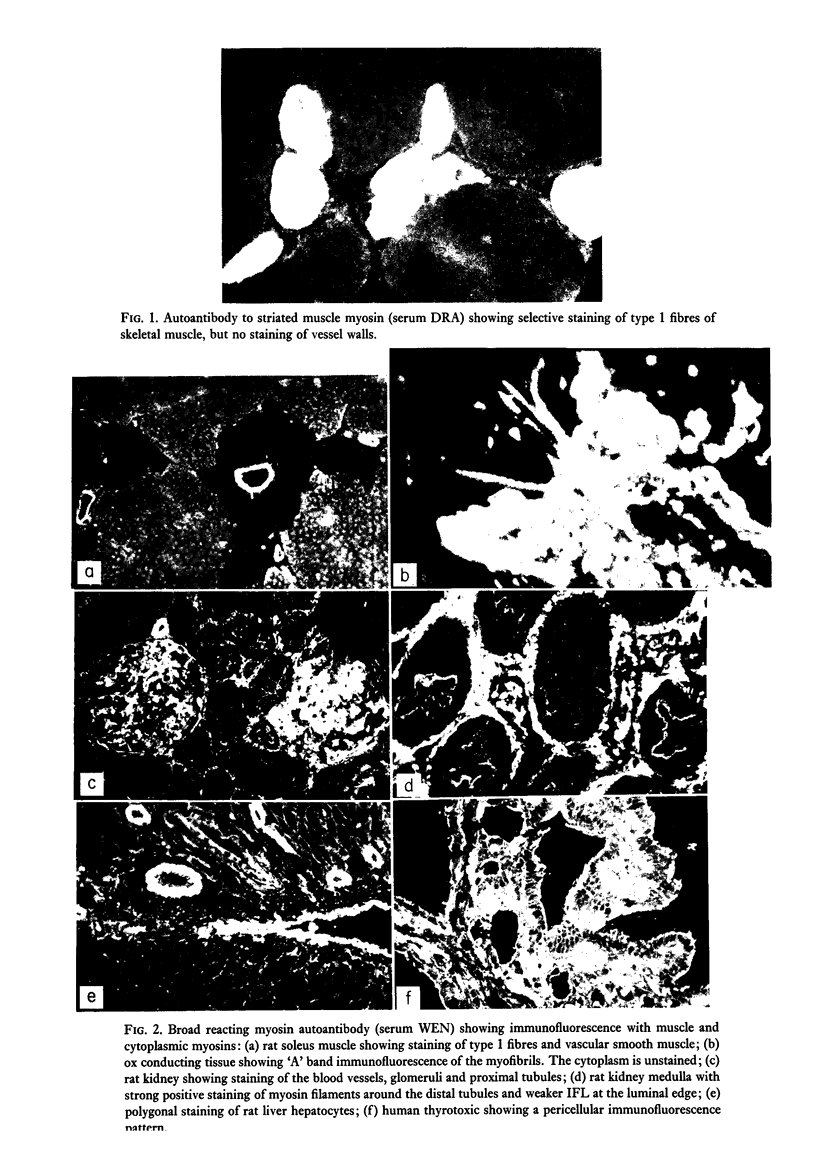
Autoantibodies to striated and smooth muscles myosins were detected by indirect immunofluorescence and confirmed by absorption with purified contractile proteins extracted from human, rabbit and chicken muscle. Myosin antibodies were rare: of fifty-five sera examined from patients with various skeletal and cardiac muscle disorders, only one serum, from a case of Coxsackie viral pericarditis, had anti-myosin activity. It reacted with cardiac muscle and type 1 fibres of skeletal muscle, staining the 'A' band of the sarcomere only. The antibody was absorbed by skeletal myosin and by skeletal heavy meromyosin fragments, but not by smooth muscle myosin. Two types of smooth muscle myosin autoantibodies are described. One is restricted to smooth muscle myosin and examples were found in polyclonal and monoclonal SMA sera. The second type of smooth muscle myosin antibody cross-reacted with skeletal and cardiac muscle and with cytoplasmic myosin in liver, kidney and thyroid cells. It was completely absorbed using either smooth or skeletal myosin and by heavy meromyosin fragments. The different types of myosin autoantibodies reflect the variety of myosins found in mammalian tissues. Cross-reacting myosin antibodies indicate epitopes on the heavy meromyosin fragment which are common to several different tissue myosins.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt I., Pepe F. A. Antigenic specificity of red and white muscle myosin. J Histochem Cytochem. 1975 Mar;23(3):159–168. doi: 10.1177/23.3.47867. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Florin-Christensen A., Fairfax A., Swana G., Doniach D., Groeschel-Stewart U. Classification of smooth muscle autoantibodies detected by immunofluorescence. J Clin Pathol. 1976 May;29(5):403–410. doi: 10.1136/jcp.29.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfax A., Doniach D. Autoantibodies to cardiac conducting tissue and their characterization by immunofluorescence. Clin Exp Immunol. 1976 Jan;23(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Feltkamp T. E., Feltkamp-Vroom T. M. Antibodies against the various types of skeletal muscle fibres. Immunology. 1965 Sep;9(3):275–279. [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Lamelin J. P., Vassalli P., Majno G., Bouvier C. A., Cruchaud A., Lüscher E. F. Human smooth muscle autoantibody. Its identification as antiactin antibody and a study of its binding to "nonmuscular" cells. Am J Pathol. 1973 Sep;72(3):473–488. [PMC free article] [PubMed] [Google Scholar]
- Gröschel-Stewart U., Chamley J. H., McConnell J. D., Burnstock G. Comparison of the reaction of cultured smooth and cardiac muscle cells and fibroblasts to specific antibodies to myosin. Histochemistry. 1975 Jun 5;43(3):215–224. doi: 10.1007/BF00499702. [DOI] [PubMed] [Google Scholar]
- Gröschel-Stewart U., Doniach D. Immunological evidence for human myosin isoenzymes. Immunology. 1969 Dec;17(6):991–994. [PMC free article] [PubMed] [Google Scholar]
- Gröschel-Stewart U., Schreiber J., Mahlmeister C. Production of specific antibodies to contractile proteins, and their use in immunofluorescence microscopy. I. Antibodies to smooth and striated chicken muscle myosins. Histochemistry. 1976 Feb 26;46(3):229–236. doi: 10.1007/BF02462786. [DOI] [PubMed] [Google Scholar]
- Horváth B. Z., Gaetjens E. Immunochemical studies on the light chains from skeletal muscle myosin. Biochim Biophys Acta. 1972 May 18;263(3):779–793. doi: 10.1016/0005-2795(72)90062-1. [DOI] [PubMed] [Google Scholar]
- Huszar G., Elzinga M. Homologous methylated and nonmethylated histidine peptides in skeletal and cardiac myosins. J Biol Chem. 1972 Feb 10;247(3):745–753. [PubMed] [Google Scholar]
- Johnson G. D., Holborow E. J., Glynn L. E. Antibody to smooth muscle in patients with liver disease. Lancet. 1965 Oct 30;2(7418):878–879. doi: 10.1016/s0140-6736(65)92505-5. [DOI] [PubMed] [Google Scholar]
- Khaw B. A., Beller G. A., Haber E., Smith T. W. Localization of cardiac myosin-specific antibody in myocardial infarction. J Clin Invest. 1976 Aug;58(2):439–446. doi: 10.1172/JCI108488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidman K., Biberfeld G., Fagraeus A., Norberg R., Torstensson R., Utter G., Carlsson L., Luca J., Lindberg U. Anti-actin specificity of human smooth muscle antibodies in chronic active hepatitis. Clin Exp Immunol. 1976 May;24(2):266–272. [PMC free article] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Lowey S., Steiner L. A. An immunochemical approach to the structure of myosin and the thick filament. J Mol Biol. 1972 Mar 14;65(1):111–126. doi: 10.1016/0022-2836(72)90495-0. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Substructure of the myosin molecule. 3. Preparation of single-headed derivatives of myosin. J Mol Biol. 1973 Mar 5;74(3):301–311. doi: 10.1016/0022-2836(73)90375-6. [DOI] [PubMed] [Google Scholar]
- Offer G., Moos C., Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973 Mar 15;74(4):653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955 May;3(3):170–195. doi: 10.1177/3.3.170. [DOI] [PubMed] [Google Scholar]
- Pepe F. A. Some aspects of the structural organization of the myofibril as revealed by antibody--staining methods. J Cell Biol. 1966 Mar;28(3):505–525. doi: 10.1083/jcb.28.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Richards E. G., Chung C. S., Menzel D. B., Olcott H. S. Chromatography of myosin on diethylaminoethyl-Sephadex A-50. Biochemistry. 1967 Feb;6(2):528–540. doi: 10.1021/bi00854a022. [DOI] [PubMed] [Google Scholar]
- Rome E. Light and X-ray diffraction studies of the filament lattice of glycerol-extracted rabbit psoas muscle. J Mol Biol. 1967 Aug 14;27(3):591–602. doi: 10.1016/0022-2836(67)90061-7. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Wager O., Räsänen J. A., Haltia K., Wasastjerna C. M components with antibody activity. Anti-smooth muscle, anti-thyroglobulin and anti-streptolysin-O activity in five M component sera. Ann Clin Res. 1971 Apr;3(2):86–97. [PubMed] [Google Scholar]
- Weeds A. G., Lowey S. Substructure of the myosin molecule. II. The light chains of myosin. J Mol Biol. 1971 Nov 14;61(3):701–725. doi: 10.1016/0022-2836(71)90074-x. [DOI] [PubMed] [Google Scholar]


